Abstract
Rationale
The leukocyte response in acute inflammation is characterized by an initial recruitment of neutrophils preceding a second wave of monocytes. Neutrophil-derived granule proteins were suggested to hold an important role in this cellular switch. The exact mechanisms by which neutrophils mediate these processes are only partially understood.
Objective
To investigate the role of neutrophils and their granule contents in the adhesion of monocyte subpopulations in acute inflammation.
Methods and Results
Here, we show that neutrophil-derived cathelicidins (human: LL37, mouse: CRAMP) induce adhesion of classical monocytes but not of non-classical monocytes in the mouse cremaster muscle and in in vitro flow chamber assays. CRAMP is released from emigrated neutrophils and then transported across the endothelium where it is presented to rolling leukocytes. Endothelial-bound cathelicidin activates FPR2 on classical monocytes, resulting in monocytic β1- and β2-integrin conformational change towards an extended, active conformation that allows for adhesion to their respective ligands VCAM-1 and ICAM-1.
Conclusions
These data elucidate a novel mechanism of neutrophil-mediated monocyte recruitment, which could be targeted in conditions where recruitment of classical monocytes plays an unfavorable role.
Keywords: Neutrophil, monocyte, inflammation, cathelicidin, recruitment
Introduction
Neutrophils dominate the initial wave of leukocytes emigrating into inflamed tissues (1). This first wave of neutrophil extravasation precedes a second wave of monocyte extravasation. Recruited neutrophils are thought to contribute to this cellular switch by several mechanisms (2, 3) including the release of soluble factors like neutrophil granule proteins which are deposited at the site of inflammation (4). Indeed, supernatants of activated neutrophils from patients with specific granule deficiency show a reduced capacity to attract monocytes despite normal monocyte chemotaxis in vitro to other stimuli (5). Following this observation, several neutrophil granule proteins with monocyte-attracting activity were identified. Among them are cathelicidin (human: LL37, mouse: CRAMP) (6), cathepsin G (7), α-defensins (8), and azurocidin (7).
Peripheral blood monocytes constitute a heterogeneous population of circulating leukocytes in both humans (9) and mice (10). Based on the expression of Gr1, classical (inflammatory, Ly6Chi) and non-classical (resident, patrolling, Ly6Clo) mouse monocytes can be discriminated. Functionally, non-classical monocytes are thought to contribute to phagocytosis, wound healing, and tissue remodeling, whereas classical monocytes dominate inflammatory processes such as the early response in infections, but also sterile inflammatory conditions such as myocardial infarction or atherosclerosis (11). In humans, monocyte subsets are discriminated based on their CD14 and CD16 expression profile with classical monocytes being CD14++CD16−, intermediate monocytes being CD14++CD16+, and nonclassical monocytes being CD14+CD16++ (12).
While several studies have shown the important chemotactic activities of neutrophil granule proteins towards monocytes, the role of neutrophil secretory products in early steps of monocyte recruitment have not been studied. Hence, we here investigated the role of neutrophils and their granule constituents in their capacity to differentially induce adhesion of monocyte subsets.
Methods
For detailed Materials and Methods, please see the supplemental materials.
Mice
Wild-type C57BL/6 and gene-targeted Lysmgfp/gfp (13), Cx3cr1eGFP/+ (14), Cramp−/− (15), Fpr1−/− (16), Fpr2−/− (17), CG−/− (18), Darc−/− (19), and Cav1−/− (20) mice were used for in vivo experiments. All genetically modified animals were on C57BL/6 background. Specific neutrophil depletion was achieved by intraperitoneal injection of monoclonal antibody 1A8 (50µg/mouse, BioXCell) 8h before injection of the stimuli (Online Table I). All animal experiments were approved by the local ethical committee for animal experimentation.
Results
Cathelicidin induces firm adhesion of classical monocytes
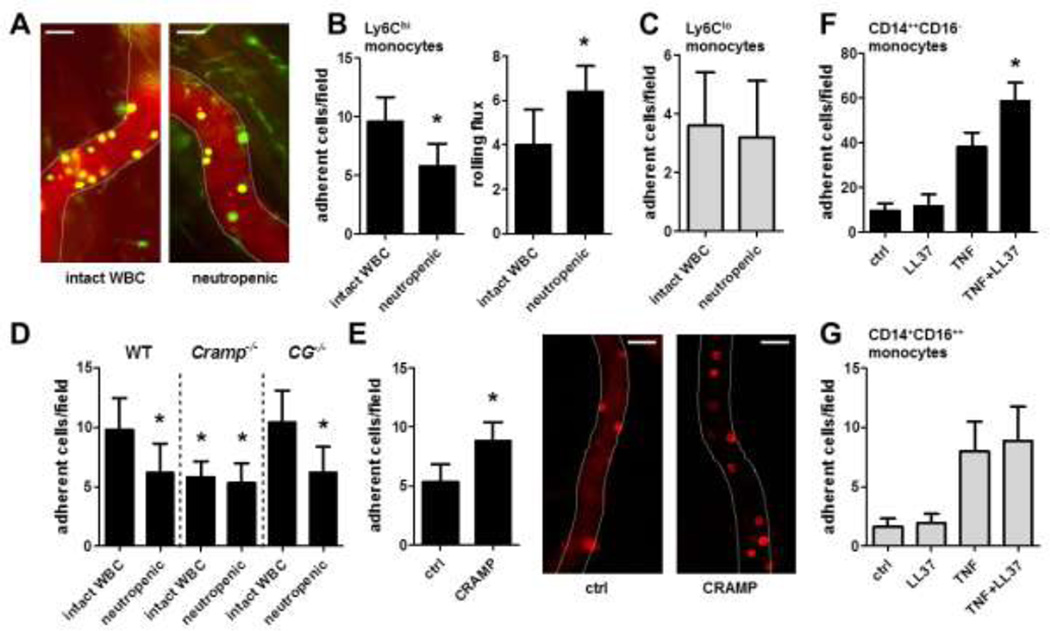
To study the importance of neutrophils in the adhesion of monocytes we injected TNF into the cremaster of Cx3cr1egfp/wt mice carrying green fluorescent monocytes and recorded the adhesion of monocytes in vivo. To discriminate between classical monocytes and non-classical monocytes, an antibody to Ly6C was introduced which labels classical monocytes (Online Figure I). In these experiments neutropenic mice displayed reduced adhesion of classical monocytes (Ly6C+/gfp+) to postcapillary venules, whereas rolling flux of classical monocytes was increased (Figure 1A,B). In contrast, adhesion of non-classical monocytes (Ly6C−/gfp+) remained unaffected (Figure 1A,C). With the reported importance of neutrophil granule proteins in the recruitment of monocytes (4) we aimed at addressing the role of granule proteins in monocyte adhesion. Neutrophil-derived azurocidin, cathepsin G, α-defensins, and cathelicidins have been suggested to be involved in neutrophil-dependent monocyte activation (2, 3). As azurocidin and defensins are absent in mouse neutrophils (21, 22), we focused on the role of cathepsin G and cathelicidin in neutrophil-mediated monocyte adhesion. Adhesion of classical monocytes in TNF-stimulated cremaster muscles was impaired in cathelicidin-deficient (Cramp−/−) mice when compared to wild type (WT) control animals, a response not further aggravated by neutrophil depletion (Figure 1D). In addition, intrascrotal injection of CRAMP restored the defect in Cramp−/− mice (Figure 1E). In contrast, cathepsin G-deficient (CG−/−) mice exhibited no impaired adhesion of classical monocytes (Figure 1D). In line, neutrophil depletion reduced classical monocyte adhesion in CG−/− mice indicating that not cathepsin G but CRAMP is relevant for neutrophil-dependent monocyte adhesion.
Figure 1. Neutrophil-derived cathelicidin enhances adhesion of inflammatory monocytes.
A–C: Neutropenia reduces adhesion of classical monocytes. Cx3cr1egfp/WT mice with intact white blood cell count or neutropenia (1A8, 50µg i.p., 12h prior to experiment) were injected intrascrotally with TNF (50ng, 12h). To discriminate between monocyte subsets an antibody to Ly6C (1µg) was introduced i.v. A: Representative images, classical monocytes appear in yellow, non-classical monocytes appear in green, scale bar 20µm. B: Adhesion and rolling flux of classical monocytes. * significant difference from respective control. C: Adhesion of non-classical monocytes. n=6. D: Comparison of adhesion of Ly6C-labeled classical monocytes in indicated mouse strains with intact white blood cell count or neutropenia following intrascrotal TNF injection (50ng, 12h). * significant difference compared to wild type (WT) mice with intact white blood cell count. n=6. E: Intrascrotal injection of CRAMP in Cramp−/− mice restores adhesion of classical monocytes. Cramp−/− were intrascrotally injected with TNF (ctrl) or with TNF and CRAMP (CRAMP, 1µg). Adhesion of Ly6C-labeled classical monocytes was assessed by intravital microscopy. n = 5. Representative images are shown. Scale bar 20µm. F/G: Human classical CD14++CD16− (F) or non-classical CD14+CD16++ monocytes (G) were perfused over TNF-activated (20ng, 12h) HUVEC monolayer and the number of adherent cells was quantified. LL37 (1µg) was deposited 15 min prior to perfusion. n=6. * significant difference compared to TNF-activated HUVEC.
To further corroborate our findings with human monocyte subsets, we performed in vitro flow chamber assays. Human classical CD14++CD16− (Figure 1F) and non-classical CD14+CD16++ monocytes (Figure 1G) were perfused over human umbilical vein endothelial cells (HUVEC). Adhesion of either subset was enhanced when HUVEC were activated with TNF. However, deposition of LL37 on HUVEC 15min prior to monocyte perfusion specifically enhanced adhesion of classical CD14++CD16− monocytes. As treatment of HUVEC with LL37 did not impact on expression of endothelial adhesion molecules (Online Figure II), we concluded that the LL37-mediated increase in monocyte adhesion is not achieved by modulating endothelial cell adhesion molecule expression.
Neutrophil-derived cathelicidin is transported in a baso-apical direction across the endothelium
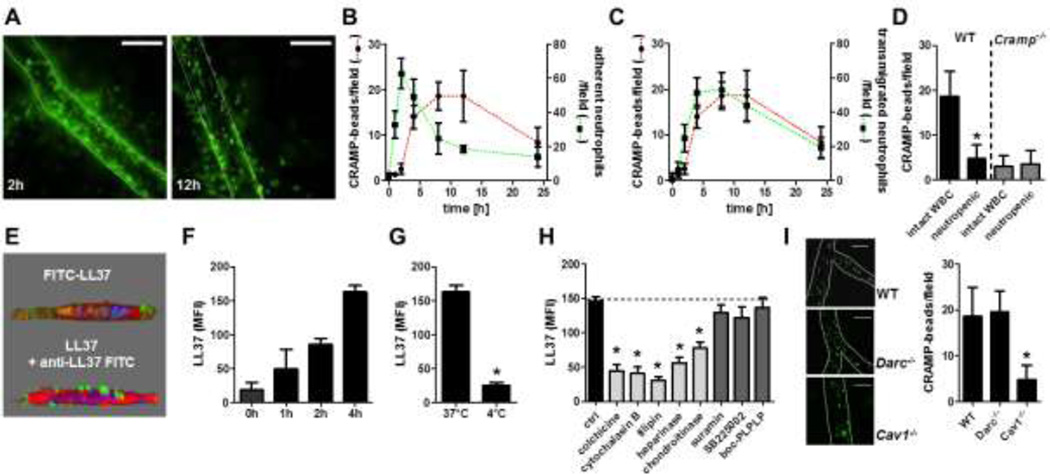
To study the dynamics of cathelicidin release and in vivo presentation, we injected monocyte-depleted Lysmgfp/gfp mice (thus creating mice with fluorescent neutrophils only) with TNF intrascrotally and recorded the adhesion and extravasation of neutrophils. In addition, microbeads conjugated with an antibody to CRAMP were injected to visualize endothelial bound CRAMP (Figure 2A). While adhesion of neutrophils occurred rapidly and peaked at 2h, endothelial-bound CRAMP was not detected in significant numbers until 4h after TNF stimulation thus correlating more closely with the number of emigrated neutrophils (Figure 2B,C). Detection of endothelial CRAMP peaks between 4 and 12h after TNF injection. During this time, neutrophil adhesion decreases, whereas the number of emigrated neutrophils reaches its maximum (Figure 2B,C). Depletion of neutrophils reduced the number of adherent anti-CRAMP-conjugated microbeads to a level observed in Cramp−/− mice thus confirming that CRAMP in this location is neutrophil derived (Figure 2D). To further investigate alternative CRAMP sources, we analyzed intracellular CRAMP expression in endothelial cells and in neutrophils obtained from enzymatically digested cremaster muscles. To discriminate between adherent and extravasated neutrophils an antibody to Ly6G was injected intravenously 5 minutes prior to sacrifice (23). In these experiments, minor amounts of CRAMP were detected in endothelial cells (figure S3). In contrast, we detected large amounts of CRAMP in adherent neutrophils that were decreased in emigrated neutrophils, suggesting discharge of CRAMP following emigration (Online Figure III).
Figure 2. Neutrophil-derived cathelicidin is transported in a baso-apical direction across the endothelium.
A–C: Luminal CRAMP accumulation parallels with neutrophil extravasation but not neutrophil adhesion. Lysmegfp/egfp mice were intrascrotally injected with TNF (50ng) and the adhesion (B, green line) and transmigration (C, green line) of neutrophils was quantified by intravital microscopy at indicated time points. To assess luminal CRAMP presentation, immobilization of G-protein coupled fluorescent beads conjugated with an antibody to CRAMP was analysed (red line in B and C). n=4 for each data point. Representative images for indicated time points are shown in A. For better visibility, immobilized beads were marked with red dots. Scale bar, 50µm. D: Wild type (WT) or Cramp−/− mice with intact white blood cell count (WBC) or neutropenia were intrascrotally injected with TNF (50ng, 12h). Luminal CRAMP presentation was assessed after injection of G-protein coupled fluorescent beads conjugated with antibodies to CRAMP. * indicates significant difference compared to respective mouse strain with intact WBC. n=5. E: LL37 is transported across HUVEC grown on transwell filter inserts. LL37 was added to the bottom well and analyses were made 4h later by 2-photon microscopy. LL37 (green) was visualized by a fluorescent tag (top) or by antibody staining of non-permeabilized endothelial cells (bottom). Cell membrane, red; Nucleus, blue. F: Antibody detection of transcytosed LL37 on the apical side of endothelial cells in time course experiments. G/H: Transcytosis experiments performed at 4 °C (G) or by pretreatment of endothelial cells with indicated inhibitors (H). n=6 for each bar. * significant difference from ctrl. I: CRAMP transportation is impaired in Cav1−/− mice. Neutropenic WT, Darc−/−, or Cav1−/− mice were intrascrotally injected with TNF (50ng, 4h) and CRAMP (1µg). Luminal CRAMP presentation was assessed after injection of G-protein coupled fluorescent beads conjugated with an antibody to CRAMP. Scale bar is 20µm in representative images. * significant difference compared to wild type mice. n=4.
Since our in vivo experiments suggested that cathelicidins are transported across the endothelium in a baso-apical direction, we aimed at studying such mechanism in vitro. HUVEC were grown on tissue culture filter inserts and stimulated with TNF. FITC-LL37 was added to the bottom well and LL37 transportation was assessed by two-photon microscopy. In these experiments LL37 was found to accumulate on the endothelial cell surface (Figure 2E). To quantify the amount of transcytosed LL37 we assessed the fluorescence resulting from antibody-stained, surface-bound LL37. In time course experiments we found that LL37 accumulated on the HUVEC surface not until 2h (figure 2F). The transendothelial transportation was abrogated at 4°C (Figure 2G) and by pretreatment with colchicine or cytochalasin B (Figure 2H). Cathelicidins were reported to act through FPR2, CXCR2, and P2X7 (7, 24, 25). However, pretreatment of HUVEC with boc-PLPLP (antagonist to FPRs), SB225002 (antagonist to CXCR2), or suramin (antagonist to P2X7) did not impact on transendothelial LL37 transportation (Figure 2H). As highly cationic polypeptide, we suspected that LL37 might interact with endothelial proteoglycans. To this end we cleaved endothelial heparansulfate or chondroitinsulfate side chains by use of heparinase or chondroitinase and recorded its effect on LL37 transcytosis. Interestingly, pretreatment with either enzyme markedly reduced the transendothelial transportation across the endothelium (Figure 2H). Finally, targeting caveolae-mediated transportation by pre-treatment with filipin resulted in clear-cut decreases of LL37 transcytosis. Since duffy antigen/receptor for chemokines (DARC) facilitates transport of chemokines across the endothelium (26) we also wanted to address its involvement in the transendothelial transportation of CRAMP. However, in our hands HUVEC activated with TNF did not express DARC and we could not detect an interaction between DARC and LL37 (Online Figure IV).
We next injected CRAMP intrascrotally into neutropenic WT mice and detected its luminal appearance by intravital microscopy. In WT mice luminal CRAMP was detected 4h after intrascrotal CRAMP injection, a response abrogated in caveolin 1-deficient (Cav−/−) mice (Figure 2I). Immobilisation of anti-CRAMP microbeads was not seen when PBS was used instead of CRAMP (not shown). However, the transportation of CRAMP across the endothelium was maintained in Darc−/− mice.
Cathelicidin-dependent recruitment requires FPR2 activation on monocytes
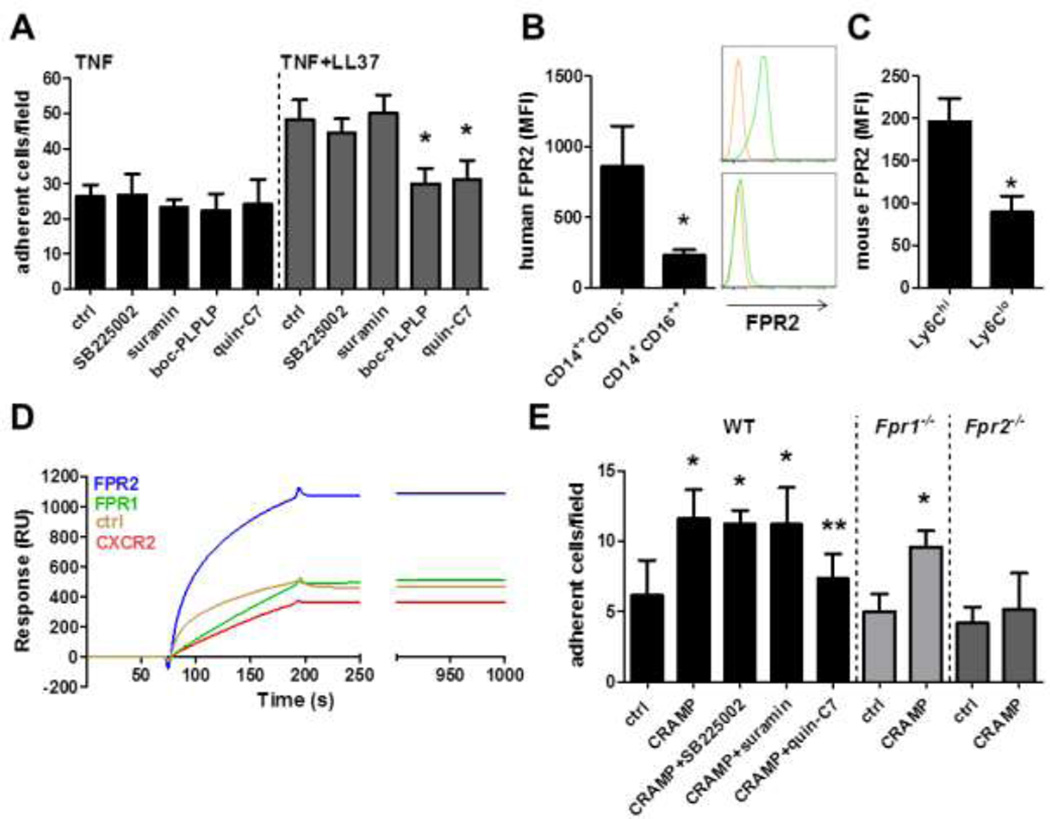
Cathelicidins were reported to activate target cells through various receptors including FPR2, CXCR2, and P2X7. To find out which of these receptors is relevant in cathelicidin-mediated adhesion of classical monocytes, we performed in vitro flow chamber assays (Figure 3A). Monocytes were pre-treated with suramin, SB225002, or boc-PLPLP and perfused over TNF-activated HUVEC. While all antagonists were without effects when classical CD14++CD16− monocytes were perfused over TNF-activated HUVEC, boc-PLPLP specifically abrogated LL37-mediated increases in classical CD14++CD16− monocyte adhesion. Since boc-PLPLP blocks activation of all FPR family members we further used specific antagonists to FPR1 and FPR2. In these experiments, the FPR2 specific antagonist quin-C7 (Figure 3A) but not the FPR1-specific antagonist spinorphin (not shown) abrogated LL37-dependent adhesion of classical CD14++CD16−monocytes.
Figure 3. Cathelicidin-induced monocyte adhesion requires FPR2.
A: Human classical CD14++CD16− monocytes were perfused over TNF-activated (20ng, 12h) HUVEC and the number of adherent cells was quantified. LL37 (1µg) was deposited 15 min prior to perfusion. Monocytes were left untreated (ctrl) or were pretreated for 15 min with SB225002 (blocks CXCR2, 100nM), suramin (blocks P2X7, 100nM), boc-PLPLP (blocks FPRs, 100nM), or quin-C7 (blocks FPR2, 1µM). * significant difference compared to respective control. n=8. B/C: FPR2 expression was analysed on human (B) or mouse (C) classical and non-classical monocytes using flow cytometry. Representative histograms in B display staining with isotype control IgG (orange) and a FPR2 antibody (green). D: Representative surface plasmon resonance sensorgrams of binding of indicated receptors expressed in proteoliposomes to immobilized LL37 on a CM4-sensorchip. Empty proteoliposomes were used as control. E: Wild type (WT), Fpr1−/−, or Fpr2−/− mice were rendered neutropenic and injected with TNF (ctrl) or with TNF and CRAMP (CRAMP, 1µg). Antagonists to CXCR2 (SB225002, 5mg/kg), P2X7 (suramin, 10mg/kg), and FPR2 (quin-C7, 10mg/kg) were administered i.v. 30 minutes prior to recording. Adhesion of Ly6C-labeled classical monocytes was assessed by intravital microscopy. n=6 for each bar. * significant difference compared to respective control group, ** significant difference compared to CRAMP-treated WT mice.
We further suspected that differences in FPR2 expression levels between monocyte subsets may explain the specificity of LL37 in enhancing adhesion of classical monocytes but not of non-classical monocytes. Analysis of FPR2 expression revealed that FPR2 is expressed to a higher degree on classical CD14++CD16− monocytes as compared to nonclassical CD14+CD16++ monocytes (Figure 3B), thus offering an explanation for the specificity of cathelicidin-dependent adhesion. Furthermore, the FPR2 expression on CD14++CD16+ monocytes (MFI 614±27) was intermediate, falling in between the FPR2 expression on classical and non-classical monocytes. In line with these observations, classical monocytes in mice also carry higher levels of FPR2 when compared to their non-classical counterpart (Figure 3C). As few data exist on direct interaction of LL37 with its putative receptors, we aimed at analyzing the interaction of LL37 and FPR1, FPR2, CXCR2, and P2X7 biochemically. For this we performed surface plasmon resonance experiments employing recombinant receptors expressed in proteoliposomes thus maintaining the three dimensional chemokine receptor structure. LL37 was immobilized on a CM4 chip and superfused with receptor-containing proteoliposomes (Figure 3D). With this approach we were able to detect a clear binding of LL37 to FPR2, while the interaction with FPR1 or CXCR2 was not different when compared to empty liposomes. For P2X7 we found no interaction with LL37 (not shown).
To further investigate if cathelicidin-mediated monocyte adhesion in vivo depends on employment of FPR2, we injected CRAMP along with TNF into the scrotum of neutropenic WT mice. Receptor utilization was initially studied by use of antagonists to FPRs, P2X7, and CXCR2. In these experiments inhibition of FPRs by quin-C7 clearly reduced CRAMP-mediated adhesion, while the other antagonists were without effects (Figure 3E). To further corroborate this, we injected neutropenic Fpr1−/− or Fpr2−/− mice with TNF and CRAMP. While CRAMP injection resulted in increased adhesion in Fpr1−/− mice, this response was not observed in Fpr2−/− mice (Figure 3E).
Cathelicidins induce integrin activation on classical monocytes
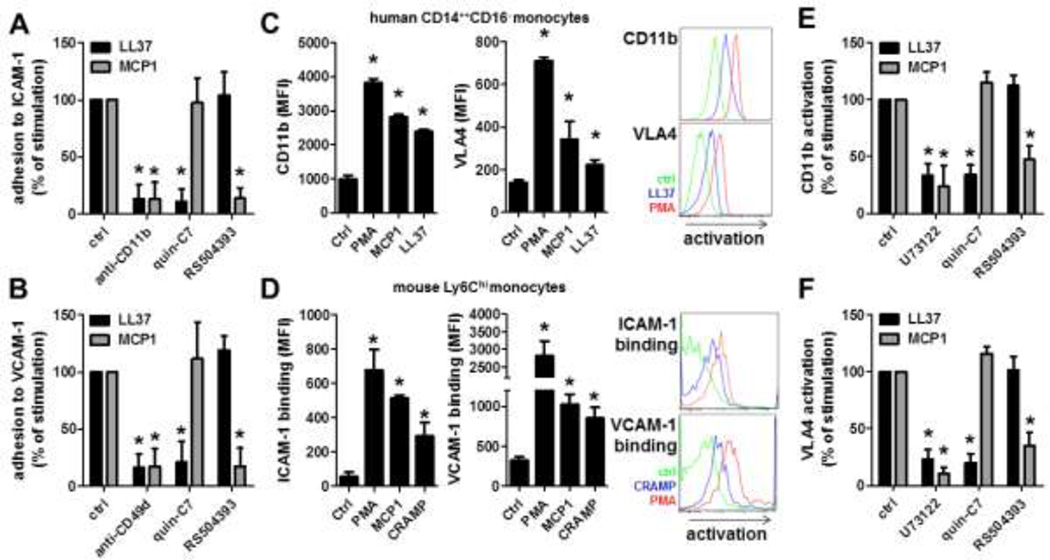
Thus far we have shown that neutrophil-derived cathelicidins resemble a functional similarity with arrest chemokines (27). The latter mediate firm adhesion through inside-out integrin signaling and subsequent changes in integrin conformation towards an extended, high affinity state. Classical ligands for VLA4 (CD49d/CD29, α4β1 integrins) and Mac1 (CD11b/CD18, αMβ2 integrin) are VCAM-1 and ICAM-1, respectively. To test the importance of these two ligands for LL37-mediated adhesion we perfused classical CD14++CD16− monocytes over plates coated with P-selectin/ICAM-1 or P-selectin/VCAM-1 (Figure 4A,B). In these experiments coimmobilization of LL37 or MCP1 strongly enhanced monocyte adhesion. This response was fully abrogated by a blocking antibody to CD11b or CD49d. While the LL37-dependent monocyte adhesion was abolished in presence of an inhibitor to FPR2, the MCP1-mediated adhesion of monocytes was blocked by an antagonist to CCR2. To further investigate the activation of β1- and β2-integrins by LL37, we used an antibody that specifically recognizes the extended conformation of these integrins. Monocytes were incubated with PMA, MCP1, or LL37 and stained for the expression of active integrins. While PMA induces integrin activation on both monocyte subsets, MCP1 and LL37 only induce significant integrin activation on classical CD14++CD16− (Figure 4C) and intermediate CD14++CD16+ (Figure S5) monocytes but not on non-classical CD14+CD16++ monocytes (Online Figure V). Similarly, PMA, MCP1, or CRAMP induced integrin activation on murine classical but not non-classical monocytes leading to increased binding of ICAM-1 or VCAM-1 (Figure 4D and Online Figure VI). In line with the adhesion experiments, antagonists to P2X7, CXCR2, and FPR1 failed to block LL37-dependent integrin activation (not shown), whereas antagonists to FPR2 fully abrogated integrin activation induced by LL37 (Figure 4E,F). In addition, we tried to elucidate if LL37 and MCP1 employ similar intracellular pathways that lead to integrin activation. To this end, monocytes were pre-treated with inhibitors to phospholipase C (PLC), which is crucial in chemokine-mediated activation of β2-integrins (28). PLC blockage reduced LL37- and MCP1-induced integrin activation on classical monocytes (Figure 4E,F). In contrast, blockade of bruton-tyrosine kinase (BTK) or Syk kinase, which are required for selectin-dependent integrin activation (28), failed to block ligand-induced integrin activation (not shown).
Figure 4. Cathelicidins induce integrin activation.
A/B: LL37 mediates Mac1 (CD11b/CD18) and VLA4 (CD49d/CD29) dependent adhesion. Classical CD14++CD16− monocytes were perfused over P-selectin/ICAM-1 (A) or P-selectin/VCAM-1 (B) coated dishes. The increase in adhesion by coimmobilization of LL37 or MCP1 was set to 100%. Classical CD14++CD16− monocytes were pretreated with anti-CD11b (1µg/ml), anti-CD49d (1µg/ml), or antagonists to FPR2 (quin-C7, 1µM) or to CCR2 (RS504393, 1µM). n=8. * significant difference compared to ctrl. C: Based on the CD16 and CD14 staining properties, human monocyte subsets were identified within peripheral blood mononuclear cells. Moreover, antibodies to activation epitopes of VLA4 (HUTS-21) and CD11b (CBRM1/5) were added. Cells were treated with PMA (50ng/ml), MCP-1 (50ng/ml), or LL37 (1µg/ml) for 15 min and the expression of activated CD11b (left) or VLA4 (right) was assessed on classical CD14++CD16− monocytes. * significant difference from control group. n=6. D: CRAMP activation enhances ICAM-1 and VCAM-1 binding to classical monocytes. Mouse peripheral leukocytes were treated with PMA (50ng/ml), MCP-1 (50ng/ml), or CRAMP (1µg/ml) in the presence of ICAM-1-Fc (left) or VCAM-1-Fc (right) and an anti-Fc antibody. Monocyte subsets were identified by additional antibody staining (CD45, CD11b, CD115, Gr1). * significant difference from control group. n = 5. E/F: Expression of activated CD11b (E) or VLA4 (F) on classical CD14++CD16− monocytes in response to LL37 (1µg/ml) or MCP1 (50ng/ml) was set to 100 %. PBMCs were pretreated with antagonists to PLC (U73122, 100nM), FPR2 (quin-C7, 1µM) or CCR2 (RS504393, 1µM). n=4. * significant difference compared to respective control group.
Mutation of LL37 abrogates its arrest function
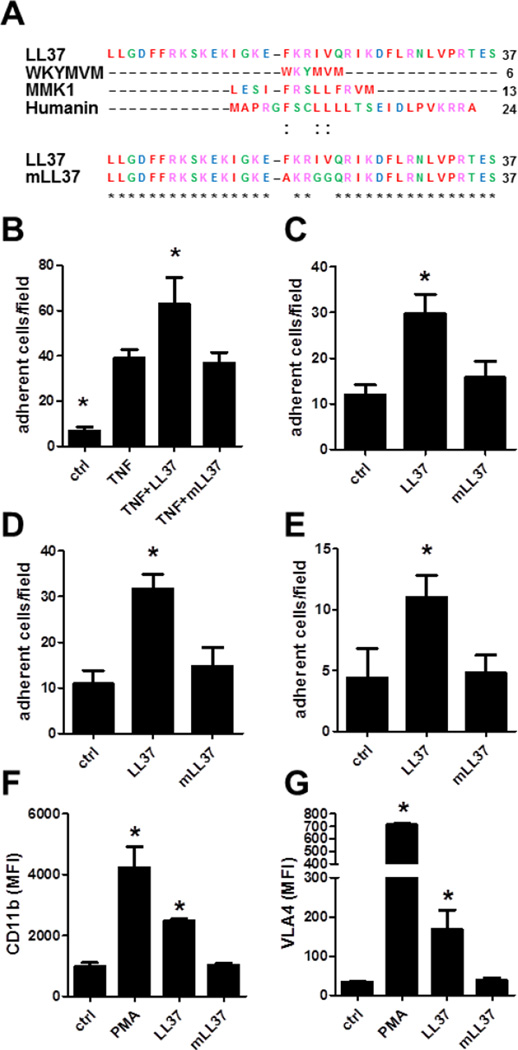
To identify the motif within LL37 that interacts with FPR2, we compared the amino acid sequences of known FPR2 ligands including MKYMVM, MMK1, and humanin with clustal Omega software. Despite the absence of any strict amino acid homology, we found a pattern of chemical properties present in all FPR2 ligands. More precisely, an aromatic amino acid and two hydrophobic residues separated by two amino acids was the only similarity between the molecules (Figure 5A). LL37 and the other FPR2 agonists are linear peptides, which simplifies the comparison of their three-dimensional structure. In their helicoid structure with a turn of 3 amino acids the aromatic and the hydrophobic residues were nearby and can form a hydrophobic domain. To test the involvement of this pattern in LL37-dependent monocyte adhesion, we synthetized a mutated LL37 (mLL37) with an alanine instead of the phenylalanine in position 17 (F17A) and glycine in replacement of the isoleucine and the valine in position 20 and 21 (I20G and V21G) (Figure 5A).
Figure 5. Mutation of LL37 abrogates its adhesive capacity.
A: Alignment of amino acid sequence of known FPR2 ligands, LL37, and mutated LL37 (mLL37). B: Human classical CD14++CD16− monocytes were perfused over TNF-activated (20ng, 12h) HUVEC and the number of adherent cells was quantified. LL37 (1µg) or mLL37 (1µg) were deposited 15 min prior to perfusion. * significant difference compared to TNF-treated sample. n=5. C/D: Classical CD14++CD16− monocytes were perfused over P-selectin/ICAM-1 (C) or P-selectin/VCAM-1 (D) coated dishes. In addition, LL37 or mLL37 were coimmobilized. n=3. * significant difference compared to ctrl. E: Wild type mice were rendered neutropenic and injected with TNF (ctrl) or with additional LL37 (1µg) or mLL37 (1µg). Adhesion of Ly6C-labeled classical monocytes was assessed by intravital microscopy. n=5. * significant difference compared to control. F/G: Activation of CD11b (F) or VLA4 (G) in response to PMA (50ng/ml), LL37 (1µg/ml), or mLL37 (1µg/ml) was assessed by flow cytometry. n=6. * significant difference compared to control.
To test the functionality of mLL37 we performed adhesion and integrin activation assays. Interestingly, mLL37 did not induce adhesion to TNF-activated HUVEC (Figure 5B) or to immobilized recombinant cell adhesion molecules (Figure 5C,D). In addition, mLL37 failed to enhance monocyte adhesion in vivo (Figure 5E) and to induce conformational changes of β1- or β2-integrin (Figure 5F,G). Thus, these data indicate the importance of the central FKRIV motif within LL37 to induce FPR2-mediated monocyte adhesion.
Discussion
The data of the here presented study identify a novel neutrophil-driven mechanism of classical monocyte adhesion. Upon neutrophil tissue infiltration cathelicidin is released and transported across the endothelium involving a caveolin 1-mediated transportation process. Cathelicidins are then presented to cells rolling along the endothelium. Classical monocytes recognize endothelial-bound cathelicidins via FPR2. This interaction triggers an intracellular signaling cascade involving PLC ultimately leading to activation of monocytic β1- and β2-integrins and subsequent adhesion of classical monocytes.
Monocyte subsets harbor crucial albeit differential functions during inflammation. Classical monocytes originate from the bone marrow and spleen to accumulate at sites of inflammation, where they differentiate into macrophages (29). In the absence of inflammation, classical monocytes are thought to convert into non-classical monocytes, although this is still under debate (30). Non-classical monocytes patrol the luminal side of post-capillary venules, where they sense damage or infection (31) and trigger inflammatory reactions. However, here we show that classical but not non-classical monocytes sense cathelicidins, which are known to be potent danger signals (32), and subsequently adhere to the endothelial lining. Classical monocytes exert potent pro-inflammatory functions including release of reactive oxygen species, TNF, IL-6, and type I interferons, which have been identified as powerful proinflammatory mediators (11). In addition, classical monocytes disturb the resolution of inflammation (33) thus perpetuating inflammatory processes.
With the discovery of monocyte subsets, a concept has emerged wherein the relative expression of adhesion molecules or chemokine receptors governs their recruitment behavior. In this context, it was shown that classical monocytes utilize CCR2, CX3CR1, CXCR2 as well as CCR5 to adhere and migrate to inflammatory sites (34). Similarly, PSGL-1 is expressed to a higher degree on classical monocytes and was hence found to be a major determinant in their recruitment (35). In the here presented study we demonstrate that FPR2 is dominantly expressed on classical monocytes and almost absent on non-classical monocytes hence explaining the exclusive adhesion of classical monocytes in response to immobilized cathelicidins. In addition, recent studies point towards the importance of FPR1 in the recruitment and locomotion of neutrophils towards danger signals (36, 37). Thus, it remains to be identified how FPR1 ligands differentially affect adhesion and recruitment of monocyte subsets.
Beyond the induction of classical monocyte adhesion as shown here, cathelicidins may promote monocyte recruitment through direct and indirect chemotactic effects. The direct chemotactic effect is primarily mediated via FPR2 with a maximum activity at concentrations of 0.5–50µg/ml (0.1–10µM) (4, 6, 38). Notably, the direct chemotactic activity of LL37 is not affected by serum (6), thus contrasting the effects of serum on the microbicidal effects of LL37 (39). This discrepancy may be due to the fact that the chemotactic activity is receptor-mediated and not dependent on a peptide-membrane interaction. Presumably, the part of the LL37 peptide that activates FPR2 is not hidden or altered by association of serum components, indicating that recruitment of leukocytes by LL37 is an important biological mechanism that is maintained along the vascular lumen. Besides its direct chemotactic activities, cathelicidins contribute to the inflammatory accumulation by stimulation of chemokine and cytokine production through effector cells. In this context, LL37 induces the production and release of IL8 from monocytes (40). In addition, LL37 was also shown to interact with the purinergic receptor P2X7, a receptor that is predominantly expressed on monocytes, macrophages and dendritic cells. LL37-stimulation via P2X7 of LPS-primed monocytes induced processing and release of the potent cytokine IL-1 β (41). This proinflammatory cytokine upregulates adhesion molecules on endothelial cells and hence promotes leukocyte adhesion.
Fundamental to the accumulation of monocytes and macrophages at sites of inflammation is, however, not just their recruitment but also the control of their mobilization from sites of production as well as their survival at the inflammatory site (42). Since counts of circulating leukocyte subsets are not altered in Cramp−/− mice (43) it is to be assumed that cathelicidins do not play a role in the mobilization of monocytes from the bone marrow. In contrast, cathelicidins were shown to exert divergent effects on apoptotic cell death. While LL37 promotes apoptosis in various T-cells, smooth muscle cells, and epithelial cells (44, 45), it was shown to inhibit apoptotic cell death in neutrophils (24). A consequence of the inhibition of apoptosis of neutrophils may be a survival strategy, leading to an increase of viable neutrophils at the site of infection, which is beneficial for the host during bacterial invasion. Although such data are not readily available for monocytes one may assume that similar mechanisms could contribute to the cathelicidin-mediated accumulation of monocytes at sites of inflammation.
As detailed above, cathelicidin-dependent mechanisms of monocyte adhesion, chemotaxis, and homeostasis may promote accumulation of classical monocytes at inflammatory sites. That such mechanisms importantly contribute to monocyte accumulation is indicated by observations from air pouch models where the instillation of cathelicidins results in pronounced accumulation of monocytes (4, 46). In diseases models of vascular inflammation, lack of CRAMP reduces the adhesion of classical monocytes as well as the number of macrophages in atherosclerotic lesions (43). In a model of arterial injury, neutrophil-derived CRAMP was found to promote adhesion of angiogenic monocytes thereby limiting neointima formation (47). In addition, CRAMP-deficient mice exhibit reduced accumulation of classical monocytes into the lungs of mice treated with hypo-chloric acid or LPS (unpublished data) and into the peritoneum upon stimulation with TNF, the latter being rescued by local application of CRAMP (unpublished data). Taken together, CRAMP may be an important facilitator of the accumulation of classical monocytes at sites of inflammation independently of the underlying stimulus. Since the chemotactic activity of cathelicidins is independent of receptors that are typically employed by classical chemokines (e.g. CCR2 for CCL2/MCP-1), we suspect that cathelicidins exert a non-redundant role in the extravasation of monocytes. In line with this concept, intracellular Ca2+ mobilization in response to neutrophil secretory products is conserved in Ccr2−/− classical monocytes (4). This notion along with the fact that many chemokines require de novo synthesis, whereas granule proteins are in general preformed led us to propose a concept where the initial monocyte efflux is primarily mediated by alarmins (i.e. preformed proteins with chemotactic activity) whereas later phases of monocyte emigration rely on chemokines (2).
Since CRAMP was found abundantly in adherent neutrophils and to far lesser extend in emigrated neutrophils, we conclude that neutrophils are the primary source of cathelicidins in the inflammation model employed in this study. However, tissue-resident cells such as endothelial cells which carry lower amounts of CRAMP may contribute to luminal CRAMP. The primary importance of neutrophil-derived CRAMP in leukocyte adhesion is further supported by a study showing that mice reconstituted with Cramp−/− bone marrow but not with WT bone marrow exhibit reduced adhesion of monocytic, angiogenic cells (47).
The here unraveled process of cathelcidin transendothelial transportation and monocyte activation offers various possibilities for therapeutic interference. Caveolin 1 mediates transportation of chemokines such as MCP1 across endothelium hence being an important facilitator of leukocyte adhesion (48). In atherosclerosis, mice lacking caveolin 1 exhibit strongly reduced atherosclerotic lesion sizes along with lower amounts of MCP1 presented on the endothelium as well as fewer adherent and transmigrating monocytes (49) thus implying that caveolin 1-dependent shuttling of chemokines is relevant to inflammatory pathologies like atherosclerosis. As similar observations regarding monocyte adhesion and recruitment are made in atherosclerotic Cramp-deficient mice (43), caveolin 1-mediated transportation of cathelicidins may not just occur in the microcirculation as shown here but also in large arteries. Hence tissue-specific targeting of such process may be an interesting approach to control the accumulation of classical monocytes.
Previous studies have shown that the antimicrobial activity of cathelicidin resides within specific domains that are different from the immune cell activating domains (50). Lack of cathelicidins in humans or mice favors the onset of infections and impairs monocyte recruitment (5, 15). This study provides evidence for the importance of the central FKRIV motif in FPR2-dependent adhesion of classical monocytes. Several groups have studied the involvement of specific amino acid domains in the LL37 sequence responsible for its antimicrobial (51) or other immunomodulatory effects (52) usually resulting in a LL37-derived peptide sequence with the FKRIV motif present in it. Even though the role of FPR2 was not investigated in these studies, our findings show that strategies specifically preventing the LL37-FPR2 interaction could be applicable in treatment of inflammatory processes where recruitment of classical monocytes plays a non-favorable role.
Taken together, our data provide a novel mechanism of monocyte recruitment involving the interaction of neutrophil-derived cathelicidin and monocytic FPR2. Therapeutic interference with transendothelial cathelicidin transportation, its endothelial presentation, or the cathelicidin-FPR2 interaction may be beneficial in circumstances where recruitment of classical monocytes plays an unfavorable role.
Supplementary Material
Novelty and Significance.
What is known?
Neutrophils infiltrate prior to classical monocytes.
Activated neutrophils secrete granule proteins which not just exert antimicrobial activities but also instruct immune cells.
Cathelicidins (CRAMP in mouse, LL37 in humans) have potent effects on recruitment and activation of immune cells, such as monocytes and dendritic cells.
What new information does this article contribute?
Neutrophils contribute to adhesion of classical monocytes an effect at least in part mediated by neutrophil-derived CRAMP.
CRAMP is released from emigrated neutrophils and then transported across the endothelium to be presented to monocytes rolling along the endothelium.
CRAMP induces adhesion of classical monocytes through ligation of FPR2.
CRAMP-induced FPR2 activation induces conformation changes of integrins towards a high affinity conformation.
Emigrating neutrophils causally contribute to monocyte recruitment, but underlying mechanisms remain unclear. Activated neutrophils secrete ready-made granule proteins, some of which induce recruitment and activation of immune cells. This study shows that neutrophil-derived cathelicidin is secreted by emigrated neutrophils, transported across the endothelium in a Caveolin 1-dependent manner and presented on the luminal side of the endothelium towards monocytes rolling along the endothelium. Endothelial-bound cathelicidin is recognized by formyl-peptide receptor 2 (FPR2), which expressed to a higher degree on classical monocytes as compared to non-classical monocytes. This interaction leads to rapid integrin activation with subsequent adhesion of classical monocytes. Given the importance of classical monocytes in many inflammatory diseases, the cathelicidin-FPR2 axis may be a novel target.
Acknowledgments
This study was supported by the German Research Foundation (SO876/3-1, SO876/4-1, HU1618/1-2, SFB914 TPB08), the German-Israeli Foundation, the Else Kröner Fresenius Stiftung, the NWO (VIDI project 91712303), and the ERC (Advanced Grant 249929). J.E.A. is recipient of a postdoctoral scholarship from the Alexander von Humboldt Foundation. The authors would like to thank Patricia Lemnitzer for excellent technical assistance, Markus Sperandio for critical data discussion, Philip M. Murphy for providing Fpr1−/− mice, and Antal Rot for DARC-MDCK cells.
List of abbreviations
- DARC
duffy antigen/receptor for chemokines
- FPR
formyl-peptide receptor
- HUVEC
human umbilical vein endothelial cells
- PMA
phorbol-12-myristate-13-acetate
- WT
wild type
Footnotes
Conflict-of-interest disclosure
The authors declare no competing financial interests.
References
- 1.Witko-Sarsat V, Rieu P, Descamps-Latscha B, Lesavre P, Halbwachs-Mecarelli L. Neutrophils: molecules, functions and pathophysiological aspects. Lab Invest. 2000;80:617–653. doi: 10.1038/labinvest.3780067. [DOI] [PubMed] [Google Scholar]
- 2.Soehnlein O, Lindbom L, Weber C. Mechanisms underlying neutrophil-mediated monocyte recruitment. Blood. 2009;114:4613–4623. doi: 10.1182/blood-2009-06-221630. [DOI] [PubMed] [Google Scholar]
- 3.Soehnlein O, Lindbom L. Phagocyte partnership during the onset and resolution of inflammation. Nat. Rev. Immunol. 2010;10:427–439. doi: 10.1038/nri2779. [DOI] [PubMed] [Google Scholar]
- 4.Soehnlein O, Zernecke A, Eriksson EE, Rothfuchs AG, Pham CT, Herwald H, Bidzhekov K, Rottenberg ME, Weber C, Lindbom L. Neutrophil secretion products pave the way for inflammatory monocytes. Blood. 2008;112:1461–1471. doi: 10.1182/blood-2008-02-139634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gallin JI, Fletcher MP, Seligmann BE, Hoffstein S, Cehrs K, Mounessa N. Human neutrophil-specific granule deficiency: a model to assess the role of neutrophil-specific granules in the evolution of the inflammatory response. Blood. 1982;59:1317–1329. [PubMed] [Google Scholar]
- 6.De Yang Chen Q, Schmidt AP, Anderson GM, Wang JM, Wooters J, Oppenheim JJ, Chertov O. LL-37, the neutrophil granule- and epithelial cell-derived cathelicidin, utilizes formyl peptide receptor-like 1 (FPRL1) as a receptor to chemoattract human peripheral blood neutrophils, monocytes, and T cells. J. Exp. Med. 2000;192:1069–1074. doi: 10.1084/jem.192.7.1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chertov O, Ueda H, Xu LL, Tani K, Murphy WJ, Wang JM, Howard OM, Sayers TJ, Oppenheim JJ. Identification of human neutrophil-derived cathepsin G and azurocidin/CAP37 as chemoattractants for mononuclear cells and neutrophils. J. Exp. Med. 1997;186:739–747. doi: 10.1084/jem.186.5.739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Territo MC, Ganz T, Selsted ME, Lehrer R. Monocyte-chemotactic activity of defensins from human neutrophils. J. Clin. Invest. 1989;84:2017–2020. doi: 10.1172/JCI114394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Passlick B, Flieger D, Ziegler-Heitbrock HW. Identification and characterization of a novel monocyte subpopulation in human peripheral blood. Blood. 1989;74:2527–2534. [PubMed] [Google Scholar]
- 10.Geissmann F, Jung S, Littman DR. Blood monocytes consist of two principal subsets with distinct migratory properties. Immunity. 2003;19:71–82. doi: 10.1016/s1074-7613(03)00174-2. [DOI] [PubMed] [Google Scholar]
- 11.Woollard KJ, Geissmann F. Monocytes in atherosclerosis: subsets and functions. Nat. Rev. Cardiol. 2010;7:77–86. doi: 10.1038/nrcardio.2009.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ziegler-Heitbrock L, Ancuta P, Crowe S, Dalod M, Grau V, Hart DN, Leenen PJ, Liu YJ, MacPherson G, Randolph GJ, Scherberich J, Schmitz J, Shortman K, Sozzani S, Strobl H, Zembala M, Austyn JM, Lutz MB. Nomenclature of monocytes and dendritic cells in blood. Blood. 2010;116:e74–e80. doi: 10.1182/blood-2010-02-258558. [DOI] [PubMed] [Google Scholar]
- 13.Faust N, Varas F, Kelly LM, Heck S, Graf T. Insertion of enhanced green fluorescent protein into the lysozyme gene creates mice with green fluorescent granulocytes and macrophages. Blood. 2000;96:719–726. [PubMed] [Google Scholar]
- 14.Jung S, Aliberti J, Graemmel P, Sunshine MJ, Kreutzberg GW, Sher A, Littman DR. Analysis of fractalkine receptor CX(3)CR1 function by targeted deletion and green fluorescent protein reporter gene insertion. Mol. Cell. Biol. 2000;20:4106–4114. doi: 10.1128/mcb.20.11.4106-4114.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nizet V, Ohtake T, Lauth X, Trowbridge J, Rudisill J, Dorschner RA, Pestonjamasp V, Piraino J, Huttner K, Gallo RL. Innate antimicrobial peptide protects the skin from invasive bacterial infection. Nature. 2001;414:454–457. doi: 10.1038/35106587. [DOI] [PubMed] [Google Scholar]
- 16.Gao JL, Lee EJ, Murphy PM. Impaired antibacterial host defense in mice lacking the N-formylpeptide receptor. J. Exp. Med. 1999;189:657–662. doi: 10.1084/jem.189.4.657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen K, Le Y, Liu Y, Gong W, Ying G, Huang J, Yoshimura T, Tessarollo L, Wang JM. A critical role for the g protein-coupled receptor mFPR2 in airway inflammation and immune responses. J. Immunol. 2010;184:3331–3335. doi: 10.4049/jimmunol.0903022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.MacIvor DM, Shapiro SD, Pham CT, Belaaouaj A, Abraham SN, Ley TJ. Normal neutrophil function in cathepsin G-deficient mice. Blood. 1999;94:4282–4293. [PubMed] [Google Scholar]
- 19.Luo H, Chaudhuri A, Zbrzezna V, He Y, Pogo AO. Deletion of the murine Duffy gene (Dfy) reveals that the Duffy receptor is functionally redundant. Mol. Cell. Biol. 2000;20:3097–3101. doi: 10.1128/mcb.20.9.3097-3101.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Razani B, Engelman JA, Wang XB, Schubert W, Zhang XL, Marks CB, Macaluso F, Russell RG, Li M, Pestell RG, Di Vizio D, Hou H, Jr, Kneitz B, Lagaud G, Christ GJ, Edelmann W, Lisanti MP. Caveolin-1 null mice are viable but show evidence of hyperproliferative and vascular abnormalities. J. Biol. Chem. 2001;276:38121–38138. doi: 10.1074/jbc.M105408200. [DOI] [PubMed] [Google Scholar]
- 21.Belaaouaj A, Moog-Lutz C, Just J, Houzel-Charavel A, Shapiro SD, Cayre Y. Genomic organization and chromosomal localization of mouse proteinase 3 (Myeloblastin) Mamm Genome. 1999;10:210–212. doi: 10.1007/s003359900974. [DOI] [PubMed] [Google Scholar]
- 22.Eisenhauer PB, Lehrer RI. Mouse neutrophils lack defensins. Infect Immun. 1992;60:3446–3447. doi: 10.1128/iai.60.8.3446-3447.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Grommes J, Alard JE, Drechsler M, Wantha S, Mörgelin M, Kuebler WM, Jacobs M, von Hundelshausen P, Markart P, Wygrecka M, Preissner KT, Hackeng TM, Koenen RR, Weber C, Soehnlein O. Disruption of platelet-derived chemokine heteromers prevents neutrophil extravasation in acute lung injury. Am J Respir Crit Care Med. 2012;185:628–636. doi: 10.1164/rccm.201108-1533OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nagaoka I, Tamura H, Hirata M. An antimicrobial cathelicidin peptide, human CAP18/LL-37, suppresses neutrophil apoptosis via the activation of formyl-peptide receptor-like 1 and P2X7. J. Immunol. 2006;176:3044–3052. doi: 10.4049/jimmunol.176.5.3044. [DOI] [PubMed] [Google Scholar]
- 25.Zhang Z, Cherryholmes G, Chang F, Rose DM, Schraufstatter I, Shively JE. Evidence that cathelicidin peptide LL-37 may act as a functional ligand for CXCR2 on human neutrophils. Eur. J. Immunol. 2009;39:3181–3194. doi: 10.1002/eji.200939496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pruenster M, Mudde L, Bombosi P, Dimitrova S, Zsak M, Middleton J, Richmond A, Graham GJ, Segerer S, Nibbs RJ, Rot A. The Duffy antigen receptor for chemokines transports chemokines and supports their promigratory activity. Nat. Immunol. 2009;10:101–108. doi: 10.1038/ni.1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ley K. Arrest chemokines. Microcirculation. 2003;10:289–295. doi: 10.1038/sj.mn.7800194. [DOI] [PubMed] [Google Scholar]
- 28.Lefort CT, Ley K. Neutrophil arrest by LFA-1 activation. Front. Immunol. 2012;3:157. doi: 10.3389/fimmu.2012.00157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Geissmann F, Manz MG, Jung S, Sieweke MH, Merad M, Ley K. Development of monocytes, macrophages, and dendritic cells. Science. 2010;327:656–661. doi: 10.1126/science.1178331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Varol C, Yona S, Jung S. Origins and tissue-context-dependent fates of blood monocytes. Immunol. Cell. Biol. 2009;87:30–38. doi: 10.1038/icb.2008.90. [DOI] [PubMed] [Google Scholar]
- 31.Auffray C, Fogg D, Garfa M, Elain G, Join-Lambert O, Kayal S, Sarnacki S, Cumano A, Lauvau G, Geissmann F. Monitoring of blood vessels and tissues by a population of monocytes with patrolling behavior. Science. 2007;317:666–670. doi: 10.1126/science.1142883. [DOI] [PubMed] [Google Scholar]
- 32.Yang D, de la Rosa G, Tewary P, Oppenheim JJ. Alarmins link neutrophils and dendritic cells. Trends Immunol. 2009;30:531–537. doi: 10.1016/j.it.2009.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Panizzi P, Swirski FK, Figueiredo JL, Waterman P, Sosnovik DE, Aikawa E, Libby P, Pittet M, Weissleder R, Nahrendorf M. Impaired infarct healing in atherosclerotic mice with Ly-6C(hi) monocytosis. J. Am. Coll. Cardiol. 2010;55:1629–1638. doi: 10.1016/j.jacc.2009.08.089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tacke F, Alvarez D, Kaplan TJ, Jakubzick C, Spanbroek R, Llodra J, Garin A, Liu J, Mack M, van Rooijen N, Lira SA, Habenicht AJ, Randolph GJ. Monocyte subsets differentially employ CCR2, CCR5, and CX3CR1 to accumulate within atherosclerotic plaques. J. Clin. Invest. 2007;117:185–194. doi: 10.1172/JCI28549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.An G, Wang H, Tang R, Yago T, McDaniel JM, McGee S, Huo Y, Xia L. P-selectin glycoprotein ligand-1 is highly expressed on Ly-6Chi monocytes and a major determinant for Ly-6Chi monocyte recruitment to sites of atherosclerosis in mice. Circulation. 2008;117:3227–3237. doi: 10.1161/CIRCULATIONAHA.108.771048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Phillipson M, Kubes P. The neutrophil in vascular inflammation. Nat Med. 2011;17:1381–1390. doi: 10.1038/nm.2514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McDonald B, Pittman K, Menezes GB, Hirota SA, Slaba I, Waterhouse CC, Beck PL, Muruve DA, Kubes P. Intravascular danger signals guide neutrophils to sites of sterile inflammation. Science. 2010;330:362–366. doi: 10.1126/science.1195491. [DOI] [PubMed] [Google Scholar]
- 38.Tjabringa GS, Ninaber DK, Drijfhout JW, Rabe KF, Hiemstra PS. Human cathelicidin LL-37 is a chemoattractant for eosinophils and neutrophils that acts via formyl-peptide receptors. Int. Arch. Allergy Immunol. 2006;140:103–112. doi: 10.1159/000092305. [DOI] [PubMed] [Google Scholar]
- 39.Johansson J, Gudmundsson GH, Rottenberg ME, Berndt KD, Agerberth B. Conformation-dependent antibacterial activity of the naturally occurring human peptide LL-37. J. Biol. Chem. 1998;273:3718–3724. doi: 10.1074/jbc.273.6.3718. [DOI] [PubMed] [Google Scholar]
- 40.Bowdish DM, Davidson DJ, Speert DP, Hancock RE. The human cationic peptide LL-37 induces activation of the extracellular signal-regulated kinase and p38 kinase pathways in primary human monocytes. J Immunol. 2004;172:3758–3765. doi: 10.4049/jimmunol.172.6.3758. [DOI] [PubMed] [Google Scholar]
- 41.Elssner A, Duncan M, Gavrilin M, Wewers MD. A novel P2X7 receptor activator, the human cathelicidin-derived peptide LL37, induces IL-1 beta processing and release. J. Immunol. 2004;172:4987–4994. doi: 10.4049/jimmunol.172.8.4987. [DOI] [PubMed] [Google Scholar]
- 42.Gautier EL, Jakubzick C, Randolph GJ. Regulation of the migration and survival of monocyte subsets by chemokine receptors and its relevance to atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2009;29:1412–1418. doi: 10.1161/ATVBAHA.108.180505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Döring Y, Drechsler M, Wantha S, Kemmerich K, Lievens D, Vijayan S, Gallo RL, Weber C, Soehnlein O. Lack of neutrophil-derived CRAMP reduces atherosclerosis in mice. Circ. Res. 2012;110:1052–1056. doi: 10.1161/CIRCRESAHA.112.265868. [DOI] [PubMed] [Google Scholar]
- 44.Lau YE, Bowdish DM, Cosseau C, Hancock RE, Davidson DJ. Apoptosis of airway epithelial cells: human serum sensitive induction by the cathelicidin LL-37. Am. J. Respir. Cell. Mol. Biol. 2006;34:399–409. doi: 10.1165/rcmb.2005-0170OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Aarbiou J, Tjabringa GS, Verhoosel RM, Ninaber DK, White SR, Peltenburg LT, Rabe KF, Hiemstra PS. Mechanisms of cell death induced by the neutrophil antimicrobial peptides alpha-defensins and LL-37. Inflamm. Res. 2006;55:119–127. doi: 10.1007/s00011-005-0062-9. [DOI] [PubMed] [Google Scholar]
- 46.Kurosaka K, Chen Q, Yarovinsky F, Oppenheim JJ, Yang D. Mouse cathelin-related antimicrobial peptide chemoattracts leukocytes using formyl peptide receptor-like 1/mouse formyl peptide receptor-like 2 as the receptor and acts as an immune adjuvant. J Immunol. 2005;174:6257–6265. doi: 10.4049/jimmunol.174.10.6257. [DOI] [PubMed] [Google Scholar]
- 47.Soehnlein O, Wantha S, Simsekyilmaz S, Döring Y, Megens RT, Mause SF, Drechsler M, Smeets R, Weinandy S, Schreiber F, Gries T, Jockenhoevel S, Möller M, Vijayan S, van Zandvoort MA, Agerberth B, Pham CT, Gallo RL, Hackeng TM, Liehn EA, Zernecke A, Klee D, Weber C. Neutrophil-derived cathelicidin protects from neointimal hyperplasia. Sci. Transl. Med. 2011;3:103ra98. doi: 10.1126/scitranslmed.3002531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ge S, Song L, Serwanski DR, Kuziel WA, Pachter JS. Transcellular transport of CCL2 across brain microvascular endothelial cells. J. Neurochem. 2008;104:1219–1232. doi: 10.1111/j.1471-4159.2007.05056.x. [DOI] [PubMed] [Google Scholar]
- 49.Engel D, Beckers L, Wijnands E, Seijkens T, Lievens D, Drechsler M, Gerdes N, Soehnlein O, Daemen MJ, Stan RV, Biessen EA, Lutgens E. Caveolin-1 deficiency decreases atherosclerosis by hampering leukocyte influx into the arterial wall and generating a regulatory T-cell response. FASEB J. 2011;25:3838–3848. doi: 10.1096/fj.11-183350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Braff MH, Hawkins MA, Di Nardo A, Lopez-Garcia B, Howell MD, Wong C, Lin K, Streib JE, Dorschner R, Leung DY, Gallo RL. Structure-function relationships among human cathelicidin peptides: dissociation of antimicrobial properties from host immunostimulatory activities. J. Immunol. 2005;174:4271–4278. doi: 10.4049/jimmunol.174.7.4271. [DOI] [PubMed] [Google Scholar]
- 51.Sigurdardottir T, Andersson P, Davoudi M, Malmsten M, Schmidtchen A, Bodelsson M. In silico identification and biological evaluation of antimicrobial peptides based on human cathelicidin LL-37. Antimicrob. Agents. Chemother. 2006;50:2983–2989. doi: 10.1128/AAC.01583-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.van der Does AM, Beekhuizen H, Ravensbergen B, Vos T, Ottenhoff TH, van Dissel JT, Drijfhout JW, Hiemstra PS, Nibbering PH. LL-37 directs macrophage differentiation toward macrophages with a proinflammatory signature. J. Immunol. 2010;185:1442–1449. doi: 10.4049/jimmunol.1000376. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.