Abstract
This paper examined whether a two-year change in fitness, body mass index (BMI) or the additive effect of change in fitness and BMI were associated with change in cardiometabolic risk factors among youth. Cardiometabolic risk factors, BMI group (normal weight, overweight or obese) were obtained from participants at the start of 6th grade and end of 8th grade. Shuttle run laps were assessed and categorized in quintiles at both time points. Regression models were used to examine whether changes in obesity, fitness or the additive effect of change in BMI and fitness were associated with change in risk factors. There was strong evidence (p < .001) that change in BMI was associated with change in cardiometabolic risk factors. There was weaker evidence of a fitness effect, with some evidence that change in fitness was associated with change in total cholesterol, HDL-C, LDL-C and clustered risk score among boys, as well as HDL-C among girls. Male HDL-C was the only model for which there was some evidence of a BMI, fitness and additive BMI*fitness effect. Changing body mass is central to the reduction of youth cardiometabolic risk. Fitness effects were negligible once change in body mass had been taken into account.
Seventeen percent of US children in the 2009–2010 National Health and Nutrition Examination Survey (NHANES) were obese (23). Childhood obesity increases the risk of adult obesity (31) and increased body mass is associated with higher levels of a number of cardiometabolic risk factors such as elevated blood pressure, lipid and glucose levels among youth (10,14,15). Cardiometabolic risk factors track from childhood into adulthood (3,22), thereby indicating the importance of lowering youth cardiometabolic risk factors.
Higher levels of adult cardiorespiratory fitness (fitness) have been inversely associated with the risk of developing cardiovascular disease and type 2 diabetes (4,5). Interestingly, the protective effects of fitness were maintained irrespective of obesity status, with fit but overweight adults having a lower risk of CVD and diabetes than unfit and normal weight adults (21). A number of studies have shown that fitness and obesity status, as indicated by body mass index (BMI) are independently associated with cardiometabolic risk factors among both children and adolescents (8,9,12). It is not clear, however, whether there is an interaction between fitness and obesity status among children and adolescents. Cross-sectional analyses from the HEALTHY study showed that both obesity status and fitness were associated with the cardiometabolic risk factors in a large sample of 11–12 year old children (14). The cross-sectional design of those analyses precluded an examination of whether change in fitness, change in obesity status or the additive effect of change in both variables (which could be further explored in a subgroup analyses), offered the greatest protection against the development of cardiometabolic risk factors over time. As such, it is important to understand how change in fitness and body mass (or the additive interaction) is associated with change in each of the individual risk factors, which might suggest targeting interventions to important outcomes or conversely targeting the overall risk profile.
This paper examines whether change in fitness, change in obesity status (as indicated by BMI) or the additive effect of change in fitness and body mass were associated with change in cardiometabolic risk among youths who participated in the 2.5 year, multicomponent HEALTHY intervention.
Methods
Sample
Data are from the HEALTHY Study, a National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) cluster randomized controlled trial, that aimed to reduce the prevalence of risk factors for type 2 diabetes mellitus among middle school children (6,28). The study design and the analysis of primary outcomes have been reported elsewhere (6,28) as well as papers describing the absence of an intervention effect on cardiometabolic variables or individual lipid levels (16,32). Briefly, participants were recruited from 42 middle schools with 6 schools (3 intervention and 3 control) recruited from each of seven field centers that were spread across the USA. Participants were recruited from schools that had at least 50% of students eligible for free or reduced-price lunch or belonging to a minority group and an annual student attrition rate from all causes ≤ 25%. All 6th grade students were invited to participate in a ‘health screening’. At baseline students were given a $50 incentive for data collection with as many students as possible followed through 8th grade when a second ‘health screening’ was conducted with a $60 incentive given for this data collection. This study was approved by the Institutional Review Boards at each field center, and written informed parental consent and child assent were obtained.
Procedures
Height and body mass were measured without shoes using the Prospective Enterprises PE-AIM-101 stadiometer and the SECA Corporation Alpha 882 electronic scale. Body mass index (BMI) was calculated (kg/m2) and converted to an age and sex specific BMI percentile using CDC 2000 criteria (7). Waist circumference was taken using a Gulick tape measure (G-tape) with a tension device on bare skin measured just above the iliac crest.
Blood pressure was recorded three times using an automated blood pressure monitor (Omron HEM-907XL, Vernon Hills IL). The initial value was recorded after the participant had been seated quietly for five minutes with each subsequent value recorded one minute after the preceding recording. The mean of the second and third recordings were used in all subsequent analyses.
Fitness was assessed using the 20-m shuttle test (20-MST) which has been shown to provide an accurate and reliable assessment of fitness among youth (18–20). As we were interested in whether the intervention effect differed by baseline obesity or body mass status, it was important to have a measure of fitness that was not expressed in relation to body mass (i.e., liters of oxygen per kilogram). Consequently, we used the actual number of shuttle run laps as an unadjusted indicator of fitness.
Participants were called the night before data collection to remind them not to eat or drink anything but water after midnight. Participants who reported eating after midnight were considered nonfasting and asked to return another day. Phlebotomists obtained fasting blood samples which were processed and then shipped to the central blood laboratory at the University of Washington Northwest Lipid Research Laboratories for all analyses. Analyses of glucose were performed on a Roche P module auto-analyzer by the hexokinase method using reagent from Roche Diagnostics. Insulin was measured by a two-site immunoenzymometric assay performed using a Tosoh 1800 auto-analyzer. The assay sensitivity level was 2.0 µU/mL, with interassay and intra-assay coefficients of variation (CV) < 10%. The assay had a high specificity as cross-reactivity with human C-peptide and proinsulin was 0% and 2%, respectively. Measurements of total plasma cholesterol, cholesterol in the lipoprotein fractions, and triglycerides were performed enzymatically on the Roche Modular-P autoanalyzer using methods standardized to the Centers for Disease Control and Prevention Reference Methods (29). Determination of high density lipoprotein (HDL) cholesterol was performed after precipitation of apolipoprotein B-containing particles by dextran sulfate Mg+2. Low density lipoprotein (LDL) cholesterol was calculated using the Friedewald equation (11). This approach for calculating LDL is clinically reliable if the measurements of total and HDL cholesterol are performed with a high level of accuracy and triglycerides are < 400 mg/dL (30). In the case of elevated triglycerides, a complete lipoprotein separation by ultracentrifugation which allows quantization of the individual lipoprotein classes was performed using the Lipid Research Clinics Beta Quantification procedure (13). The inter-assay CVs are consistently < 1.5% for total cholesterol and triglycerides and < 2% for HDL cholesterol.
Pubertal status was individually self-reported at eighth grade in private using the Pubertal Development Scale (25) and converted to pubertal stage groups that are consistent with the five pubertal stages that have been outlined by Tanner (27). Ethnicity was self-reported and household education were obtained via parental report.
Statistical Analysis
A total of 4603 students were followed from baseline (early sixth grade) to the end of study (second semester of eighth grade). As we wanted to examine the natural progression of both fitness and body mass the sample included participants from both intervention and control arms. Of these participants, 3812 had complete data for anthropometric assessments, blood values, 20-m shuttle test, eighth grade pubertal stage survey, and parental or guardian report of highest level of household education. However, 298 of these participants reported other or mixed race and were dropped from all analysis since the results for this ethnic group are too heterogeneous to interpret. Therefore, analyses are presented for the remaining 3514 participants (1842 females). In light of the small number of participants who were classified as pubertal stage 5 at baseline the pubertal data were collapsed into three groups, prepubertal (stage 1), early pubertal (stages 2 and 3) and pubertal (stages 4 and 5).
As noted above the goal of this paper was to assess how change in fitness, body mass and the additive effect of fitness and body mass change were associated with change in both the individual risk factors and the overall risk profile. However, as less than 10% of the participants were classified as having metabolic syndrome using the International Diabetes Federation criteria (33), we created a clustered risk score using the methods of Andersen and colleagues (2). In this approach, z-scores were obtained for triglycerides, HDL-C (reverse scored), systolic blood pressure, diastolic blood pressure, glucose and waist circumference. The z-scores for the first 5 items were summed to create one clustered risk score and all the items were summed to create a second clustered risk score that is not totally independent of BMI since waist circumference and BMI are related.
Youth with BMI ≥ 85th but < 95th percentile were classified as overweight, those ≥ 95th percentile as obese and those < 85th percentile were healthy weight (1). In this study, the 3-level BMI-based obesity status is used as a surrogate for fatness. From baseline to end of study, each participant’s BMI category could improve (moved from obese to overweight or normal weight, overweight to normal weight), get worse (move from overweight to obese, normal weight to overweight or obese), or remain unchanged (remained in same category). To provide an equal spread of participants in categories and maximize variance between groups’ sex specific fitness quintiles were determined at both baseline and end of study. If a participant remained in the same fitness quintile, they were classified as Fitness Unchanged, if a participant had their fitness quintile go up by at least one/ ≥ 1 quintile (e.g.,: Q1 to Q2–Q5, Q2 to Q3–Q5, etc.), they were classified as Fitness Improved, and if a participants fitness quintile decreased by at least one/or ≥ 1 quintile (e.g.,: Q5 to Q4-Q1, Q3 to Q2-Q1, etc.), they were classified as Fitness declined.
Frequencies of participants in the nine BMI change fitness change groups were tabulated. Analyses were conducted to evaluate whether shifts in BMI category and fitness category over time differed by school intervention status using generalized linear mixed models that took into account sources of variability both within and between schools. Since these analyses showed no intervention effect, the full sample was used for analysis, rather than just the sample from control schools, but intervention status was included in all subsequent models as a covariate.
Regression models were fit for each cardiometabolic risk factor using the PROC MIXED procedure with BMI category change, fitness category change as well as the additive effect of change in BMI and fitness. For all models change in risk factor was the outcome variable. The regression coefficients and their associated 95% confidence intervals along with the p-values for the test of coefficient equal to zero are presented. However, since both BMI category change and fitness category change are categorical variables only the first two levels (worse and unchanged) have regression coefficients since both lower groups are compared with the third (improved) group. To adjust for the clustering of participants within schools, a random effect for school was included in the models. As one way analysis of variance tests indicated that there were sex differences for both fitness category change (p = .0110) and BMI category change (p = .0004), models were run separately for males and females. All models were adjusted for eighth grade pubertal stage group as this was the age when the outcome variable for each model was assessed. The models were also adjusted for (race, household education, baseline value for the cardiometabolic risk factor, and intervention group with the overall models also adjusted for sex. In light of the number of different analyses that were conducted an arbitrary alpha value was not set but rather analyses were interpreted in light of the strength of evidence of associations with p-values of 0.05 interpreted as some evidence against the null hypothesis, p = .01 interpreted as increasing evidence against the null hypothesis and p < .001 interpreted as strong evidence against the null hypothesis (26).
Where there was some evidence (p < .05) of an additive effect of change in BMI and fitness further subgroup analyses including graphical methods and pairwise comparisons were used to identify the strength of differences between subgroups. To account for the number of tests performed in these subgroup analyses Bonferroni p-value adjustments were performed. All analyses were performed using SAS version 9.2 (SAS Institute, Cary, NC).
Results
Descriptive statistics with means and standard deviations for continuous measures and frequency distributions for categorical variables are presented in Table 1, both overall and by sex. The sample was 60% Hispanic, 21% non-Hispanic White and 19% non-Hispanic Black. By the 8th grade assessment, 41% of the boys and 94% of the girls were Tanner stage 4 or 5. The mean BMI percentile (which facilitates comparison with other studies), of all participants dropped from 73.3 at 6th grade to 72.8 at 8th grade with the mean number of fitness laps increasing from 21.3 at 6th grade to 27.6 at 8th grade. The unadjusted mean changes from sixth grade to eighth grade for each cardiometabolic risk factor, both overall and by sex, were found to significantly differ from zero except for diastolic blood pressure in males.
Table 1.
Participant Characteristics, Baseline, End-of-Study, and Change Means ± SD or Frequency and Percent (N = 3514)
| Overall | Boys | Girls | ||||
|---|---|---|---|---|---|---|
| N | % | N | % | N | % | |
| Race/Ethnicity | ||||||
| Hispanic | 2113 | 60.1% | 1002 | 59.9% | 1111 | 60.3% |
| Black | 663 | 18.9% | 298 | 17.8% | 365 | 19.8% |
| White | 738 | 21.0% | 372 | 22.2% | 366 | 19.9% |
| 8th Grade Tanner Stage | ||||||
| Tanner 1 | 24 | 0.7% | 22 | 1.3% | 2 | 0.1% |
| Tanner 2 or 3 | 1073 | 30.5% | 957 | 57.2% | 116 | 6.3% |
| Tanner 4 or 5 | 2417 | 68.8% | 693 | 41.4% | 1724 | 93.6% |
| Highest Household Education | ||||||
| No HS Diploma | 983 | 28.0% | 463 | 27.7% | 520 | 28.2% |
| Some college | 1873 | 53.3% | 869 | 52.0% | 1004 | 54.5% |
| College grad or higher | 658 | 18.7% | 340 | 20.3% | 318 | 17.3% |
| 6th Grade | 8th Grade | 8th-6th Change | ||||
| Mean ± SD | Mean ± SD | Mean ± SD | p-value | |||
| BMI Percentile | 73.32 ± 27.73 | 72.78 ± 26.33 | −0.54 ± 12.88 | 0.0132 | ||
| Boys | 74.56 ± 27.96 | 72.34 ± 27.65 | −2.22 ± 12.79 | <.0001 | ||
| Girls | 72.20 ± 27.48 | 73.19 ± 25.08 | 0.99 ± 12.77 | 0.0009 | ||
| Fitness (number of laps) | 21.27 ± 12.16 | 27.55 ± 17.36 | 6.28 ± 13.91 | <.0001 | ||
| Boys | 23.90 ± 14.07 | 34.95 ± 19.61 | 11.05 ± 15.91 | <.0001 | ||
| Girls | 18.88 ± 9.52 | 20.83 ± 11.44 | 1.95 ± 10.00 | <.0001 | ||
| Insulin (µU/mL) | 13.28 ± 11.44 | 17.29 ± 14.53 | 4.01 ± 13.51 | <.0001 | ||
| Boys | 12.22 ± 12.15 | 16.85 ± 16.21 | 4.63 ± 15.01 | <.0001 | ||
| Girls | 14.24 ± 10.68 | 17.70 ± 12.82 | 3.46 ± 11.96 | <.0001 | ||
| Glucose (mg/dL) | 93.63 ± 6.54 | 94.04 ± 8.51 | 0.40 ± 8.32 | 0.0041 | ||
| Boys | 94.53 ± 6.51 | 95.98 ± 8.11 | 1.45 ± 8.07 | <.0001 | ||
| Girls | 92.82 ± 6.45 | 92.27 ± 8.48 | −0.55 ± 8.44 | 0.0053 | ||
| Total Cholesterol (mg/dL) | 157.18 ± 27.54 | 148.07 ± 26.77 | −9.12 ± 21.28 | <.0001 | ||
| Boys | 159.17 ± 28.60 | 145.32 ± 27.65 | −13.85 ± 21.36 | <.0001 | ||
| Girls | 155.38 ± 26.42 | 150.56 ± 25.69 | −4.82 ± 20.28 | <.0001 | ||
| HDL-C (mg/dL) | 52.35 ± 12.22 | 51.45 ± 12.36 | −0.90 ± 9.17 | <.0001 | ||
| Boys | 52.80 ± 12.39 | 49.44 ± 11.95 | −3.36 ± 8.77 | <.0001 | ||
| Girls | 51.94 ± 12.05 | 53.27 ± 12.45 | 1.33 ± 8.95 | <.0001 | ||
| LDL-C (mg/dL) | 86.92 ± 23.20 | 80.04 ± 22.33 | −6.88 ± 17.15 | <.0001 | ||
| Boys | 88.74 ± 23.89 | 78.92 ± 23.06 | −9.82 ± 17.53 | <.0001 | ||
| Girls | 85.26 ± 22.43 | 81.05 ± 21.60 | −4.22 ± 16.36 | <.0001 | ||
| Triglycerides (mg/dL) | 89.89 ± 52.08 | 83.22 ± 47.75 | −6.67 ± 46.60 | <.0001 | ||
| Boys | 88.40 ± 52.37 | 85.40 ± 56.02 | −3.00 ± 50.15 | 0.0144 | ||
| Girls | 91.24 ± 51.79 | 81.24 ± 38.64 | −9.99 ± 42.86 | <.0001 | ||
| Systolic Blood Pressure (mmHg) | 107.45 ± 10.16 | 111.18 ± 10.50 | 3.73 ± 10.96 | <.0001 | ||
| Boys | 108.04 ± 10.26 | 114.36 ± 10.42 | 6.32 ± 10.59 | <.0001 | ||
| Girls | 106.91 ± 10.05 | 108.28 ± 9.71 | 1.37 ± 10.75 | <.0001 | ||
| Diastolic Blood Pressure (mmHg) | 63.75 ± 8.68 | 64.67 ± 7.94 | 0.91 ± 9.35 | <.0001 | ||
| Boys | 63.67 ± 8.66 | 63.97 ± 7.97 | 0.31 ± 9.32 | 0.1807 | ||
| Girls | 63.83 ± 8.69 | 65.30 ± 7.87 | 1.47 ± 9.35 | <.0001 | ||
| Waist Circumference (cm) | 76.06 ± 14.60 | 80.87 ± 14.65 | 4.81 ± 6.64 | <.0001 | ||
| Boys | 76.41 ± 15.47 | 80.91 ± 15.59 | 4.51 ± 6.78 | <.0001 | ||
| Girls | 75.74 ± 13.77 | 80.82 ± 13.73 | 5.08 ± 6.49 | <.0001 | ||
The average insulin, glucose, blood pressure and waist circumference levels increased from 6th to 8th grade, while total cholesterol, HDL-C, LDL-C and triglycerides decreased. Comparisons indicated that there were no differences between the participants who were included in the analysis and excluded participants, in terms of sex, ethnicity, 6th or 8th grade BMI category or 8th grade fitness levels. There was, however, increasing evidence of a difference in the baseline fitness levels of the participants, with the included sample achieving slightly more laps on the shuttle test than the excluded participants (21.3 vs. 20.8, p = .009).
The frequencies of participants in the nine BMI*fitness change subgroups are presented in Table 2. The table indicates that at the alternative ends of the distribution, 7.3% of participants BMI and fitness improved while 2.7% of participants BMI and fitness worsened. The largest group was the 30.5% of participants who’s BMI and fitness group classification did not change.
Table 2.
Frequencies of Change in BMI Category and Sex Specific Fitness Quintile Category
| Sex Specific Quintile Fitness Change | ||||
|---|---|---|---|---|
| Fitness Quintile Worsened |
Fitness Quintile Unchanged |
Fitness Quintile Improved |
Total | |
| BMI Change | ||||
| BMI Category Worsened | 93 (2.65%) | 70 (1.99%) | 54 (1.54%) | 217 (6.18%) |
| BMI Category Unchanged | 883 (25.13%) | 1071 (30.48%) | 809 (23.02%) | 2763 (78.63%) |
| BMI Category Improved | 115 (3.27%) | 164 (4.67%) | 255 (7.26%) | 534 (15.20%) |
| Total | 1091 (31.05%) | 1305 (37.14%) | 1118 (31.82%) | 3514 |
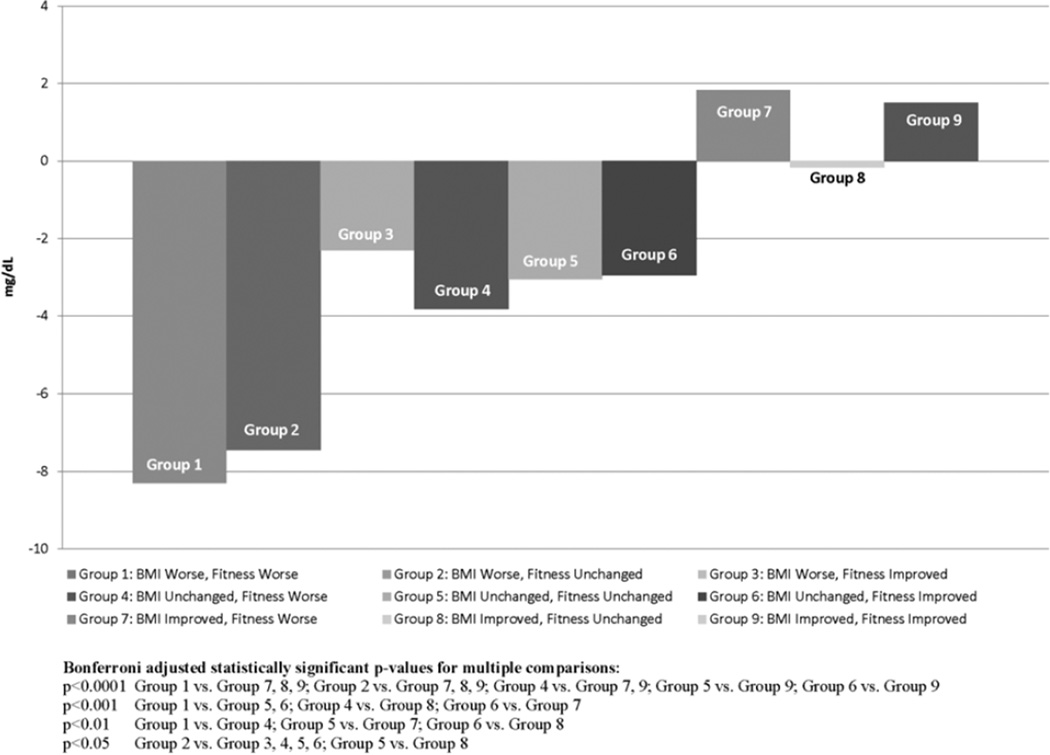
The results of the regression models predicating change in risk factors for boys are presented in Table 3. The Table includes the beta and 95%CI for each of the model parameters (i.e., BMI category worsened and BMI category improved in relation to BMI category improved) and the overall model p-value for BMI, fitness and the additive effect of BMI fitness. There was strong evidence of a BMI effect in all of the models. There was weaker evidence of a fitness effect with some evidence that change with fitness was associated with change in total cholesterol, HDL-C, LDL-C, and the clustered risk factor scores. The model for HDL-C in boys was the only model for which there was some evidence of a BMI, fitness and additive BMI*fitness effect. This additive effect was further explored in Figure 1 which indicates that HDL-C only improved in the groups in which BMI improved, with these beneficial effects evident for both the group whose fitness worsened and fitness improved. The table also shows that the largest decline in HDL-C was in the group of participants whose BMI and fitness declined. It is also interesting to note that there was some evidence that the group in which BMI and fitness group declined was different from all of the BMI unchanged and BMI improved groups.
Table 3.
Regression Coefficients, Standard Errors and p Values of BMI Category and Fitness Quintile Category for Boys in Relation to Change in Cardiometabolic Disease Risk Factors
| Fitness Worsened |
Fitness Unchanged |
Model Parameters | |||||||
|---|---|---|---|---|---|---|---|---|---|
| β | p-value | β | p-value | β | p-value |
BMI p-value |
Fitness p-value |
BMI*Fitness p-value |
|
| Insulin (µU/mL) | 0.55 | 0.8098 | 0.91 | 0.6234 | <.0001 | 0.3019 | 0.2451 | ||
| BMI Category Worsened | 7.05 | 0.0506 | 2.79 | 0.5454 | −0.27 | 0.9539 | |||
| BMI Category Unchanged | 3.43 | 0.0178 | 2.25 | 0.3666 | 4.07 | 0.0522 | |||
| Glucose (mg/dL) | 1.19 | 0.3141 | 1.93 | 0.0442 | 0.0024 | 0.3119 | 0.6550 | ||
| BMI Category Worsened | 2.86 | 0.1251 | 0.29 | 0.9026 | −0.59 | 0.8083 | |||
| BMI Category Unchanged | 2.30 | 0.0021 | −0.67 | 0.6045 | −1.64 | 0.1300 | |||
| Total Cholesterol (mg/dL) | 3.95 | 0.2036 | 4.35 | 0.0858 | <.0001 | 0.0495 | 0.7888 | ||
| BMI Category Worsened | 10.82 | 0.0277 | 2.74 | 0.6618 | −4.66 | 0.4697 | |||
| BMI Category Unchanged | 6.21 | 0.0016 | 1.10 | 0.7464 | −1.31 | 0.6470 | |||
| HDL-C (mg/dL) | 0.32 | 0.7943 | −1.67 | 0.0968 | <.0001 | 0.0150 | 0.0362 | ||
| BMI Category Worsened | −3.80 | 0.0509 | −6.32 | 0.0110 | −3.48 | 0.1738 | |||
| BMI Category Unchanged | −4.45 | <.0001 | −1.20 | 0.3727 | 1.56 | 0.1681 | |||
| LDL-C (mg/dL) | 3.83 | 0.1321 | 4.42 | 0.0329 | <.0001 | 0.0091 | 0.4025 | ||
| BMI Category Worsened | 9.23 | 0.0218 | 4.39 | 0.3925 | −3.55 | 0.5013 | |||
| BMI Category Unchanged | 6.84 | <.0001 | 0.06 | 0.9842 | −3.03 | 0.1948 | |||
| Triglycerides (mg/dL) | −1.49 | 0.8369 | 7.42 | 0.2074 | <.0001 | 0.0428 | 0.4674 | ||
| BMI Category Worsened | 25.30 | 0.0268 | 24.50 | 0.0932 | 11.13 | 0.4578 | |||
| BMI Category Unchanged | 17.89 | <.0001 | 11.87 | 0.1330 | 2.65 | 0.6902 | |||
| Systolic Blood Pressure (mmHg) | −1.17 | 0.4277 | −0.69 | 0.5670 | 0.0003 | 0.3185 | 0.7439 | ||
| BMI Category Worsened | 2.92 | 0.2087 | 1.65 | 0.5788 | 3.02 | 0.3222 | |||
| BMI Category Unchanged | 0.64 | 0.4908 | −1.20 | 0.4530 | 0.06 | 0.9655 | |||
| Diastolic Blood Pressure (mmHg) | −0.71 | 0.5453 | 0.11 | 0.9122 | 0.0012 | 0.4749 | 0.5968 | ||
| BMI Category Worsened | 1.25 | 0.5012 | 2.74 | 0.2466 | 1.37 | 0.5735 | |||
| BMI Category Unchanged | 0.72 | 0.3317 | 1.69 | 0.1869 | 1.27 | 0.2405 | |||
| Clustered Risk Score1 | −0.16 | 0.6659 | 0.51 | 0.0969 | <.0001 | 0.0417 | 0.2841 | ||
| BMI Category Worsened | 1.75 | 0.0033 | 1.52 | 0.0442 | 0.69 | 0.3771 | |||
| BMI Category Unchanged | 1.22 | <.0001 | 0.32 | 0.4280 | −0.22 | 0.5318 | |||
| Clustered Risk Score2 | −0.03 | 0.9377 | 0.68 | 0.0376 | <.0001 | 0.0117 | 0.2474 | ||
| BMI Category Worsened | 2.67 | <.0001 | 1.67 | 0.0384 | 0.70 | 0.3993 | |||
| BMI Category Unchanged | 1.78 | <.0001 | 0.31 | 0.4743 | −0.26 | 0.4837 | |||
Clustered risk score for metabolic syndrome constructed from triglycerides, HDL-C, systolic blood pressure, diastolic blood pressure, and glucose.
Clustered risk score for metabolic syndrome constructed from triglycerides, HDL-C, systolic blood pressure, diastolic blood pressure, glucose, and waist circumference.
Figure 1.
Adjusted mean change in HDL-C for boys for the interaction groups and adjusted p-values.
The results of the regression models predicating change in risk factors for girls are presented in Table 4. There was strong evidence of a BMI effect with increases in BMI group associated with increases in insulin, LDL-C, triglycerides, and the clustered risk factor score with some or increasing BMI evidence in all of the other models. There was some evidence of a fitness effect of HDL-C but there were no models in which there was support for a BMI, fitness and BMI plus fitness additive effect among girls.
Table 4.
Regression Coefficients, Standard Errors and p Values of BMI Category and Fitness Quintile Category for Girls in Relation to Change in Cardiometabolic Disease Risk Factors
| Fitness Worsened |
Fitness Unchanged |
Model Parameters | |||||||
|---|---|---|---|---|---|---|---|---|---|
| β | p-value | β | p-value | β | p-value |
BMI p-value |
Fitness p-value |
BMI*Fitness p-value |
|
| Insulin (µU/mL) | 2.60 | 0.1299 | 1.90 | 0.2647 | <.0001 | 0.6611 | 0.1711 | ||
| BMI Category Worsened | 9.91 | <.0001 | −7.27 | 0.0132 | −3.53 | 0.2363 | |||
| BMI Category Unchanged | 4.88 | <.0001 | −2.59 | 0.1647 | −1.54 | 0.4017 | |||
| Glucose (mg/dL) | 2.41 | 0.0433 | 0.07 | 0.9540 | 0.0266 | 0.4702 | 0.2423 | ||
| BMI Category Worsened | 1.67 | 0.2425 | −2.11 | 0.2971 | 0.81 | 0.6946 | |||
| BMI Category Unchanged | 2.12 | 0.0058 | −2.53 | 0.0488 | 0.57 | 0.6504 | |||
| Total Cholesterol (mg/dL) | −0.59 | 0.8360 | 4.57 | 0.1026 | 0.0008 | 0.1617 | 0.4215 | ||
| BMI Category Worsened | 9.36 | 0.0058 | −2.03 | 0.6739 | −4.62 | 0.3461 | |||
| BMI Category Unchanged | 2.82 | 0.1232 | 3.85 | 0.2084 | −0.65 | 0.8305 | |||
| HDL-C (mg/dL) | −2.23 | 0.0753 | −0.40 | 0.7486 | <.0001 | 0.0173 | 0.3335 | ||
| BMI Category Worsened | −6.37 | <.0001 | −0.72 | 0.7352 | 0.61 | 0.7778 | |||
| BMI Category Unchanged | −5.44 | <.0001 | 1.89 | 0.1608 | 0.60 | 0.6549 | |||
| LDL-C (mg/dL) | 0.91 | 0.6919 | 4.14 | 0.0666 | <.0001 | 0.1174 | 0.8313 | ||
| BMI Category Worsened | 9.09 | 0.0009 | 0.08 | 0.9836 | −2.75 | 0.4871 | |||
| BMI Category Unchanged | 4.13 | 0.0053 | 1.77 | 0.4731 | −1.23 | 0.6120 | |||
| Triglycerides (mg/dL) | 3.92 | 0.4057 | 1.00 | 0.8304 | <.0001 | 0.8883 | 0.3750 | ||
| BMI Category Worsened | 27.66 | <.0001 | −9.26 | 0.2490 | −9.71 | 0.2355 | |||
| BMI Category Unchanged | 13.28 | <.0001 | −2.32 | 0.6491 | 3.24 | 0.5193 | |||
| Systolic Blood Pressure (mmHg) | −0.41 | 0.7613 | −1.64 | 0.2196 | 0.0032 | 0.0940 | 0.6227 | ||
| BMI Category Worsened | 3.45 | 0.0327 | −2.25 | 0.3270 | 1.16 | 0.6201 | |||
| BMI Category Unchanged | 1.04 | 0.2352 | −1.68 | 0.2484 | 0.44 | 0.7616 | |||
| Diastolic Blood Pressure (mmHg) | 2.22 | 0.0468 | 0.11 | 0.9239 | 0.0006 | 0.3992 | 0.0277 | ||
| BMI Category Worsened | 5.22 | <.0001 | −3.75 | 0.0471 | −2.83 | 0.1423 | |||
| BMI Category Unchanged | 2.03 | 0.0047 | −2.00 | 0.0126 | 0.12 | 0.9184 | |||
| Clustered Risk Score1 | 0.83 | 0.0201 | −0.13 | 0.7237 | <.0001 | 0.4157 | 0.0209 | ||
| BMI Category Worsened | 2.52 | <.0001 | −1.29 | 0.0341 | −0.44 | 0.4751 | |||
| BMI Category Unchanged | 1.38 | <.0001 | −1.09 | 0.0048 | 0.15 | 0.6953 | |||
| Clustered Risk Score2 | 1.03 | 0.0072 | 0.052 | 0.8902 | <.0001 | 0.5967 | 0.0096 | ||
| BMI Category Worsened | 3.59 | <.0001 | −1.64 | 0.0117 | −0.70 | 0.2879 | |||
| BMI Category Unchanged | 1.92 | <.0001 | −1.28 | 0.0019 | 0.044 | 0.9138 | |||
Clustered risk score for metabolic syndrome constructed from triglycerides, HDL-C, systolic blood pressure, diastolic blood pressure, and glucose.
Clustered risk score for metabolic syndrome constructed from triglycerides, HDL-C, systolic blood pressure, diastolic blood pressure, glucose, and waist circumference.
Discussion
Increase in BMI group from 6th to 8th grade was associated with increases in the risk factors and clustered risk factor score at 8th grade for both boys and girls. Fitness, however, was only associated with very few of the risk factors and there was only an additive effect of the change fitness and obesity for HDL-C. Collectively, these findings indicate that changing obesity status is central to the reduction of youth cardiometabolic risk. The beneficial effects of improvements on fitness are however, negligible once change in body mass index has been taken into account.
Previous studies have reported that fitness was independently associated with cardiometabolic risk factors among children and adolescents. For example, fitness was independently associated with glucose, HDL-C, insulin and clustered metabolic syndrome risk factor score among 9 and 15 year old European children in a cross-sectional model (9). The European study used a submaximal cycle ergometry test, whereby the results were expressed in relation to fat free mass. We did not adopt this approach because we needed a field measure that could be used for large groups of participants. The shuttle test met this criteria, while also providing an indication of fitness that has been closely associated with laboratory derived energy expenditure (19). Thus, the dissonance between our findings and others could be a function of the fitness assessment as the shuttle run assessed running-related fitness while the bike test assessed cycling fitness. Alternatively, the difference could be a function of how the fitness variable was calculated: when fitness is expressed in relation to body mass some element of the associations between fitness and cardiometabolic risk factors may be accounted for by the effect of body mass which includes fat mass. Equally, as we have also reported in baseline, cross-sectional models (14) that fitness was associated with cardiometabolic effects after controlling for BMI group, it may be the case that fitness effects are attenuated in longitudinal analyses.
We found additive fitness and obesity status effects for HDL-C among boys. Further examination of this effect indicated that HDL-C only improved when BMI improved with no evidence of a difference between the subgroups of participants whose BMI improved and fitness improved when compared with the participants whose BMI improved and fitness declined. The examination also showed that the biggest decline in HDL-C was among the participants whose fitness and BMI group declined. Thus, our findings suggest that the BMI change and not fitness change may have been most critical to improvement in HDL-C levels.
Our findings are not consistent with the well established adult literature which indicates that overweight but physically fit individuals have a lower risk of heart disease and all cause mortality than their unfit, normal weight counterparts (17). Since the participants in our study were on average 14 years of age, whereas the bulk of the studies that have fitness*fatness additive effects have included middle aged adults, our dissonant findings may simply be a function of age and suggest that fitness may become more important in adult life. Support for this concept is provided by Ondrak and colleagues who found that, among 1800, 8–16 yr old children, associations between body fatness and CVD risk factors declined as children aged (24), while the association between fitness and CVD risk factors tended to increase. Thus, maintaining normal weight appears to be of primary importance during youth, with fitness becoming more important during the move to adulthood.
Strengths and Limitations
The major strength of this study was the direct assessment of obesity status (BMI), fitness and cardiometabolic risk factors in a large, ethnically diverse group of US adolescents who were assessed in 6th grade and again 2.5 years later when in 8th grade. The study also benefits from the use of a fitness assessment that was not expressed in relation to body mass, thereby allowing us to test the independent effects of fitness and body mass. It is, however, important to note that, although the shuttle run test has been shown to be closely associated with directly measured fitness (19), the shuttle run only provides an approximation of fitness. In addition, the risk factor score provides an estimate of the overall level of cardiometabolic risk factors but unlike the IDF metabolic syndrome risk criteria the risk factor score does not provide a score that can be used by clinicians to advocate specific treatment. It is also important to highlight that multiple comparisons were conducted in this paper. As such it is possible that any findings could be a function of chance. To mitigate the risk that we interpreted a chance finding as important we have not applied an arbitrary p-value to indicate statistical significance but have presented the strength of evidence for each test, thereby allowing the reader to apply his or her own interpretation.
Conclusion
Increase in obesity group from 6th to 8th grade in this study was associated with higher cardiometabolic risk in both boys and girls. Fitness effects were negligible once change in body mass had been taken into account. Results suggest that among youth change in body mass rather than fitness change appears to have a greater effect on achieving improvements in cardiometabolic risk factors.
Acknowledgments
We wish to thank the administration, faculty, staff, students, and their families at the middle schools and school districts that participated in the HEALTHY study. This work was completed with funding from NIDDK/NIH grant numbers U01-DK61230, U01-DK61249, U01-DK61231, and U01-DK61223, with additional support from the American Diabetes Association.
Contributor Information
Russell Jago, School for Policy Studies, University of Bristol, Bristol, UK.
Kimberly L. Drews, Biostatistics Center, George Washington University, Washington, DC
Robert G. McMurray, Dept. of Exercise and Sport Science, University of North Carolina at Chapel Hill, Chapel Hill, NC
Tom Baranowski, Children’s Nutrition Research Center, Baylor College of Medicine, Houston, TX.
Pietro Galassetti, Pediatrics & Institute for Clinical and Translational Science, University of California-Irvine, Irvine, CA.
Gary D. Foster, Center for Obesity Research and Education, Temple University, Philadelphia, PA
Ester Moe, Oregon Health and Sciences University, Portland, OR.
John B. Buse, Dept. of Medicine, University of North Carolina at Chapel Hill, Chapel Hill, NC
References
- 1.American Medical Association. Expert Committee Recommendations on the Assessment, Prevention, and Treatment of Child and Adolescent Overweight and Obesity. [Accessed 11/02/2007];2007 Available at: www.ama-assn.org/ama1/pub/upload/mm/433/ped_obesity_recs.pdf.
- 2.Andersen LB, Harro M, Sardinha LB, et al. Physical activity and clustered cardiovascular risk in children: a cross-sectional study (The European Youth Heart Study) Lancet. 2006;368:299–304. doi: 10.1016/S0140-6736(06)69075-2. [DOI] [PubMed] [Google Scholar]
- 3.Bao W, Threefoot SA, Srinivasan SR, Berenson GS. Essential hypertension predicted by tracking of elevated blood pressure from childhood to adulthood: The Bogalusa Heart Study. Am. J. Hypertens. 1995;8:657–665. doi: 10.1016/0895-7061(95)00116-7. [DOI] [PubMed] [Google Scholar]
- 4.Blair SN, Cheng Y, Holder S. Is physical activity or physical fitness more important in defining health benefits? Med. Sci. Sports Exerc. 2001;33:S379–S399. doi: 10.1097/00005768-200106001-00007. [DOI] [PubMed] [Google Scholar]
- 5.Blair SN, Kohl HW, 3rd, Barlow CE, Paffenbarger RS, Jr, Gibbons LW, Macera CA. Changes in physical fitness and all-cause mortality. A prospective study of healthy and unhealthy men. JAMA. 1995;273:1093–1098. [PubMed] [Google Scholar]
- 6.Buse J, Hirst K. The HEALTHY study: introduction. Int J Obes (Lond) 2009;33(Suppl. 4):S1–S2. doi: 10.1038/ijo.2009.110. [DOI] [PubMed] [Google Scholar]
- 7.Centers for Disease Control National Center for Health Statistics. 2000 CDC growth charts for the United States. [Accessed, 2009]; Available at: http://www.cdc.gov/growthcharts.
- 8.Ekelund U, Anderssen S, Andersen LB, et al. Prevalence and correlates of the metabolic syndrome in a population-based sample of European youth. Am. J. Clin. Nutr. 2009;89:90–96. doi: 10.3945/ajcn.2008.26649. [DOI] [PubMed] [Google Scholar]
- 9.Ekelund U, Anderssen SA, Froberg K, Sardinha LB, Andersen LB, Brage S. Independent associations of physical activity and cardiorespiratory fitness with metabolic risk factors in children: the European youth heart study. Diabetologia. 2007;50:1832–1840. doi: 10.1007/s00125-007-0762-5. [DOI] [PubMed] [Google Scholar]
- 10.Freedman DS, Dietz WH, Srinivasan SR, Berenson GS. The relation of overweight to cardiovascular risk factors among children and adolescents: the Bogalusa Heart Study. Pediatrics. 1999;103:1175–1182. doi: 10.1542/peds.103.6.1175. [DOI] [PubMed] [Google Scholar]
- 11.Fridewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin. Chem. 1972;18:499–502. [PubMed] [Google Scholar]
- 12.Froberg K, Andersen LB. Mini review: physical activity and fitness and its relations to cardiovascular disease risk factors in children. Int J Obes (Lond) 2005;29(Suppl. 2):S34–S39. doi: 10.1038/sj.ijo.0803096. [DOI] [PubMed] [Google Scholar]
- 13.Hainline AJ, Karon J, K K. Manual of laboratory operations: Lipid research clinics program, lipid and lipoprotein analysis. 2nd ed. US Department of Health & Human Services; 1983. pp. 75–628. [Google Scholar]
- 14.Jago R, Drews KL, McMurray RG, et al. Fatness, Fitness, and Cardiometabolic Risk Factors Among Sixth Grade Youth. Med. Sci. Sports Exerc. 2010;42:1502–1510. doi: 10.1249/MSS.0b013e3181d322c4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jago R, Harrell JS, McMurray RG, Edelstein S, El Ghormli L, Bassin S. Prevalence of abnormal lipid and blood pressure values among an ethnically diverse population of eighth-grade adolescents and screening implications. Pediatrics. 2006;117:2065–2073. doi: 10.1542/peds.2005-1716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jago R, McMurray RG, Drews KL, et al. HEALTHY Intervention: Fitness, Physical Activity, and Metabolic Syndrome Results. Med. Sci. Sports Exerc. 2011;43:1513–1522. doi: 10.1249/MSS.0b013e31820c9797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee CD, Blair SN, Jackson AS. Cardiorespiratory fitness, body composition, and all-cause and cardiovascular disease mortality in men. Am. J. Clin. Nutr. 1999;69:373–380. doi: 10.1093/ajcn/69.3.373. [DOI] [PubMed] [Google Scholar]
- 18.Leger LA, Lambert J. A maximal multistage 20-m shuttle run test to predict VO2 max. Eur. J. Appl. Physiol. Occup. Physiol. 1982;49:1–12. doi: 10.1007/BF00428958. [DOI] [PubMed] [Google Scholar]
- 19.Leger LA, Mercier D, Gadoury C, Lambert J. The multistage 20 metre shuttle run test for aerobic fitness. J Sports Sci. 1988;6:93–101. doi: 10.1080/02640418808729800. [DOI] [PubMed] [Google Scholar]
- 20.Liu NY, Plowman SA, Looney MA. The reliability and validity of the 20-meter shuttle test in American students 12 to 15 years old. Res. Q. Exerc. Sport. 1992;63:360–365. doi: 10.1080/02701367.1992.10608757. [DOI] [PubMed] [Google Scholar]
- 21.McAuley PA, Sui X, Church TS, Hardin JW, Myers JN, Blair SN. The joint effects of cardiorespiratory fitness and adiposity on mortality risk in men with hypertension. Am. J. Hypertens. 2009;22:1062–1069. doi: 10.1038/ajh.2009.122. [DOI] [PubMed] [Google Scholar]
- 22.Nicklas TA, von Duvillard SP, Berenson GS. Tracking of serum lipids and lipoproteins from childhood to dyslipidemia in adults: The Bogalusa heart study. Int. J. Sports Med. 2002;23:s39–s43. doi: 10.1055/s-2002-28460. [DOI] [PubMed] [Google Scholar]
- 23.Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of obesity in the United States, 2009–2010. NCHS Data Brief. 2012:1–8. [PubMed] [Google Scholar]
- 24.Ondrak KS, McMurray RG, Bangdiwala SI, Harrell JS. Influence of aerobic power and percent body fat on cardiovascular disease risk in youth. J. Adolesc. Health. 2007;41:146–152. doi: 10.1016/j.jadohealth.2007.03.008. [DOI] [PubMed] [Google Scholar]
- 25.Petersen AC, Crockett L, Richards M, Boxer A. A self-report measure of pubertal status: reliability, validity, and initial norms. Youth Adol. 1988;17:117–133. doi: 10.1007/BF01537962. [DOI] [PubMed] [Google Scholar]
- 26.Sterne JA, Davey Smith G. Sifting the evidence-what’s wrong with significance tests? BMJ. 2001;322:226–231. doi: 10.1136/bmj.322.7280.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tanner JM. Growth at adolescence. Oxford: Blackwell; 1962. p. 340. [Google Scholar]
- 28.The Healthy Study Group. A School-Based Intervention for Diabetes Risk Reduction. N. Engl. J. Med. 2010;363:445–453. doi: 10.1056/NEJMoa1001933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Warnick GR. Enzymatic methods for quantification of lipoprotein lipids. Methods Enzymol. 1986;129:101–123. doi: 10.1016/0076-6879(86)29064-3. [DOI] [PubMed] [Google Scholar]
- 30.Warnick GR, Knopp RH, Fitzpatrick V, Branson L. Estimating low-density lipoprotein cholesterol by the Fridewald equation is adequate for classifying patients on the basis of nationally recommended cutpoints. Clin. Chem. 1990;36:15–19. [PubMed] [Google Scholar]
- 31.Whitaker RC, Wright JA, Pepe MS, Seidel KD. Predicting obesity in young adulthood from childhood and parental obesity. N. Engl. J. Med. 1997;337:869–873. doi: 10.1056/NEJM199709253371301. [DOI] [PubMed] [Google Scholar]
- 32.Willi SM, Hirst K, Jago R, et al. Cardiovascular risk factors in multi-ethnic middle school students: the HEALTHY primary prevention trial. Pediatr Obes. 2012;7:230–239. doi: 10.1111/j.2047-6310.2011.00042.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zimmet P, Alberti G, Kaufman F, et al. The metabolic syndrome in children and adolescents. Lancet. 2007;369:2059–2061. doi: 10.1016/S0140-6736(07)60958-1. [DOI] [PubMed] [Google Scholar]