Abstract
Objectives
This study evaluated the association of timing of lipid levels and lipid genetic risk score (GRS) with subclinical atherosclerosis.
Background
Atherosclerosis is a slowly progressive disorder influenced by suboptimal lipid levels. Long-term versus contemporary lipid levels may more strongly impact the development of coronary artery calcium (CAC).
Methods
Framingham Heart Study (FHS) Offspring Cohort participants (n=1156, 44%M, 63±9 years) underwent serial fasting lipids [low-density lipoprotein (LDL-C), high-density lipoprotein, and triglycerides], Exam 1 (1971–1975) – Exam 7 (1998–2001). FHS Third Generation Cohort participants (n=1954, 55%M, 45±6 years) had fasting lipid profiles assessed, 2002–2005. Computed tomography (2002–2005) measured CAC. Lipid GRSs were computed from significantly associated single nucleotide polymorphisms. The association between early, long-term average, and contemporary lipids, and lipid GRS, with elevated CAC was assessed using logistic regression.
Results
In FHS Offspring, Exam 1 and long-term average versus Exam 7 lipid measurements, including untreated lipid levels, were strongly associated with elevated CAC. In the FHS Third Generation, contemporary lipids were associated with CAC. The LDL-C GRS was associated with CAC (age/sex-adjusted OR 1.14, 95%CI 1.00–1.29, p=0.04). However, addition of the GRS to the lipid models did not result in a significant increase in the OR or C-statistic for any lipid measure.
Conclusions
Early and long-term average lipid levels, as compared with contemporary measures, are more strongly associated with elevated CAC. Lipid GRS was associated with lipid levels but did not predict elevated CAC. Adult early and long-term average lipid levels provide important information when assessing subclinical atherosclerosis and cardiovascular risk.
Keywords: Lipids, Genetic risk score, Coronary artery calcium
INTRODUCTION
Coronary artery calcium (CAC), a measure of calcified coronary atherosclerotic plaque, is strongly and consistently associated with cardiovascular morbidity and mortality (1–7). Dyslipidemia is an atherosclerotic risk factor (8) and remote and long-term averaged lipid levels in young adulthood are associated with the presence and extent of CAC by middle age (9,10). The relationship of lipid levels with CAC may be modulated by the time course and duration of elevated lipid levels (1,2,4,9–12). Few studies have examined the association of timing and duration of lipid levels with CAC in an older population.
Recent genome-wide association studies (GWAS) have discovered 95 genetic loci associated with low-density lipoprotein cholesterol (LDL-C), high-density lipoprotein cholesterol (HDL-C), and triglycerides (TG) in multiple adult populations (13). TG genetic risk score was found to improve risk prediction over long-term TG level for elevated TG in adulthood (14), lending support to the impact of the genetic risk score (GRS) on ultimate lipid levels. Given the association of lipid levels with atherosclerosis, GWAS provides a unique tool to examine the impact of lipid GRS on measures of atherosclerosis such as CAC. We hypothesized that elevated lipid levels over many years, reflected in both long-term lipid levels and lipid GRS, would be more strongly associated with CAC than contemporary lipid levels. We thus sought to examine the association between early, long-term average, and contemporary measures of LDL-C, HDL-C, and TG, and their corresponding lipid GRS, with extent of CAC in a broad community population.
METHODS
Study Population
The Framingham Heart Study (FHS) is a longitudinally-followed prospective cohort study of community-dwelling adults evaluated every 4–6 years, as described previously (15,16). This study included Offspring Cohort (original FHS cohort members’ children and their spouses) and Third Generation Cohort (children of the Offspring) participants. Included FHS Offspring attended at least three of seven serial Examinations, Exam 1 (1971–75) through Exam 7 (1998–2001), inclusive of Exams 1 and 7. Third Generation participants were evaluated at Examination 1 (2002–2005). A subset of participants from the Offspring and Third Generation Cohorts, weighted towards larger FHS families, received multi-detector computed tomography (MDCT) to assess coronary artery calcium (CAC) in 2002 to 2005. Participants were excluded for weight ≥ 160 kilograms, age <35 years in men or <40 in women, and pregnancy. A total of 3529 FHS members (1422 Offspring and 2093 Third Generation) participated in the MDCT sub-study. Individuals were excluded if they had missing data for CAC measurements, lipid measurements, or prevalent coronary heart disease (myocardial infarction, angina, coronary insufficiency, coronary artery bypass graft, or angioplasty). A total of 3110 individuals (n=1156 Offspring 63±9 years of age, and n=1954 Third Generation, 45±6 years of age) were included in the analysis.
Measurement of Coronary Artery Calcium
CAC was imaged using an eight-slice MDCT scanner (LightSpeed Ultra, General Electric, Milwaukee, WI) (17). Forty-eight contiguous 2.5-mm thick slices were acquired. Each participant was scanned twice. Using a dedicated offline workstation (Aquarius, Terarecon, San Mateo, CA), an experienced reader assessed the presence and amount of CAC. A calcified lesion was identified as an area of ≥ 3 connected pixels of attenuation >130 Hounsfield units, and an Agatston score (AS) was calculated as described (18). For the present analysis, we defined CAC using a ≥ 75th percentile age-, sex-, and cohort-specific cut-points based on a healthy referent sample (19).
Measurement of Lipid Levels
At each study visit, each participant underwent a routine physical examination, medical history interview, and fasting laboratory tests, including total cholesterol, HDL-C, and TG. LDL-C was calculated according to the Friedewald equation (20). For the present analysis, we considered lipid measurements at three time points: 1) Early, at Offspring Examination 1, 2) Long-Term Average, the mean of all available lipid levels from Offspring Examinations 1–7, and 3) Contemporary, at both Offspring Examination 7 (1998–2001) and Third Generation Examination 1 (2002–2005), contemporaneously with MDCT. The use of lipid lowering medication was assessed at each examination.
Genotyping Methods and Lipid Genetic Risk Scores
Framingham Heart Study participants in the Offspring and Third Generation cohorts with available genomic DNA with cell line backups and with prior genotyping in the SHARe project were genotyped for lipid SNPs using an Illumina Golden Gate assay (21). Subjects also had 550K SNPs available from the Affymetrix platform and imputation to 2.5 million HapMap SNPs, as described (13,22). SNP genotypes were available in all n=3110 participants in this study. SNPs included in the genetic risk scores are shown in Supplemental Table 1.
Lipid GRSs were created from the significant SNP associations reported by Global Lipids Genetic Consortium Supplemental Table 2 (best SNP) (13). Scores were calculated for each individual in our sample, separately for HDL-C (47 SNP score), LDL-C (37 SNP score), and TG (32 SNP score) by weighted summation of genotypes (coded additively for the risk allele, increasing for LDL and TG and decreasing for HDL) multiplied by the reported beta estimates for each trait. Genotypes from “second-tier” genotyping filled in missing genotypes.
Statistical Analysis
All values for triglycerides were natural logarithm transformed. Values for LDL-C, triglycerides, and each of the lipid GRSs were standardized to a mean of zero and one standard deviation (SD) to facilitate comparisons between the measures. HDL-C was standardized to a mean of zero and one SD separately for each sex due to known sex differences in HDL-C. Pearson correlation coefficients were calculated between the GRSs and the lipid levels. Logistic regression models were constructed to calculate odds ratios (OR) and 95% confidence intervals (CI) for the association between each lipid measure and elevated CAC. Models were first adjusted for age at CAC measurement and sex, and then adjusted for lipid levels and lipid treatment status at corresponding time points. Because lipid-lowering therapy may confound the association of LDL-C and other lipid levels with CAC, we imputed LDL-C to approximate untreated levels in participants on lipid-lowering therapy, as previously described (23). C-statistics were calculated to assess the impact of both untreated lipid levels at each Exam cycle and the addition of lipid GRSs to models on the associations of lipid levels with elevated CAC. All analyses were performed using SAS v.9.1 (SAS Institute, Cary, NC). A p-value of <0.05 was considered statistically significant.
RESULTS
Offspring and Third Generation Cohort
Baseline characteristics of the Offspring and Third Generation cohorts are presented in Table 1. Compared to participants with CAC<75th percentile, those with CAC≥75th percentile had higher LDL-C and TG, lower HDL-C, and were more likely to be on lipid-lowering medication. The overall GRSs were similar between those with and without CAC≥75th percentile.
Table 1.
Study sample characteristics by coronary artery calcium score percentile in the Framingham Heart Study Offspring (n=1156) and Third Generation (n=1954) cohorts
| Offspring Cohort (n=1156) | Third Generation Cohort (n=1954) | |||
|---|---|---|---|---|
|
| ||||
| Characteristic | Coronary Artery Calcium Score* | Coronary Artery Calcium Score* | ||
|
| ||||
| <75th Percentile (N=763) | ≥75th Percentile (N=393) | <75th Percentile (N=1570) | ≥75th Percentile (N=384) | |
| Women, n (%) | 430 (56.4) | 212 (53.9) | 777 (49.5) | 104 (27.1) |
| Age at CAC measurement, mean (SD) | 63 (9) | 64 (9) | 45 (6) | 47 (6) |
| Lipid Measures | ||||
| LDL cholesterol (mg/dL), mean (SD) | ||||
| Exam 1 | 113 (29) | 124 (32) | 115 (31) | 124 (31) |
| Exam 7 | 122 (32) | 122 (32) | --- | --- |
| Cumulative average of exams 1–7 | 121 (26) | 130 (27) | --- | --- |
| HDL cholesterol (mg/dL), mean (SD) | ||||
| Exam 1 | 52 (14) | 50 (15) | 55 (17) | 49 (16) |
| Exam 7 | 55 (16) | 52 (16) | --- | --- |
| Cumulative average of exams 1–7 | 53 (13) | 50 (13) | --- | --- |
| Log triglycerides (mg/dL), mean (SD) | ||||
| Exam 1 | 4.2 (0.6) | 4.4 (0.6) | 4.6 (0.5) | 4.8 (0.6) |
| Exam 7 | 4.7 (0.5) | 4.8 (0.6) | --- | --- |
| Cumulative average of exams 1–7 | 4.6 (0.4) | 4.7 (0.5) | --- | --- |
| Lipid treatment, n (%) | ||||
| Exam 1 | 0 (0.0) | 3 (0.8) | 129 (8.2) | 63 (16.4) |
| Exam 7 | 85 (11.1) | 91 (23.2) | --- | --- |
| At any exam attended (1–7) | 93 (12.2) | 98 (24.9) | --- | --- |
| Lipid Genetic Risk Scores | ||||
| LDL-C genetic risk score | ||||
| Mean (SD) | 78 (8) | 79 (7) | 79 (8) | 79 (8) |
| Minimum, maximum | 47, 105 | 54, 99 | 49, 100 | 59, 98 |
| HDL-C genetic risk score | ||||
| Mean (SD) | 34 (3) | 34 (4) | 34 (4) | 34 (3) |
| Minimum, maximum | 24, 45 | 25, 44 | 23, 47 | 21, 42 |
| Triglyceride genetic risk score | ||||
| Mean (SD) | 161 (17) | 162 (16) | 160 (16) | 160 (15) |
| Minimum, maximum | 112, 209 | 112, 204 | 111, 217 | 116, 208 |
| Coronary artery calcium (HU) | ||||
| Mean (SD) | 51 (115) | 590 (840) | 2 (13) | 153 (329) |
| Median (25th, 75th percentile) | 1 (0, 39) | 247 (79, 690) | 0 (0, 0) | 43 (11, 146) |
Age and sex-specific CAC percentile cutpoints
Correlation of Lipid Measures with Genetic Risk Score
Lipid GRSs and corresponding lipid levels were modestly associated (Supplemental Table 2). For all lipid GRSs, the correlation was strongest with the long-term average lipid measure in the total study sample. Imputed LDL-C and TG were similarly modestly associated with their respective GRSs (data not shown). Among lipid GRSs, the correlation was strongest between HDL-C GRS and TG GRS.
Relationship of LDL-C measures and LDL-C GRS with CAC
The OR of CAC≥75th percentile per SD increment of LDL-C in Offspring is shown in Table 2a. Every SD unit increase in LDL-C at Exam 1 was associated with a 42% increased risk of CAC ≥ 75th percentile in age- and sex-adjusted analyses (95% CI 1.24–1.63, p<0.0001). The OR remained similar after further adjustment for LDL-C GRS. In contrast, Exam 7 LDL-C was not significantly associated with CAC (OR 1.06 per SD increase in LDL-C, 95% CI: 0.93–1.20; p=0.41). The Exam 1–7 averaged LDL-C was less strongly associated with elevated CAC than that in Exam 1 (per SD increase in LDL-C, OR 1.24, 95% CI: 1.09–1.42; p=0.002) and remained significant after adjustment for lipid treatment and LDL-C GRS. In the Third Generation Cohort (Table 3, top panel) and pooled analysis of the Offspring and Third Generation Cohorts (Table 4, top panel), contemporary LDL-C was positively associated with CAC ≥ 75th percentile in fully adjusted models (per SD increase in LDL-C, OR 1.22, 95% CI 1.08–1.38, p=0.001 in Third Generation; OR 1.17, 95% CI 1.07–1.27, p=0.0006 in the pooled analysis).
Table 2a.
Odds ratio of CAC≥75th percentile per SD increment of LDL-C measure or LDL-C genetic risk score in the Framingham Heart Study Offspring cohort
| Model | OR (95% CI) | P-value |
|---|---|---|
| LDL cholesterol (Exam 1) | ||
| Age + sex | 1.42 (1.24–1.63) | <0.0001 |
| Age + sex + LDL-C genetic risk score | 1.41 (1.23–1.62) | <0.0001 |
| LDL cholesterol (Exam 7) | ||
| Age + sex + lipid treatment (Exam 7) | 1.06 (0.93–1.20) | 0.41 |
| Age + sex + lipid treatment (Exam 7) + LDL-C genetic risk score | 1.04 (0.91–1.18) | 0.56 |
| LDL cholesterol (average, Exams 1–7) | ||
| Age + sex + lipid treatment (any) | 1.24 (1.09–1.42) | 0.002 |
| Age + sex + lipid treatment (any) + LDL-C genetic risk score | 1.23 (1.07–1.42) | 0.004 |
| LDL-C genetic risk score | ||
| Age + sex | 1.14 (1.00–1.29) | 0.04 |
| Age + sex + LDL-C (Exam 1) | 1.03 (0.91–1.18) | 0.62 |
| Age + sex + LDL-C (Exam 7) + lipid treatment (Exam 7) | 1.07 (0.94–1.22) | 0.30 |
| Age + sex + LDL-C (average, Exams 1–7) + lipid treatment (any) | 1.03 (0.90–1.18) | 0.65 |
Table 3.
Odds ratio of CAC≥75th percentile per SD increment of lipid measure or genetic risk score in the Framingham Heart Study Third Generation Cohort (n=1954)
| Model | OR (95% CI) | P-value |
|---|---|---|
| LDL-C cholesterol | ||
| Age + sex | 1.19 (1.06–1.34) | 0.004 |
| Age + sex + lipid treatment | 1.23 (1.09–1.38) | 0.0007 |
| Age + sex + lipid treatment + LDL-C genetic risk score | 1.22 (1.08–1.38) | 0.001 |
| LDL-C genetic risk score | ||
| Age + sex | 1.06 (0.94–1.19) | 0.33 |
| Age + sex + lipid treatment | 1.04 (0.92–1.17) | 0.52 |
| Age + sex + LDL-C + lipid treatment | 1.02 (0.91–1.16) | 0.72 |
|
| ||
|
| ||
| HDL cholesterol | ||
| Age + sex | 0.80 (0.71–0.90) | 0.0003 |
| Age + sex + lipid treatment | 0.81 (0.72–0.92) | 0.0007 |
| Age + sex + lipid treatment + HDL-C genetic risk score | 0.82 (0.72–0.93) | 0.002 |
| HDL-C genetic risk score | ||
| Age + sex | 1.09 (0.97–1.22) | 0.17 |
| Age + sex + lipid treatment | 1.08 (0.96–1.21) | 0.19 |
| Age + sex + HDL-C + lipid treatment | 1.02 (0.91–1.16) | 0.70 |
|
| ||
| TG cholesterol | ||
| Age + sex | 1.32 (1.17–1.48) | <0.0001 |
| Age + sex + lipid treatment | 1.30 (1.16–1.46) | <0.0001 |
| Age + sex + lipid treatment + TG genetic risk score | 1.32 (1.17–1.49) | <0.0001 |
| TG genetic risk score | ||
| Age + sex | 1.02 (0.90–1.14) | 0.80 |
| Age + sex + lipid treatment | 1.01 (0.90–1.13) | 0.92 |
| Age + sex + TG + lipid treatment | 0.94 (0.84–1.06) | 0.35 |
Lipid levels and lipid treatment were assessed at Exam 1 in the Third Generation Cohort.
Table 4.
Odds ratio of CAC≥75th percentile per SD increment of lipid measure or genetic risk score in the pooled Framingham Heart Study Offspring and Third Generation Cohorts (n=3110)
| Model | OR (95% CI) | P-value |
|---|---|---|
| LDL-C cholesterol | ||
| Age + sex | 1.12 (1.03–1.22) | 0.007 |
| Age + sex + lipid treatment | 1.17 (1.08–1.28) | 0.0003 |
| Age + sex + lipid treatment + LDL-C genetic risk score | 1.17 (1.07–1.27) | 0.0006 |
| LDL-C genetic risk score | ||
| Age + sex | 1.09 (1.00–1.18) | 0.05 |
| Age + sex + lipid treatment | 1.06 (0.97–1.15) | 0.22 |
| Age + sex + LDL-C + lipid treatment | 1.03 (0.94–1.13) | 0.51 |
|
| ||
| HDL cholesterol | ||
| Age + sex | 0.85 (0.78–0.92) | 0.0001 |
| Age + sex + lipid treatment | 0.86 (0.79–0.94) | 0.001 |
| Age + sex + lipid treatment + HDL-C genetic risk score | 0.87 (0.80–0.95) | 0.003 |
| HDL-C genetic risk score | ||
| Age + sex | 1.07 (0.98–1.16) | 0.11 |
| Age + sex + lipid treatment | 1.06 (0.98–1.16) | 0.15 |
| Age + sex + HDL-C + lipid treatment | 1.03 (0.94–1.12) | 0.51 |
|
| ||
| TG cholesterol | ||
| Age + sex | 1.30 (1.19–1.41) | <0.0001 |
| Age + sex + lipid treatment | 1.27 (1.16–1.38) | <0.0001 |
| Age + sex + lipid treatment + TG genetic risk score | 1.27 (1.17–1.39) | <0.0001 |
| TG genetic risk score | ||
| Age + sex | 1.04 (0.96–1.13) | 0.33 |
| Age + sex + lipid treatment | 1.03 (0.95–1.13) | 0.44 |
| Age + sex + TG + lipid treatment | 0.98 (0.90–1.07) | 0.67 |
Lipid levels and lipid treatment were assessed at Exam 7 for Offspring and Exam 1 for 3rd Generation Cohort.
The LDL-C GRS was modestly associated with increasing CAC. In the Offspring, each SD increase in the LDL-C GRS conferred a 1.14 odds for CAC ≥ 75th percentile (95% CI 1.00–1.29, p=0.04) in the age- and sex-adjusted model. The significance was attenuated after further adjustment for LDL-C at Exam 1, Exam 7, averaged Exams 1–7, and lipid treatment (Table 2a). The LDL-C GRS did not attain significance in combined analysis of both cohorts (Table 4, top panel).
In sensitivity analyses, we further evaluated the association between untreated LDL-C and CAC ≥ 75th percentile using an imputed estimate of the predicted untreated LDL-C for the minority of participants on lipid-lowering therapy. Exam 1 and long-term average, but not Exam 7, LDL-C were significantly associated with elevated CAC (Supplemental Table 3). Similarly, the marginal association of LDL-C GRS with CAC was attenuated in further multivariable adjustment. Every 20 mg/dl increase in LDL-C at Exams 1–2 were associated with a 1.22 odds of elevated CAC (95% CI 1.12–1.33, p<0.0001 in age and sex-adjusted analysis), with the greatest C-statistics at these Exams (Table 5). In contrast, Exam 7 lipid levels were not associated with elevated CAC.
Table 5.
Association between untreated LDL-C level and CAC ≥ 75th percentile at each Exam Cycle in the Framingham Heart Study Offspring Cohort
| Exam Cycle | OR (95% CI) [per 20 mg/dL] | P-value | C-statistic |
|---|---|---|---|
| 1 | 1.22 (1.12–1.33) | <0.0001 | 0.600 |
| 2 | 1.22 (1.13–1.33) | <0.0001 | 0.608 |
| 3 | 1.13 (1.05–1.22) | 0.0009 | 0.591 |
| 4 | 1.18 (1.09–1.27) | <0.0001 | 0.595 |
| 5 | 1.21 (1.11–1.31) | <0.0001 | 0.599 |
| 6 | 1.13 (1.05–1.22) | 0.002 | 0.582 |
| 7 | 1.05 (0.97–1.14) | 0.22 | 0.563 |
Models are adjusted for age at CT and sex.
Relationship of HDL-C measures and HDL-C GRS with CAC
Table 2b shows the OR of CAC ≥ 75th percentile per SD increment of HDL-C measure in the Offspring. Increased HDL-C at Exam 1 and average HDL-C over Exams 1–7 were associated with a decreased risk of CAC ≥ 75th percentile (per SD increase in HDL-C, OR 0.84, 95% CI 0.74–0.95, p=0.007 for both). The ORs remained significant and similar after further adjustment for the HDL-C GRS. Exam 7 HDL-C was not associated with increased CAC in the Offspring (OR 0.91 per SD increase in HDL-C, 95% CI 0.80–1.03, p=0.13). In the Third Generation Cohort alone and the combined cohort, however, increases of contemporary HDL-C were significantly associated with decreased risk for CAC in all models (per SD increase in HDL-C, OR 0.82, 95% CI 0.72–0.93, p=0.002 in Third Generation and OR 0.87 95% CI 0.80–0.95, p=0.003 in pooled analysis, middle panels of Tables 3 and 4, respectively). The HDL-C GRS was not significantly associated with CAC≥75th percentile in either Cohort (Tables 2b and 3).
Table 2b.
Odds ratio of CAC≥75th percentile per SD increment of HDL-C measure or HDL-C genetic risk score in the Framingham Heart Study Offspring cohort
| Model | OR (95% CI) | P-value |
|---|---|---|
| HDL cholesterol (Exam 1) | ||
| Age + sex | 0.84 (0.74–0.95) | 0.007 |
| Age + sex + HDL-C genetic risk score | 0.85 (0.75–0.97) | 0.01 |
| HDL cholesterol (Exam 7) | ||
| Age + sex + lipid treatment (Exam 7) | 0.91 (0.80–1.03) | 0.13 |
| Age + sex + lipid treatment (Exam 7) + HDL-C genetic risk score | 0.92 (0.80–1.05) | 0.19 |
| HDL cholesterol (average, Exams 1–7) | ||
| Age + sex + lipid treatment (any) | 0.84 (0.74–0.95) | 0.007 |
| Age + sex + lipid treatment (any) + HDL-C genetic risk score | 0.84 (0.74–0.96) | 0.01 |
| HDL-C genetic risk score | ||
| Age + sex | 1.06 (0.94–1.20) | 0.34 |
| Age + sex + HDL-C (Exam 1) | 1.05 (0.92–1.19) | 0.51 |
| Age + sex + HDL-C (Exam 7) + lipid treatment (Exam 7) | 1.04 (0.91–1.19) | 0.54 |
| Age + sex + HDL-C (average, Exams 1–7) + lipid treatment (any) | 1.01 (0.88–1.15) | 0.92 |
Relationship of TG measures and TG GRS with CAC
The OR of CAC≥75th percentile per SD increment of TG in the Offspring is shown in Table 2c. Every SD increase in TG at Exam 1 and averaged over Exams 1–7 was associated with a 36% increased risk of significant CAC in fully adjusted models (95% CI 1.19–1.56, p<0.0001 in Exam 1 and 95% CI 1.18–1.57, p<0.0001 in averaged Exams 1–7). TG measured at Exam 7 was also significantly associated with high CAC, though the magnitude of association was less than seen for Exam 1 and averaged Exams 1–7 (per SD increment of TG, OR 1.18, 95% CI 1.03–1.34; p=0.01 in the fully adjusted model). The ORs remained similar after further adjustment for the TG GRS. Similarly to results for LDL-C and HDL-C, contemporary TG levels were significantly associated with CAC in the Third Generation (OR 1.32 per SD increment of TG, 95% CI 1.17–1.48, p<0.0001) and pooled cohorts (OR 1.30 per SD increment of TG, 95% CI 1.19–1.41, p<0.0001) (bottom panels of Tables 3 and 4, respectively). The TG GRS was not significantly associated with CAC ≥ 75th percentile in either Cohort (Tables 2c, 3, and 4, respectively).
Table 2c.
Odds ratio of CAC≥75th percentile per standard deviation increment of TG measure or TG genetic risk score in the Framingham Heart Study Offspring Cohort
| Model | OR (95% CI) | P-value |
|---|---|---|
| Log triglycerides (Exam 1) | ||
| Age + sex | 1.37 (1.19–1.56) | <0.0001 |
| Age + sex + triglyceride genetic risk score | 1.36 (1.19–1.56) | <0.0001 |
| Log triglycerides (Exam 7) | ||
| Age + sex + lipid treatment (Exam 7) | 1.19 (1.05–1.35) | 0.008 |
| Age + sex + lipid treatment (Exam 7) + TG genetic risk score | 1.18 (1.03–1.34) | 0.01 |
| Log triglycerides (average, Exams 1–7) | ||
| Age + sex + lipid treatment (any) | 1.36 (1.18–1.56) | <0.0001 |
| Age + sex + lipid treatment (any) + TG genetic risk score | 1.36 (1.18–1.57) | <0.0001 |
| Triglyceride genetic risk score | ||
| Age + sex | 1.07 (0.94–1.20) | 0.31 |
| Age + sex + log TG (Exam 1) | 1.02 (0.89–1.15) | 0.82 |
| Age + sex + log TG (Exam 7) + lipid treatment (Exam 7) | 1.03 (0.91–1.17) | 0.62 |
| Age + sex + log TG (average, Exams 1–7) + lipid treatment (any) | 0.98 (0.86–1.12) | 0.81 |
Longitudinal Lipid Levels and Impact of Lipid-Lowering Therapy
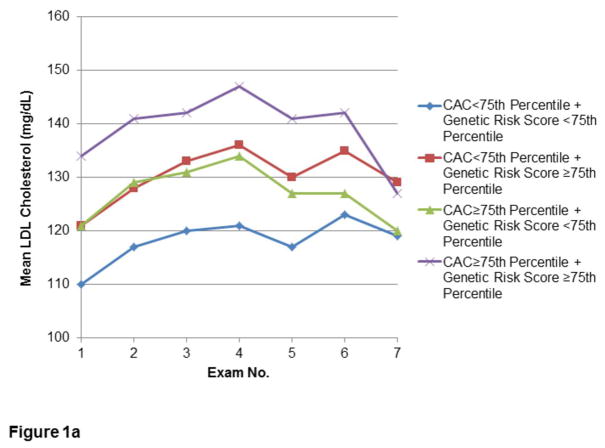
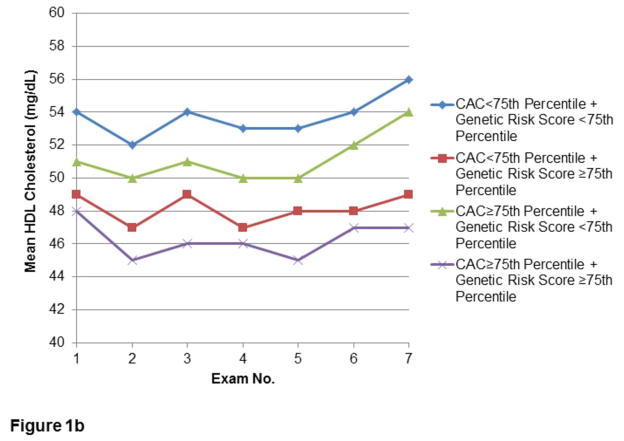
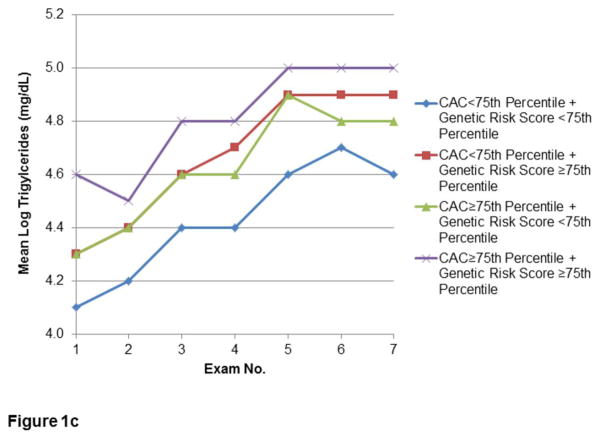
The relationships of CAC and lipid GRS to longitudinal measures of LDL-C, HDL-C, and TG in the Offspring are shown in Figures 1a, 1b, and 1c, respectively. Lipid levels progressed towards unfavorable levels through the early Exam cycles, with trends towards improvement in Exams 5–7. Corresponding use of lipid-lowering therapy in this Cohort rose from minimal (0.3–0.5%) at Exams 1–3, to 2.1% at Exam 4, approximately doubling each subsequent Exam to 15.2% at Exam 7. In Exams 6–7, use of statin therapy (6.6% and 13.8%, respectively) comprised the majority of lipid-lowering treatment. However, similar time trends of lipids were noted after conducting secondary analyses excluding Offspring on lipid-lowering therapy (Supplemental Figure 1).
Figure 1.
Figures 1a–c. Longitudinal measures of lipid levels.
Measures of LDL-C (1a), HDL-C (1b), and TG (1c) over the first seven Examination cycles in the FHS Offspring Cohort, stratified by extent of CAC and lipid GRS. Included participants attended at least three of the seven Examinations, including both Exams 1 and 7.
Incremental Impact of Lipid GRS
For both strata of CAC, those with lipid GRS ≥ 75th percentile had greater mean LDL-C. The mean LDL-C difference between the lowest group (CAC and GRS both <75th percentile) and the highest group (CAC and GRS both ≥ 75th percentile) was greatest in earlier Exam cycles, with a decrease at Exam 7 in the mean LDL-C in the group with both CAC and LDL-C GRS ≥ 75th percentile. By Exam 7, there were no differences in LDL-C between those with CAC ≥ 75th percentile or <75th percentile, regardless of GRS stratum. However, even at Exam 7, the presence of high GRS defined separate strata of LDL-C levels for individuals above and below a high cut-point for CAC (Figure 1a). Similarly, high (≥75th percentile) CAC and high GRS was associated with less favorable HDL-C and TG levels, with the lowest HDL-C and highest TG in those with greatest lipid GRS (Figures 1b and 1c, respectively). In a secondary analysis of participants who attended Exam 1 and at least two of the final three Exams, the pattern of serum lipids according to CAC≥75th percentile or <75th percentile appeared similar (data not shown).
The C-statistics for LDL-C, HDL-C, and TG adjusted models to predict CAC ≥ 75th percentile were modest (Supplemental Table 4). Overall, addition of the GRS to models resulted in very small increases in the C-statistic that were not statistically significant for any of the lipid measures.
DISCUSSION
Our longitudinal study of middle-aged to elderly adults, with initial lipid measurements beginning up to 30 years earlier in the Offspring cohort, reveals the effect of long-term measures of lipids and the possible effect of age at lipid measurement on the association of lipids with elevated CAC. In the older age population of FHS Offspring, early and long-term average levels of LDL-C, HDL-C, and TG are strongly associated with elevated CAC score. With the exception of TG, however, contemporary lipid levels were not associated with elevated CAC. In contrast, in the younger Third Generation Cohort and pooled cohort, contemporary measures of all lipids were associated with CAC ≥ 75th percentile. The GRSs for each of the lipid types are modestly associated with early and long-term levels of their respective lipid types. However, while the presence of high GRS and high CAC divided individuals into clear strata of long-term lipid levels, the GRS were not associated with elevated CAC in multivariable-adjusted models.
Remote, Long-term, and Contemporary Lipid Measures and Cardiovascular Risk
LDL-C particles initiate inflammation and atherosclerosis in the vessel wall (24). This process starts early in life, and childhood LDL-C and total cholesterol levels correlate with adult cardiovascular risk burden (25–27). Thus, a long-term average of LDL-C may be a better measure of cardiovascular risk than a single contemporary assessment. Indeed, we found that both remote and long-term averaged, but not contemporary, LDL-C were strongly predictive of elevated CAC by middle to elderly age. While contemporary statin use may be partially implicated in findings, our multivariable models adjusted for lipid-lowering therapy. In addition, in analysis of untreated lipid levels, we noted similar time trend of lipid levels, similar associations of LDL-C with CAC, and a consistent strong association of the earliest LDL-C with elevated CAC. Moreover, the results of other population studies lend support to our findings. In the Coronary Artery Risk Development in Young Adults (CARDIA) study, contemporary lipid levels were less strongly associated with CAC compared with remote levels, despite a low (2%) prevalence of lipid-lowering therapy (9). In the Multi-Ethnic Study of Atherosclerosis (MESA), untreated contemporary lipid levels were not significantly associated with prevalent CAC (11). Together with CARDIA (9,10) and MESA (4), our results collectively provide support for the longitudinal impact of dyslipidemia on atherosclerosis. Thus, the estimation of cardiovascular risk in older adult populations should include remote lipid measures, as elevated CAC is not well predicted by contemporary lipid measures.
The association between contemporary lipids and elevated CAC differed between the two FHS cohorts. While contemporary LDL-C and HDL-C were not associated with elevated CAC in the Offspring (mean age 63 years), all contemporary lipid measures were associated with elevated CAC in the Third Generation cohort (mean age 45 years). Similar associations in the pooled analysis likely reflect weighting by the significantly greater population size of the Third Generation Cohort. Our findings are consistent with a differential association of contemporaneous lipid measures with CAC in younger, rather than older, adults. In CARDIA (mean age 42 years), contemporaneous LDL-C was associated with prevalent CAC (9). In addition, MESA investigators reported progressive attenuation of the association between lipid measures and CAC with increasing decade of middle to elderly age (11). Interestingly, in CARDIA, while contemporaneous LDL-C was associated with prevalent CAC, remote and long-term averaged LDL-C were more strongly associated with CAC (9). While the explanation for the age effect is not clear, the lack of association of contemporaneous lipids with elevated CAC could be due to the interplay with and contributions of non-pharmacological lipid-lowering lifestyle interventions or other cardiovascular risk factors for CAC at older ages, which were not measured or adjusted for in our analyses.
SNP Scores, Lipids and Cardiovascular Risk
One of the major theoretical advantages of genetic risk prediction is that genes are present at birth and remain largely unchanged over lifetime. Hence, genetic polymorphisms affecting serum lipids can be expected to correlate with lifelong average (or integrated) lipid levels. Indeed, we found that our SNP scores for LDL-C, HDL-C, and triglycerides correlated more consistently with 30-year average lipids than with single measurements. However, this was particularly apparent for LDL-C where lipid-lowering therapy may confound the association between unadjusted contemporary serum lipid levels and genetic background.
Reflecting a lifelong burden of a particular phenotype, genetic scores may be a superior to single risk factor measurements for assessing subclinical disease. However, our data do not support the current use of genetic assessment of lipid profiles. In age- and sex-adjusted models, the LDL-C GRS showed only modest associations with CAC which were attenuated in fully-adjusted models. Our findings are consistent with previous studies that found no significant improvement in CAD prediction models after incorporating SNP score information (21,28,29). One possibility is that SNPs captured in the lipid genetic risk score, while associated with lipid levels, may not necessarily reflect the best combination of SNPs predisposing to development of CAC. Another explanation is that SNP scores generally explain only a small proportion of the inter-individual variance of an associated trait. In our data, the SNP scores accounted for 6.2%, 8.6% and 3.8% of the variance in early, long-term average and contemporary LDL-C, respectively. Similar proportions were found for HDL-C (4.7%, 5.8%, and 5.7%) and triglycerides (2.8%, 6.0%, and 5.8%). These values are low, given that these lipid traits have estimated heritabilities of 40–70% (30,31). This obvious mismatch (the “missing heritability”) has been observed in almost any disease or trait where GWAS have been performed, and may be attributed to several factors, including a large number of yet undiscovered SNPs with low minor allele frequencies and/or weak effect sizes, suboptimal fit of SNP effect estimates, gene-gene and gene-environment interactions, structural genetic variation not captured by SNPs, epigenetic modification, and familial / social clustering of cardiovascular risk behaviors (29,32).
Limitations
Our study has some limitations. First, because our study is cross-sectional and we did not acquire CAC data at baseline Exam 1, our results preclude causal inference of the lipid measures directly on the development of CAC. In addition, our work was based on the currently available GWAS data. It is likely that refined genotyping techniques and improved biostatistical tools will augment our collective knowledge of the genetic regulation of lipid traits, increasing the diagnostic power of future GRSs. Finally, the study population consists largely of Caucasians, which may limit generalizability to other races and/or ethnicities.
CONCLUSIONS
In this longitudinal study of the associations between timing of exposure to lipids, lipid GRSs, and subclinical coronary atherosclerosis, remote (~30 year), followed by long-term averaged, lipids were most strongly associated with CAC in our middle aged to elderly adult population, though contemporary lipid measures were associated with elevated CAC in our younger adults. While lipid GRSs were modestly correlated with their corresponding lipid measures, overall lipid GRSs were not significantly associated with elevated CAC. Our findings support assessments of early adulthood and long-term lipid profile measurements to assist in determination of cardiovascular risk. With further detailed DNA sequencing studies, the resulting characterization of the complete spectrum of common and uncommon alleles implicated in lipid levels may improve the GRS and its usefulness in prediction of cardiovascular risk.
Supplementary Material
Acknowledgments
The Framingham Heart Study (FHS) is supported by the National Heart, Lung, and Blood Institute (contract N01-HC-25195). This work was partially supported by the National Heart, Lung, and Blood Institute’s contract with Affymetrix, Inc, for genotyping services (contract N02-HL-6-4278). Analyses are partially based on resources from Framingham Heart Study investigators in the SNP Health Association Resource project. Dr. Tsao is partially supported by an award from the ACCF/Merck.
Abbreviations and Acronyms
- CAC
coronary artery calcium
- FHS
Framingham Heart Study
- GRS
genetic risk score
- GWAS
genome-wide association study
- HDL-C
high-density lipoprotein cholesterol
- LDL-C
low-density lipoprotein cholesterol
- TG
triglycerides
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Allison MA, Pavlinac P, Wright CM. The differential associations between HDL, non-HDL and total cholesterols and atherosclerotic calcium deposits in multiple vascular beds. Atherosclerosis. 2007;194:e87–94. doi: 10.1016/j.atherosclerosis.2006.10.006. [DOI] [PubMed] [Google Scholar]
- 2.Allison MA, Wright CM. A comparison of HDL and LDL cholesterol for prevalent coronary calcification. Int J Cardiol. 2004;95:55–60. doi: 10.1016/j.ijcard.2003.04.013. [DOI] [PubMed] [Google Scholar]
- 3.Detrano R, Guerci AD, Carr JJ, et al. Coronary calcium as a predictor of coronary events in four racial or ethnic groups. N Engl J Med. 2008;358:1336–45. doi: 10.1056/NEJMoa072100. [DOI] [PubMed] [Google Scholar]
- 4.Kronmal RA, McClelland RL, Detrano R, et al. Risk factors for the progression of coronary artery calcification in asymptomatic subjects: results from the Multi-Ethnic Study of Atherosclerosis (MESA) Circulation. 2007;115:2722–30. doi: 10.1161/CIRCULATIONAHA.106.674143. [DOI] [PubMed] [Google Scholar]
- 5.Wong ND, Hsu JC, Detrano RC, Diamond G, Eisenberg H, Gardin JM. Coronary artery calcium evaluation by electron beam computed tomography and its relation to new cardiovascular events. Am J Cardiol. 2000;86:495–8. doi: 10.1016/s0002-9149(00)01000-6. [DOI] [PubMed] [Google Scholar]
- 6.Greenland P, Alpert JS, Beller GA, et al. 2010 ACCF/AHA guideline for assessment of cardiovascular risk in asymptomatic adults: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2010;122:e584–636. doi: 10.1161/CIR.0b013e3182051b4c. [DOI] [PubMed] [Google Scholar]
- 7.Polonsky TS, McClelland RL, Jorgensen NW, et al. Coronary artery calcium score and risk classification for coronary heart disease prediction. JAMA. 2010;303:1610–6. doi: 10.1001/jama.2010.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Third Report of the Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (ATP III Final Report) Bethesda, MD: National Heart, Lung, and Blood Institute; [February 29, 2012]. 2004. Accessed at www.nhlbi.nih.gov/guidelines/cholesterol on. [Google Scholar]
- 9.Loria CM, Liu K, Lewis CE, et al. Early adult risk factor levels and subsequent coronary artery calcification: the CARDIA Study. J Am Coll Cardiol. 2007;49:2013–20. doi: 10.1016/j.jacc.2007.03.009. [DOI] [PubMed] [Google Scholar]
- 10.Pletcher MJ, Bibbins-Domingo K, Liu K, et al. Nonoptimal lipids commonly present in young adults and coronary calcium later in life: the CARDIA (Coronary Artery Risk Development in Young Adults) study. Ann Intern Med. 2010;153:137–46. doi: 10.1059/0003-4819-153-3-201008030-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Paramsothy P, Katz R, Owens DS, Burke GL, Probstfield JL, O’Brien KD. Age-modification of lipoprotein, lipid, and lipoprotein ratio-associated risk for coronary artery calcium (from the Multi-Ethnic Study of Atherosclerosis [MESA]) Am J Cardiol. 2010;105:352–8. doi: 10.1016/j.amjcard.2009.09.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Paramsothy P, Knopp RH, Bertoni AG, et al. Association of combinations of lipid parameters with carotid intima-media thickness and coronary artery calcium in the MESA (Multi-Ethnic Study of Atherosclerosis) J Am Coll Cardiol. 2010;56:1034–41. doi: 10.1016/j.jacc.2010.01.073. [DOI] [PubMed] [Google Scholar]
- 13.Teslovich TM, Musunuru K, Smith AV, et al. Biological, clinical and population relevance of 95 loci for blood lipids. Nature. 2010;466:707–13. doi: 10.1038/nature09270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tikkanen E, Tuovinen T, Widen E, et al. Association of known loci with lipid levels among children and prediction of dyslipidemia in adults. Circ Cardiovasc Genet. 2011;4:673–80. doi: 10.1161/CIRCGENETICS.111.960369. [DOI] [PubMed] [Google Scholar]
- 15.Kannel WB, Feinleib M, McNamara PM, Garrison RJ, Castelli WP. An investigation of coronary heart disease in families. The Framingham offspring study. Am J Epidemiol. 1979;110:281–90. doi: 10.1093/oxfordjournals.aje.a112813. [DOI] [PubMed] [Google Scholar]
- 16.Splansky GL, Corey D, Yang Q, et al. The Third Generation Cohort of the National Heart, Lung, and Blood Institute’s Framingham Heart Study: design, recruitment, and initial examination. Am J Epidemiol. 2007;165:1328–35. doi: 10.1093/aje/kwm021. [DOI] [PubMed] [Google Scholar]
- 17.Parikh NI, Hwang SJ, Larson MG, et al. Parental occurrence of premature cardiovascular disease predicts increased coronary artery and abdominal aortic calcification in the Framingham Offspring and Third Generation cohorts. Circulation. 2007;116:1473–81. doi: 10.1161/CIRCULATIONAHA.107.705202. [DOI] [PubMed] [Google Scholar]
- 18.Agatston AS, Janowitz WR, Hildner FJ, Zusmer NR, Viamonte M, Jr, Detrano R. Quantification of coronary artery calcium using ultrafast computed tomography. J Am Coll Cardiol. 1990;15:827–32. doi: 10.1016/0735-1097(90)90282-t. [DOI] [PubMed] [Google Scholar]
- 19.Hoffmann U, Massaro JM, Fox CS, Manders E, O’Donnell CJ. Defining normal distributions of coronary artery calcium in women and men (from the Framingham Heart Study) Am J Cardiol. 2008;102:1136–41. 41, e1. doi: 10.1016/j.amjcard.2008.06.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clinical chemistry. 1972;18:499–502. [PubMed] [Google Scholar]
- 21.Thanassoulis G, Peloso GM, Pencina MJ, et al. A genetic risk score is associated with incident cardiovascular disease and coronary artery calcium: the Framingham Heart Study. Circ Cardiovasc Genet. 2012;5:113–21. doi: 10.1161/CIRCGENETICS.111.961342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Psaty BM, O’Donnell CJ, Gudnason V, et al. Cohorts for Heart and Aging Research in Genomic Epidemiology (CHARGE) Consortium: Design of prospective meta-analyses of genome-wide association studies from 5 cohorts. Circ Cardiovasc Genet. 2009;2:73–80. doi: 10.1161/CIRCGENETICS.108.829747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kathiresan S, Manning AK, Demissie S, et al. A genome-wide association study for blood lipid phenotypes in the Framingham Heart Study. BMC Med Genet. 2007;8 (Suppl 1):S17. doi: 10.1186/1471-2350-8-S1-S17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ross R. Atherosclerosis--an inflammatory disease. N Engl J Med. 1999;340:115–26. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- 25.Mahoney LT, Burns TL, Stanford W, et al. Coronary risk factors measured in childhood and young adult life are associated with coronary artery calcification in young adults: the Muscatine Study. J Am Coll Cardiol. 1996;27:277–84. doi: 10.1016/0735-1097(95)00461-0. [DOI] [PubMed] [Google Scholar]
- 26.Napoli C, D’Armiento FP, Mancini FP, et al. Fatty streak formation occurs in human fetal aortas and is greatly enhanced by maternal hypercholesterolemia. Intimal accumulation of low density lipoprotein and its oxidation precede monocyte recruitment into early atherosclerotic lesions. J Clin Invest. 1997;100:2680–90. doi: 10.1172/JCI119813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Davis PH, Dawson JD, Riley WA, Lauer RM. Carotid intimal-medial thickness is related to cardiovascular risk factors measured from childhood through middle age: The Muscatine Study. Circulation. 2001;104:2815–9. doi: 10.1161/hc4601.099486. [DOI] [PubMed] [Google Scholar]
- 28.Ripatti S, Tikkanen E, Orho-Melander M, et al. A multilocus genetic risk score for coronary heart disease: case-control and prospective cohort analyses. Lancet. 2010;376:1393–400. doi: 10.1016/S0140-6736(10)61267-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Thanassoulis G, Vasan RS. Genetic cardiovascular risk prediction: will we get there? Circulation. 2010;122:2323–34. doi: 10.1161/CIRCULATIONAHA.109.909309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Heller DA, de Faire U, Pedersen NL, Dahlen G, McClearn GE. Genetic and environmental influences on serum lipid levels in twins. N Engl J Med. 1993;328:1150–6. doi: 10.1056/NEJM199304223281603. [DOI] [PubMed] [Google Scholar]
- 31.Kaess B, Fischer M, Baessler A, et al. The lipoprotein subfraction profile: heritability and identification of quantitative trait loci. J Lipid Res. 2008;49:715–23. doi: 10.1194/jlr.M700338-JLR200. [DOI] [PubMed] [Google Scholar]
- 32.Manolio TA, Collins FS, Cox NJ, et al. Finding the missing heritability of complex diseases. Nature. 2009;461:747–53. doi: 10.1038/nature08494. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.