Abstract
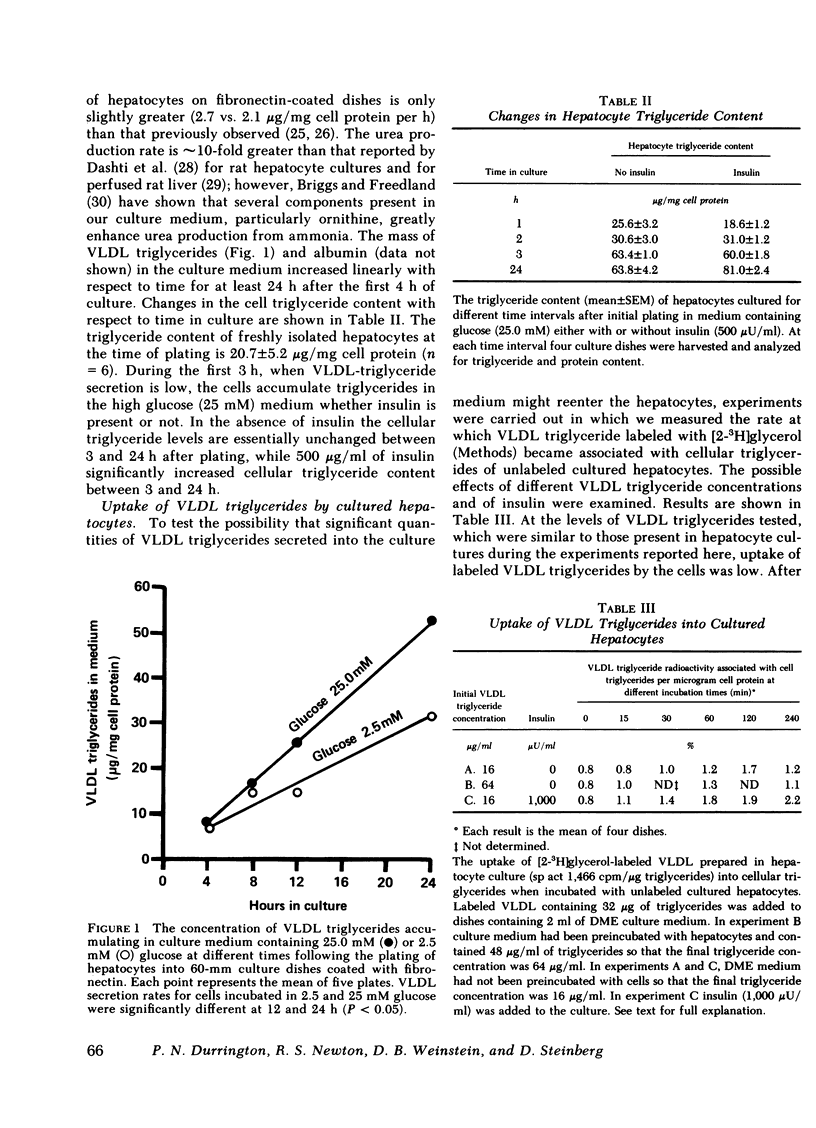
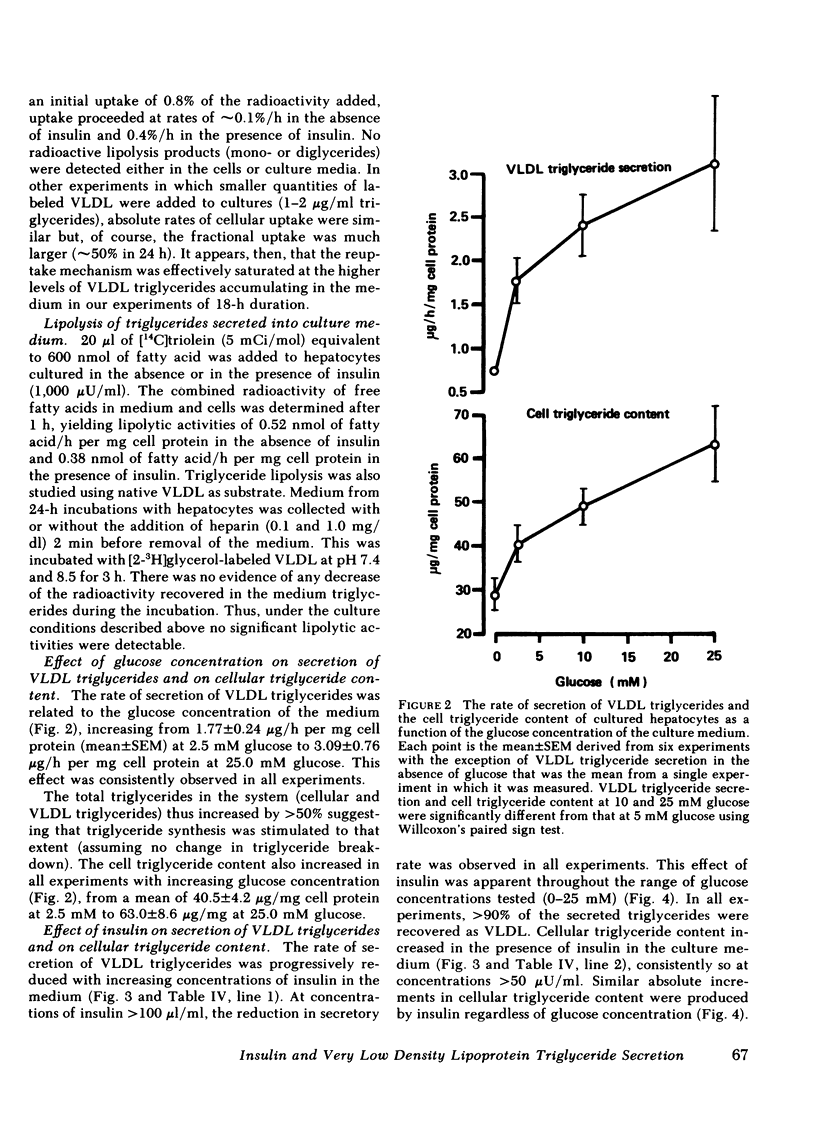
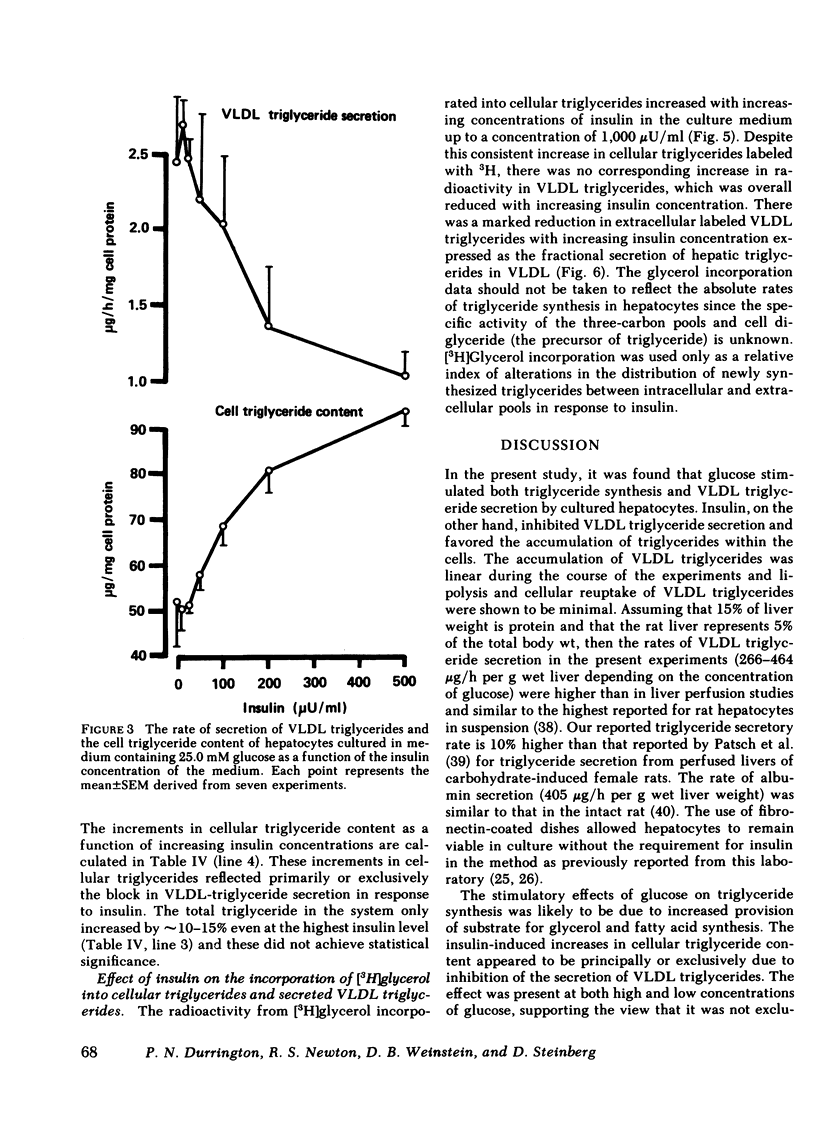
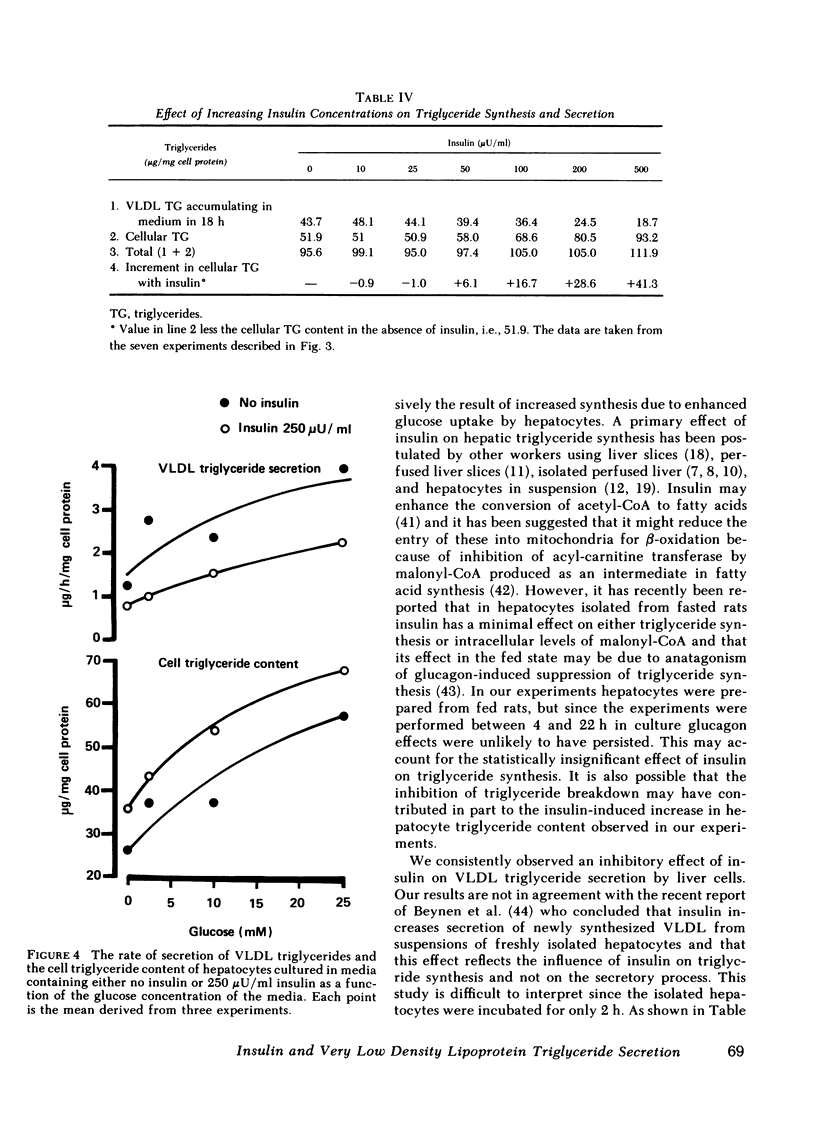
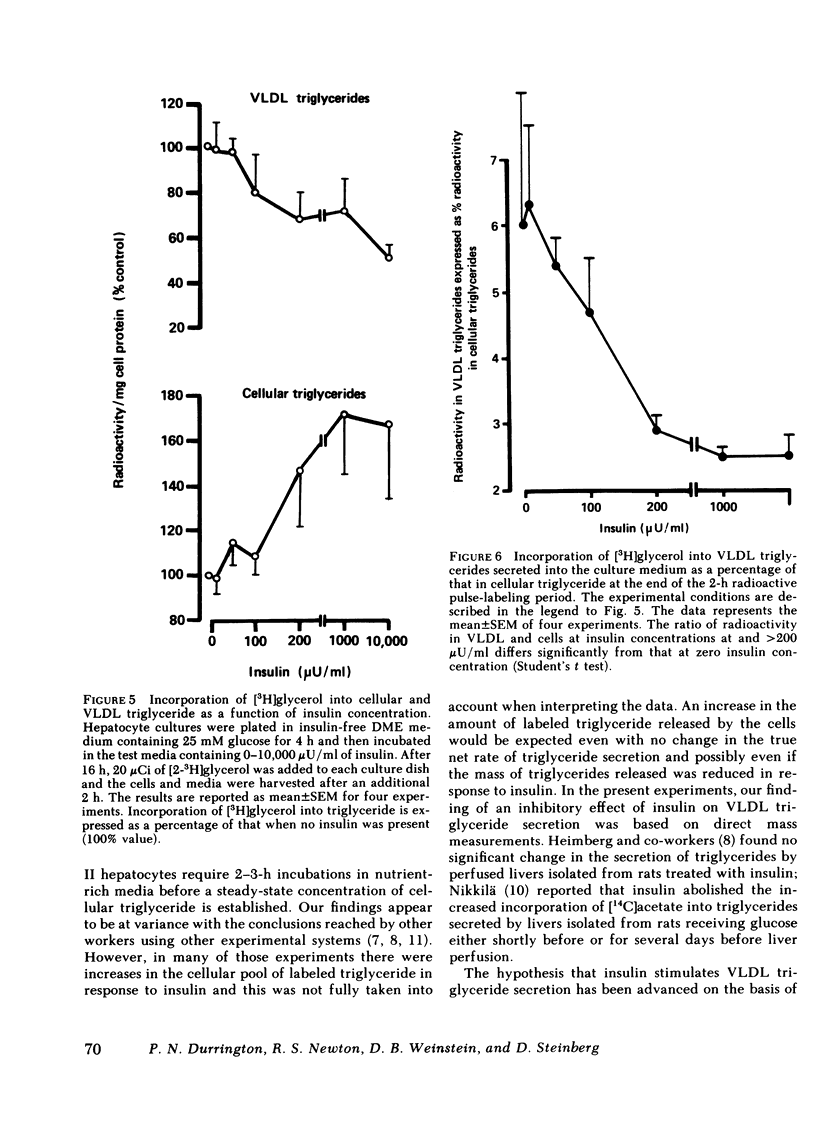
The effect of insulin on hepatic triglyceride synthesis and secretion is controversial. Previously, we have described a cell culture system of adult rat hepatocytes that synthesize and secrete very low density lipoprotein (VLDL) triglycerides with small and irreproducible effects of insulin on triglyceride metabolism. To study the primary effects of insulin on hepatic triglyceride metabolism a method was developed utilizing fibronectin-coated culture dishes that allowed adhesion, spreading, and maintenance of hepatocytes for 2-3 d in the absence of serum and insulin. This culture system allowed mass measurements of both cellular and secreted VLDL triglycerides for long time periods after the addition of physiological concentrations of insulin to hormone-free culture medium. In the absence of insulin and after an initial 4 h in culture, the medium was replenished and triglyceride mass was measured at the end of 18-h incubations. VLDL triglyceride accumulated in the culture medium at a linear rate over this time-course with increasing accumulation as the medium glucose concentration was raised from 2.5 to 25 mM glucose (1.77±0.24 to 3.09±0.76 μg triglyceride/mg cell protein per h). There was no apparent significant lipolysis or hepatocellular reuptake of secreted VLDL triglycerides. In the absence of insulin cellular triglyceride levels were unchanged between 3 and 24 h in culture while insulin (50-500 μU/ml) significantly increased cellular triglyceride content at all glucose concentrations tested (0-25 mM). The addition of insulin to the culture medium progressively reduced the rate of VLDL triglyceride secretion accompanied by an increase in cellular triglyceride at insulin concentrations > 50 μU/ml. Most or all of the observed increase in cell triglyceride content could in all experiments be accounted for by the insulin-induced inhibition of VLDL secretion. Incorporation of [2-3H]glycerol into cellular and VLDL triglycerides as a function of insulin concentration was also measured. Glycerol incorporation data at 20-22 h after plating of the cells closely paralleled the insulin-induced changes in cellular and VLDL triglyceride as determined by mass analysis. The observed effects of insulin occurred at concentrations close to the physiological range and suggest that the direct hepatic effect is to suppress VLDL secretion although the net effect in vivo will clearly reflect many additional accompanying changes.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alberti K. G., Johnston D. G., Gill A., Barnes A. J., Orskov H. Hormonal regulation of ketone-body metabolism in man. Biochem Soc Symp. 1978;(43):163–182. [PubMed] [Google Scholar]
- Axén R., Porath J., Ernback S. Chemical coupling of peptides and proteins to polysaccharides by means of cyanogen halides. Nature. 1967 Jun 24;214(5095):1302–1304. doi: 10.1038/2141302a0. [DOI] [PubMed] [Google Scholar]
- BELOGORSKY J. B., SLAUGHTER D. Administration of BAL in selenium poisoning. Proc Soc Exp Biol Med. 1949 Oct;72(1):196–198. doi: 10.3181/00379727-72-17376. [DOI] [PubMed] [Google Scholar]
- Balasse E. O., Bier D. M., Havel R. J. Early effects of anti-insulin serum on hepatic metabolism of plasma free fatty acids in dogs. Diabetes. 1972 May;21(5):280–288. doi: 10.2337/diab.21.5.280. [DOI] [PubMed] [Google Scholar]
- Basso L. V., Havel R. J. Hepatic metabolism of free fatty acids in normal and diabetic dogs. J Clin Invest. 1970 Mar;49(3):537–547. doi: 10.1172/JCI106264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry M. N., Friend D. S. High-yield preparation of isolated rat liver parenchymal cells: a biochemical and fine structural study. J Cell Biol. 1969 Dec;43(3):506–520. doi: 10.1083/jcb.43.3.506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beynen A. C., Haagsman H. P., Van Golde L. M., Geelen M. J. The effects of insulin and glucagon on the release of triacylglycerols by isolated rat hepatocytes are mere reflections of the hormonal effects on the rate of triacylglycerol synthesis. Biochim Biophys Acta. 1981 Jul 24;665(1):1–7. doi: 10.1016/0005-2760(81)90224-1. [DOI] [PubMed] [Google Scholar]
- Beynen A. C., Vaartjes W. J., Geelen M. J. Opposite effects of insulin and glucagon in acute hormonal control of hepatic lipogenesis. Diabetes. 1979 Sep;28(9):828–835. doi: 10.2337/diab.28.9.828. [DOI] [PubMed] [Google Scholar]
- Boyd M. E., Albright E. B., Foster D. W., McGarry J. D. In vitro reversal of the fasting state of liver metabolism in the rat. Reevaluation of the roles of insulin and glucose. J Clin Invest. 1981 Jul;68(1):142–152. doi: 10.1172/JCI110230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briggs S., Freedland R. A. Effect of ornithine and lactate on urea synthesis in isolated hepatocytes. Biochem J. 1976 Nov 15;160(2):205–209. doi: 10.1042/bj1600205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown M. S., Kovanen P. T., Goldstein J. L. Evolution of the LDL receptor concept-from cultured cells to intact animals. Ann N Y Acad Sci. 1980;348:48–68. doi: 10.1111/j.1749-6632.1980.tb21290.x. [DOI] [PubMed] [Google Scholar]
- Carlson L. A. Regulation of endogenous plasma triglyceride concentration: can we measure the rate of production or of removal of endogenous plasma triglycerides in man? Eur J Clin Invest. 1980 Feb;10(1):5–7. doi: 10.1111/j.1365-2362.1980.tb00002.x. [DOI] [PubMed] [Google Scholar]
- Crespin S. R., Greenough W. B., 3rd, Steinberg D. Stimulation of insulin secretion by infusion of free fatty acids. J Clin Invest. 1969 Oct;48(10):1934–1943. doi: 10.1172/JCI106160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DANNENBURG W. N., BURT R. L. THE EFFECT OF INSULIN AND GLUCOSE ON PLASMA LIPIDS DURING PREGNANCY AND THE PUERPERIUM. Am J Obstet Gynecol. 1965 May 15;92:195–201. doi: 10.1016/s0002-9378(65)80007-2. [DOI] [PubMed] [Google Scholar]
- DULBECCO R., FREEMAN G. Plaque production by the polyoma virus. Virology. 1959 Jul;8(3):396–397. doi: 10.1016/0042-6822(59)90043-1. [DOI] [PubMed] [Google Scholar]
- DULBECCO R., VOGT M. Plaque formation and isolation of pure lines with poliomyelitis viruses. J Exp Med. 1954 Feb;99(2):167–182. doi: 10.1084/jem.99.2.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dashti N., McConathy W. J., Ontko J. A. Production of apolipoproteins E and A-I by rat hepatocytes in primary culture. Biochim Biophys Acta. 1980 May 28;618(2):347–358. doi: 10.1016/0005-2760(80)90041-7. [DOI] [PubMed] [Google Scholar]
- Davis R. A., Engelhorn S. C., Pangburn S. H., Weinstein D. B., Steinberg D. Very low density lipoprotein synthesis and secretion by cultured rat hepatocytes. J Biol Chem. 1979 Mar 25;254(6):2010–2016. [PubMed] [Google Scholar]
- Davis R. A., Engelhorn S. C., Weinstein D. B., Steinberg D. Very low density lipoprotein secretion by cultured rat hepatocytes. Inhibition by albumin and other macromolecules. J Biol Chem. 1980 Mar 10;255(5):2039–2045. [PubMed] [Google Scholar]
- Eisenberg S. Plasma lipoprotein conversions: the origins of low-density and high-density lipoproteins. Ann N Y Acad Sci. 1980;348:30–47. doi: 10.1111/j.1749-6632.1980.tb21289.x. [DOI] [PubMed] [Google Scholar]
- Engvall E., Ruoslahti E. Binding of soluble form of fibroblast surface protein, fibronectin, to collagen. Int J Cancer. 1977 Jul 15;20(1):1–5. doi: 10.1002/ijc.2910200102. [DOI] [PubMed] [Google Scholar]
- FOLCH J., LEES M., SLOANE STANLEY G. H. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957 May;226(1):497–509. [PubMed] [Google Scholar]
- Farquhar J. W., Frank A., Gross R. C., Reaven G. M. Glucose, insulin, and triglyceride responses to high and low carbohydrate diets in man. J Clin Invest. 1966 Oct;45(10):1648–1656. doi: 10.1172/JCI105472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster L. B., Hochholzer J. M. A single-reagent manual method for directly determining urea nitrogen in serum. Clin Chem. 1971 Sep;17(9):921–925. [PubMed] [Google Scholar]
- Freeman C. P., West D. Complete separation of lipid classes on a single thin-layer plate. J Lipid Res. 1966 Mar;7(2):324–327. [PubMed] [Google Scholar]
- Geelen M. J., Beynen A. C., Christiansen R. Z., Lepreau-Jose M. J., Gibson D. M. Short-term effects of insulin and glucagon on lipid synthesis in isolated rat hepatocytes. Covariance of acetyl-CoA carboxylase activity and the rate of 3H2O incorporation into fatty acids. FEBS Lett. 1978 Nov 15;95(2):326–330. doi: 10.1016/0014-5793(78)81022-9. [DOI] [PubMed] [Google Scholar]
- Glueck C. J., Levy R. I., Fredrickson D. S. Immunoreactive insulin, glucose tolerance, and carbohydrate inducibility in types II, 3, IV, and V hyperlipoproteinemia. Diabetes. 1969 Nov;18(11):739–747. doi: 10.2337/diab.18.11.739. [DOI] [PubMed] [Google Scholar]
- HAAHTI E. Effect of insulin in a case of essential hyperlipemia. Scand J Clin Lab Invest. 1959;11:305–306. doi: 10.3109/00365515909060455. [DOI] [PubMed] [Google Scholar]
- Hayford J. T., Danney M. M., Thompson R. G. Triglyceride-integrated concentration: relationship to insulin-integrated concentration. Metabolism. 1979 Nov;28(11):1078–1085. doi: 10.1016/0026-0495(79)90145-8. [DOI] [PubMed] [Google Scholar]
- Jones D. P., Arky R. A. Effects of insulin on triglyceride and free fatty acid metabolism in man. Metabolism. 1965 Dec;14(12):1287–1293. doi: 10.1016/s0026-0495(65)80010-5. [DOI] [PubMed] [Google Scholar]
- Kabara J. J., Chen J. S. Microdetermination of lipid classes after thin-layer chromatography. Anal Chem. 1976 May;48(6):814–817. doi: 10.1021/ac60370a019. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laurell C. B. Quantitative estimation of proteins by electrophoresis in agarose gel containing antibodies. Anal Biochem. 1966 Apr;15(1):45–52. doi: 10.1016/0003-2697(66)90246-6. [DOI] [PubMed] [Google Scholar]
- Lewis B., Mancini M., Mattock M., Chait A., Fraser T. R. Plasma triglyceride and fatty acid metabolism in diabetes mellitus. Eur J Clin Invest. 1972 Nov;2(6):445–453. doi: 10.1111/j.1365-2362.1972.tb00676.x. [DOI] [PubMed] [Google Scholar]
- Nikkilä E. A. Control of plasma and liver triglyceride kinetics by carbohydrate metabolism and insulin. Adv Lipid Res. 1969;7:63–134. doi: 10.1016/b978-0-12-024907-7.50009-9. [DOI] [PubMed] [Google Scholar]
- Nikkilä E. A. Triglyceride metabolism in diabetes mellitus. Prog Biochem Pharmacol. 1973;8:271–299. [PubMed] [Google Scholar]
- Olefsky J. M., Farquhar J. W., Reaven G. M. Reappraisal of the role of insulin in hypertriglyceridemia. Am J Med. 1974 Oct;57(4):551–560. doi: 10.1016/0002-9343(74)90006-0. [DOI] [PubMed] [Google Scholar]
- Ontko J. A. Metabolism of free fatty acids in isolated liver cells. Factors affecting the partition between esterification and oxidation. J Biol Chem. 1972 Mar 25;247(6):1788–1800. [PubMed] [Google Scholar]
- Patsch W., Kim K., Wiest W., Schonfeld G. Effects of sex hormones on rat lipoproteins. Endocrinology. 1980 Oct;107(4):1085–1094. doi: 10.1210/endo-107-4-1085. [DOI] [PubMed] [Google Scholar]
- Pittman R. C., Khoo J. C., Steinberg D. Cholesterol esterase in rat adipose tissue and its activation by cyclic adenosine 3':5'-monophosphate-dependent protein kinase. J Biol Chem. 1975 Jun 25;250(12):4505–4511. [PubMed] [Google Scholar]
- RANDLE P. J., GARLAND P. B., HALES C. N., NEWSHOLME E. A. The glucose fatty-acid cycle. Its role in insulin sensitivity and the metabolic disturbances of diabetes mellitus. Lancet. 1963 Apr 13;1(7285):785–789. doi: 10.1016/s0140-6736(63)91500-9. [DOI] [PubMed] [Google Scholar]
- Reaven G. M., Lerner R. L., Stern M. P., Farquhar J. W. Role of insulin in endogenous hypertriglyceridemia. J Clin Invest. 1967 Nov;46(11):1756–1767. doi: 10.1172/JCI105666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlierf G., Kinsell L. W. Effect of insulin in hypertriglyceridemia. Proc Soc Exp Biol Med. 1965 Oct;120(1):272–274. doi: 10.3181/00379727-120-30509. [DOI] [PubMed] [Google Scholar]
- Schreiber G., Urban J. The synthesis and secretion of albumin. Rev Physiol Biochem Pharmacol. 1978;82:27–95. doi: 10.1007/BFb0030497. [DOI] [PubMed] [Google Scholar]
- Soler-Argilaga C., Heimberg M. Comparison of metabolism of free fatty acid by isolated perfused livers from male and female rats. J Lipid Res. 1976 Nov;17(6):605–615. [PubMed] [Google Scholar]
- Topping D. L., Mayes P. A. The immediate effects of insulin and fructose on the metabolism of the perfused liver. Changes in lipoprotein secretion, fatty acid oxidation and esterification, lipogenesis and carbohydrate metabolism. Biochem J. 1972 Jan;126(2):295–311. doi: 10.1042/bj1260295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tulloch B. R., Dyal K., Fraser T. R. Increased lipid synthesis by liver slice in a superfusion system following raised glucose or insulin concentration. Diabetologia. 1972 Aug;8(4):267–273. doi: 10.1007/BF01225570. [DOI] [PubMed] [Google Scholar]



