Abstract
Subconjunctival hemorrhage is a benign disorder that is a common cause of acute ocular redness. The major risk factors include trauma and contact lens usage in younger patients, whereas among the elderly, systemic vascular diseases such as hypertension, diabetes, and arteriosclerosis are more common. In patients in whom subconjunctival hemorrhage is recurrent or persistent, further evaluation, including workup for systemic hypertension, bleeding disorders, systemic and ocular malignancies, and drug side effects, is warranted.
Keywords: subconjunctival hemorrhage, contact lens, hypertension, red eye
What is a subconjunctival hemorrhage?
Subconjunctival hemorrhage (SCH) is a common benign condition of the eye that has characteristic features, such as the painless acute appearance of a sharply circumscribed redness of bleeding underneath the conjunctiva in the absence of discharge, and inflammation in contagious areas.1 Reduction in visual acuity is not expected. It can vary from dot-blot hemorrhages to extensive areas of bleeding that render the underlying sclera invisible.2 Histologically, SCH can be defined as hemorrhage between the conjunctiva and episclera, and the blood elements are found in the substantia propria of the conjunctiva when a subconjunctival vessel breaks.3,4 The incidence of SCH was reported as 2.9% in a study with 8726 patients, and increase with age was observed, particularly over 50 years of age.5 It is thought that this significant increase depends on the increase of prevalence of systemic hypertension after the age of 50 years; also, diabetes mellitus, hyperlipidemia, and the use of anticoagulation therapy becomes more frequent with aging.4 Generally, SCH is most often seen in the inferior and temporal areas of the conjunctiva, but trauma causes localized hemorrhage at the site of injury, especially in the temporal areas.4 The fibrous connections under the conjunctiva, including elastic and connective tissues, become more fragile with age, and this can be the reason for easy spread of hemorrhage in older patients.4 Traumatic SCH is more likely to remain localized around the site of impact compared to diffuse SCH-associated systemic vascular disorders (Figure 1).4 SCHs are observed more often in summer, and this is related to the high frequency of local traumas in this season.5,6
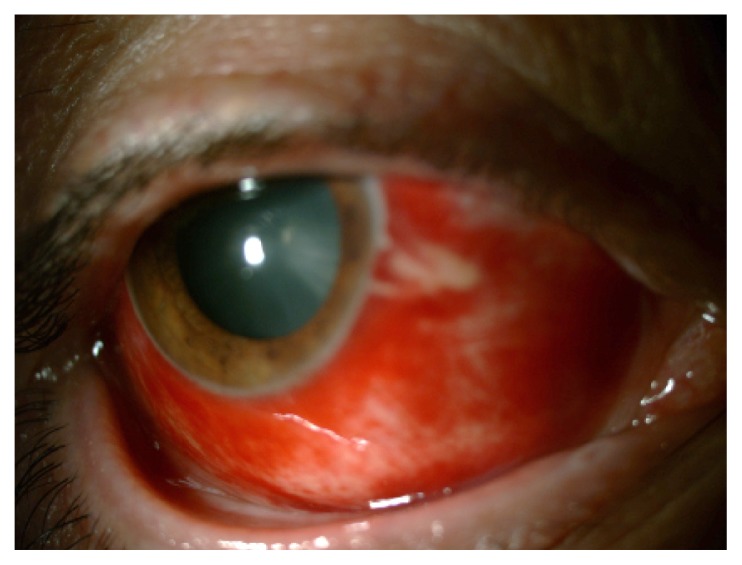
Figure 1.
This patient with diffuse subconjunctival hemorrhage had uncontrolled hypertension.
What are the causes of subconjunctival hemorrhage?
The majority of cases are mostly considered to be idiopathic, since it is usually impossible and impractical to define the main cause of SCH. However, the clinician must have a systematic review scheme in mind, and major causes can be classified under ocular and systemic conditions, respectively.
The first study on the risk factors was reported by Fukuyama et al5 in 1990, who showed that local trauma, systemic hypertension, acute conjunctivitis, and diabetes mellitus were the main causes or associated conditions of SCH. On the other hand, the cause of SCH was undetermined in about half of the patients. The relationship between age, local trauma, and systemic hypertension was assessed, and it was demonstrated that hypertension was seen more often in patients older than 50 years; however, local trauma was an important cause in all age-groups.5,6 Since the 1980s, the order of the risk factors of SCH has changed, and the number of patients with acute hemorrhagic conjunctivitis has decreased, whereas contact lens usage and ocular surgery have become more common as underlying causes.6 Mimura et al6 showed that the major risk factors for SCH are trauma and contact lens usage in younger patients, and among older patients it is mostly associated with systemic vascular disorders, such as systemic hypertension, diabetes, and arteriosclerosis, which causes the walls of the blood vessels to become fragile.
Ocular causes include local trauma to the globe, injuries to the orbit, acute inflammation of the conjunctiva, conjunctival tumors, conjunctivochalasis, ocular amyloidosis, contact lens usage, ocular surgery, and ocular adnexal tumors.
Local trauma
Various types of local injuries to the globe constitute the common cause of SCH, spanning from a minor trauma originating from a foreign body or eye rubbing to major traumas, such as blunt or penetrating injuries of the globe, which can cause SCH at all levels.2 Traumatic SCH tends to be more often in temporal areas than in the nasal areas.4 At this point, it should always be kept in mind that the patient may not recall minor trauma until questioned in detail. Therefore, all patients presenting with SCH should be thoroughly asked about any possible trauma in the last few days.
Orbital injuries
SCH may develop 12–24 hours after the fracture of orbital bones and results from influent leakage of blood under the conjunctiva.2,7 Another similar phenomenon may be observed in cases of fractures of the base of the skull.7 Hemorrhage under the conjunctiva can be located on the nasal side, coming from the fornix and in the absence of globe trauma; this appearance of the hemorrhage after 24 hours or more after a head injury is pathognomonic for basilar fractures.7
Acute inflammation of the conjunctiva
Acute hemorrhagic conjunctivitis, caused by enterovirus type 70, Coxsackie virus A24 variant, and less commonly adenovirus types 8, 11, and 19, is characterized by sudden onset of follicular conjunctivitis with mucoid discharge, epiphora, photophobia, eyelid edema, and conjunctival chemosis.8,9 It is often associated with multiple petechial hemorrhages of the upper palpebral and superior bulbar conjunctiva or widely extended SCH, especially localized to the temporal side.10,11
SCH was seen in 22.9% of 61 young immunocompetent males during the course of a measles epidemic in addition to conjunctivitis, which is a well-known diagnostic sign of measles.12 A patient with chickenpox and normal platelet count was reported to develop unilateral SCH after the onset of typical cutaneous eruptions, without any other ocular complications.13
Conjunctival tumors
Sometimes, SCH may result from vascular tumors of conjunctiva such as conjunctival lymphangiectasia, lymphangioma, cavernous hemangioma, and Kaposi’s sarcoma (Figure 2).14–16 Cavernous hemangioma may be one of the factors that causes recurrent SCH, particularly in early adulthood.16 Spontaneous rupture of conjunctival aneurysms that are associated with hereditary hemochromatosis patients can lead to recurrent SCHs.17
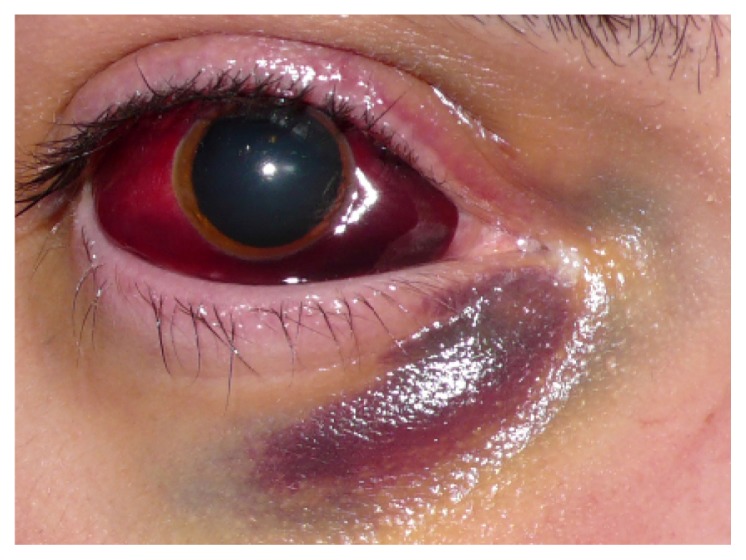
Figure 2.
This massive subconjunctival hemorrhage accompanied acute intralesional bleeding of an orbital arteriovenous malformation following strenuous physical exercise.
Conjunctivochalasis
In recent years, there have been few reports evaluating the association between conjunctivochalasis and SCH.18–21 Mimura et al18 reported that conjunctivochalasis-related parameters were more severe in SCH than in control patients, especially the grade of conjunctivochalasis, which was higher in the SCH patients at the nasal and temporal conjunctiva. According to these results, the authors suggested that conjunctivochalasis might contribute to the pathogenesis of SCH. In this report, they could not comment on the role of dry eyes in their patients but Liu et al19 evaluated the tear film of spontaneous SCH patients by noninvasive interferometry. They demonstrated that Schirmer’s test I values of spontaneous SCH patients were lower than controls and that this could be related to elevation of conjunctiva and impairment of ocular surface wetting by SCH.19 Another study reported by Wells et al20 demonstrated that conjunctivochalasis resulting from circumferential drainage blebs following trabeculectomy might prompt SCH. The authors explained the possible mechanisms as damage of conjunctival vessels from the bulge of bullous conjunctiva, and degeneration of fibrous connections between the conjunctiva and Tenon’s capsule.20,21
Ocular amyloidosis
Conjunctival amyloidosis may be one of the unusual causes of spontaneous SCH. At this point, it is worth considering the simple classification of amyloidosis: (1) primary localized amyloidosis, (2) primary systemic amyloidosis, (3) secondary localized amyloidosis, and (4) secondary systemic amyloidosis.22 In the eye, it usually presents as a painless, nodular mass or swelling of the eyelid and chemosis of the conjunctiva, and most commonly develops after inflammatory conditions.23 A patient with primary localized conjunctival amyloidosis may present with recurrent SCH.24,25 Further evaluation for systemic disease is needed for these patients, although positive results are not often expected. Although the association of conjunctival amyloidosis with monoclonal gammopathies and multiple myeloma is not common, there is a case, reported by Higgins et al,26 presenting with recurrent SCH and periorbital hemorrhage as the first sign of systemic amyloid light-chain amyloidosis. In patients with systemic disease such as multiple myeloma, which can be associated with amyloidosis, recurrent SCHs may occur even in the absence of prominent amyloid deposits. The possible pathogenesis of these hemorrhages can be explained as amyloid deposition within the walls of the vessels, leading to increase in the fragility of the vessels.27
Contact lens usage
Contact lens-induced hemorrhages have been increasingly encountered in recent years as much as the other complications of contact lens wear. SCH in contact lens wearers can be related to contact lenses themselves or to other factors independent of contact lens usage (Figure 3).28 Tears resulting from improper lens insertion or removal are often the cause of the SCH, and frequently detailed examination of conjunctiva with slit-lamp biomicroscopy reveals a small tear near the limbus. Devices used for lens insertion or removal or long fingernails can promote this kind of injury in contact lens wearers.28 The other important cause of SCH in these patients is a defect of the rim of the lens resulting from long wear of disposable lenses or material defects, especially in hard lenses, or surface deposits, which can be seen because of inadequate hygiene or improper storage conditions. The incidence of contact lens-related SCH was reported to be 5.0%.6 A prospective study evaluating the clinical features of contact lens-induced SCH demonstrated that the hemorrhage was limited to temporal areas of the conjunctiva, whereas another study showed that the hemorrhage associated with systemic disorders tended to be seen haphazardly in more extensive areas.28 This can be related to various factors, the most important being that contact lens usage and related injuries are more common in younger patients who usually do not have any systemic vascular disorders. Also, the connective tissue under the conjunctiva is still strong in young individuals, preventing the spread of hemorrhage.
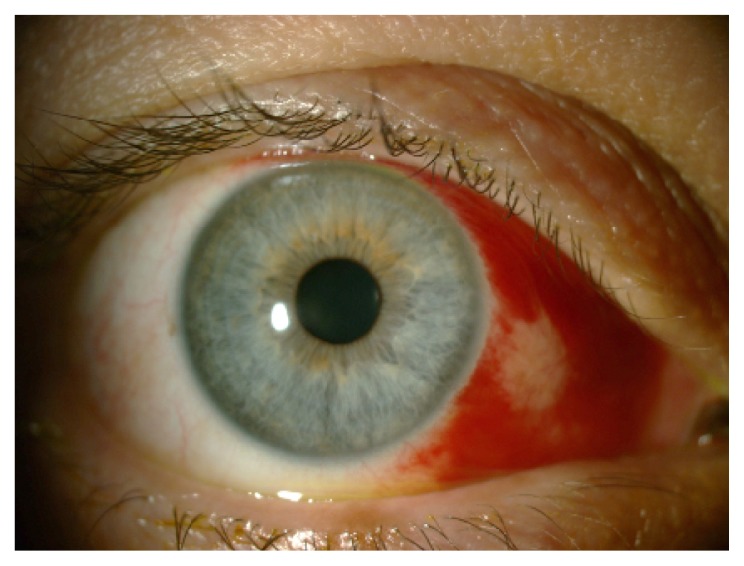
Figure 3.
Traumatic subconjunctival hemorrhage involving the nasal half of the bulbar conjunctiva caused by soft contact lens wear.
It should not be forgotten that although SCH in contact lens users can be related to the contact lenses most of the time, other ocular or systemic factors must also be considered. The contact lens should be inspected thoroughly, and recurrent hemorrhages should be accepted as a sign for further systemic evaluation. Patients with hematologic disorders should not wear contact lenses.28,29
Ocular surgery
Many ocular and nonocular surgical procedures may prompt SCH by different mechanisms. Cataract surgery, filtration surgery, refractive surgery, and local anesthesia techniques, such as sub-Tenon’s anesthetic injection and peribulbar block, might be the cause of recurrent SCH in the postoperative period.30–34
SCH may appear at each step of ocular surgery, especially starting with anesthesia. SCH during the conjunctival incision is one of the disadvantages of sub-Tenon’s anesthetic injection, and incidence of SCH during sub-Tenon’s anesthesia has been reported to be 7%–56%.32,33 Generally, it is limited to the area of conjunctival dissection. Although it does not have any effect on postoperative visual status of the eye, the patient may remain cosmetically unsatisfied.
There have been many reports suggesting that patients on anticoagulant or antiplatelet therapy did not show an increased rate of hemorrhagic complications during cataract surgery or local anesthesia, although some studies have reported that there was an increase in minor hemorrhagic complications in patients taking warfarin.30,35–37 SCH was reported as the most frequent hemorrhagic complication in patients undergoing phacoemulsification and lens implantation who were treated with aspirin and warfarin.30 It is widely accepted that anticoagulation and antiplatelet agents should be continued before cataract surgery. Patients on aspirin should continue taking the drug before cataract surgery and international normalized ratio (INR) should be checked in all patients on warfarin medication to maintain the therapeutic level.
Lalchan31 reported a case of patient on aspirin prophylaxis who had Fuchs’s heterochromic cyclitis (FCH) complicated by secondary open-angle glaucomatous optic neuropathy in his past ocular history and who presented with SCH as an intrableb hemorrhage. In that case, prolonged bleeding time was identified as the possible mechanism. Previously, Noda and Hayasaka38 reported two cases of FCH associated with recurrent spontaneous SCH two to four times per year, and the relationship between FCH and spontaneous recurrent SCH was unclear. Although the association of FCH with hyphema is well recognized, it was the first report demonstrating co-occurrence of FCH and spontaneous recurrent SCH.31
A case of subconjunctival ecchymosis appearing after extraction of maxillary teeth has been reported.39 The incidence of subconjunctival ecchymosis was found to be 19.1% after rhinoplasty in a study involving 73 patients.40 SCH may occur during intraoperative positioning for lumbar spinal surgery as a rare complication, and also there have been reported cases of patients showing that SCH may occur during endoscopy, particularly in thrombocytopenic patients.41,42
Ocular adnexal tumors
Recurrent SCHs have been reported as the initial sign of anaplastic carcinoma of the lacrimal gland.43 Ocular adnexal lymphoma can cause a set of signs and symptoms including ptosis, proptosis, and salmon-colored mass in the conjunctiva. Although not a common presenting sign, ocular adnexal lymphoma can be an underlying condition of recurrent SCH.44
Systemic factors
Systemic factors that may lead to SCH can be classified as systemic vascular diseases, sudden severe venous congestion, hematological dyscrasias, systemic trauma, acute febrile systemic diseases, drugs, carotid cavernous fistulas (CCFs), menstruation, and delivery in newborns.
Systemic vascular diseases
The fragility of conjunctival vessels, as well as every other vessel elsewhere in the body, increases with age and as a result of arteriosclerosis, systemic hypertension, and diabetes.2 Patients with vascular diseases may present with SCH repetitively, and the association of SCH and systemic hypertension has been investigated many times.45 Severe SCH can result from uncontrolled hypertension, but it is also known that systemic hypertension may cause SCH even if it is controlled with drugs, because patients with hypertension tend to have microvascular changes in small vessels and in conjunctival vessels.6,45,46 These findings make it necessary to check the blood pressure of each patient presenting with SCH. A study by Pitts et al47 demonstrated that blood pressure checked at initial presentation and 1 week and 4 weeks after first presentation was higher in patients presenting with SCH than healthy controls; therefore, the incidence of hypertension was higher in patients with SCH. It is recommended that all patients with SCH have their systemic blood pressure checked.
Sudden severe venous congestion
SCH may occur after sudden severe venous congestion to the head, such as in a Valsalva maneuver, whooping cough, vomiting, sneezing, weight lifting, crush injuries, or spontaneously (without any apparent cause).2 Compression of the thorax and abdomen as in accidents or explosions may act in the same way, and raised venous pressure can cause severe SCH.2 Also, nonaccidental trauma should be seriously considered in infants presenting with bilateral isolated SCHs, particularly in the presence of facial petechia. This condition may be part of traumatic asphyxia syndrome caused by severe compression of the child’s thorax and abdomen or as a result of child abuse. The patient should be examined by a pediatrician from the perspective of high suspicion of abuse in the case of unexplained isolated bilateral SCHs.48,49
Asthmatic patients may face severe bilateral SCH at the peak of their fulminant attacks of severe asthma. A possible mechanism could be intrathoracic airway pressure rising to overcome airway obstruction, causing sudden congestion of blood into the superior vena cava.50 Although uncommon, asthma may be an etiological factor in SCH, as well as pertussis infection causing coughing paroxysms.51 Also with the same mechanism, there is a case report presenting with bilateral SCH resulting from voluntary breath-holding, an example of self-inflicted injury in psychiatric patients.52
Hematological dyscrasias
Pathologies of the coagulation system, including the disorders associated with thrombocytopenia and platelet dysfunction, such as thrombocytopenic purpura, anemia, leukemia, splenic disorders, anticoagulant or antiplatelet therapy, and uremia, may cause bleeding in conjunctival vessels.2,53,54–59
Parmeggiani et al60 conducted a study to determine whether FXIII Val34Leu polymorphism, thought to be a predisposing risk factor for primary intracerebral hemorrhages in a previous study, might increase the risk of SCH, and showed that frequency of FXIII-mutated allele was higher in patients with SCH than in controls.60,61 These findings suggest that FXIII Val34Leu polymorphism can be considered a potential risk factor for spontaneous SCH, which needs to be validated by further studies.
An unusual bilateral massive spontaneous SCH can be an initial sign of acute lymphoblastic leukemia as a result of blood dyscrasia.54 Another example for one of the same underlying serious conditions is idiopathic thrombocytopenic purpura, which can present with isolated unilateral SCH.53 It must be borne in mind that any disorder that can cause hemostatic failure may be the reason for SCH.
Anticoagulant and antiplatelet therapies, including aspirin, dipyridamole, clopidogrel, warfarin, and dabigatran (direct thrombin inhibitor), may prompt recurrent SCHs. It is important to take a detailed drug history to determine the usage of these drugs, as they may increase the risk of spontaneous or perioperative SCHs.55–58 Warfarin is the most commonly used anticoagulant in North America to treat venous and pulmonary thromboembolism and reduce the incidence of life-threatening thromboembolic events.62 Bleeding is the most frequent adverse effect of warfarin use, and SCH is one of the minor bleedings that may be seen under warfarin medication.63,64 In an effort to identify patients with SCH on warfarin therapy, Leiker et al58 reported that after evaluating 4334 patients, they noted 15 with SCH, – only 0.35% of patients. Only three patients were not in their targeted range of INR (INRs were greater than individual patient target range).58 These findings were comparable with Superstein et al,64 who found a rate of ocular bleeding of 4.8% (five of 126 patients on anticoagulation therapy), with two of those five patients with SCH.64 It is important to determine the cause of SCH in this group of patients, as secondary causes previously mentioned, such as trauma, systemic hypertension, or blood dyscrasias, may prompt SCH besides anticoagulant therapy. Although supratherapeutic INRs have not been related to increased risk of SCH, patients on warfarin medication should have their INR checked.58,63
Systemic trauma
Splinter SCHs may be seen in the upper fornix, due to fat emboli originating from fractures of long bones in remote injuries.2
Acute febrile systemic diseases
Petechial SCHs can be seen in febrile systemic infections, such as zoonosis (tsutsugamushi disease, scrub typhus, leptospirosis), enteric fever, malaria, meningococcal septicemia, subacute bacterial endocarditis, scarlet fever, diphtheria, influenza, smallpox, and measles.2,65–68
Drugs
In addition to anticoagulant and antiplatelet medication, there are some drugs reported in the literature related to SCH. It should be kept in mind that interferon therapy in chronic viral hepatitis patients may give rise to SCH, and retinopathy and antiviral therapy, including polyethylene gycolated interferon plus ribavirin, can cause SCH in addition to vascular ophthalmological side effects.69,70
Carotid cavernous fistulas
SCH was one of the presenting signs of CCFs in two case reports. One of them was direct CCF presenting with sudden onset and pulsatile exophthalmos, SCH, ophthalmoplegia, and increased intraocular pressure.71 The other CCF case was a patient with spontaneous unilateral SCH complaining of a right periorbital swelling.72 Those two observations suggest that SCH may be a part of the clinical picture of CCF patients.
Miscellaneous conditions
Newborns may show SCH after normal vaginal delivery. In a study of 3573 healthy full-term newborns who had undergone an eye examination, the number of patients who showed SCH was reported as 50 (1.40%).73
Spontaneous SCHs may be seen in menstruation, whereas hemorrhages from the conjunctiva occur more frequently in these cases.2
An ophthalmologist, a general practitioner, or a physician may face patients with SCH many times in each step of daily clinical practice. The key point is to decide whether further investigation is necessary or not. In most cases, SCHs do not require specific treatment, but the patient should be reassured that the hemorrhage will disperse in 2–3 weeks, with blood turning from red to brown and then to yellow (Figure 4).1,2
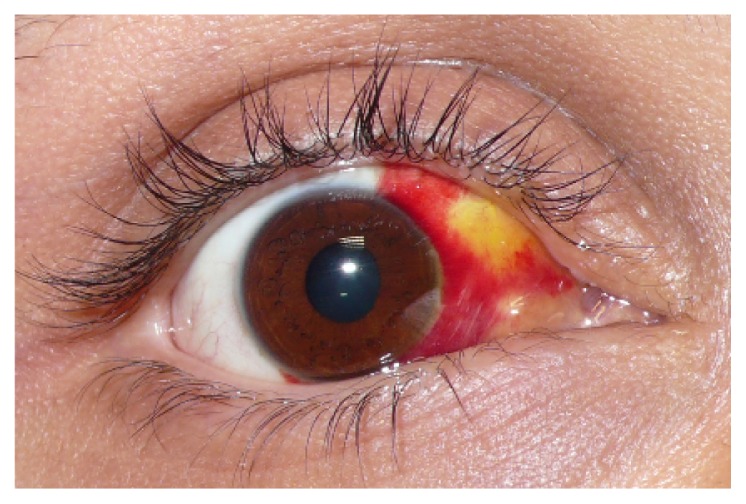
Figure 4.
An island of yellow discoloration on the nasal part of the bulbar conjunctiva indicating absorption of the subconjunctival hemorrhage.
There is not any approved treatment to accelerate the resolution and absorption of SCH. The first treatment reported in the literature was air therapy.74 A patient with a severe SCH caused by acute hemorrhagic conjunctivitis was treated with nasal and temporal subconjunctival injection of tissue plasminogen activator.75 SCH was a new area of usage for tissue plasminogen activator alongside its use in vitreous, anterior chamber, and glaucoma filter bleb to induce the clearance of fibrin clots.76–78 Moon et al79 evaluated the effect of subconjunctival injection of liposome-bound, low-molecular-weight heparin (LMWH) on the absorption rate of SCHs in rabbits. The report concluded that the subconjunctival injection of liposome-bound LMWH had a significant influence on facilitating SCH absorption in rabbits in comparison to only liposome and liposome-free form of LMWH.79 Another two forms of the same molecule – liposome-encapsulated streptokinase and free-form streptokinase – were injected into the subconjunctival area to enhance the rate of SCH absorption in rabbits by Baek et al,80 and they found that SCH absorption rate in the liposome-capsulated form was faster than the free-form streptokinase injection group, particularly in the early phases, which were described as 24–48 hours after SCH induction.
Failure to resolve hemorrhage in persistent or recurrent cases suggests a serious underlying cause. A careful history is the most important step in identifying whether there is a serious underlying condition that may require more detailed examination and treatment. A detailed history may provide clues to the underlying conditions. It is important to obtain a thorough medication, medical, and ocular history from patients presenting with SCH, including any possible trauma, ocular surgery, contact lens wear, drugs, and heritable conditions. First, a careful slit-lamp examination is essential to determine if there has been any trauma to the eye, and also to rule out any local ocular condition that can lead to SCH, as mentioned previously. After excluding ocular factors, further systemic evaluation is necessary. Blood pressure should be checked routinely in all patients with SCH, particularly in older patients. In recurrent cases, a workup for bleeding disorders and hypocoagulable states is required. The INR should be checked if the patient is taking warfarin.
In conclusion, only recurrent or persistent SCH mandates further systemic evaluation, and no treatment is required unless it is associated with certain serious conditions.
Footnotes
Disclosure
The authors have no conflicts of interest and no commercial interests in any products or services used in this study.
References
- 1.Leibowitz HM. The red eye. N Engl J Med. 2000;343(5):345–351. doi: 10.1056/NEJM200008033430507. [DOI] [PubMed] [Google Scholar]
- 2.Duke-Elder S. System of Ophthalmology Diseases of the Outer Eye. VIII. London: Henry Kimpton; 1965. Conjunctival diseases; pp. 34–39. [Google Scholar]
- 3.Yanoff M, Fine BS. Ocular Pathology. Maryland Heights (MO): Mosby; 1996. Conjunctiva; pp. 206–207. [Google Scholar]
- 4.Mimura T, Yamagami S, Usui T, et al. Location and extent of subconjunctival hemorrhage. Ophthalmologica. 2010;224(2):90–95. doi: 10.1159/000235798. [DOI] [PubMed] [Google Scholar]
- 5.Fukuyama J, Hayasaka S, Yamada K, Setogawa T. Causes of subconjunctival hemorrhage. Ophthalmologica. 1990;200(2):63–67. doi: 10.1159/000310079. [DOI] [PubMed] [Google Scholar]
- 6.Mimura T, Usui T, Yamagami S, et al. Recent causes of subconjunctival hemorrhage. Ophthalmologica. 2010;224(3):133–137. doi: 10.1159/000236038. [DOI] [PubMed] [Google Scholar]
- 7.King AB, Walsh FB. Trauma to the head with particular reference to the ocular signs; injuries involving the hemispheres and brain stem; miscellaneous conditions; diagnostic principles; treatment. Am J Ophthalmol. 1949;32(3):379–398. [PubMed] [Google Scholar]
- 8.Asbell PA, DeLuise VP, Bartolomei A. Viral conjunctivitis. In: Tabbara KF, Hyndiuk RA, editors. Infections of the Eye. Boston: Listen Brown; 1996. pp. 462–463. [Google Scholar]
- 9.Chiu CH, Chuang YY, Siu LH. Subconjunctival haemorrhage and respiratory distress. Lancet. 2001;358(9283):724. doi: 10.1016/S0140-6736(01)05841-X. [DOI] [PubMed] [Google Scholar]
- 10.Sklar VE, Patriarca PA, Onorato IM, et al. Clinical findings and results of treatment in acute hemorrhagic conjunctivitis in Southern Florida. Am J Ophthalmol. 1983;95(1):45–54. doi: 10.1016/0002-9394(83)90332-x. [DOI] [PubMed] [Google Scholar]
- 11.Bhatia V, Swami HM. An epidemic of acute haemorrhagic conjunctivitis in school children. Indian J Pediatr. 1999;66(1):158–159. doi: 10.1007/BF02752379. [DOI] [PubMed] [Google Scholar]
- 12.Kayikçioglu O, Kir E, Söyler M, Güler C, Irkeç M. Ocular findings in a measles epidemic among young adults. Ocul Immunol Inflamm. 2000;8(1):59–62. doi: 10.1076/0927-3948(200003)811-sft059. [DOI] [PubMed] [Google Scholar]
- 13.Gaver-Shavit A, Minouni M. Subconjunctival hemorrhage in chickenpox. Pediatr Infect Dis J. 1991;10(3):253–254. [PubMed] [Google Scholar]
- 14.Shields CL, Shields JA. Tumors of the conjunctiva and cornea. Surv Ophthalmol. 2004;49(1):3–24. doi: 10.1016/j.survophthal.2003.10.008. [DOI] [PubMed] [Google Scholar]
- 15.Shields JA, Mashayekhi A, Kligman BE, et al. Vascular tumors of the conjunctiva in 140 cases. Ophthalmology. 2011;118(9):1747–1753. doi: 10.1016/j.ophtha.2011.04.034. [DOI] [PubMed] [Google Scholar]
- 16.Kiratli H, Uzun S, Tarlan B, Tanas Ö. Recurrent subconjunctival hemorrhage due to cavernous hemangioma of the conjunctiva. Can J Ophthalmol. 2012;47(3):318–320. doi: 10.1016/j.jcjo.2012.02.002. [DOI] [PubMed] [Google Scholar]
- 17.Tong JW, Sawamura MH. Subconjunctival hemorrhages: presenting sign for hereditary hemochromatosis. Optom Vis Sci. 2011;88(9):1133–1139. doi: 10.1097/OPX.0b013e3182223683. [DOI] [PubMed] [Google Scholar]
- 18.Mimura T, Usui T, Yamagami S, et al. Subconjunctival hemorrhage and conjunctivochalasis. Ophthalmology. 2009;116(10):1880–1886. doi: 10.1016/j.ophtha.2009.03.021. [DOI] [PubMed] [Google Scholar]
- 19.Liu W, Li H, Qiao J, et al. The tear film characteristics of spontaneous subconjunctival hemorrhage patients detected by Schirmer test I and tear interferometry. Mol Vis. 2012;18:1952–1954. [PMC free article] [PubMed] [Google Scholar]
- 20.Wells AP, Marks J, Khaw PT. Spontaneous inferior subconjunctival hemorrhages in association with circumferential drainage blebs. Eye (Lond) 2005;19(3):269–272. doi: 10.1038/sj.eye.6701496. [DOI] [PubMed] [Google Scholar]
- 21.Schmitz J. Conjunctivochalasis and subconjunctival hemorrhage. Ophthalmology. 2010;117(12):2444. doi: 10.1016/j.ophtha.2010.08.026. [DOI] [PubMed] [Google Scholar]
- 22.Brownstein MH, Elliott R, Helwig EB. Ophthalmologic aspects of amyloidosis. Am J Ophthalmol. 1970;69(3):423–430. doi: 10.1016/0002-9394(70)92276-2. [DOI] [PubMed] [Google Scholar]
- 23.Smith ME, Zimmerman LE. Amyloidosis of the eyelid and conjunctiva. Arch Ophthalmol. 1966;75(1):42–51. doi: 10.1001/archopht.1966.00970050044009. [DOI] [PubMed] [Google Scholar]
- 24.Lee HM, Naor J, DeAngelis D, Rootman DS. Primary localized conjunctival amyloidosis presenting with recurrence of subconjunctival hemorrhage. Am J Ophthalmol. 2000;129(2):245–247. doi: 10.1016/s0002-9394(99)00421-3. [DOI] [PubMed] [Google Scholar]
- 25.Cheong-Leen R. Primary localised conjunctival amyloidosis presenting as subconjunctival haemorrhage. Eye (Lond) 2001;15(5):679–680. doi: 10.1038/eye.2001.218. [DOI] [PubMed] [Google Scholar]
- 26.Higgins GT, Olujohungbe A, Kyle G. Recurrent subconjunctival and periorbital haemorrhage as the first presentation of systemic amyloidosis secondary to myeloma. Eye (Lond) 2006;20(4):512–515. doi: 10.1038/sj.eye.6701923. [DOI] [PubMed] [Google Scholar]
- 27.Felipe AF, Nottage JM, Rapuano CJ. Recurrent bilateral subconjunctival hemorrhage as an initial presentation of multiple myeloma. Oman J Ophthalmol. 2012;5(2):133–134. doi: 10.4103/0974-620X.99384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Roth HW. Contact Lens Complications. New York: Thieme; 2003. Pathologic findings; pp. 42–44. [Google Scholar]
- 29.Mimura T, Yamagami S, Funatsu H, et al. Contact lens-induced subconjunctival hemorrhage. Am J Ophthalmol. 2010;150(5):656–665. doi: 10.1016/j.ajo.2010.05.028. [DOI] [PubMed] [Google Scholar]
- 30.Carter K, Miller KM. Phacoemulsification and lens implantation in patients treated with aspirin or warfarin. J Cataract Refract Surg. 1998;24(10):1361–1364. doi: 10.1016/s0886-3350(98)80229-0. [DOI] [PubMed] [Google Scholar]
- 31.Lalchan SA. Spontaneous hyphaema and intra-bleb subconjunctival haemorrhage in a patient with previous trabeculectomy. Eye (Lond) 2006;20(7):853–854. doi: 10.1038/sj.eye.6702026. [DOI] [PubMed] [Google Scholar]
- 32.Guise PA. Sub-Tenon anesthesia: a prospective study of 6,000 blocks. Anesthesiology. 2003;98(4):964–968. doi: 10.1097/00000542-200304000-00025. [DOI] [PubMed] [Google Scholar]
- 33.Roman SJ, Chong Sit DA, Boureau CM, Auclin FX, Ullern MM. Sub-Tenon’s anaesthesia: an effect and safe technique. Br J Ophthalmol. 1997;81(8):673–676. doi: 10.1136/bjo.81.8.673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Calenda E, Lamothe L, Genevois O, Cardon A, Muraine M. Peribulbar block in patients scheduled for eye procedures and treated with clopidogrel. J Anesth. 2012;26(5):779–782. doi: 10.1007/s00540-012-1406-6. [DOI] [PubMed] [Google Scholar]
- 35.Katz J, Feldman MA, Bass EB, et al. Study of medical testing for cataract surgery team. Risks and benefits of anticoagulant and antiplatelet medication use before cataract surgery. Ophthalmology. 2003;110(9):1784–1788. doi: 10.1016/S0161-6420(03)00785-1. [DOI] [PubMed] [Google Scholar]
- 36.Morris A, Elder MJ. Warfarin therapy and cataract surgery. Clin Experiment Ophthalmol. 2000;28(6):419–422. doi: 10.1046/j.1442-9071.2000.00350.x. [DOI] [PubMed] [Google Scholar]
- 37.Robinson GA, Nylander A. Warfarin and cataract extraction. Br J Ophthalmol. 1989;73(9):702–703. doi: 10.1136/bjo.73.9.702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Noda S, Hayasaka S. Recurrent subconjunctival hemorrhages in patients with Fuchs’ heterochromic iridocyclitis. Ophthalmologica. 1995;209(5):289–291. doi: 10.1159/000310634. [DOI] [PubMed] [Google Scholar]
- 39.Kumar RA, Moturi K. Subconjunctival ecchymosis after extraction of maxillary molar teeth: a case report. Dent Traumatol. 2010;26(3):298–300. doi: 10.1111/j.1600-9657.2010.00870.x. [DOI] [PubMed] [Google Scholar]
- 40.Kara CO, Kara IG, Yaylali V. Subconjunctival ecchymosis due to rhinoplasty. Rhinology. 2001;39(3):166–168. [PubMed] [Google Scholar]
- 41.Akhaddar A, Boucetta M. Subconjunctival hemorrhage as a complication of intraoperative positioning for lumbar spinal surgery. Spine J. 2012;12(3):274. doi: 10.1016/j.spinee.2012.02.007. [DOI] [PubMed] [Google Scholar]
- 42.Rajvanshi P, McDonald GB. Subconjunctival hemorrhage as a complication of endoscopy. Gastrointest Endosc. 2001;53(2):251–253. doi: 10.1067/mge.2001.109586. [DOI] [PubMed] [Google Scholar]
- 43.Rodgers IR, Jakobiec FA, Gingold MP, Hornblass A, Krebs W. Anaplastic carcinoma of the lacrimal gland presenting with recurrent subconjunctival hemorrhages and displaying incipient sebaceous differentiation. Ophthal Plast Reconstr Surg. 1991;7(4):229–237. doi: 10.1097/00002341-199112000-00001. [DOI] [PubMed] [Google Scholar]
- 44.Hicks D, Mick A. Recurrent subconjunctival hemorrhages leading to the discovery of ocular adnexal lymphoma. Optometry. 2010;81(10):528–532. doi: 10.1016/j.optm.2009.11.008. [DOI] [PubMed] [Google Scholar]
- 45.Kittisupamongkol W. Blood pressure in subconjunctival hemorrhage. Ophthalmologica. 2010;224(5):332. doi: 10.1159/000313835. [DOI] [PubMed] [Google Scholar]
- 46.Gondim FA, Leacock RO. Subconjunctival hemorrhages secondary to hypersympathetic state after a small diencephalic hemorrhage. Arch Neurol. 2003;60(12):1803–1804. doi: 10.1001/archneur.60.12.1803. [DOI] [PubMed] [Google Scholar]
- 47.Pitts JF, Jardine AG, Murray SB, Barker NH. Spontaneous subconjunctival haemorrhage – a sign of hypertension? Br J Ophthalmol. 1992;76(5):297–299. doi: 10.1136/bjo.76.5.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Spitzer SG, Luorno J, Noël LP. Isolated subconjunctival hemorrhages in nonaccidental trauma. J AAPOS. 2005;9(1):53–56. doi: 10.1016/j.jaapos.2004.10.003. [DOI] [PubMed] [Google Scholar]
- 49.DeRidder CA, Berkowitz CD, Hicks RA, Laskey AL. Subconjunctival hemorrhages in infants and children: a sign of nonaccidental trauma. Pediatr Emerg Care. 2013;29(2):222–226. doi: 10.1097/PEC.0b013e318280d663. [DOI] [PubMed] [Google Scholar]
- 50.Rodriguez-Roisin R, Torres A, Agustí AG, Ussetti P, Agustí-Vidal A. Subconjunctival haemorrhage: a feature of acute severe asthma. Postgrad Med J. 1985;61(7):579–581. doi: 10.1136/pgmj.61.717.579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Paysse EA, Coats DK. Bilateral eyelid ecchymosis and subconjunctival hemorrhage associated with coughing paroxysms in pertussis infection. J AAPOS. 1998;2(2):116–119. doi: 10.1016/s1091-8531(98)90075-1. [DOI] [PubMed] [Google Scholar]
- 52.Chow LY, Lee JS, Leung CM. Voluntary breath-holding leading to bilateral subconjunctival haemorrhaging in a patient with schizophrenia. Hong Kong Med J. 2010;16(3):232. [PubMed] [Google Scholar]
- 53.Sodhi PK, Jose R. Subconjunctival hemorrhage: the first presenting clinical feature of idiopathic trombocytopenic purpura. Jpn J Ophthalmol. 2003;47(3):316–318. doi: 10.1016/s0021-5155(03)00017-0. [DOI] [PubMed] [Google Scholar]
- 54.Taamallah-Malek I, Chebbi A, Bouladi M, Nacef L, Bouguila H, Ayed S. Massive bilateral subconjunctival hemorrhage revealing acute lymphoblastic leukemia. J Fr Ophtalmol. 2013;36(3):e45–e48. doi: 10.1016/j.jfo.2012.03.013. French. [DOI] [PubMed] [Google Scholar]
- 55.Benzimra JD, Johnstin RL, Jaycock P, et al. The Cataract National Dataset electronic multicentre audit of 55,567 operations: antiplatelet and anticoagulant medications. Eye (Lond) 2009;23(1):10–16. doi: 10.1038/sj.eye.6703069. [DOI] [PubMed] [Google Scholar]
- 56.Bodack MI. A warfarin-induced subconjunctival hemorrhage. Optometry. 2007;78(3):113–118. doi: 10.1016/j.optm.2006.10.015. [DOI] [PubMed] [Google Scholar]
- 57.Nguyen TM, Phelan MP, Werdich XQ, Rychwalski PJ, Huff CM. Subconjunctival hemorrhage in a patient on dabigatran (Pradaxa) Am J Emerg Med. 2013;31(2):445. e3–e5. doi: 10.1016/j.ajem.2012.07.021. [DOI] [PubMed] [Google Scholar]
- 58.Leiker LL, Mehta BH, Pruchnicki MC, Rodis JL. Risk factors and complications of subconjunctival hemorrhages in patients taking warfarin. Optometry. 2009;80(5):227–231. doi: 10.1016/j.optm.2008.10.018. [DOI] [PubMed] [Google Scholar]
- 59.Chijioke A. Uremic bleeding with pericardial and subconjunctival hemorrhage. Saudi J Kidney Dis Transpl. 2011;22(6):1246–1248. [PubMed] [Google Scholar]
- 60.Parmeggiani F, Costagliola C, Incorvaia C, et al. Prevalence of factor XIII Val34Leu polymorphism in patients affected by spontaneous subconjunctival hemorrhage. Am J Ophthalmol. 2004;138(3):481–484. doi: 10.1016/j.ajo.2004.03.017. [DOI] [PubMed] [Google Scholar]
- 61.Incorvaia C, Costagliola C, Parmeggiani F, Gemmati D, Scapoli GL, Sebastiani A. Recurrent episodes of spontaneous subconjunctival hemorrhage in patients with factor XIII Val34Leu mutation. Am J Ophthalmol. 2002;134(6):927–929. doi: 10.1016/s0002-9394(02)01812-3. [DOI] [PubMed] [Google Scholar]
- 62.Haines ST, Racine E, Zeolla M. Venous thromboembolism. In: DiPiro JT, Talber RL, Yee GC, Matzke GR, Wells BG, Posey LM, editors. Pharmacotherapy: A Pathophysiologic Approach. 5th ed. New York: McGraw-Hill; 2002. pp. 337–373. [Google Scholar]
- 63.Bodack MI. A warfarin-induced subconjunctival hemorrhage. Optometry. 2007;78(3):113–118. doi: 10.1016/j.optm.2006.10.015. [DOI] [PubMed] [Google Scholar]
- 64.Superstein R, Gomolin JE, Hammouda W, Rosenberg A, Overbury O, Arsenault C. Prevalence of ocular hemorrhage in patients receiving warfarin therapy. Can J Ophthalmol. 2000;35(7):385–389. doi: 10.1016/s0008-4182(00)80126-x. [DOI] [PubMed] [Google Scholar]
- 65.Kato T, Watanabe K, Katori M, Terada Y, Hayasaka S. Conjunctival injection, episcleral vessel dilation, and subconjunctival hemorrhage in patients with new tsutsugamushi disease. Jpn J Ophthalmol. 1997;41(3):196–199. doi: 10.1016/s0021-5155(97)00002-6. [DOI] [PubMed] [Google Scholar]
- 66.Dass R, Deka NM, Duwarah SG, et al. Characteristics of pediatric scrub typhus during an outbreak in the North Eastern region of India: peculiarities in clinical presentation, laboratory findings and complications. Indian J Pediatr. 2011;78(11):1365–1370. doi: 10.1007/s12098-011-0470-5. [DOI] [PubMed] [Google Scholar]
- 67.Lin CY, Chiu NC, Lee CM. Leptospirosis after typhoon. Am J Trop Med Hyg. 2012;86(2):187–188. doi: 10.4269/ajtmh.2012.11-0518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Thapa R, Banerjee P, Jain TS. Bilateral subconjunctival haemorrhage in childhood enteric fever. Singapore Med J. 2009;50(10):1038–1039. [PubMed] [Google Scholar]
- 69.Hayasaka S, Fujii M, Yamamoto Y, Noda S, Kurome H, Sasaki M. Retinopathy and subconjunctival haemorrhage in patients with chronic viral hepatitis receiving interferon alfa. Br J Ophthalmol. 1995;79(2):150–152. doi: 10.1136/bjo.79.2.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Andrade RJ, González FJ, Vázques L, et al. Vascular ophthalmological side effects associated with antiviral therapy for chronic hepatitis C are related to vascular endothelial growth factor levels. Antivir Ther. 2006;11(4):491–498. [PubMed] [Google Scholar]
- 71.Razeghinejad MR, Tehrani MJ. Sudden onset and blinding spontaneous direct carotid-cavernous fistula. J Ophthalmic Vis Res. 2011;6(1):50–53. [PMC free article] [PubMed] [Google Scholar]
- 72.Pong JC, Lam DK, Lai JS. Spontaneous subconjunctival haemorrhage secondary to carotid-cavernous fistula. Clin Experiment Ophthalmol. 2008;36(1):90–91. doi: 10.1111/j.1442-9071.2007.01655.x. [DOI] [PubMed] [Google Scholar]
- 73.Li LH, Li N, Zhao JY, et al. Findings of perinatal ocular examination performed on 3573, healthy full-term newborns. Br J Ophthalmol. 2013;97(5):588–591. doi: 10.1136/bjophthalmol-2012-302539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Richards RD. Subconjunctival hemorrhage: treatment with air therapy. Eye Ear Nose Throat Mon. 1965;44:59. [PubMed] [Google Scholar]
- 75.Mimura T, Yamagami S, Funatsu H, et al. Management of subconjunctival haematoma by tissue plasminogen activator. Clin Experiment Ophthalmol. 2005;33(5):541–542. doi: 10.1111/j.1442-9071.2005.01080.x. [DOI] [PubMed] [Google Scholar]
- 76.Lambrou FH, Snyder RW, Williams GA, Lewandowski M. Treatment of experimental intravitreal fibrin with tissue plasminogen activator. Am J Ophthalmol. 1987;104(6):619–623. doi: 10.1016/0002-9394(87)90175-9. [DOI] [PubMed] [Google Scholar]
- 77.Lambrou FH, Snyder RW, Wiliams GA. Use of tissue plasminogen activator in experimental hyphema. Arch Ophthalmol. 1987;105(7):995–997. doi: 10.1001/archopht.1987.01060070139044. [DOI] [PubMed] [Google Scholar]
- 78.Szymanski A. Promotion of glaucoma filter bleb with tissue plasminogen activator after sclerotomy under a clot. Int Ophthalmol. 1992;16(4–5):387–390. doi: 10.1007/BF00917997. [DOI] [PubMed] [Google Scholar]
- 79.Moon JW, Song YK, Jee JP, Kim CK, Choung HK, Hwang JM. Effect of subconjunctivally injected, liposome-bound, low-molecular-weight heparin on the absorption rate of subconjunctival hemorrhage in rabbits. Invest Ophthalmol Vis Sci. 2006;47(9):3968–3974. doi: 10.1167/iovs.05-1345. [DOI] [PubMed] [Google Scholar]
- 80.Baek SH, Park SJ, Jin SE, Kim JK, Kim CK, Hwang JM. Subconjunctivally injected, liposome-encapsulated streptokinase enhances the absorption rate of subconjunctival hemorrhages in rabbits. Eur J Pharm Biopharm. 2009;72(3):546–551. doi: 10.1016/j.ejpb.2009.03.010. [DOI] [PubMed] [Google Scholar]