Abstract
Inflammation contributes to secondary injury and neuronal loss after intracerebral hemorrhage, but the role of individual immune populations in these processes is unclear. In a mouse model, the injection of autologous blood into the striatum was associated with an intense inflammatory cell infiltrate composed of neutrophils, monocytes, and dendritic cells. Selective depletion of neutrophils resulted in decreased infiltration of monocytes and improved functional outcomes at day 3 post-hemorrhage. These findings indicate that neutrophil infiltration into the site of hemorrhage contributes to brain injury either by direct cellular damage or the recruitment of monocytes.
Keywords: Intracerebral hemorrhage, Inflammation, Neutrophils, Monocytes
Introduction
Intracerebral hemorrhage (ICH) is a devastating stroke subtype without specific treatment. Activation of the innate immune system after ICH leads to perihematomal inflammation and neuronal loss over the first 3 days [1]. As neurons have limited regeneration, neuronal loss likely contributes to poor functional outcome. More detailed understanding of the triggers of the inflammatory response and the particular effector immune cells involved may lead to development of therapies that can mitigate the secondary injury response after ICH and improve outcome.
At the site of the hemorrhage, there is an intense inflammatory response evident by activation of resident microglia and an acute influx of neutrophils that accumulate at the edge of the hematoma [1-5]. Similarly, neutrophils are also observed in models of acute cerebral ischemia and have been associated with poor outcome. For example, inhibition of neutrophil elastase reduced infarct volume and blood-brain barrier permeability in transient middle cerebral artery occlusion [6]. The protective role of cannabinoid 2 receptors after ischemia has been shown to be mediated solely through inhibited neutrophil recruitment [7]. In addition, depletion of neutrophils with vinblastine reduced hemorrhagic transformation of ischemic brain after tPA administration [8]. Thus, there is accumulating evidence of deleterious effects of infiltrating neutrophils in the sterile brain injury models of cerebral ischemia and hemorrhage.
Although previous studies have documented the presence of perihematomal neutrophils after ICH, the specific role of neutrophils in the pathogenesis of secondary injury has not been investigated. This study used immunohistochemistry and flow cytometry to quantify the degree of neutrophil, monocyte, and dendritic cell infiltration after experimental hemorrhage. Moreover, as neutrophils may serve a pathogenic role in this sterile injury model, the effects of neutrophil depletion on the composition of the inflammatory infiltrate and on functional outcome in the first 3 days after ICH were determined.
Methods and Materials
Mice
C3H/HeOuJ mice were obtained from Jackson Laboratories (Bar Harbor, ME). Male mice aged 14–18 weeks and weighing 28–35 g were used for all experiments. All procedures were carried out with the approval of the Institutional Animal Use and Care Committee of the University of Pennsylvania.
Intracerebral hemorrhage surgery
Mice were anesthetized with inhaled 70% N2O, 30% O2, and 1–5% Isoflurane, and given buprenorphine 0.1 mg/kg subcutaneously for analgesia. Autologous blood from the ventral tail artery was injected at 0.5 μl/min for a total of 15 μl by microinfusion pump (World Precision Instruments, Sarasota, FL) with a 5-min pause midway through the infusion, at coordinates 2.5 mm right of the bregma, angled 5° medial and 3 mm deep. The needle was left in place for 30 additional minutes to allow blood clotting and then withdrawn at 1 mm/min. Incisions were closed using Vetbond (3 M, St. Paul, MN). Body core temperature was maintained at 37 ± 0.5°C by rectal thermometer using a thermistor-controlled heating pad throughout the procedure. Sham surgeries were also performed that included all procedures (including needle insertion) except blood injection.
Immediately after sacrifice, the brain was inspected for ICH success based on gross inspection of a coronal section at the needle insertion site, blinded to mouse treatment. Hemorrhages that tracked down to the base of the brain, up the needle track past the corpus callosum, or into the ventricles were deemed unsuccessful and that mouse was eliminated from all analyses. Three mice were eliminated after ICH resulting in a success rate 81%.
Neutrophil depletion
Mice were injected intraperitoneally with rat anti-mouse Ly6G monoclonal antibody (clone 1A8, BD Biosciences, San Jose, CA), 5 mg/kg, n = 6, to deplete circulating neutrophils or control rat IgG (5 mg/kg, n = 6) 12 h prior to ICH surgery and then every 36–48 h until sacrifice on post-ICH day 3.
Quantification of neurobehavioral deficit
Cylinder testing was performed postoperatively each morning, blinding to treatment, and videotaped for review of the scoring. Each mouse was placed in a 12-cm-diameter clear glass cylinder and observed for 20 rears. The initial placement of the forelimbs on the wall of the cylinder was scored per rear. Subsequent movements (such as lateral exploration) were not scored until the mouse returned to the ground; the next rear was then scored. The laterality index was calculated as (number of right forelimb placements on the side of the cylinder – number of left forelimb placements)/(number of right + number of left + number of both), where 0 indicates no forelimb preference, and 1 indicates only the right forelimb was used.
Immunohistochemistry
Mice were euthanized at 72 ± 2 h after ICH; their brains were removed and immediately frozen in Tissue-tek O.C.T. (Andwin Scientific, Addison, IL), and stored at −80°C until analysis. Then 6-μm sections were fixed with 75% acetone/25% ethanol and blocked with 2% normal goat serum. Slides were incubated with rat anti-mouse Ly6G (5 μg/ml) or rat anti-mouse CD11b (2.5 μg/ml) (eBioscience, San Diego, CA) followed by secondary antibody [Cy3 Affinipure goat anti-rat IgG (Jackson Immunoresearch, West Grove, PA)] at 1:500. DAPI was used at 0.5 μg/ml (Roche Diagnostics, Mannheim, Germany).
Images were acquired using a Nikon E600 fluorescence microscope equipped with a CoolSNAP CCD camera (Photometrics, Tucson, AZ) and processed with NIS Elements software (Nikon, Melville, NY). Neutrophil infiltration was quantified by summing the number of perihematomal neutrophils in five perihematomal 40× fields per mouse to yield the neutrophil count for each mouse. CD11b-positive cells were quantified by summing the number of positive cells in five 20× fields.
Tissue preparation for flow cytometry
Immediately following sacrifice, 1 ml of venous blood was withdrawn and mixed with heparin 200 U/ml. Mice were then perfused with 50 mL of ice cold PBS, and the brains and spleens removed. The two cerebral hemispheres were divided along the inter-hemispheric fissure so that the ipsilateral and contralateral hemispheres could be analyzed separately. Each hemisphere was placed in 4 ml of complete RPMI 1640 (Life Technologies, Gaithersburg, MD) medium supplemented with 10% fetal calf serum, 1% sodium pyruvate, 1% non-essential amino acids, 0.1% β-mercaptoethanol, 100 U penicillin/mL, and 100 μg/ml streptomycin (all Gibco, Invitrogen Incorporation, Grand Island, NY). Tissues were mechanically dissociated and incubated with 100 μl of collagenase/dispase (10 mg/ml, Roche Diagnostics, Indianapolis, IN) and 300 μl DNase (10 mg/ml, Sigma) for 45 min at 37°C. The suspension was then passed through a 70-μm cell strainer, pelleted at 2,000 × g for 10 min, and resuspended in 60% isotonic Percoll (GE Healthcare, Pittsburgh, PA) solution, overlaid with 30%, and centrifuged at 1,000 × g for 25 min. Brain mononuclear cells were harvested at the 60% and 30% inter-phase layer.
Peripheral blood leukocytes were overlaid on 4 ml Lympholyte-M and centrifuged at 800 ×g for 20 min. Leukocytes at the interface were harvested and washed with complete RPMI.
Flow cytometry
Cells were washed in PBS and then blocked with 50 μl Fc block [10% CD16/CD32 10 μg/ml, BD Biosciences, 0.5% normal rat IgG in FACS buffer (1× PBS, 0.2% BSA, and 2 mM EDTA)] for 15 min prior to staining with CD45-APC, CD11b-PerCp Cy5.5, Ly6G-Pacific Blue, CD11c-PECy7, CD3-FITC, CD19-FITC, NK1.1-FITC, and Gr-1-PE (eBioscience) for 15 min. Data were acquired on a BD Canto II using FACsDIVA 6.0 software (BD Biosciences). Analysis was performed using FlowJo software (Treestar Inc., Ashland, OR). Microglia were identified as CD45intCD11b+Gr-1- cells. Neutrophils were identified as CD45hiCD3-CD19-NK1.1-CD11b+Ly6G+ F4/80- cells. Monocytes were identified as CD45hiCD3-CD19-NK1.1-CD11b+Ly6G-CD11c-F4/80int cells. Dendritic cells were identified as CD45hiCD3-CD19-NK1.1-CD11b+Ly6G-CD11c+ cells.
Statistical analysis
Cell counts by immunohistochemistry and flow cytometry were tested for normality, and differences between treatment groups were compared by two-sided t-test or Wilcoxon rank sum, as appropriate. Cells counts in peripheral blood were compared by t-test. The ratios of ipsilateral/contralateral neutrophils, monocytes, and dendritic cells in the brain were calculated to account for differences in the success of perfusion in each mouse and compared by t-test. Cylinder test results on each day were compared by ANOVA followed by t-tests for each pair of cohorts since the comparisons were pre-specified and distribution was normal. Analysis was performed with Stata IC/10 (College Station, TX).
Results
Localized Inflammatory Response After Experimental ICH
In order to better understand how inflammation contributes to the pathogenesis of ICH, a murine model of autologous blood injection was used. In these studies, mice were infused with 15 μl of blood into the right striatum or a sham injection, and at 3 days post injection brain tissue was removed and used for immunohistochemistry and flow cytometry. Neutrophils were identified immunohistochemically by characteristic bright Ly6G staining and multi-lobed nuclei. Rare neutrophils were found in the needle track after sham surgery (Fig. 1a). In contrast, after ICH, neutrophils were readily identified at the rim of the lesion but did not extend more than 50 μm from the edge of the hematoma (Fig. 1b). The median number of neutrophils in five 40× perihematomal fields was 21.5 neutrophils (IQR 16–22) after ICH and two [2, 9, 10] after sham surgery, p = 0.006.
Fig. 1.
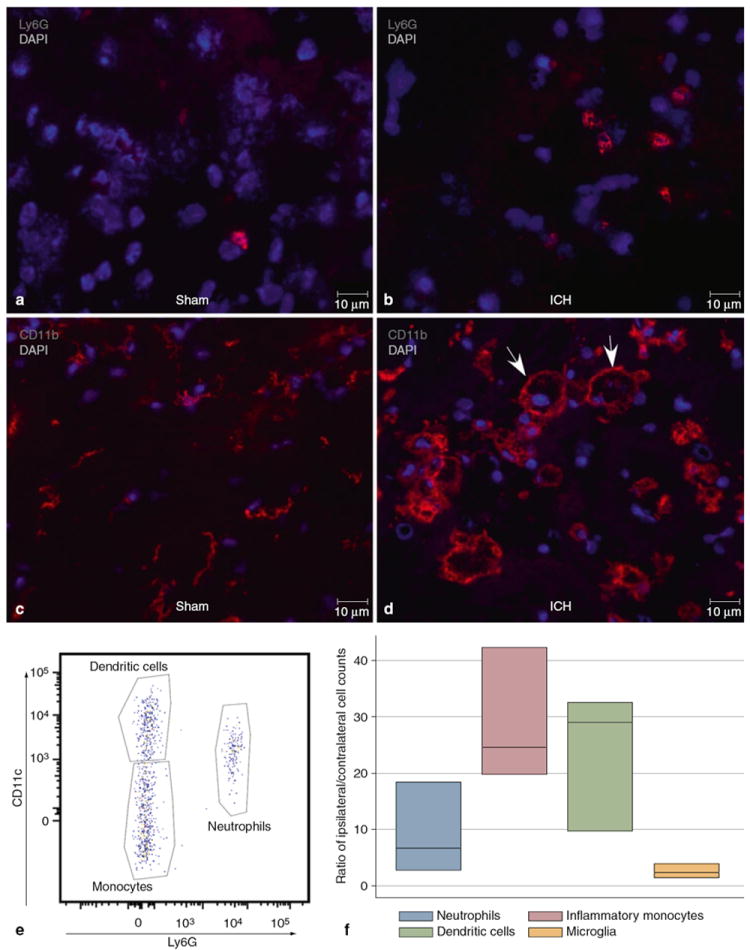
Immunohistochemistry of perihematomal brain post-ICH day 3 in an untreated mouse. (a) Ly6G staining (red) identifies rare neutrophils after sham surgery. (b) Increased numbers of infiltrating neutrophils are seen at the periphery of the hematoma. (c) After sham surgery, most CD11b+ (myeloid-derivative) cells are small and resemble resting microglia. (d) After ICH, CD11b+ cells have differing morphologies both within and at the periphery of the hematoma. Larger, vacuolated cells are indicated by the arrows. (e) Flow cytometry plot of CD45hiCD3-CD19-NK1.1-CD11b+ cells plotted Ly6G against CD11c to show three separate populations of dendritic cells (CD11c+Ly6G-), inflammatory monocytes (CD11c-Ly6G-), and neutrophils (Ly6G+). (f) Ratios of ipsilateral/contralateral cell counts in brain, n=3
Also present in both cohorts were CD11b + cells of different morphologies. After sham surgery most CD11b + cells had small cell bodies with multiple processes, resembling resting microglia (Fig. 1c). At the hematoma there were also large CD11b + cells, with at least 1 diameter greater than 10 μm, and vacuolated cytoplasm, which were present within the hemorrhage and extended 50–100 μm from the edge of the hematoma, and smaller cells with processes and without vacuolated cytoplasm (Fig. 1d). Quantification of the populations of CD11b + cells revealed that the ICH resulted in approximately a 60% increase in the numbers, with a mean of 255.6 ± 77.3 cells in five 20× fields after ICH and 161.7 ± 22.5 cells after sham, p > 0.05.
Consistent with the immunohistochemistry, flow cytometric analysis of the mononuclear cell preparations revealed that the inflammatory infiltrate consisted of neutrophils, monocytes, dendritic cells, and microglia (gating shown in Fig. 1e). The ratios of cells in the ipsilateral/contralateral hemispheres are shown in Fig. 1f. In the ipsilateral hemisphere, there was a 9-fold increase in neutrophils, a 29-fold increase in monocytes, a 24-fold increase in dendritic cells, and a 3-fold increase in microglia after ICH. These results confirm an intense, localized inflammation associated with experimental ICH.
Neutrophil Depletion Reduced Monocyte Infiltration into Perihematomal Brain
The presence of perihematomal neutrophils after ICH and their association with poor outcome in other models of sterile brain injury led to experiments to assess the contribution of these cells to the development of inflammation. Therefore, prior to ICH surgery, mice were treated with a neutrophil-specific (anti-Ly6G) antibody that spared other myeloid cell lines [10], but led to the depletion of 96.5% of blood neutrophils (data not shown). In these experiments, neutrophil depletion did not alter the twofold increase in ipsilateral microglia observed after ICH. However, this treatment did result in a significant reduction in the number of monocytes that infiltrated into perihematomal brain (neutrophil-depleted mean ratio 2.8 ± 1.7 vs. control 10.2 ± 6.6, p < 0.05). There was also a trend toward fewer dendritic cells in the neutrophil-depleted mice, although this did not reach significance. The ratios of cell counts by treatment are shown in Fig. 2a. These results indicate an important role for neutrophils in recruiting monocytes to the injured brain.
Fig. 2.
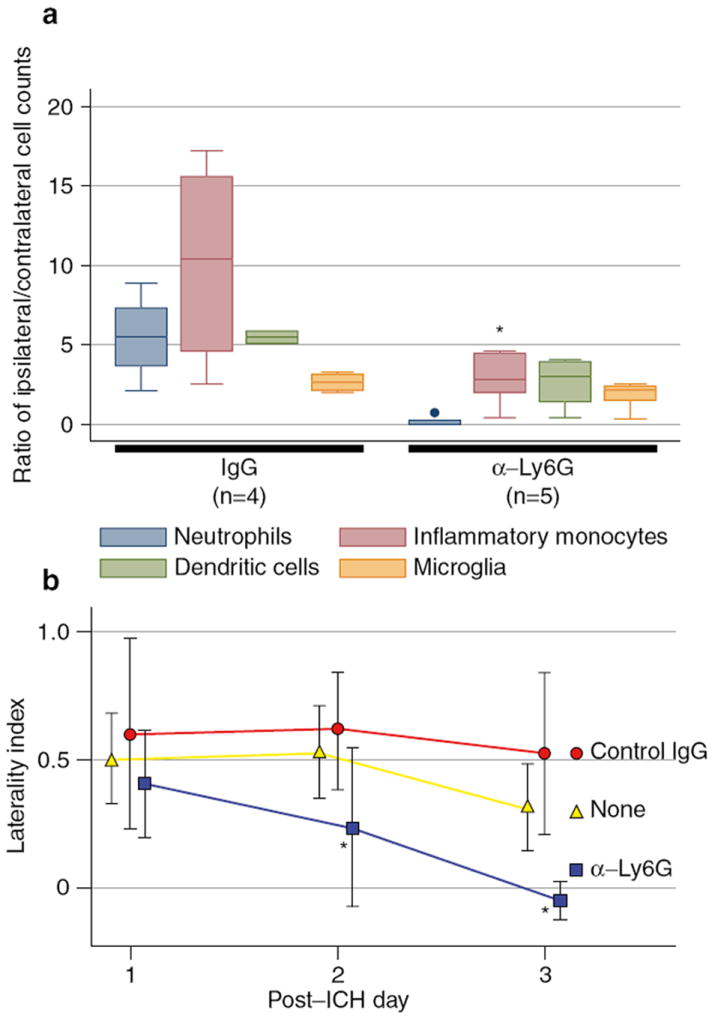
Neutrophil depletion reduced inflammatory response and improved functional outcome. (a) Ratios of ipsilateral/contralateral cell counts in brain after neutrophil depletion or control IgG treatment. (b) Cylinder score results on post-ICH days 1-3 by treatment
Neutrophil Depletion Improved Functional Outcome
To assess the importance of the modified inflammatory response on injury and recovery, the laterality index on the cylinder test was compared among neutrophil-depleted, control IgG-treated, and untreated mice. There was no difference in the laterality indices among untreated (n = 5), control IgG-treated (n = 5), and neutrophil-depleted mice (n = 6) on post-ICH day 1 (Fig. 2b). However, the neutrophil-depleted mice significantly improved each day (post-ICH day 2: p = 0.03, post-ICH day 3: p = 0.002, laterality indices compared by ANOVA). On post-ICH day 3 they had no measurable forelimb preference (laterality index mean −0.14 ± 0.23), whereas the other two groups remained with significant left-sided weakness (untreated laterality index 0.32 ± 0.17; control IgG-treated 0.52 ± 0.31). There was no difference in the laterality indices between untreated and IgG-treated mice at any time point. Thus, neutrophil depletion resulted in improved functional recovery after ICH, indicating that neutrophils are a mediator of secondary injury and a potential target for therapeutic intervention.
Discussion
A substantial neutrophil infiltration was identified in the perihematomal brain on day 3 after ICH by immunohistochemistry and flow cytometry. Thus, we hypothesized that neutrophil infiltration into brain after sterile injury contributes to secondary brain injury. Neutrophil depletion prior to ICH with a selective (anti-Ly6G) antibody led to significantly fewer neutrophils and monocytes infiltrating into the perihematomal region and improved functional outcome. The basis for this protection is unclear, but possible mechanisms include decreased direct brain injury by neutrophil degranulation and decreased monocyte recruitment. The timing of granule release from neutrophils is coordinated with the stage of migration into tissues, with primary and secondary granules being released after successful extravasation into tissue [11]. Contents of these granules include collagenase, elastase, and myeloperoxidase, among others, which may cause direct injury to neurons and astrocytes and contribute to the poor outcomes seen after ICH. Neutrophil-derived serine proteases also regulate the immune response via cytokine activation and release, including IL-8 [12], IL-6 [13], TNF-α, and IL-1β [9, 14], which may contribute to additional injury [15-18]
Neutrophils have a key role in the recruitment of monocytes to infected or injured tissue. When neutrophils adhere to the endothelial surface, the contents of secretory granules are released, including cationic antimicrobial protein of 37 kd (CAP37)/azurocidin and proteinase 3, leading to endothelial cell activation, increased cellular adhesion molecule expression, and increased monocyte adhesion [19-21]. Neutrophils also contribute to monocyte chemotaxis by releasing LL-37 [22] and cathepsin G [23], and enhancing endothelial cell release of monocyte chemoattractant protein-1 [24]. Thus, our observation that neutrophil depletion led to decreased monocyte infiltration after ICH is consistent with a model in which the early recruitment of neutrophils establishes a local environment that allows monocytes to enter the injured brain. Nevertheless, it is unclear based on this study whether the diminished monocyte response contributed to the benefit on functional outcome. Monocytes may contribute to acute injury by releasing inflammatory cytokines and chemokines. Alternatively, monocytes may contribute to recovery by aiding in phagocytosis of red blood cells and cellular debris or by shifting response to a less inflammatory one. Additional studies will be required to elucidate the role of monocytes in the perihematomal region.
An early study on the detrimental effect of circulating leukocytes after ICH demonstrated decreased cerebral edema after whole body irradiation and increased edema after brain irradiation [25]. The irradiation depleted all leukocyte cell lines and platelets and used a microballoon (inert) model of ICH, indicating that blood-derived cells contribute to local inflammation after tissue disruption in the brain. More recently, CD18 deficiency has been shown to reduce brain edema after ICH, confirming the role of leukocyte migration in the pathogenesis of inflammation after ICH [26]. Identification of which immune cells are pathogenic in the acute phase after ICH may lead to new potential therapies in blocking specific cell trafficking and signaling while leaving beneficial responses intact. Thus, the finding of an injurious role of neutrophils after ICH invites further investigation of potential therapies to mitigate their effect. While antagonizing neutrophil migration or function may leave critically ill patients at high risk of infection, selective inhibition of neutrophil trafficking across the blood-brain barrier or antagonism of specific neutrophil-mediated detrimental processes may offer more specific/tangible benefits.
Acknowledgments
Funded by a fellowship from the Institute for Translational Medicine and Therapeutics, University of Pennsylvania (LHS), T32-AI055400 and AI081478 (TH), and AI41158 (CAH).
Footnotes
Disclosure/conflict of interest statement We declare that we have no conflict of interest.
Contributor Information
Lauren H. Sansing, Email: lauren.sansing@uphs.upenn.edu, Department of Neurology, University of Pennsylvania Medical Center, 3 W Gates, 3400 Spruce Street, Philadelphia, PA, USA.
Tajie H. Harris, Department of Pathobiology, School of Veterinary Medicine, University of Pennsylvania, Philadelphia, PA, USA
Scott E. Kasner, Department of Neurology, University of Pennsylvania Medical Center, Philadelphia, PA, USA
Christopher A. Hunter, Department of Pathobiology, School of Veterinary Medicine, University of Pennsylvania, Philadelphia, PA, USA
Katalin Kariko, Department of Neurosurgery, University of Pennsylvania Medical Center, Philadelphia, PA, USA.
References
- 1.Wasserman JK, Schlichter LC. Neuron death and inflammation in a rat model of intracerebral hemorrhage: effects of delayed minocycline treatment. Brain Res. 2007;1136:208–218. doi: 10.1016/j.brainres.2006.12.035. [DOI] [PubMed] [Google Scholar]
- 2.Bigio MRD, Yan HJ, Buist R, Peeling J, del Zoppo GJ. Experimental intracerebral hemorrhage in rats: magnetic resonance imaging and histopathological correlates. Stroke. 1996;27:2312–2320. doi: 10.1161/01.str.27.12.2312. [DOI] [PubMed] [Google Scholar]
- 3.Gong C, Hoff JT, Keep RF. Acute inflammatory reaction following experimental intracerebral hemorrhage in rat. Brain Res. 2000;871:57–65. doi: 10.1016/s0006-8993(00)02427-6. [DOI] [PubMed] [Google Scholar]
- 4.Guo FQ, Li XJ, Chen LY, Yang H, Dai HY, Wei YS, Huang YL, Yang YS, Sun HB, Xu YC, Yang ZL. Study of relationship between inflammatory response and apoptosis in perihematoma region in patients with intracerebral hemorrhage. Zhongguo Wei Zhong Bing Ji Jiu Yi Xue. 2006;18:290–293. [PubMed] [Google Scholar]
- 5.Xue M, Del Bigio MR. Intracerebral injection of autologous whole blood in rats: time course of inflammation and cell death. Neurosci Lett. 2000;283:230–232. doi: 10.1016/s0304-3940(00)00971-x. [DOI] [PubMed] [Google Scholar]
- 6.Stowe AM, Adair-Kirk TL, Gonzales ER, Perez RS, Shah AR, Park TS, Gidday JM. Neutrophil elastase and neurovascular injury following focal stroke and reperfusion. Neurobiol Dis. 2009;35:82–90. doi: 10.1016/j.nbd.2009.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Murikinati S, Juttler E, Keinert T, Ridder DA, Muhammad S, Waibler Z, Ledent C, Zimmer A, Kalinke U, Schwaninger M. Activation of cannabinoid 2 receptors protects against cerebral ischemia by inhibiting neutrophil recruitment. FASEB J. 2010;24:788–798. doi: 10.1096/fj.09-141275. [DOI] [PubMed] [Google Scholar]
- 8.Gautier S, Ouk T, Petrault O, Caron J, Bordet R. Neutrophils contribute to intracerebral haemorrhages after treatment with recombinant tissue plasminogen activator following cerebral ischaemia. Br J Pharmacol. 2009;156:673–679. doi: 10.1111/j.1476-5381.2009.00068.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Coeshott C, Ohnemus C, Pilyavskaya A, Ross S, Wieczorek M, Kroona H, Leimer AH, Cheronis J. Converting enzyme- independent release of tumor necrosis factor Î ± and IL-1Î2 from a stimulated human monocytic cell line in the presence of activated neutrophils or purified proteinase 3. Proc Natl Acad Sci USA. 1999;96:6261–6266. doi: 10.1073/pnas.96.11.6261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Daley JM, Thomay AA, Connolly MD, Reichner JS, Albina JE. Use of Ly6G-specific monoclonal antibody to deplete neutrophils in mice. J Leukoc Biol. 2008;83:64–70. doi: 10.1189/jlb.0407247. [DOI] [PubMed] [Google Scholar]
- 11.Soehnlein O, Weber C, Lindbom L. Neutrophil granule proteins tune monocytic cell function. Trends Immunol. 2009;30:538–546. doi: 10.1016/j.it.2009.06.006. [DOI] [PubMed] [Google Scholar]
- 12.Padrines M, Wolf M, Walz A, Baggiolini M. Interleukin-8 processing by neutrophil elastase, cathepsin G and proteinase-3. FEBS Lett. 1994;352:231–235. doi: 10.1016/0014-5793(94)00952-x. [DOI] [PubMed] [Google Scholar]
- 13.Li T, Wang H, He S. Induction of interleukin-6 release from monocytes by serine proteinases and its potential mechanisms. Scand J Immunol. 2006;64:10–16. doi: 10.1111/j.1365-3083.2006.01772.x. [DOI] [PubMed] [Google Scholar]
- 14.Elssner A, Duncan M, Gavrilin M, Wewers MD. A novel P2X7 receptor activator, the human cathelicidin-derived peptide LL37, induces IL-1{beta} processing and release. J Immunol. 2004;172:4987–4994. doi: 10.4049/jimmunol.172.8.4987. [DOI] [PubMed] [Google Scholar]
- 15.Fang HY, Ko WJ, Lin CY. Inducible heat shock protein 70, interleukin-18, and tumor necrosis factor alpha correlate with outcomes in spontaneous intracerebral hemorrhage. J Clin Neurosci. 2007;14:435–441. doi: 10.1016/j.jocn.2005.12.022. [DOI] [PubMed] [Google Scholar]
- 16.Holmin S, Mathiesen T. Intracerebral administration of inter-leukin-1beta and induction of inflammation, apoptosis, and vasogenic edema. J Neurosurg. 2000;92:108–120. doi: 10.3171/jns.2000.92.1.0108. [DOI] [PubMed] [Google Scholar]
- 17.Hua Y, Wu J, Keep RF, Nakamura T, Hoff JT, Xi G. Tumor necrosis factor-alpha increases in the brain after intracerebral hemorrhage and thrombin stimulation. Neurosurgery. 2006;58:542–550. doi: 10.1227/01.NEU.0000197333.55473.AD. discussion 542–550. [DOI] [PubMed] [Google Scholar]
- 18.Mayne M, Ni W, Yan HJ, Xue M, Johnston JB, Del Bigio MR, Peeling J, Power C. Antisense oligodeoxynucleotide inhibition of tumor necrosis factor-alpha expression is neuroprotective after intracerebral hemorrhage. Stroke. 2001;32:240–248. doi: 10.1161/01.str.32.1.240. [DOI] [PubMed] [Google Scholar]
- 19.Lee TD, Gonzalez ML, Kumar P, Grammas P, Pereira HA. CAP37, a neutrophil-derived inflammatory mediator, augments leukocyte adhesion to endothelial monolayers. Microvasc Res. 2003;66:38–48. doi: 10.1016/s0026-2862(03)00010-4. [DOI] [PubMed] [Google Scholar]
- 20.Soehnlein O, Xie X, Ulbrich H, Kenne E, Rotzius P, Flodgaard H, Eriksson EE, Lindbom L. Neutrophil-derived heparin-binding protein (HBP/CAP37) deposited on endothelium enhances monocyte arrest under flow conditions. J Immunol. 2005;174:6399–6405. doi: 10.4049/jimmunol.174.10.6399. [DOI] [PubMed] [Google Scholar]
- 21.Taekema-Roelvink MEJ, Van Kooten C, Heemskerk E, Schroeijers W, Daha MR. Proteinase 3 interacts with a 111-kD membrane molecule of human umbilical vein endothelial cells. J Am Soc Nephrol. 2000;11:640–648. doi: 10.1681/ASN.V114640. [DOI] [PubMed] [Google Scholar]
- 22.Yang D, Chen Q, Schmidt AP, Anderson GM, Wang JM, Wooters J, Oppenheim JJ, Chertov O. Ll-37, the neutrophil granule- and epithelial cell-derived cathelicidin, utilizes formyl peptide receptor-like 1 (FPRL1) as a receptor to chemoattract human peripheral blood neutrophils, monocytes, and T cells. J Exp Med. 2000;192:1069–1074. doi: 10.1084/jem.192.7.1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sun R, Iribarren P, Zhang N, Zhou Y, Gong W, Cho EH, Lockett S, Chertov O, Bednar F, Rogers TJ, Oppenheim JJ, Wang JM. Identification of neutrophil granule protein cathepsin G as a novel chemotactic agonist for the G protein-coupled formyl peptide receptor. J Immunol. 2004;173:428–436. doi: 10.4049/jimmunol.173.1.428. [DOI] [PubMed] [Google Scholar]
- 24.Taekema-Roelvink MEJ, Kooten CV, Kooij SVD, Heemskerk E, Daha MR. Proteinase 3 enhances endothelial monocyte chemoattractant protein-1 production and induces increased adhesion of neutrophils to endothelial cells by upregulating intercellular cell adhesion molecule-1. J Am Soc Nephrol. 2001;12:932–940. doi: 10.1681/ASN.V125932. [DOI] [PubMed] [Google Scholar]
- 25.Kane PJ, Modha P, Strachan RD, Cook S, Chambers IR, Clayton CB, Mendelow AD. The effect of immunosuppression on the development of cerebral oedema in an experimental model of intracerebral haemorrhage: whole body and regional irradiation. J Neurol Neurosurg Psychiatry. 1992;55:781–786. doi: 10.1136/jnnp.55.9.781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Titova E, Kevil CG, Ostrowski RP, Rojas H, Liu S, Zhang JH, Tang J. Deficiency of CD18 gene reduces brain edema in experimental intracerebral hemorrhage in mice. Acta Neurochir Suppl. 2008;105:85–87. doi: 10.1007/978-3-211-09469-3_17. [DOI] [PubMed] [Google Scholar]


