Abstract
In this issue of Molecular Cell, He and colleagues (2013) unveil a high-resolution structure of a key regulatory interface in cell-cycle control: the destruction box sequence bound to the anaphase-promoting complex.
A little amino acid sequence can go a long way. Consider the ‘destruction box’ (D box), with its simple core sequence of an arginine followed two residues later by a leucine. This ‘RxxL’ sequence lies at the heart of cell-cycle control, and its presence on a protein can have a profound impact on that protein’s existence. Proteins containing a D box interact with a ubiquitin-protein ligase called the anaphase-promoting complex or cyclosome (APC/C), resulting in protein ubiquitination and then destruction by the proteasome. The D box-dependent destruction of securin and cyclins triggers chromosome segregation and the completion of mitosis (Primorac and Musacchio, 2013). Despite its clear biological importance, however, the molecular details of D box-APC/C binding have been unclear––until now, with the report in this issue of a crystallographic structure of a D box in its binding site on the APC/C (He et al., 2013).
Like other ubiquitin-protein ligases or E3s of the ‘RING’ subfamily, the APC/C is a large, multisubunit complex with binding sites for two reactants: (1) a ubiquitin-conjugating enzyme or E2, coupled to the C terminus of the small protein ubiquitin; and (2) the protein substrate, positioned so that a lysine side chain can attack the E2-ubiquitin conjugate to effect ubiquitin transfer (Barford, 2011). The APC/C binds substrates with high affinity and specificity. It also possess the remarkable ability to modify multiple lysines on the substrate during a single substrate-binding event. Specificity and processivity depend on an a broad, open-faced active site, in which a disordered substrate is held tightly at one or two specific sequence motifs but otherwise waves freely near the E2 to allow modification of multiple lysines.
Two major sequence motifs have been found in APC/C substrates. The first to be discovered, over twenty years ago, was the D box, some version of which is found in most, if not all, APC/C substrates (Glotzer et al., 1991). A nine-residue consensus sequence was defined in early work (RxALGxIxN), but RxxL has emerged as the most conserved feature. Later work unveiled a second motif called the KEN box, named for its core sequence (Pfleger and Kirschner, 2000). Many APC/C substrates contain both a D box and a KEN box, distributed in poorly conserved regions of predicted disorder.
APC/C substrates are recruited to the enzyme by an interchangeable, cell-cycle regulated ‘coactivator’ subunit. The two major coactivators are Cdc20 and Cdh1, each containing a WD40 domain that is the main site of substrate interaction. A secondary substrate-binding site is also found on an APC/C subunit called Apc10/Doc1, and structural studies suggest that the substrate D box is sandwiched between Apc10 and the coactivator WD40 domain (da Fonseca et al., 2011; Buschhorn et al., 2011).
The first high-resolution snapshots of coactivator structure and substrate binding came from recent crystallographic studies of the coactivator Cdc20 bound to a protein containing a KEN box (Chao et al., 2012; Tian et al., 2012). The WD40 domain of Cdc20 forms a seven-bladed β-propeller, and the three residues of the KEN box interact with residues in the center of its upper face. These studies also revealed a conserved channel along one side of the WD40 domain, containing an acidic groove adjacent to a deep hydrophobic pocket––highly suggestive of a binding site for the arginine and leucine of the D box. Further hints that this site binds the D box came from fortuitous interactions between the hydrophobic pocket and a leucine side chain from a neighboring protein in the crystal. In another case, the pocket was occupied by a small chemical used in the crystallization procedure.
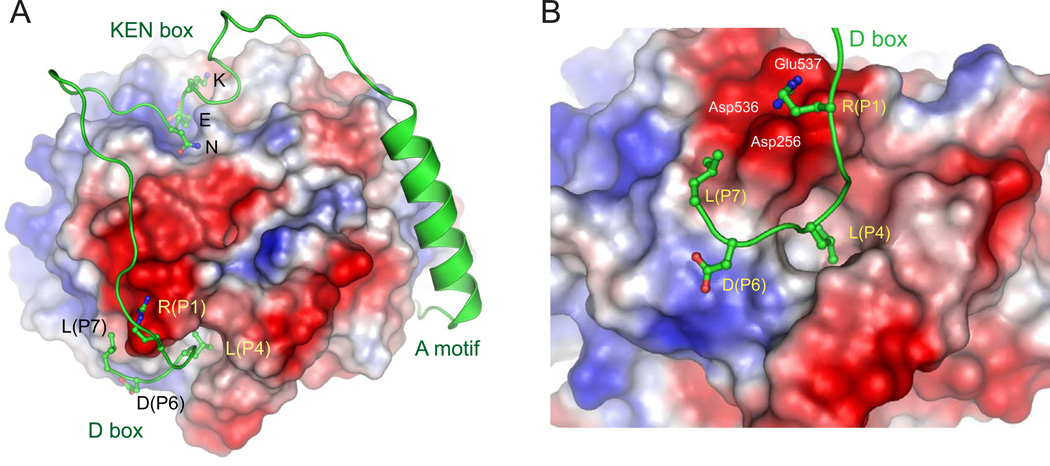
In this issue, the Barford group now provides the picture we’ve been waiting for: a crystallographic structure of a D box bound at the proposed site on the side of the coactivator––in this case the coactivator Cdh1, bound to a D box in a pseudosubstrate inhibitor called Acm1 (Figure 1) (He et al., 2013). Sure enough, the arginine interacts with the conserved acidic residues, and the leucine side chain is inserted in the hydrophobic pocket. The structure also reveals numerous other interesting features of the APC/C-substrate interaction. First, it provides insights into the function of the poorly conserved residues downstream of the RxxL motif, which interact with specific sites on the coactivator surface. Based on an extensive alignment of known D box sequences, the authors use the structure to explain the prevalence of certain residues in specific positions of the D box sequence––providing clues about the varying affinities that different D boxes have for the same coactivator, or that the same D box can have for different coactivators.
Figure 1. Binding sites for the D box, KEN box, and A motif on the coactivator Cdh1.
(A) The three sequence motifs of the pseudosubstrate Acm1 (green) are shown at their binding sites on the surface of the Cdh1 WD40 domain, which is colored according to electrostatic potential (red is negative, blue is positive). This region of Acm1 begins at its N terminus with the A motif, preceding the alpha helix; the first residues of this motif bind on the lower surface of Cdh1 and are not seen here. In the D box, the arginine at position 1 (P1) interacts with nearby acidic residues, while the leucine at position P4 is buried in a hydrophobic pocket. The most C-terminal residue resolved in the structure is a leucine at position 7.
(B) A close-up of the D box in its binding site. Acidic amino acids that interact with the P1 arginine are labeled. Images courtesy of David Barford.
The last two residues of the D box are disordered and not visible in the structure. An intriguing possibility is that the unresolved residues interact with Apc10, explaining the previous evidence that the D box is bound between Apc10 and coactivator. Furthermore, the D box begins to make a U-turn after the RxxL motif, suggesting perhaps that the D box region enters and exits on the same side of the space between coactivator and Apc10.
A handful of APC/C substrates contain ‘non-canonical’ destruction motifs that are required for degradation but don’t look like a typical D or KEN box. The authors show that some of these unusual motifs interact with the D box binding site, and that, in some cases, the core motif of the D box might work in reverse: a substrate containing an LxxK motif can interact with the RxxL-binding site on Cdh1. It was already difficult to predict functional D boxes on the basis of the common RxxL motif, and these new results will make such predictions nearly impossible.
Upstream of the D box, Acm1 contains a KEN box that binds the previously identified site on the top face of Cdh1 (Figure 1). The KEN and D boxes are spaced ideally to allow both to bind the same coactivator. Many APC/C substrates contain both motifs, and the new structure will help us understand how the spacing and ordering of the two motifs can influence their cooperative binding to the same coactivator––thereby generating high binding affinity.
The structure includes an extra bonus that opens up a potentially exciting new avenue in APC/C-substrate interactions. In addition to its KEN and D boxes, Acm1 contains a sequence called the ‘A motif’, which is known to enhance Cdh1 binding (Burton et al., 2011). The A motif binds along the side and bottom of Cdh1 on the opposite face from the D box (Figure 1). The A motif has thus far been identified only in Acm1 and binds only Cdh1 and not Cdc20, but it seems likely that related motifs exist in other APC/C regulators or substrates. Three motif-binding sites are thus aligned on the coactivator surface, and it is easy to imagine that other low-affinity binding sites are lurking among the other bumps and grooves of the structure. The APC/C coactivator is bristling with binding sites, suggesting that multiple low-affinity interactions provide the high avidity and specificity required for processive and selective substrate modification.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Barford D. Q. Rev. Biophys. 2011;44:153–190. doi: 10.1017/S0033583510000259. [DOI] [PubMed] [Google Scholar]
- Burton JL, Xiong Y, Solomon MJ. EMBO J. 2011;30:1818–1829. doi: 10.1038/emboj.2011.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buschhorn BA, Petzold G, Galova M, Dube P, Kraft C, Herzog F, Stark H, Peters JM. Nat. Struct. Mol. Biol. 2011;18:6–13. doi: 10.1038/nsmb.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao WC, Kulkarni K, Zhang Z, Kong EH, Barford D. Nature. 2012;484:208–213. doi: 10.1038/nature10896. [DOI] [PubMed] [Google Scholar]
- da Fonseca PC, Kong EH, Zhang Z, Schreiber A, Williams MA, Morris EP, Barford D. Nature. 2011;470:274–278. doi: 10.1038/nature09625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glotzer M, Murray AW, Kirschner MW. Nature. 1991;349:132–138. doi: 10.1038/349132a0. [DOI] [PubMed] [Google Scholar]
- He J, Chao WCH, Zhang Z, Yang J, Cronin N, Barford D. Mol. Cell. 2013 doi: 10.1016/j.molcel.2013.04.024. this issue. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfleger CM, Kirschner MW. Genes Dev. 2000;14:655–665. [PMC free article] [PubMed] [Google Scholar]
- Primorac I, Musacchio A. J. Cell Biol. 2013;201:177–189. doi: 10.1083/jcb.201301130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian W, Li B, Warrington R, Tomchick DR, Yu H, Luo X. Proc. Natl. Acad. Sci. USA. 2012;109:18419–18424. doi: 10.1073/pnas.1213438109. [DOI] [PMC free article] [PubMed] [Google Scholar]