Abstract
Coccidioidomycosis consists of a spectrum of disease, ranging from a mild, self-limited, febrile illness to severe, life-threatening infection. It is caused by the soil-dwelling fungi, Coccidioides immitis and C. posadasii, which are present in diverse endemic areas. Climate changes and environmental factors affect the Coccidioides lifecycle and influence infection rates. The incidence of coccidioidomycosis has risen substantially over the past two decades. The vast majority of Coccidioides infections occur in the endemic zones, such as California, Arizona, Mexico, and Central America. Infections occurring outside those zones appear to be increasingly common, and pose unique clinical and public health challenges. It has long been known that elderly persons, pregnant women, and members of certain ethnic groups are at risk for severe or disseminated coccidioidomycosis. In recent years, it has become evident that persons with immunodeficiency diseases, diabetics, transplant recipients, and prisoners are also particularly vulnerable.
Keywords: coccidioidomycosis, Coccidioides, epidemiology, incidence, risk factors, geography
Introduction
Coccidioides spp. are dimorphic, soil-dwelling, fungi known to cause a broad spectrum of disease, ranging from a mild febrile illness to severe pulmonary manifestations or disseminated disease.1,2 The genus Coccidioides is comprised of two genetically distinct species: Coccidioides immitis and C. posadasii. These two species cause similar clinical diseases, however they are present in different geographic regions of endemicity.3–5 This review addresses the history of Coccidioides spp. and summarizes the current knowledge of their ecologic environment and geographic distribution. The risk factors for disease acquisition and recent epidemiologic trends, including coccidioidomycosis in non-endemic areas, the enlarging at-risk populations, and current public health challenges are highlighted.
History
The causative agent of coccidioidomycosis was first identified by a medical intern, Alejandro Posadas, in Buenos Aires in 1892.6 During his medical training, Posadas evaluated an Argentine soldier who had skin lesions previously attributed to mycosis fungoides. Microscopic examination of the soldier’s skin biopsy specimens revealed organisms that appeared similar to the protozoan Coccidia, leading to a misconception that the microbe was a parasite. A few years later, a manual laborer from the San Joaquin Valley, California, was evaluated for similar skin lesions.7 Upon his death, autopsy specimens from multiple organs were notable for granulomas containing the same type of “protozoal” organisms. A collaborative evaluation of this patient’s specimens by Emmet Rixford, a surgeon at Cooper Medical College in San Francisco, and T Caspar Gilchrist and CW Stiles, pathologists at Johns Hopkins Medical School, resulted in the decision that the organism was, indeed, a protozoan. Consequently, in 1896, it was given the name Coccidioides (derived from its morphologic appearance of “resembling Coccidia”) immitis (“not mild”, because it was believed that the organism caused lethal disease).6,7 Just a few years later, William Ophüls and Herbert C Moffitt, accurately classified C. immitis as a fungus.8 These investigators satisfied Koch’s postulates by inoculating material from a case patient into guinea pigs, which subsequently developed signs of infection, culturing the fungal organism from the animals’ organs, and ultimately injecting mycelia from this culture into a rabbit, which developed Coccidioides-containing nodules in tissue.
Important advances in the understanding of Coccidioides pathogenesis and natural history were made during the first half of the twentieth century. In 1929, the conventional notion that coccidioidal infections were rare and consistently fatal was questioned after Harold Chope, a medical student, accidentally inhaled Coccidioides spores from a culture plate in the Stanford University laboratory of Ernest Dickson.6 Chope developed pneumonia and, despite the grim expectation that death was imminent, he survived. This surprising outcome, along with the fact that the fungus was isolated from Chope’s respiratory specimen, sparked a surge of investigations by Dickson and others. In 1938, Dickson linked “San Joaquin Fever” or “Valley Fever”, the self-resolving illness of cough, chest pain, fever, and erythema nodosum, to dust exposure in patients who had positive reactions to Coccidioides skin testing.6,9 A few years later, Charles E Smith collected information from over 400 patients with a history of Valley Fever and found that the majority of infections were mild and that there was no evidence for human-to-human transmission.10 A decade later, Smith and others published the details of a cluster of coccidioidal infections in a group of students in Kern County, California.11 It was determined that the students had been exposed to Coccidioides while digging a rattlesnake out of a ground squirrel hole. These reports led to the present-day understanding that coccidioidomycosis is acquired via inhalation of contaminated dust or soil and that it generally does not cause lethal disease.
Ecology
Coccidioides spp. are found in warm, arid, desert regions in the Western Hemisphere. They thrive in an ecological zone that has hot summers, gentle winters without harsh freezes, and annual rainfall of 10–50 cm.5,12 The organisms are found within alkaline, sandy soil, commonly about 10–30 cm beneath the surface, though they can have a sporadic or patchy distribution within a given locality.5,13
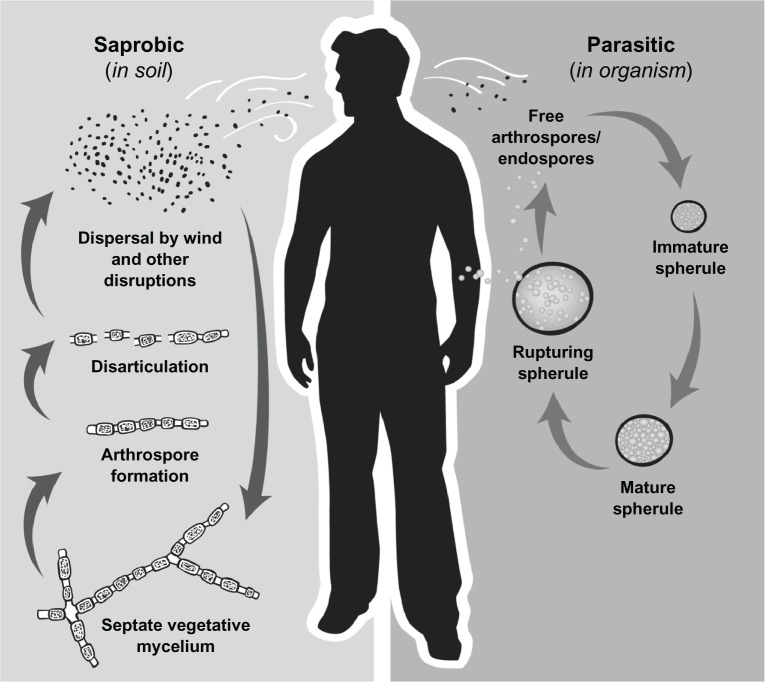
The Coccidioides spp. lifecycle is closely intertwined with changes in climate conditions. The fungal mycelia require moisture in the soil to grow.14,15 Hence the fungus is usually recovered in greatest abundance in the spring after the heavy, winter rainfall has ended. The hyphae then need a dry period to promote desiccation and maturation into arthroconidia, which can be aerosolized and inhaled (Figure 1).5,14–16 This requirement partially explains why the rate of Coccidioides infections tends to rise in the drier months of the year. Other subtle factors in the ecological milieu, such as variations in the chemical components of the soil, may also affect fungal subsistence. In an early study, Elconin and colleagues evaluated the soil in a parcel of land in the San Joaquin Valley where coccidioidomycosis was common; more than 5000 soil samples were collected at monthly intervals over an 8-year period (1955–1962).17 After controlling for other variables, including yearly temperature and rainfall levels, the authors found that a higher concentration of soluble salts (eg, sodium, calcium, magnesium, sulfates, and chlorides) in the soil was significantly correlated with the presence of C. immitis. Thus, differences in soil constitution may account for the patchy distribution of these two Coccidioides species within a particular region, as well as for their divergence in geographical distribution.
Figure 1.
Life cycle of Coccidioides spp.
Notes: In the environment Coccidioides exists as a mycelium and following periods of low precipitation arthroconidia are formed and are easily aerosolized when disturbed. Arthroconidia can be inhaled or return to the soil and again grow to vegetative mycelia. However if inhaled, arthroconidia undergo a morphologic change and become immature spherules which divide internally until filled with endospores and subsequently rupture. Endospores are dispersed into the surrounding tissue and are then able to form new spherules and repeat the cycle.
Ecology – role of an animal vector?
Interestingly, some reports describe higher concentrations of the organisms around archaic Indian burial sites or animal burrows.18,19 The later observation has led to speculation that there may be a rodent host reservoir for Coccidioides; both the kangaroo rat and the Arizona pocket mouse have been proposed as possible animal reservoirs. However, to date, zoonotic transmission to humans has not been reported. Although carcasses or excretions from infected rodents have been hypothesized to play a role in the environmental life cycle of the fungus,14,18 the extent to which rodents or other animals such as bats20 or armadillos21 act as a reservoir for Coccidioides or influence its geographic distribution is still unknown. Comparative whole genome sequencing data suggest that Coccidioides have evolved in response to interaction with an animal host.22
Coccidioidomycosis has been shown to affect other non-human mammals, including domestic and non-domestic animals in the wild and in captivity.23 It is especially common among domestic dogs, with an estimated annual incidence of 4% among dogs in Pima and Maricopa Counties, Arizona.24 Because dogs presumably share similar exposures to their human counterparts, studies of canine coccidioidomycosis may be useful for assessing the risk for human infection, particularly in suspected or known, but broadly-defined, endemic areas.25
Geographic range
Historically, methods to isolate Coccidioides from the soil across wide-ranging regions have been neither feasible nor practical. As a result, the geographic range for Coccidioides spp. has been largely extrapolated from epidemiologic studies of persons diagnosed with coccidioidomycosis or from population surveys via spherulin or coccidioidin skin testing. One early investigation, that used skin testing to map the distribution of Coccidioides in the USA, was conducted by Edwards and Palmer in 1957.26 The results of this and similar studies established that the south-central valley of California and the deserts of southern Arizona, were the most highly endemic for Coccidioides.3,18,27 In the future, culture-independent methods, such as multiplex polymerase chain reaction testing of bulk soil samples, may prove to be useful tools to identify Coccidioides locations.28
Presently, it is known that Coccidioides spp. are endemic to specific regions in the Western Hemisphere, primarily those located between the north and south 40° latitudes (Figure 2).5,29 The two species, C. immitis and C. posadasii, populate two distinct and divergent geographic regions. C. immitis is found in central and southern California, with the San Joaquin Valley being the region of greatest endemicity.3,5,13 The rate of positive skin tests ranges as high as 50%–70% in Kern County, including the city of Bakersfield, and its neighboring Tulare and Kings Counties.26 Compared with C. immitis, C. posadasii has a broader region of endemicity, from central and southern Arizona to western Texas and southern New Mexico. The most concentrated region for C. posadasii is in Arizona, where the majority of positive skin tests and coccidioidomycosis cases occur in Maricopa County (including the city of Phoenix), Pima County (including the city of Tucson), and Pinal County.3 C. posadasii is also present in sporadic sites in southern Utah and Nevada.13,30
Figure 2.
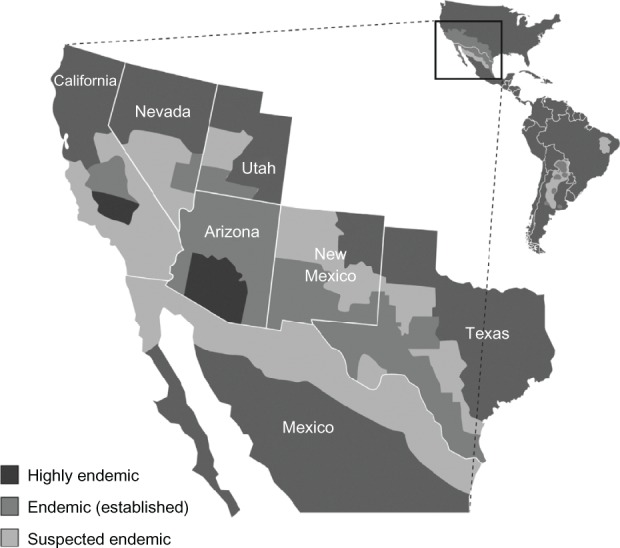
Endemic areas for coccidioidomycosis.
Beyond the USA, C. posadasii is present in parts of Mexico and Central and South America.4,5,31–33 On the basis of surveys using coccidioidin testing conducted in the 1960s in Mexico, it was concluded that Coccidioides-endemic regions include the Northern zone bordering the USA, (eg, the Mojave, Sonoran, and Chihuahuan Desert regions), the Pacific Coast zone (eg, Nayarit, Jalisco, and Michoacan), and the Central Zone (eg, Durango).5,33 More limited skin surveys, performed in the 1990s, found rates of positivity ranging from 40% to over 90% in the state of Coahuila.34 The mapping of Coccidioides endemicity in Central and South America is less complete. This reflects the fact that, in most countries, coccidioidomycosis is not considered a reportable disease, and nationwide skin testing surveys have not been conducted. In Central America, skin testing has identified regions of endemicity in the Montagua River Valley in Guatemala and in Comayagua, Honduras.4,5,31,33 In South America, endemic regions have been found in northern Venezuela and northeastern Brazil.5,33 Argentina, Bolivia, Paraguay, and Nicaragua also may have some endemic areas.4,5,33 The precision of these surveys is unknown, though, since skin tests may cross-react with other mycoses such as Histoplasma capsulatum, Blastomyces dermatitidis, and Paracoccidioides brasiliensis, which may also be endemic in these regions.
Coccidioidomycosis in non-endemic areas
While the vast majority of the burden of coccidioidomycosis exists in Arizona and California, there is evidence to suggest that the disease also poses specific clinical and public health challenges outside the endemic areas. The majority of cases observed outside the southwestern USA occur among persons who visit or temporarily relocate to endemic areas and seek medical care after returning to their permanent residence. In many instances, travelers become symptomatic shortly after returning home, although latent or reactivation infection presenting months to years later can also occur in immunosuppressed persons.35,36 As the infectious dose of Coccidioides arthroconidia is presumed to be very small, even a very brief exposure in an endemic area may result in symptomatic infection.
Clusters of coccidioidomycosis cases among groups of travelers have been observed, including clusters in Pennsylvania37 and Washington State38 among church group members who had visited Mexico. Another cluster occurred in US Marine reserves from Tennessee who completed 3 weeks of training exercises in southern California.39 In each of these reports, nearly all case-patients had extensive exposure to soil or dusty environments. In addition, the travelers were unaware of their risk for acquiring coccidioidomycosis, and many case-patients were initially misdiagnosed with bacterial or viral respiratory infections.38,39 Several series of isolated coccidioidomycosis cases (not occurring as part of a cluster) outside endemic areas have been described, including 23 cases at an Ohio hospital during 1980–1998,40 161 cases identified from New York State hospital discharge records during 1992–1997,41 two cases with musculoskeletal involvement in Chicago,42 and four cases in New Orleans.43 Travel-associated coccidioidomycosis cases have also occurred among visitors from other countries,44 including an outbreak after a model airplane flying event in Kern County, California in 2001, which over 300 persons from more than 30 countries attended.45,46 These reports of coccidioidomycosis in non-endemic areas highlight the importance of educating travelers and healthcare providers in non-endemic areas about the disease so that delays in diagnosis and treatment can be avoided or minimized.
Recent evidence suggests that some coccidioidomycosis cases occurring outside the endemic area may be locally-acquired, including three cases in eastern Washington (one case of primary cutaneous coccidioidomycosis and two cases of pneumonia, one of which progressed to meningeal disease).47 Another case occurred in a 14-year old Chinese boy who had never visited, or been exposed to materials imported from, an endemic area. Interestingly, he had reported choking on seawater,48 an environment in which Coccidioides can survive.49 These occurrences suggest that the geographic range of Coccidioides may be expanding or is wider than that previously recognized.
Risk factors for infection
Any person who resides in or travels to a Coccidioides endemic region can become infected with Coccidioides spp. following inhalation of airborne arthroconidia. Although infections contracted from non-respiratory routes (eg, in utero exposure, animal bites) have been reported, these are rare.50,51
Populations with intense exposure to aerosolized arthroconidia are at greater risk for infection. These groups include agricultural or construction workers, or persons who participate in outdoor activities such as hunting or digging in the soil.5,11,52,53 Outbreaks of coccidioidomycosis have been linked to military training exercises, model airplane competitions, earthquakes, windstorms, and armadillo hunting adventures.38,54–58 Though uncommon, infections acquired in persons with minimal exposure (eg, changing airplanes at the Phoenix airport or briefly driving through the San Joaquin Valley) have also been reported.41,59
After inhaling Coccidioides spores, 60% of infected persons remain asymptomatic. The rest develop mild-to-severe symptomatic pulmonary infections. About 1% of infected persons develop disseminated disease, which can involve the skin, joints, bones, central nervous system, or other organs. Many factors are correlated with an increased risk for severe or disseminated disease (Table 1).
Table 1.
Risk factors for severe or disseminated coccidioidomycosis
| Filipino or African ethnicity |
| HIV/AIDS |
| Immunosuppressive medications |
| Prednisone |
| TNF-α inhibitors |
| Chemotherapy |
| Organ transplantation (tacrolimus, etc) |
| Diabetes mellitus |
| Pregnancy |
| Cardiopulmonary disease |
Abbreviations: AIDS, acquired immunodeficiency syndrome; HIV, human immunodeficiency virus; TNF, tumor necrosis factor.
Age
Although coccidioidomycosis affects all age groups, the highest incidence rates are consistently documented among adults, typically among those aged 40–49 years in California66 and among those aged ≥65 in Arizona.60,61 Several studies have examined the factors associated with the disproportionate burden of coccidioidomycosis among the elderly in Arizona. For example, a case-control study showed that among coccidioidomycosis case-patients aged ≥60 years, the risk for acquiring symptomatic coccidioidomycosis declined by 5% for each year lived in the endemic area.60 After controlling for duration of residence, congestive heart failure, corticosteroid use, smoking, cancer, and male sex were independently associated with developing symptomatic coccidioidomycosis among cases compared to geographically-matched controls.60 This suggests that coccidioidomycosis among the elderly in Arizona may be related, at least in part, to the high prevalence of seasonal or permanent relocation of retirement-age individuals (many of whom have comorbid conditions) from non-endemic areas.
While an older population at risk may explain the high rates among the elderly, other factors may more fully account for the risk of developing adverse outcomes. A retrospective medical record review of Arizona coccidioidomycosis cases found no significant differences in disease manifestations between patients aged ≥60 years and those aged <60 years when controlling for comorbid conditions; regardless of age or ethnicity, immunosuppression was the only factor associated with poor outcomes including hospitalization, disseminated infection, or death.62 Similarly, a study of Kern County, California coccidioidomycosis cases found that older age was independently associated with developing severe pulmonary disease, but not with developing disseminated disease.63 Altogether, these studies suggest that additional research into the role of age as a risk factor for infection and for the development of adverse outcomes is warranted.
Gender
Historically, coccidioidomycosis has been more common among men than women. Surveillance data from Arizona from 1993–2006 have consistently shown a preponderance of cases in men (55%–66%) compared with women, although more cases have occurred among females in Arizona since 2009.60,61,64 In California, from 2000–2007, men had an average annual rate of 7.6 Coccidioides infections per 100,000 compared with a rate of 4.0 per 100,000 in women.65 This gender distribution may be partially explained by the greater participation by men in high-risk activities or cigarette smoking. Certain human sex hormones, which have been shown to stimulate the growth of Coccidioides in vitro, may also contribute to the elevated incidence in males.66
Severe or disseminated disease may occur more often in men than in women. In the 1940s, Smith and colleagues performed serial coccidioidin skin testing and clinical evaluations of military personnel at US Army airfields in the San Joaquin Valley.67 Though the number of women in the study was small, the frequency of disseminated disease in women was one-fifth the rate found in men. Durry et al evaluated 128 cases of coccidioidomycosis that occurred in 1991 in Tulare County, California, and found that male sex was associated with a higher rate of hospitalization for severe disease.68 In contrast, among the aforementioned coccidioidomycosis cases (n = 380) in Kern County, there was no correlation between male gender and severe or disseminated disease.63
Race
Extrapulmonary dissemination of Coccidioides develops more frequently in certain ethnic groups, including those of African or Filipino descent.69 A study by Smith et al, in the 1940s, found that black persons had rates of dissemination 8–10 times higher than those in white persons.67 Though the age of the subjects in these studies was not reported, all subjects worked in similar conditions and received the same nutrition, housing, and medical care. Decades later, after a severe dust storm led to an outbreak of coccidioidomycosis in California, a disproportionate increase in the incidence of disseminated infection was noted in the non-Caucasian population; the rate of dissemination among African American men was 23.8 cases per 100,000 versus 2.5 cases per 100,000 for Caucasian men.53 Similar findings were reported from Kern County where, in 1995–1996, large numbers of coccidioidomycosis cases occurred in Filipinos, Asians, Hispanics, and blacks.63 Although black race was not significantly associated with severe pulmonary disease, it was associated with disseminated coccidioidomycosis. The other ethnic groups were not linked with either elevated rates of severe pulmonary disease or extrapulmonary dissemination, though the low numbers of Filipinos or Asians in the study limited the analysis. Others have confirmed these findings using geomorphic and demographic strata to control for differences in group-level exposure – making the results more externally valid than prior epidemiologic studies conducted solely on military personnel, clinic or hospitalized patients, or student population.70,71
The causes for racial disparities for Coccidioides infections are not completely understood. Genetic influences, such as the ABO blood group type and human leukocyte antigen (HLA) alleles (eg, the HLA class II DRB1* 1301 allele has been associated with the development of severe, disseminated disease), may play a role,69,72 although these may simply be surrogate markers for at-risk ethnic populations.73
Pregnancy
Pregnant women are especially vulnerable to coccidioidal infection, and their risk of developing severe or disseminated disease rises when infection is acquired in the later stages of pregnancy.74,75 Also, severe coccidioidomycosis is more likely to occur during the immediate postpartum period if infection is acquired in the third trimester.74,76 Two retrospective reviews of Kern County birth records from the 1940s to the 1960s were notable for a rate of 7.7–11 coccidioidomycosis cases per 10,000 pregnancies, with a striking rate of death (33%) among women who had disseminated disease.77,78 A more recent review of 81 coccidioidomycosis cases in pregnant women found that disseminated disease occurred in 50% of the cases diagnosed in the first trimester, 62% in the second trimester, and 96% in the third trimester.75 These extremely high rates of dissemination, which have not been seen in other investigations, may reflect a component of reporting bias. In contrast, Wack et al, in a 1988 review of over 47,000 deliveries from three health care centers in Tucson, Arizona, found only 10 cases of coccidioidomycosis (rate of 2.1 cases per 10,000 pregnancies).76 The authors did, however, find an association of severe disease with increasing stage of pregnancy; of the seven cases diagnosed in the first or second trimester, illness resolved in all, whereas two-thirds of the cases diagnosed in the third trimester developed disseminated infection. More recently, among 32 cases of coccidioidomycosis in pregnant women in Kern County, dissemination occurred in about 10% of cases.79 Sex hormone influences, combined with diminished cell-mediated immunity, may explain this predilection for disseminated coccidioidomycosis to rise as pregnancy progresses.66,80
Immunosuppression
Persons that are especially vulnerable to developing severe or disseminated coccidioidomycosis are those with diseases that impair T-cell function. Such conditions include hematologic malignancies, inflammatory arthritis, and diabetes.2,63,81–83 In one review of 55 patients with hematologic malignancies and coccidioidomycosis close to half of all cases occurred in patients with non-Hodgkin lymphoma (NHL) or chronic lymphocytic leukemia (CLL).81 As compared to other hematologic malignancies, NHL and CLL did not appear to be disproportionately represented since these two hematologic malignancies comprised the majority of the malignancies in their cancer registry. Nonetheless, a striking percentage (20%) of patients did experience extrapulmonary dissemination and 50% of those with disseminated infection died; patients with NHL had the highest rate of dissemination (35%). In patients with diabetes, poor glycemic control (blood glucose levels ≥220 mg/dL) may increase the risk of dissemination.83 In addition, medical therapies that impair cellular immunity, such as antineoplastic agents, high dose corticosteroid therapy (equivalent to a prednisone dose of >20 mg/day), or tumor necrosis factor-α antagonists heighten the risk for severe or disseminated coccidioidomycosis.84–86 Other immunodeficiency states that confer a significant risk for Coccidioides infection are HIV infection and immunosuppression following hematopoietic stem cell or solid organ transplantation.87
Transplant
Coccidioidomycosis has been described as a serious infectious complication among organ transplant recipients in endemic areas; infection is believed to most commonly result from reactivation of previously acquired coccidioidomycosis.88 Risk factors for post-transplant coccidioidomycosis include a history of coccidioidomycosis, positive serology at the time of transplantation, and the use of antirejection therapy; however, concurrent illnesses such as cytomegalovirus, diabetes, and hepatitis C virus infection have not been shown to be associated with coccidioidomycosis among transplant recipients.88–91
Early retrospective studies of coccidioidomycosis among solid organ transplant recipients in Arizona calculated incidence rates as high as 6.9% among kidney transplant recipients during 1970–197992 and 8.7% among heart transplant recipients during 1979–1984,93 with high rates of dissemination (75%) and overall mortality (63%).92 A more recent report from one large Arizona institution found lower rates during 1999–2006 of 1.2% to 2.4% among hematologic and liver transplant recipients respectively, possibly related to their use of targeted antifungal prophylaxis among patients at high risk for post-transplant coccidioidomycosis.94 Other reports of small case series indicate that considerable rates (>30%) of disseminated coccidioidomycosis and mortality still occur among transplant recipients.89,90
Several instances of coccidioidomycosis acquired from donated organs have also been documented in persons with no history of exposure to endemic areas, including lung,95–98 liver,99,100 kidney,96,99–101 pancreas,96 and heart,99 transplant recipients; the majority of these reports describe disseminated and/or fatal cases. The exact risk for coccidioidomycosis transmission through an infected organ is unknown. A prospective study of 568 healthy potential liver or kidney donors in Arizona found that 12 (2.1%) had a positive coccidioidal serology, four of whom proceeded to donate an organ, but none of the recipients developed coccidioidomycosis.102 Nevertheless, donor-derived coccidioidomycosis is likely to be under-recognized in endemic areas, due to the reasonable assumption that infection in a transplant patient is the result of re-activation of prior infection or acquired from the local environment.94,102 Further research is needed to better understand if screening donors or recipients with a serological test can reduce the risk of donor-derived Coccidioides transmission.102
HIV/AIDS
Coccidioidomycosis is also a well-recognized opportunistic infection among HIV-infected individuals, though its incidence and severity in this population appears to have declined in the USA with the broader use of combination antiretroviral therapy (cART) beginning around 1996.103,104 A prospective study at an Arizona HIV clinic in 1988 showed a cumulative incidence of active coccidioidomycosis of 25% during 41 months of follow-up, corresponding to an average annual incidence of 7.3%.105 In contrast, a retrospective review at the same clinic during 2003–2008 found an annual incidence of only 0.9% and a decrease in severity of disease compared to the previous study.87 As with other HIV-associated opportunistic infections, CD4 cell counts are the most important risk factors for coccidioidomycosis. Studies conducted both before and after the advent of cART have shown CD4 count to be the only predictor for developing active coccidioidomycosis; factors such as a history of coccidioidomycosis and duration of residence in an endemic area105 or age, sex, race, ethnicity, plasma HIV RNA level, or receipt of cART were not associated with increased risk for coccidioidomycosis.87 Another study matched HIV-infected patients based on CD4 count, age, sex, and county of residence, and found that black race, a history of oropharyngeal or esophageal candidiasis, and not receiving any medication were independent risk factors for developing coccidioidomycosis, while use of protease inhibitors was protective; in patients with a history of oropharyngeal or esophageal candidiasis, receipt of an azole drug in the 90 days preceding the diagnosis of coccidioidomycosis was associated with a reduced risk for developing coccidioidomycosis.104
Prisons
Coccidioidomycosis has been described in a number of prisons in endemic areas, with a large degree of associated morbidity. In California, a large burden of disease occurs in state and federal correctional institutions in the San Joaquin Valley,106 and recently, incidence has been increasing in some prisons, with rates as high as 7% during 2006–2010.107 The reasons for high rates among prisoners are not entirely clear, but may be due, at least in part, to large numbers of immune-naïve persons who have been relocated from other, less-endemic areas. Public health officials have instituted aggressive measures to reduce the morbidity and mortality of this disease, including widespread education of prisoners and staff and exclusion of immunosuppressed individuals from prisons located in highly endemic areas. Still, the annual cost of coccidioidomycosis care and treatment in California prisons is estimated to exceed $23 million.107 Further research into additional methods aimed at reducing the numbers of infected prisoners is needed. One promising strategy may be to risk stratify prisoners via the spherulin skin test (Spherusol, Allermed Laboratories, Inc. San Diego, CA, USA, is FDA-approved but not yet commercially available) prior to entry in a prison in a highly endemic region.
Recent epidemiologic trends
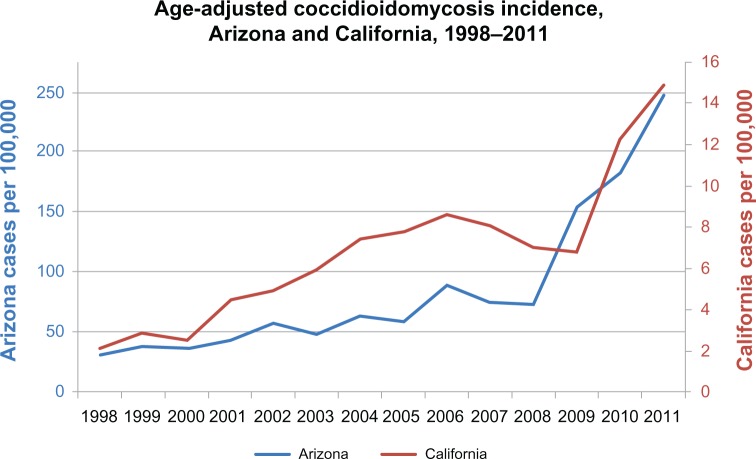
The incidence of coccidioidomycosis has risen substantially during the past two decades (Figure 3). In Arizona, this increase first became apparent during the early 1990s, when the annual incidence more than doubled from 7/100,000 persons in 1990 to 15/100,000 in 1995.108 Coccidioidomycosis became a mandatory reportable disease in Arizona in 1997; the incidence rose from 21/100,000 in 1997 to 37/100,000 in 1999 and continued to rise to 91/100,000 in 2006.61 Incidence decreased slightly during 2007–2008,64 but then increased dramatically during 2009 (154/100,000) to 2011 (248/100,000).109 Reasons for the observed increase since 2009 are not known, but may be due in part to a major commercial laboratory changing its reporting practices to conform with the other laboratories in Arizona, by reporting positive enzyme immunoassay (EIA) results as cases without confirmation by immunodiffusion.64 An enhanced surveillance study suggests that the use of laboratory-only criteria for case reporting, including a single positive EIA test, accurately reflects the burden of disease in Arizona.110 Other reports show that EIA testing has a high false-positivity rate111 and more recent evidence has suggested the performance characteristics of EIA testing varies among different patient groups.112
Figure 3.
Incidence of coccidioidomycosis in Arizona and California, 1998–2011.
Diagnostic practices in coccidioidomycosis vary between institutions with some facilities employing highly sensitive but nonspecific testing, such as the aforementioned EIA, while others perform more labor intensive methodologies such as complement fixation and immunodiffusion testing that are available only at reference laboratories.113 Recently coccidioidal antigen testing has become commercially available, however this test has thus far proven useful only in cases of widely disseminated infection114 and has a poor sensitivity in veterinary samples.115
Although it is likely that differences in testing methodology have contributed in part to a reported increase in Arizona, a similar incidence trend has occurred in California. Average statewide annual incidence increased from 2.5/100,000 during 1995–2000 to 8/100,000 in 2006,65 and after a slight decline during 2007–2009, increased during 2010 and 2011 (14/100,000).109 During this time, the only major change to California’s surveillance was a transition to a laboratory-based reporting system in 2010. Coccidioidomycosis incidence also rose substantially during 1998–2011 in other endemic areas (where reporting practices have remained relatively consistent), including New Mexico, Nevada, and Utah,116 perhaps indicating improved awareness of the disease, changes in testing practices, increased travel or relocation to endemic areas, and growth of the “at-risk” immunosuppressed population.117 Other hypotheses include increased spore dispersal due to environmental changes (ie, temperature, moisture) or human activity such as construction.118
Analyses of hospitalization data further suggest that the recent increase reflects an increase in actual disease. In Arizona, the number of persons discharged with a primary or secondary diagnosis of coccidioidomycosis increased from 69 in 1998 to 598 in 2001, with the highest hospitalization rate (29/100,000) occurring among persons ≥65 years.119 An analysis of national pediatric coccidioidomycosis hospitalizations showed that rates were stable during 2003–2005 but increased significantly during 2005–2006.120 In California, coccidioidomycosis-related hospitalization rates remained fairly stable during 1997–2002, with an average annual rate of 3.7/100,000.121 A review of California hospital discharge data from 2001–2008, however, showed an overall increase in coccidioidomycosis-related hospitalizations during 2001–2006, peaking at 6/100,000 in 2006 before decreasing in 2007 and 2008.64 Overall, data on coccidioidomycosis-related hospitalizations in recent years are limited. Given that one study estimated that over 40% of reported Arizona coccidioidomycosis case-patients require hospitalization,110 this topic deserves further study.
In contrast to the dramatic recent increase in incidence, coccidioidomycosis-associated mortality has remained largely unchanged. Based on death certificate data, the mortality rate in Arizona actually decreased slightly from 0.9/100,000 in 1996 to 0.5/100,000 in 2005.61 A review of US death certificates identified a total of 3089 coccidioidomycosis-associated deaths during 1990–2008, but found that mortality rates have remained fairly stable since 1997, with an overall age-adjusted mortality rate of 0.59 per 1 million person-years during the study period.122 Possible reasons for the stable mortality rates despite the overall increase in incidence include treatment advancements or a disproportionate increase in primary pulmonary coccidioidomycosis diagnoses compared to the more severe forms of disease.61 Although mortality rates have remained stable, the number of documented coccidioidomycosis-related deaths is likely underestimated.122
Conclusion
The public health burden of coccidioidomycosis is substantial and has been increasing in recent years. Current prevention messages focus on common-sense methods to reduce exposure to soil or dust where Coccidioides is common in the environment, such as wearing a dust mask, wetting soil before participating in soil-disturbing activities, or limiting these types of activities altogether, particularly among people at risk for severe disease. However, it is important to point out that few to no data exist that demonstrate the effectiveness of any of these measures. Wide-scale measures to reduce airborne dispersal of Coccidioides such as watering construction sites, paving roads, planting grass, or other vegetation have also been proposed, but these methods are not likely to be overly effective because airborne conidia can travel for miles.
Determination of prior Coccidioides exposure to evaluate the risk for disease represents a promising public health strategy. Measurement of coccidioidal cellular immunity using skin tests has been a valuable clinical and epidemiologic tool since the 1940s; however, Coccidioides skin test reagents have not been commercially available in the USA for over a decade.123 The return of widespread access to coccidioidal skin tests in endemic areas could help identify and thus better manage specific groups of people at risk for infection.
Because there are no proven methods to prevent coccidioidomycosis, additional research into strategies to reduce the associated morbidity is required. Continued efforts to promote awareness among the public may help to reduce delays in diagnosis and treatment, as evidence suggests that persons with coccidioidomycosis who knew about the disease before seeking healthcare were more likely to request testing and be diagnosed sooner than those who were unfamiliar with the disease.110 Similarly, increased awareness among healthcare providers about coccidioidomycosis diagnosis and treatment is needed,124 especially because the symptoms are often indistinguishable from those of other community-acquired respiratory infections. Admittedly, it is not definitively known whether earlier diagnosis and treatment can lead to improved outcomes, but other benefits of diagnosis, such as reduced anxiety or unnecessary medical treatment or procedures, make recognition of coccidioidomycosis essential.
Further research into the optimal antifungal treatment regimen for coccidioidomycosis is also warranted, particularly with regard to the role of antifungal treatment for primary pulmonary disease.2,125 Currently, guidelines from the Infectious Diseases Society of America recommend treatment of primary pulmonary disease in persons who are at risk for developing severe or disseminated disease.2 However, the role of antifungal medications in this clinical syndrome is controversial. Some experts recommend treatment of all persons with symptomatic respiratory disease, while others prefer to observe these patients closely. More research is needed to determine if existing or newly-developed antifungal agents can reduce the severity or duration of disease. Finally, efforts to create a preventive vaccine are ongoing;126 and if developed, a vaccine could prove to be a cost-effective strategy to reduce the burden of disease among some at risk populations.127
Footnotes
Disclosure
The authors have no conflicts of interest to declare.
References
- 1.Thompson GR., 3rd Pulmonary coccidioidomycosis. Semin Respir Crit Care Med. 2011;32(6):754–763. doi: 10.1055/s-0031-1295723. [DOI] [PubMed] [Google Scholar]
- 2.Galgiani JN, Ampel NM, Blair JE, et al. Coccidioidomycosis. Clin Infect Dis. 2005;41(9):1217–1223. doi: 10.1086/496991. [DOI] [PubMed] [Google Scholar]
- 3.Barker BM, Jewell KA, Kroken S, Orbach MJ. The population biology of coccidioides: epidemiologic implications for disease outbreaks. Ann N Y Acad Sci. 2007;1111:147–163. doi: 10.1196/annals.1406.040. [DOI] [PubMed] [Google Scholar]
- 4.Colombo AL, Tobon A, Restrepo A, Queiroz-Telles F, Nucci M. Epidemiology of endemic systemic fungal infections in Latin America. Medi Mycol. 2011;49(8):785–798. doi: 10.3109/13693786.2011.577821. [DOI] [PubMed] [Google Scholar]
- 5.Laniado-Laborin R. Expanding understanding of epidemiology of coccidioidomycosis in the Western hemisphere. Ann N Y Acad Sci. 2007;1111:19–34. doi: 10.1196/annals.1406.004. [DOI] [PubMed] [Google Scholar]
- 6.Hirschmann JV. The early history of coccidioidomycosis: 1892–1945. Clin Infect Dis. 2007;44(9):1202–1207. doi: 10.1086/513202. [DOI] [PubMed] [Google Scholar]
- 7.Rixford EGT. Two cases of protozoan (coccidioidal) infection of the skin and other organs. Johns Hopkins Hosp Rep. 1896;10:209–268. [Google Scholar]
- 8.Ophuls W. Further observations on a pathogenic mould formerly described as a protozoan (Coccidioides immitis, Coccioides pyogenes) J Exp Med. 1905;6(4–6):443–485. doi: 10.1084/jem.6.4-6.443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dickson EC GM. Coccidioides Infection (Coccidioidomycosis): ii. The primary type of infection. Arch Intern Med. 1938;62(5):853–871. [Google Scholar]
- 10.Smith CE. Epidemiology of Acute Coccidioidomycosis with Erythema Nodosum (“San Joaquin” or “Valley Fever”) Am J Public Health Nations Health. 1940;30(6):600–611. doi: 10.2105/ajph.30.6.600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Davis BL, SR, Smith CE. An epidemic of coccidioidal infection (coccidioidomycosis) JAMA. 1942;118:1182–1186. [Google Scholar]
- 12.Pappagianis D. Epidemiology of coccidioidomycosis. Curr Top Med Mycol. 1988;2:199–238. doi: 10.1007/978-1-4612-3730-3_6. [DOI] [PubMed] [Google Scholar]
- 13.Saubolle MA, McKellar PP, Sussland D. Epidemiologic, clinical, and diagnostic aspects of coccidioidomycosis. J Clin Microbiol. 2007;45(1):26–30. doi: 10.1128/JCM.02230-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tamerius JD, Comrie AC. Coccidioidomycosis incidence in Arizona predicted by seasonal precipitation. PloS One. 2011;6(6):e21009. doi: 10.1371/journal.pone.0021009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kolivras KN, Comrie AC. Modeling valley fever (coccidioidomycosis) incidence on the basis of climate conditions. Int J Biometeorol. 2003;47(2):87–101. doi: 10.1007/s00484-002-0155-x. [DOI] [PubMed] [Google Scholar]
- 16.Talamantes J, Behseta S, Zender CS. Fluctuations in climate and incidence of coccidioidomycosis in Kern County, California: a review. Ann N Y Acad Sci. 2007;1111:73–82. doi: 10.1196/annals.1406.028. [DOI] [PubMed] [Google Scholar]
- 17.Elconin AF, Egeberg RO, Egeberg MC. Significance of soil salinity on the ecology of coccidioides immitis. J Bacteriol. 1964;87:500–503. doi: 10.1128/jb.87.3.500-503.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Maddy KCH. Establishment of coccidioides immitis in negative soil following burial of infected animals and animal tissues. In: Ajello L, editor. Papers from the Second Symposium on Coccidioidomycosis. Tuscon, AZ: University of Arizona Press; 1967. pp. 309–312. [Google Scholar]
- 19.Egeberg RO, Ely AF. Coccidioides immitis in the soil of the southern San Joaquin Valley. Am J Med Sci. 1956;231(2):151–154. [PubMed] [Google Scholar]
- 20.Cordeiro Rde A, e Silva KR, Brilhante RS, et al. Coccidioides posadasii infection in bats, Brazil. Emerg Infect Dis. 2012;18(4):668–670. doi: 10.3201/eid1804.111641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Eulalio KD, de Macedo RL, Cavalcanti MA, Martins LM, Lazera MS, Wanke B. Coccidioides immitis isolated from armadillos (Dasypus novemcinctus) in the state of Piaui, northeast Brazil. Mycopathologia. 2001;149(2):57–61. doi: 10.1023/a:1007273019647. [DOI] [PubMed] [Google Scholar]
- 22.Sharpton TJ, Stajich JE, Rounsley SD, et al. Comparative genomic analyses of the human fungal pathogens Coccidioides and their relatives. Genome Res. 2009;19(10):1722–1731. doi: 10.1101/gr.087551.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shubitz LF. Comparative aspects of coccidioidomycosis in animals and humans. Ann N Y Acad Sci. 2007;1111:395–403. doi: 10.1196/annals.1406.007. [DOI] [PubMed] [Google Scholar]
- 24.Shubitz LE, Butkiewicz CD, Dial SM, Lindan CP. Incidence of coccidioides infection among dogs residing in a region in which the organism is endemic. J Am Vet Med Assoc. 2005;226(11):1846–1850. doi: 10.2460/javma.2005.226.1846. [DOI] [PubMed] [Google Scholar]
- 25.Gautam R, Srinath I, Clavijo A, et al. Identifying areas of high risk of human exposure to coccidioidomycosis in Texas using serology data from dogs. Zoonoses Public Health. 2013;60(2):174–181. doi: 10.1111/j.1863-2378.2012.01526.x. [DOI] [PubMed] [Google Scholar]
- 26.Edwards PQ, Palmer CE. Prevalence of sensitivity to coccidioidin, with special reference to specific and nonspecific reactions to coccidioidin and to histoplasmin. Dis Chest. 1957;31(1):35–60. doi: 10.1378/chest.31.1.35. [DOI] [PubMed] [Google Scholar]
- 27.Beadenkopf WG, Loosli CG, et al. Tuberculin, coccidioidin, and histoplasmin sensitivity in relation to pulmonary calcifications; a survey among 6,000 students at the University of Chicago. Public Health Rep. 1949;64(1):17–32. [PubMed] [Google Scholar]
- 28.Lauer A, Baal JD, Baal JC, Verma M, Chen JM. Detection of Coccidioides immitis in Kern County, California, by multiplex PCR. Mycologia. 2012;104(1):62–69. doi: 10.3852/11-127. [DOI] [PubMed] [Google Scholar]
- 29.Burt A, Dechairo BM, Koenig GL, Carter DA, White TJ, Taylor JW. Molecular markers reveal differentiation among isolates of Coccidioides immitis from California, Arizona and Texas. Mol Ecol. 1997;6(8):781–786. doi: 10.1046/j.1365-294x.1997.00245.x. [DOI] [PubMed] [Google Scholar]
- 30.Centers for Disease Control and Prevention (CDC) Coccidioidomycosis – United States, 1991–1992. MMWR Morb Mortal Wkly Rep. 1993;42(2):21–24. [PubMed] [Google Scholar]
- 31.Rios-Fabra A, Moreno AR, Isturiz RE. Fungal infection in Latin American countries. Infect Dis Clin North Am. 1994;8(1):129–154. [PubMed] [Google Scholar]
- 32.Rios-Olivares EO. 1st human case of coccidioidomycosis in Nicaragua. Rev Latinoam Microbiol. 1979;21(4):215–218. [PubMed] [Google Scholar]
- 33.Ajello L. Comparative ecology of respiratory mycotic disease agents. Bacteriol Rev. 1967;31(1):6–24. doi: 10.1128/br.31.1.6-24.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Padua y Gabriel A, Martinez-Ordaz VA, Velasco-Rodreguez VM, Lazo-Saenz JG, Cicero R. Prevalence of skin reactivity to coccidioidin and associated risks factors in subjects living in a northern city of Mexico. Arch Med Res. 1999;30(5):388–392. doi: 10.1016/s0188-0128(99)00048-2. [DOI] [PubMed] [Google Scholar]
- 35.D’Avino A, Di Giambenedetto S, Fabbiani M, Farina S. Coccidioidomycosis of cervical lymph nodes in an HIV-infected patient with immunologic reconstitution on potent HAART: a rare observation in a nonendemic area. Diagn Microbiol Infect Dis. 2012;72(2):185–187. doi: 10.1016/j.diagmicrobio.2011.10.002. [DOI] [PubMed] [Google Scholar]
- 36.Kotton CN, Marconi VC, Fishman JA, Chung RT, Elias N, Hertl M. Coccidioidal meningitis after liver transplantation in a nonendemic region: a case report. Transplantation. 2006;81(1):132–134. doi: 10.1097/01.tp.0000184755.03306.14. [DOI] [PubMed] [Google Scholar]
- 37.Centers for Disease Control and Prevention (CDC) Coccidioidomycosis in Travelers Returning From Mexico – Pennsylvania, 2000. MMWR. 2000;49(44):1004–1006. [PubMed] [Google Scholar]
- 38.Cairns L, Blythe D, Kao A, et al. Outbreak of coccidioidomycosis in Washington state residents returning from Mexico. Clin Infect Dis. 2000;30(1):61–64. doi: 10.1086/313602. [DOI] [PubMed] [Google Scholar]
- 39.Standaert SM, Schaffner W, Galgiani JN, et al. Coccidioidomycosis among visitors to a Coccidioides immitis-endemic area: an outbreak in a military reserve unit. J Infect Dis. 1995;171(6):1672–1675. doi: 10.1093/infdis/171.6.1672. [DOI] [PubMed] [Google Scholar]
- 40.Desai SA, Minai OA, Gordon SM, O’Neil B, Wiedemann HP, Arroliga AC. Coccidioidomycosis in non-endemic areas: a case series. Respir Med. 2001;95(4):305–309. doi: 10.1053/rmed.2000.1039. [DOI] [PubMed] [Google Scholar]
- 41.Chaturvedi V, Ramani R, Gromadzki S, Rodeghier B, Chang HG, Morse DL. Coccidioidomycosis in New York State. Emerg Infect Dis. 2000;6(1):25–29. doi: 10.3201/eid0601.000104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Taxy JB, Kodros S. Musculoskeletal coccidioidomycosis: unusual sites of disease in a nonendemic area. Am J Clin Pathol. 2005;124(5):693–696. doi: 10.1309/KRNY-U4RN-7Q12-WEYD. [DOI] [PubMed] [Google Scholar]
- 43.Desai NR, McGoey R, Troxclair D, Simeone F, Palomino J. Coccidioidomycosis in nonendemic area: case series and review of literature. J La State Med Soc. 2010;162(2):97–103. [PubMed] [Google Scholar]
- 44.Panackal AA, Hajjeh RA, Cetron MS, Warnock DW. Fungal infections among returning travelers. Clin Infect Dis. 2002;35(9):1088–1095. doi: 10.1086/344061. [DOI] [PubMed] [Google Scholar]
- 45.Centers for Disease Control and Prevention (CDC) Coccidioidomycosis among persons attending the world championship of model airplane flying – Kern County, California, Oct 2001. MMWR Morbidity and Mortality Weekly Report. 2001;50(49):1106–1107. [PubMed] [Google Scholar]
- 46.Clark TA, HS Johnson E, Mark K, et al. Coccidioidomycosis associated with the World Championship of Model Airplane Free Flight – Lost Hills, California, 2001; Proceedings of the Annual Coccidioidomycosis Study Group Meeting; 2002. [Google Scholar]
- 47.Marsden-Haug N, Goldoft M, Ralston C, et al. Coccidioidomycosis acquired in Washington State. Clin Infect Dis. 2013;56(6):847–850. doi: 10.1093/cid/cis1028. [DOI] [PubMed] [Google Scholar]
- 48.Lan F, Tong YZ, Huang H, Xiong WN, Xu YJ, Xiong SD. Primary pulmonary coccidioidomycosis in China. Respirology. 2010;15(4):722–725. doi: 10.1111/j.1440-1843.2010.01747.x. [DOI] [PubMed] [Google Scholar]
- 49.Dzawachiszwili N, Landau JW, Newcomer VD, Plunkett OA. The effect of sea water and sodium chloride on the growth of fungi pathogenic to man. J Invest Dermat. 1964;43:103–109. [PubMed] [Google Scholar]
- 50.Charlton V, Ramsdell K, Sehring S. Intrauterine transmission of coccidioidomycosis. Pediatr Infect Dis J. 1999;18(6):561–563. doi: 10.1097/00006454-199906000-00021. [DOI] [PubMed] [Google Scholar]
- 51.Gaidici A, Saubolle MA. Transmission of coccidioidomycosis to a human via a cat bite. J Clin Microbiol. 2009;47(2):505–506. doi: 10.1128/JCM.01860-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Werner SB, Pappagianis D, Heindl I, Mickel A. An epidemic of coccidioidomycosis among archeology students in northern California. N Engl J Med. 1972;286(10):507–512. doi: 10.1056/NEJM197203092861003. [DOI] [PubMed] [Google Scholar]
- 53.Cummings KC, McDowell A, Wheeler C, et al. Point-source outbreak of coccidioidomycosis in construction workers. Epidemiol Infect. 2010;138(4):507–511. doi: 10.1017/S0950268809990999. [DOI] [PubMed] [Google Scholar]
- 54.Flynn NM, Hoeprich PD, Kawachi MM, et al. An unusual outbreak of windborne coccidioidomycosis. N Engl J Med. 1979;301(7):358–361. doi: 10.1056/NEJM197908163010705. [DOI] [PubMed] [Google Scholar]
- 55.Pappagianis D, Einstein H. Tempest from Tehachapi takes toll or Coccidioides conveyed aloft and afar. West J Med. 1978;129(6):527–530. [PMC free article] [PubMed] [Google Scholar]
- 56.Schneider E, Hajjeh RA, Spiegel RA, et al. A coccidioidomycosis outbreak following the Northridge, Calif, earthquake. JAMA. 1997;277(11):904–908. [PubMed] [Google Scholar]
- 57.Wanke B, Lazera M, Monteiro PC, et al. Investigation of an outbreak of endemic coccidioidomycosis in Brazil’s northeastern state of Piaui with a review of the occurrence and distribution of Coccidioides immitis in three other Brazilian states. Mycopathologia. 1999;148(2):57–67. doi: 10.1023/a:1007183022761. [DOI] [PubMed] [Google Scholar]
- 58.Crum N, Lamb C, Utz G, Amundson D, Wallace M. Coccidioidomycosis outbreak among United States Navy SEALs training in a Coccidioides immitis-endemic area – Coalinga, California. J Infect Dis. 2002;186(6):865–868. doi: 10.1086/342409. [DOI] [PubMed] [Google Scholar]
- 59.Vartivarian SE, Coudron PE, Markowitz SM. Disseminated coccidioidomycosis. Unusual manifestations in a cardiac transplantation patient. Am J Med. 1987;83(5):949–952. doi: 10.1016/0002-9343(87)90657-7. [DOI] [PubMed] [Google Scholar]
- 60.Leake JA, Mosley DG, England B, et al. Risk factors for acute symptomatic coccidioidomycosis among elderly persons in Arizona, 1996–1997. J Infect Dis. 2000;181(4):1435–1440. doi: 10.1086/315400. [DOI] [PubMed] [Google Scholar]
- 61.Sunenshine RH, Anderson S, Erhart L, et al. Public health surveillance for coccidioidomycosis in Arizona. Ann N Y Acad Sci. 2007;1111:96–102. doi: 10.1196/annals.1406.045. [DOI] [PubMed] [Google Scholar]
- 62.Blair JE, Mayer AP, Currier J, Files JA, Wu Q. Coccidioidomycosis in elderly persons. Clin Infect Dis. 2008;47(12):1513–1518. doi: 10.1086/593192. [DOI] [PubMed] [Google Scholar]
- 63.Rosenstein NE, Emery KW, Werner SB, et al. Risk factors for severe pulmonary and disseminated coccidioidomycosis: Kern County, California, 1995–1996. Clin Infect Dis. 2001;32(5):708–715. doi: 10.1086/319203. [DOI] [PubMed] [Google Scholar]
- 64.Hector RF, Rutherford GW, Tsang CA, et al. The public health impact of coccidioidomycosis in Arizona and California. Int J Environ Res Public Health. 2011;8(4):1150–1173. doi: 10.3390/ijerph8041150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Centers for Disease Control and Prevention (CDC) Increase in Coccidioidomycosis – California, 2000–2007. MMWR Morb Mortal Wkly Rep. 2009;58(5):105–109. [PubMed] [Google Scholar]
- 66.Drutz DJ, Huppert M, Sun SH, McGuire WL. Human sex hormones stimulate the growth and maturation of Coccidioides immitis. Infect Immun. 1981;32(2):897–907. doi: 10.1128/iai.32.2.897-907.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Smith CE, Beard RR, et al. Varieties of coccidioidal infection in relation to the epidemiology and control of the diseases. Am J Public Health Nations Health. 1946;36(12):1394–1402. doi: 10.2105/ajph.36.12.1394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Durry E, Pappagianis D, Werner SB, et al. Coccidioidomycosis in Tulare County, California, 1991: reemergence of an endemic disease. J Med Vet Mycol. 1997;35(5):321–326. doi: 10.1080/02681219780001361. [DOI] [PubMed] [Google Scholar]
- 69.Louie L, Ng S, Hajjeh R, et al. Influence of host genetics on the severity of coccidioidomycosis. Emerg Infect Dis. 1999;5(5):672–680. doi: 10.3201/eid0505.990508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Tabor JA, O’Rourke MK. A risk factor study of coccidioidomycosis by controlling differential misclassifications of exposure and susceptibility using a landscape ecology approach. Sci Total Environ. 2010;408(10):2199–2207. doi: 10.1016/j.scitotenv.2010.02.013. [DOI] [PubMed] [Google Scholar]
- 71.Tabor JA, O’Rourke MK, Lebowitz MD, Harris RB. Landscape-epidemiological study design to investigate an environmentally based disease. J Exp Sci Environ Epidemiol. 2011;21(2):197–211. doi: 10.1038/jes.2009.67. [DOI] [PubMed] [Google Scholar]
- 72.Deresinski SC, Pappagianis D, Stevens DA. Association of ABO blood group and outcome of coccidioidal infection. Sabouraudia. 1979;17(3):261–264. doi: 10.1080/00362177985380381. [DOI] [PubMed] [Google Scholar]
- 73.Vinh DC, Masannat F, Dzioba RB, Galgiani JN, Holland SM. Refractory disseminated coccidioidomycosis and mycobacteriosis in interferon-gamma receptor 1 deficiency. Clin Infect Dis. 2009;49(6):e62–e65. doi: 10.1086/605532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Bercovitch RS, Catanzaro A, Schwartz BS, Pappagianis D, Watts DH, Ampel NM. Coccidioidomycosis during pregnancy: a review and recommendations for management. Clin Infect Dis. 2011;53(4):363–368. doi: 10.1093/cid/cir410. [DOI] [PubMed] [Google Scholar]
- 75.Crum NF, Ballon-Landa G. Coccidioidomycosis in pregnancy: case report and review of the literature. Am J Med. 2006;119(11):993. e911–e997. doi: 10.1016/j.amjmed.2006.04.022. [DOI] [PubMed] [Google Scholar]
- 76.Wack EE, Ampel NM, Galgiani JN, Bronnimann DA. Coccidioidomycosis during pregnancy. An analysis of ten cases among 47,120 pregnancies. Chest. 1988;94(2):376–379. doi: 10.1378/chest.94.2.376. [DOI] [PubMed] [Google Scholar]
- 77.Vaughan JE, Ramirez H. Coccidioidomycosis as a complication of pregnancy. Calif Med. 1951;74(2):121–125. [PMC free article] [PubMed] [Google Scholar]
- 78.Smale LE, Waechter KG. Dissemination of coccidioidomycosis in pregnancy. Am J Obstet Gynecol. 1970;107(3):356–361. doi: 10.1016/0002-9378(70)90557-0. [DOI] [PubMed] [Google Scholar]
- 79.Caldwell JW, Arsura EL, Kilgore WB, Garcia AL, Reddy V, Johnson RH. Coccidioidomycosis in pregnancy during an epidemic in California. Obstet Gynecol. 2000;95(2):236–239. doi: 10.1016/s0029-7844(99)00484-6. [DOI] [PubMed] [Google Scholar]
- 80.Weinberg ED. Pregnancy-associated depression of cell-mediated immunity. Rev Infect Dis. 1984;6(6):814–831. doi: 10.1093/clinids/6.6.814. [DOI] [PubMed] [Google Scholar]
- 81.Blair JE, Smilack JD, Caples SM. Coccidioidomycosis in patients with hematologic malignancies. Arch Intern Med. 2005;165(1):113–117. doi: 10.1001/archinte.165.1.113. [DOI] [PubMed] [Google Scholar]
- 82.Mertz LE, Blair JE. Coccidioidomycosis in rheumatology patients: incidence and potential risk factors. Ann N Y Acad Sci. 2007;1111:343–357. doi: 10.1196/annals.1406.027. [DOI] [PubMed] [Google Scholar]
- 83.Santelli AC, Blair JE, Roust LR. Coccidioidomycosis in patients with diabetes mellitus. Am J Med. 2006;119(11):964–969. doi: 10.1016/j.amjmed.2006.03.033. [DOI] [PubMed] [Google Scholar]
- 84.Bergstrom L, Yocum DE, Ampel NM, et al. Increased risk of coccidioidomycosis in patients treated with tumor necrosis factor alpha antagonists. Arthritis Rheum. 2004;50(6):1959–1966. doi: 10.1002/art.20454. [DOI] [PubMed] [Google Scholar]
- 85.Rutala PJ, Smith JW. Coccidioidomycosis in potentially compromised hosts: the effect of immunosuppressive therapy in dissemination. Am J Med Sci. 1978;275(3):283–295. doi: 10.1097/00000441-197805000-00006. [DOI] [PubMed] [Google Scholar]
- 86.Galgiani JN. Coccidioidomycosis: a regional disease of national importance. Rethinking approaches for control. Ann Intern Med. 1999;130(4 Pt 1):293–300. doi: 10.7326/0003-4819-130-4-199902160-00015. [DOI] [PubMed] [Google Scholar]
- 87.Masannat FY, Ampel NM. Coccidioidomycosis in patients with HIV-1 infection in the era of potent antiretroviral therapy. Clin Infect Dis. 2010;50(1):1–7. doi: 10.1086/648719. [DOI] [PubMed] [Google Scholar]
- 88.Blair JE, Logan JL. Coccidioidomycosis in solid organ transplantation. Clin Infect Dis. 2001;33(9):1536–1544. doi: 10.1086/323463. [DOI] [PubMed] [Google Scholar]
- 89.Blair JE. Coccidioidomycosis in liver transplantation. Liver Transpl. 2006;12(1):31–39. doi: 10.1002/lt.20654. [DOI] [PubMed] [Google Scholar]
- 90.Braddy CM, Heilman RL, Blair JE. Coccidioidomycosis after renal transplantation in an endemic area. Am J Transplant. 2006;6(2):340–345. doi: 10.1111/j.1600-6143.2005.01169.x. [DOI] [PubMed] [Google Scholar]
- 91.Vikram HR, Dosanjh A, Blair JE. Coccidioidomycosis and lung transplantation. Transplantation. 2011;92(7):717–721. doi: 10.1097/TP.0b013e31822e6e9a. [DOI] [PubMed] [Google Scholar]
- 92.Cohen IM, Galgiani JN, Potter D, Ogden DA. Coccidioidomycosis in renal replacement therapy. Arch Intern Med. 1982;142(3):489–494. [PubMed] [Google Scholar]
- 93.Calhoun D, Galgiani J, Zukoski C. Coccidioidomycosis in recent renal or cardiac transplant recipients; Coccidioidomycosis: proceedings of the 4th International Conference on Coccidioidomycosis; 1984; San Diego. [Google Scholar]
- 94.Blair JE. Coccidioidomycosis in patients who have undergone transplantation. Ann N Y Acad Sci. 2007;1111:365–376. doi: 10.1196/annals.1406.009. [DOI] [PubMed] [Google Scholar]
- 95.Brugiere O, Forget E, Biondi G, et al. Coccidioidomycosis in a lung transplant recipient acquired from the donor graft in France. Transplantation. 2009;1588(11):1319–1320. doi: 10.1097/TP.0b013e3181bc22e1. [DOI] [PubMed] [Google Scholar]
- 96.Dierberg KL, Marr KA, Subramanian A, et al. Donor-derived organ transplant transmission of coccidioidomycosis. Transpl Infect Dis. 2012;14(3):300–304. doi: 10.1111/j.1399-3062.2011.00696.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Miller MB, Hendren R, Gilligan PH. Posttransplantation disseminated coccidioidomycosis acquired from donor lungs. J Clin Microbiol. 2004;42(5):2347–2349. doi: 10.1128/JCM.42.5.2347-2349.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Tripathy U, Yung GL, Kriett JM, Thistlethwaite PA, Kapelanski DP, Jamieson SW. Donor transfer of pulmonary coccidioidomycosis in lung transplantation. Ann Thorac Surg. 2002;73(1):306–308. doi: 10.1016/s0003-4975(01)02723-0. [DOI] [PubMed] [Google Scholar]
- 99.Blodget E, Geiseler PJ, Larsen RA, Stapfer M, Qazi Y, Petrovic LM. Donor-derived Coccidioides immitis fungemia in solid organ transplant recipients. Transplant Infect Dis. 2012;14(3):305–310. doi: 10.1111/j.1399-3062.2011.00698.x. [DOI] [PubMed] [Google Scholar]
- 100.Wright PW, Pappagianis D, Wilson M, et al. Donor-related coccidioidomycosis in organ transplant recipients. Clin Infect Dis. 2003;37(9):1265–1269. doi: 10.1086/378741. [DOI] [PubMed] [Google Scholar]
- 101.Carvalho C, Ferreira I, Gaiao S, et al. Cerebral coccidioidomycosis after renal transplantation in a non-endemic area. Transplant Infect Dis. 2010;12(2):151–154. doi: 10.1111/j.1399-3062.2009.00456.x. [DOI] [PubMed] [Google Scholar]
- 102.Blair JE, Mulligan DC. Coccidioidomycosis in healthy persons evaluated for liver or kidney donation. Transplant Infect Dis. 2007;9(1):78–82. doi: 10.1111/j.1399-3062.2006.00179.x. [DOI] [PubMed] [Google Scholar]
- 103.Ampel NM. Coccidioidomycosis in persons infected with HIV-1. Ann N Y Acad Sci. 2007;1111:336–342. doi: 10.1196/annals.1406.033. [DOI] [PubMed] [Google Scholar]
- 104.Woods CW, McRill C, Plikaytis BD, et al. Coccidioidomycosis in human immunodeficiency virus-infected persons in Arizona, 1994–1997: incidence, risk factors, and prevention. J Infect Dis. 2000;181(4):1428–1434. doi: 10.1086/315401. [DOI] [PubMed] [Google Scholar]
- 105.Ampel NM, Dols CL, Galgiani JN. Coccidioidomycosis during human immunodeficiency virus infection: results of a prospective study in a coccidioidal endemic area. Am J Med. 1993;94(3):235–240. doi: 10.1016/0002-9343(93)90054-s. [DOI] [PubMed] [Google Scholar]
- 106.Pappagianis D. Coccidioidomycosis in California state correctional institutions. Ann N Y Acad Sci. 2007;1111:103–111. doi: 10.1196/annals.1406.011. [DOI] [PubMed] [Google Scholar]
- 107.Coccidioidomycosis in California’s Adult Prisons 2006–2010. California Correctional Health Care Services Public Health Unit and Quality Management; 2012. [Google Scholar]
- 108.Centers for Disease Control and Prevention (CDC) Coccidioidomycosis – Arizona, 1990–1995. MMWR Morb Mortal Wkly Rep. 1996;45(49):1069–1073. [PubMed] [Google Scholar]
- 109.Centers for Disease Control and Prevention (CDC) Increase in reported coccidioidomycosis – United States, 1998–2011. MMWR Morb Mortal Wkly Rep. 2013;62(12):217–221. [PMC free article] [PubMed] [Google Scholar]
- 110.Tsang CA, Anderson SM, Imholte SB, et al. Enhanced surveillance of coccidioidomycosis, Arizona, USA, 2007–2008. Emerg Infect Dis. 2010;16(11):1738–1744. doi: 10.3201/eid1611.100475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Kuberski T, Herrig J, Pappagianis D. False-positive IgM serology in coccidioidomycosis. J Clin Microbiol. 2010;48(6):2047–2049. doi: 10.1128/JCM.01843-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Blair JE, Mendoza N, Force S, Chang YH, Grys TE. Clinical specificity of the enzyme immunoassay test for coccidioidomycosis varies according to the reason for its performance. Clin Vaccine Immunol. 2013;20(1):95–98. doi: 10.1128/CVI.00531-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Pappagianis D. Serologic studies in coccidioidomycosis. Semin Respir Infect. 2001;16(4):242–250. doi: 10.1053/srin.2001.29315. [DOI] [PubMed] [Google Scholar]
- 114.Durkin M, Connolly P, Kuberski T, et al. Diagnosis of coccidioidomycosis with use of the Coccidioides antigen enzyme immunoassay. Clin Infect Dis. 2008;47(8):e69–e73. doi: 10.1086/592073. [DOI] [PubMed] [Google Scholar]
- 115.Kirsch EJ, Greene RT, Prahl A, et al. Evaluation of Coccidioides antigen detection in dogs with coccidioidomycosis. Clin Vaccine Immunol. 2012;19(3):343–345. doi: 10.1128/CVI.05631-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Benedict K, Park BJ. The re-emergence and changing epidemiology of coccidioidomycosis, United States, 1998–2010 (poster); Paper presented at: International Conference on Emerging Infectious Diseases; 2012; Atlanta, GA. [Google Scholar]
- 117.Ampel NM. What’s behind the increasing rates of coccidioidomycosis in Arizona and California? Curr Infect Dis Rep. 2010;12(3):211–216. doi: 10.1007/s11908-010-0094-3. [DOI] [PubMed] [Google Scholar]
- 118.Park BJ, Sigel K, Vaz V, et al. An epidemic of coccidioidomycosis in Arizona associated with climatic changes, 1998–2001. J Infect Dis. 2005;191(11):1981–1987. doi: 10.1086/430092. [DOI] [PubMed] [Google Scholar]
- 119.Centers for Disease Control and Prevention (CDC) Increase in coccidioidomycosis – Arizona 1998–2001. MMWR Morb Mortal Wkly Rep. 2003;52(6):109–112. [PubMed] [Google Scholar]
- 120.Fisher BT, Chiller TM, Prasad PA, Beveridge M, Walsh TJ, Zaoutis TE. Hospitalizations for coccidioidomycosis at forty-one children’s hospitals in the United States. Pediatr Infect Dis J. 2010;29(3):243–247. doi: 10.1097/INF.0b013e3181bcfd7f. [DOI] [PubMed] [Google Scholar]
- 121.Flaherman VJ, Hector R, Rutherford GW. Estimating severe coccidioidomycosis in California. Emerg Infect Dis. 2007;13(7):1087–1090. doi: 10.3201/eid1307.061480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Huang JY, Bristow B, Shafir S, Sorvillo F. Coccidioidomycosis-associated Deaths, United States, 1990–2008. Emerg Infect Dis. 2012;18(11):1723–1728. doi: 10.3201/eid1811.120752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Ampel NM. Measurement of cellular immunity in human coccidioidomycosis. Mycopathologia. 2003;156(4):247–262. doi: 10.1023/b:myco.0000003580.93839.71. [DOI] [PubMed] [Google Scholar]
- 124.Chen S, Erhart L, Anderson S, et al. Coccidioidomycosis: knowledge, attitudes, and practices among healthcare providers – Arizona, 2007. Med Mycol. 2011;49(6):649–656. doi: 10.3109/13693786.2010.547995. [DOI] [PubMed] [Google Scholar]
- 125.Ampel NM, Giblin A, Mourani JP, Galgiani JN. Factors and outcomes associated with the decision to treat primary pulmonary coccidioidomycosis. Clin Infect Dis. 2009;48(2):172–178. doi: 10.1086/595687. [DOI] [PubMed] [Google Scholar]
- 126.Hector R, Rutherford GW. The public health need and present status of a vaccine for the prevention of coccidioidomycosis. Ann N Y Acad Sci. 2007;1111:259–268. doi: 10.1196/annals.1406.035. [DOI] [PubMed] [Google Scholar]
- 127.Barnato AE, Sanders GD, Owens DK. Cost-effectiveness of a potential vaccine for Coccidioides immitis. Emerg Infect Dis. 2001;7(5):797–806. doi: 10.3201/eid0705.010505. [DOI] [PMC free article] [PubMed] [Google Scholar]