Abstract
Injectable, “smart” microspheres that are sensitive to both temperature and pH have been fabricated and tested for controlled delivery of therapeutic proteins to ischemic skeletal muscle. A library of copolymers composed of N-isopropyl acrylamide (NIPAAm), propyl acrylic acid (PAA), and butyl acrylate (BA) was used to fabricate microspheres using a double emulsion method, and an optimal formulation made from copolymers composed of 57 mol% NIPAAm, 18 mol% PAA, and 25 mol% BA copolymers was identified. At 37°C and pH representative of ischemic muscle (i.e., pH 5.2–7.2), these microspheres produced sustained, diffusion-controlled release, and at normal, physiologic pH (i.e. pH 7.4), they underwent dissolution and rapid clearance. Delivery of fibroblast growth factor 2 (FGF-2) was used to confirm that protein bioactivity was retained following microsphere encapsulation/release based on a dose-dependent increase in NIH3T3 fibroblast proliferation in vitro. Microsphere-loaded or free Cy5.5-labeled albumin was injected into ischemic and control gastrocnemii of mice following unilateral induction of hind limb ischemia to model peripheral arterial disease. In the ischemic limb at day 3.5 and day 7, there was higher local retention of the protein delivered via microspheres relative to injected free protein (p<0.05). However, clearance of protein delivered via microspheres was equivalent to free protein at later time points that correspond to ischemic recovery in this model. Finally, histological analysis of the gastrocnemius revealed that the polymeric microspheres did not produce any microscopic signs of toxicity near the injection site. These combined results suggest that the pH- and temperature-responsive microspheres presented herein are a promising technological platform for controlled protein delivery to ischemic tissue.
Keywords: Reversible addition fragmentation chain transfer (RAFT) polymerization, pH-responsive polymer, temperature-responsive polymer, LCST, environmentally-responsive microspheres, Controlled Release, Hind limb Ischemia
1.0 Introduction
Peripheral arterial disease (PAD) is estimated to affect 29% of the population over 70 and an even higher percentage of diabetics [1–3]. PAD results in a gradual decline in quality of life due to loss of function of the lower extremities [4, 5] and triples the 5-year mortality rate in patients with intermittent claudication [6]. Although the prevalence of PAD is high and its consequences severe, most cases go untreated [7], indicating the need for improved PAD therapies. Delivery of protein growth factors is one promising approach to stimulate development of collateral vessels that can functionally compensate for obstructed arteries and restore limb function in patients with PAD [8]. For example, fibroblast growth factor 2 (FGF-2) is a mitogen for endothelial and smooth muscle cells and can stimulate arteriogenesis in vivo [9]. However, clinical trials that have tested delivery of FGF-2 have yielded disappointing results, likely due to the short half-life of FGF-2 (7.6h) [10]. Thus, delivery depots that can stabilize and provide well-controlled and sustained local delivery of FGF-2 may produce a more potent therapeutic effect on ischemic disease [11].
Controlled release of therapeutic proteins has been attempted using a variety of delivery approaches including hydrogels [12, 13], nanoparticles [14], and microspheres [15, 16]. Microsphere delivery is non-invasive and enables the administration of larger effective dosages of a variety of encapsulated drugs and is a powerful approach to sustained drug release. Most microsphere delivery systems primarily consist of ester-containing polymers that release their cargo at a rate dictated by hydrolytic degradation, rather than based on logical environmental stimuli related to the disease. These systems are often characterized by a rapid burst release of the drug, which can potentially cause undesirable systemic effects and lead to rapid degradation and/or distribution throughout the body [17]. Furthermore, commonly-utilized poly(lactic-co-glycolic acid) (PLGA) generates degradation products that can acidify the local environment, which can destroy protein growth factor activity and exacerbate local inflammation [18]. This can also activate auto-catalytic degradation mechanisms that make kinetics of drug release difficult to control [18, 19]. Therefore, a new platform to deliver drugs or proteins to ischemic sites in an optimized, sustained manner would potentially be of high impact.
Utilization of “smart” materials that undergo a phase transition in response to small changes in environmental stimuli that serve as surrogate markers of presence/recovery of local ischemia presents one potentially viable route for controlling release to sites of PAD. Micro- and nanoparticles have been reported that are responsive to factors such as pH [20–23], temperature [24–26], reactive oxygen species [27, 28], and magnetic fields [29]. Temperature is one of the most commonly leveraged stimuli for systems that “gel” once injected from room temperature into in vivo conditions, but polymers that form irreversible gels risk leading to chronic inflammation or foreign body reactions. Formulation of delivery carriers that are sensitive to dual stimuli may present an opportunity for more finely-tuned properties that enable both controlled release and ultimately, dissociation and clearance of the matrix that forms the drug depot [30, 31].
Mild tissue acidosis is a defining characteristic at sites of ischemia, resulting from anaerobic cellular metabolism [32–35]. In this study, we have fabricated and tested a dual temperature- and pH-responsive microsphere delivery system for sustained protein delivery to ischemic environments. This formulation leverages pH as an indicator of presence/recovery of ischemia and results in microspheres that break down and enable clearance once ischemia is resolved. These microspheres are fabricated from a random copolymer consisting of temperature-responsive N-isopropyl acrylamide (NIPAAm), pH-responsive propyl acrylic acid (PAA), and hydrophobic butyl acrylate (BA), which can be utilized to tune the polymer lower critical solution temperature (LCST). Polymers with similar composition have been previously pursued as injectable hydrogels that form in situ [36, 37]. However, this approach requires that a polymer and protein solution must rapidly undergo a phase change and form mechanically robust hydrogels, which requires very high polymer concentrations and makes retention at the ischemic site a challenge. Hence, we sought to fabricate microspheres that are compatible with percutaneous tissue injection but that do not require an instantaneous phase change at the time of tissue injection. These dual-responsive microspheres have been optimized to retain their solid form upon injection into slightly acidic ischemic sites and to undergo gradual dissolution as they release their payload and the ischemic site returns to physiological pH. This report describes the synthesis and characterization of microspheres from a small library of NIPAAm, PAA, and BA random copolymers, encapsulation of proteins using a water–in–oil–in–water (W1/O/W2) double emulsion method, demonstration of microsphere delivery of bioactive FGF-2 in vitro, and proof-of-concept analysis of controlled release and biocompatibility at sites of PAD in vivo.
2.0 Materials and Methods
2.1 Materials
All chemicals were purchased from Sigma-Aldrich (St. Louis, MO) and used as received unless otherwise described. N-isopropylacrylamide (NIPAAm) was recrystallized in hexane. Butyl acrylate (BA) was purified by vacuum distillation. 2,2’-Azobisisobutyronitrile (AIBN) was recrystallized in methanol. Propyl acrylic acid was synthesized in-house and purified by distillation as described previously [38].
2.2 Copolymer Synthesis
Random copolymers comprised of the monomers NIPAAm, PAA, and BA were synthesized using RAFT polymerization. The NIPAAm:PAA:BA molar feed ratio was varied as follows: P1(90:10:0), P2(87.5:10:2.5), P3(85:10:5), P4(77.5:10:12.5), P5(75:10:15), P6(72.5:10:17.5) in order to optimize the pH and temperature dependent LCST behavior. 2-dodecylsulfanylthiocarbonylsulfanyl-2-methyl propionic acid (DMP) was used as the RAFT chain transfer agent (CTA), and 2,2’-Azobis(2-methylpropionitrile) (AIBN) was used as the free radical initiator. The CTA to initiator molar ratio was 10:1, the degree of polymerization was 200, and the overall monomer to solvent weight ratio was 1:1 in dimethylformamide (DMF). The solution was purged with high purity nitrogen (A-L Compressed Gases, Nashville, TN), and the reaction was carried out at 60 °C for 24h, yielding a light-yellow viscous product. The polymer was precipitated twice into cold diethyl ether from DMF. The polymers were then dissolved in cold DPBS (Gibco) adjusted to a pH of 8.0 and dialyzed in dH2O (Mw cut off 6000–8000, Fisher). After dialysis, the polymer was lyophilized yielding the final product.
2.3 Copolymer Characterization
Composition of the copolymers was characterized by proton nuclear magnetic resonance spectroscopy (1H NMR, Bruker 400 MHz Spectrometer equipped with a 9.4 T Oxford magnet) in CDCL3 and d6-DMSO. Composition was calculated by comparing the NIPAAm amide signal at 7.2 ppm, the total peak area between 3.7 and 4.1 ppm (corresponding to the sum of the NIPAAM isopryl C-H and the BA ester OCH2 proton peaks), and the total area between 0.7 and 2.2 ppm encompassing the remaining CH protons (Supplementary information).
The polymer molecular weight was analyzed by gel permeation chromatography (GPC, Shimadzu Corp., Kyoto, Japan) using DMF + 0.1 M LiBR mobile phase. An inline Wyatt miniDAWN TREOS light scattering detector (Wyatt Technology Corp., Santa Barbara, CA) and a Shimadzu RID-10A refractive index detector were used to calculate absolute molecular weight and polydispersity based on dη/dc values experimentally determined by offline injections into the RI detector.
Lower critical solution temperature (LCST) was determined by measuring absorbance at 500 nm of copolymer solutions as a function of temperature using a Varian UV-VIS spectrophotometer equipped with a Peltier single cell temperature controller. The temperature was ramped from 2 °C to 101 °C at a heating rate of 2 °C every 5 min. The LCST behavior of each polymer was investigated at different pH values in the ischemic to physiologic tissue range (pH 5.5, 6.2, 6.5, 6.8, 7.0, and 7.4). Each polymer’s LCST was defined as the temperature where the absorbance reached 10% of the maximum saturation value [39].
2.4 Microsphere Preparation
Microspheres were fabricated from P4, P5, and P6 polymers (see Table 1 and Table 2) using the water-in-oil-in-water (W1/O/W2) emulsion method [40]. FITC-labeled BSA (10 mg) was dissolved in 0.2 mL dH2O at pH 5.0 (W1 phase). 125 mg of P4, P5, or P6 was dissolved in 1.5 mL dichloromethane (DCM, O Phase). The W1 phase was added to the O phase and emulsified for 30 s using an Ultra-Turrax TP 18–10 (Janke and Kunkel KG, IKA-WERK) at 20,000 rpm. This primary emulsion was then added to 30 mL of 1% w/v Polyvinyl alcohol (1%PVA, W2 phase) and emulsified again for 30 s. The organic solvent was allowed to evaporate on a rotary shaker for 3 h. The resulting microspheres were washed twice with deionized water at pH 5 and lyophilized for 24 h.
Table 1.
Series of random copolymers with increasing BA content
Characterization of P1-P6 series of random NIPAAm-co-PAA-co-BA polymers
| Polymer code |
NIPAAm:PAA:BA % feed ratio |
NIPAAm:PAA:BA % ratio obtained by NMR |
Target Mn |
Obtained Mn |
PDI | Polymer yield % |
|---|---|---|---|---|---|---|
| P1 | 90.0: 10.0: 0.00 | 82.4: 17.6: 0.00 | 22650 | 25230 | 1.07 | 55 |
| P2 | 87.5: 10.0: 2.50 | 74.1: 18.9: 7.00 | 22720 | 20930 | 1.09 | 37 |
| P3 | 85.0: 10.0: 5.00 | 72.5: 19.9: 7.60 | 22800 | 19310 | 1.08 | 21 |
| P4 | 77.5: 10.0: 12.5 | 66.7: 16.7: 16.6 | 23020 | 24030 | 1.05 | 18 |
| P5 | 75.0: 10.0: 15.0 | 59.0: 16.9: 24.0 | 23100 | 19870 | 1.06 | 31 |
| P6 | 72.5: 10.0: 17.5 | 57.2: 17.8: 25.0 | 23170 | 25670 | 1.09 | 44 |
Table 2.
pH dependent LCST of P1–P6
| LCST (°C) | |||
|---|---|---|---|
| Polymer | pH 6.5 | pH 7.0 | pH 7.5 |
| P1 | 51 | 63 | - |
| P2 | 59 | 65 | 85 |
| P3 | 59 | 71 | 101 |
| P4 | 28 | 74 | 84 |
| P5 | <4 | 47.5 | 76 |
| P6 | <4 | 32 | 64 |
2.5 Microsphere Characterization
Microspheres were characterized for size and morphology by scanning electron microscopy (SEM, Hitachi S4200, Tokyo, Japan) and fluorescence microscopy (Nikon Instruments Inc., Melville, NY). For SEM, the microspheres were suspended in a water drop and dried onto double sided carbon tape that was then attached to an aluminum SEM stub (Ted Pella Inc. Redding, CA). After drying, the samples were sputter coated with gold prior to imaging. For fluorescent microscopy, microspheres were suspended in water, placed on a glass clover slip, and imaged using the encapsulated FITC-labeled BSA. The microsphere size was quantified using ImageJ version 1.45s (Freeware, NIH, Bethesda, MD) by measuring 150 microspheres. Loading capacity and encapsulation efficiency were determined by dissolving the FITC-BSA-loaded microspheres in DCM and quantification of FITC-BSA concentration using a fluorescence standard curve of FITC-BSA in DCM. Loading capacity was calculated based on the weight of the encapsulated FITC-BSA per total weight of the microspheres. Encapsulation efficiency was calculated based on the weight of the encapsulated FITC-BSA per weight of the total FITC-BSA added during the emulsion fabrication procedure.
2.6 pH-dependent Release of FITC-BSA Model Protein from Microspheres
In vitro protein release was quantified by measuring the fluorescence of the FITC-BSA released from the microspheres. Microspheres suspensions of 1 mg solids per mL of PBS of pH 5.5, 6.2, 6.5, and 7.4 were placed on a shaker in an incubator at 37 °C. At periodic time points, the microspheres were centrifuged at 16100 × g for 3 min, and 100 µL of the supernatant was removed. The samples were then replaced with 100 µL of fresh PBS of respective pH and returned to the shaker. The removed supernatant was used to quantify the amount of FITC-BSA released based on FITC-BSA fluorescence standard curve generated in a buffer with pH matching the release condition being tested. Fluorescent measurements were taken using 485 nm excitation and 535 nm emission wavelengths on a plate reader (Infinite F500, Tecan Group Ltd., Mannedorf, Switzerland). All samples were stored in the dark, and the cumulative percentage release of FITC-BSA was quantified over a period of three weeks. An additional release experiment was completed for P6 microspheres in order to mimic the change from ischemic to physiologic pH that would occur during ischemic tissue revascularization. To do so, 1mg/mL microspheres were suspended in pH 6.2 PBS for 11 d, changed to pH 6.8 for 11 d, and finally placed in pH 7.4 until 100% release was reached.
2.7 Cell Culture and Bioactivity of FGF-loaded Microspheres
Mouse Embryonic Fibroblasts (NIH3T3) were cultured in Dulbecco’s Modified Eagle’s Medium (DMEM, Gibco Cell Culture, Carlsbad, CA) supplemented with 10% Bovine Calf Serum (BCS, Gibco) and 1% penicillin-streptomycin (Gibco). NIH3T3 cells were modified to constitutively express firefly luciferase and red fluorescent protein (RFP) (Supplemental Information). The resulting luciferase reporter 3T3s (LR3T3s) were used for the longitudinal and noninvasive readout of cell growth in culture through bioluminescent imaging. Microspheres were prepared as described above using 75 mg of P6 to encapsulate 500 µg of fibroblast growth factor 2 (FGF-2, Creative BioMart, Shirley, NY). To verify that bioactivity of the protein was not destroyed during microsphere encapsulation, LR3T3s were seeded at a density of 12,500 cells/cm2 in 96 well plates and left to adhere overnight (37°C, 5% CO2). For these experiments, media was reduced to 1% BCS in order to slow cell growth and to enable discrimination of the proliferative effect of FGF-2 from that of the serum. Cells were treated with free FGF-2 at concentrations ranging from 0.25 – 50 ng/mL, control microspheres loaded with albumin (0.25 – 9 ng/mL, Sigma), or microspheres loaded with FGF-2 (0.25 – 9 ng/mL). Note that these protein concentrations were determined based on encapsulation efficiency measurements and represent the total protein concentration and are reported as if the total dose were released. Cell number was longitudinally assessed out to 72 h through bioluminescence quantification on an IVIS (Caliper, Hopkinton, MA).
2.8 Femoral Artery Ligation
The animal studies were conducted in adherence to the guidelines for the Care and Use of Laboratory Animals of the National Institutes of Health (NIH). All protocols involving animals were approved by the Animal Care and Use Committee of Vanderbilt University. 8–10 week male A/J mice were purchased from Jackson Laboratories (Bar Harbor, ME). The animals were fed a standard chow diet ad libitum and had free access to water. Established methods were used to induce hind limb ischemia by femoral artery ligation and transection in n = 6 mice [41–43]. Mice were anesthetized with 1.5–2.5% isoflurane and maintained at 37 °C. Mice were treated with pre-operative analgesia (ketoprofen, 10 mg/kg), and the surgical site was shaved and cleaned with iodine followed by chlorhexidine. A 5 mm unilateral incision was made over the right medial thigh of the mouse. The femoral artery and vein were dissected away from the femoral nerve and ligated with 6-0 silk sutures immediately distal to the origins of the superficial epigastic artery and deep branch of the femoral artery. A second ligation was placed proximal to the origin of the nearest distal branch. The artery and vein were transected and excised between the two ligation sites leaving a 2–3 mm gap. The wound was irrigated with sterile saline and the incision was closed with interrupted 5-0 nylon sutures. The left limb served as a contralateral control. Post-treatment analgesia (ketoprofen, 5–10 mg/kg) was administered every 18–24 h for 72 h or until animals exhibited normal appearance and behavior.
2.9 In vivo Release Kinetics
Albumin (1 mg) was fluorescently labeled with Cy5.5 bis-reactive dye (GE Healthcare, Waukesha, WI, USA) following the manufacturer’s protocol. The product was then purified using a PD-10 column (GE Healthcare), yielding 662 µg of Cy5.5-albumin with 1.6 Cy5.5 molecules per protein. The protein was lyophilized and encapsulated into 75 mg of microspheres made with P6 using the method described above. At 4 h after induction of hind limb ischemia, mice were administered 5 × 20 µL bilateral injections into the gastrocnemius (both ischemic and contralateral control) containing protein microspheres or free protein in sterile DPBS (30 µg/mL protein, 3.75 mg/mL polymer). Each mouse was imaged immediately after the injection and then longitudinally at 1, 4, 7, 9, 11, and 14 d using non-invasive fluorescent imaging with a MaestroTM (Caliper), with all imaging settings kept constant throughout (2 s exposure). At the end of 14 d, mice were anesthetized with isoflurane and sacrificed by cervical dislocation. Each gastrocnemius was removed, fixed with 10% formalin for 24 h, and embedded in paraffin. Histological sections (4 µm thick) were cut and then stained with hematoxylin and eosin (H&E) in order to examine host response to the microspheres.
3.0 Results and Discussion
3.1 Synthesis and Characterization of p(NIPAAM-co-PAA-co-BA) Copolymers
A small library of random copolymers comprised of NIPAAm, PAA, and BA was successfully synthesized using RAFT polymerization following the scheme in Fig. 1. This library was designed to create a series of polymers with gradually increasing hydrophobic content, which is well known to decrease the LCST of NIPAAm-based polymers [44]. The PAA monomer was incorporated at a constant 10 mol% in order to impart pH-responsiveness, as it is optimized to respond in a physiologic range due to its pKa of 6.7–6.8 [45]. P(NIPAAm-co-PAA-coBA) polymers have been assessed previously [36, 37], but pH-dependent LCST behavior has never been methodically optimized for drug delivery applications based on incorporating a constant amount of PAA (10 mol%) and studying the effect of titrating the BA percent composition.
Figure 1.
Synthesis of p(NIPAAm-co-PAA-co-BA) using RAFT polymerization
Results from analysis of copolymer composition, molecular weight, and polydispersity are shown in Table 1. The molar composition of each monomer in the polymers was confirmed by 1H NMR (Supplementary Figure S-1) and was proportional to the feed ratio. There were some consistent discrepancies between experimentally-determined compositions and the feed ratios as noted previously [36], which is likely due to the different reactivity of each monomer [46]. Based on GPC, the number average molecular weight (Mn) of the final copolymers was consistent with the targeted molecular weights. The polydispersity (PDI - Mw/Mn, Mw - weight average molecular weight) was less than 1.1 for all polymers, indicating that the polymerizations were well-controlled by the RAFT technique. This series of polymers provides a good point of comparison for previous copolymer studies because P4, a polymer with intermediate BA content, had a similar composition to a 67 mol% NIPAAm, 18 mol% PAA, and 15 mol% BA polymer previously tested for injectable hydrogel formation in rat myocardial infarcts [37].
This library was synthesized in order to identify a polymer candidate with optimal pH-dependent LCST behavior. We specifically sought polymer(s) with an LCST below 37°C at ischemic pH (5.9–7.2) and above 37°C at physiologic pH (7.4) in order to provide the optimal delivery and microsphere dissolution profile. It has been observed that p(NIPAAm-co-PAA – 13 mol%PAA) does not form a gel at pH 6 below 45 °C, likely due to insufficient hydrophobic aggregation [36]. In order to increase the pKa and facilitate stable microsphere formation at higher pH values, increased hydrophobic content was incorporated into the copolymers via addition of BA. LCST behavior is driven by polymer collapse and self-aggregation, and the LCST can be lowered by incorporation of BA due to its alkyl side group that enhances hydrophobic interactions. In order to initially screen the polymers, the LCST was measured by absorbance and presented in Table 2. As expected, the titration of increased hydrophobic content in the copolymer through incorporation of more of the BA monomer correlated with decreased LCST values. P6 exhibited the most desirable pH-dependent LCST behavior (Fig. 2) because its LCST at pH 7.0 was below physiologic temperature of 37°C and its LCST at pH 7.5 was higher than physiologic temperature of 37°C. All other polymers in the library had LCSTs above 37°C at pH 7.0. These data indicate that higher mol% BA than previously tested may be better optimized for delivery applications in order to ensure that polymer dissolution does not occur prematurely and result in full therapeutic protein release and clearance prior to full ischemic recovery.
Figure 2. LCST Determination.
LCST Determination for P6 by UV-VIS absorbance demonstrates ideal pH dependent LCST
3.2 Microsphere Morphological Characterization
The most promising candidate polymers, P4, P5, and P6, were utilized to fabricate microspheres encapsulating the model protein FITC-labeled BSA using the W1/O/W2 double emulsion method. Microspheres fabricated with P6 had a loading capacity of 19.5% and an encapsulation efficiency of 89.0%. The encapsulation of FITC-BSA within the microspheres was confirmed using fluorescent microscopy (Fig 3A), while blank microspheres prepared using the same procedure showed no green fluorescence (not shown). SEM showed successful fabrication of spherical particles using the W1/O/W2 double emulsion techinique (Fig. 3B). The diameters of P4-P6 were quantified using ImageJ 1.45s, and an average diameter of 1.28 ± 0.66 µm was found for P6 microspheres (Fig. 3C). The polydispersity in size of the microspheres is due to the use of conventional W1/O/W2 method, as it is known that the properties of microspheres formed by this method can be difficult to control [47]. However, more controlled particle fabrication techniques [48] will be considered for future applications beyond the current proof-of-concept studies. Porosity of microspheres could also be observed with SEM, and there appeared to potentially be an inverse correlation between mol% BA in the polymer and microsphere pore size (Supplemental Info S-2).
Figure 3. Optical and electron microscopy confirmation of microsphere size and morphology.
A) Microsphere fluorescence confirms model protein FITC-BSA encapsulation (Scale = 200 µm). B) SEM of microspheres shows the size distribution and morphology of microspheres (Scale = 10µm). C) The size distribution for P6 was quantified with a histogram representing the distribution of the diameter. i) Table of microspheres sizes.
3.3 In Vitro Release of FITC-BSA from Microspheres
The dependence of pH on the rate of release of FITC-BSA from P4, P5, and P6 microspheres was characterized in vitro in buffers that mimic ischemic (pH < 7.0) and physiologic (pH = 7.4) conditions. For all polymers tested, the rate of release was slower and more sustained in acidic (ischemia-mimicking) conditions, while the microspheres released their cargo more rapidly at normal physiological pH of 7.4 (Fig. 4A–C). The kinetics of protein release from the microspheres was also inversely proportional to the BA content of the polymers, and the rate of release was P4 > P5 > P6 for all pH values tested (Fig. 4A–C). Generally, there was a strong relationship between rate of release and polymer LCST for a given pH. For example, at pH 7.0, P4 and P5 had LCST > 37 °C, while P6 had an LCST of 32 °C, implying that P6 microspheres would remain in a stable, collapsed state at body temperature at this slightly sub-physiologic pH. Thus, it is predicted that the pH dependent LCST behavior of P6 microspheres would lead to release kinetics that are ideal for sustained release in ischemic tissues and that dissolution and clearance of the therapeutic and polymer would occur upon restoration to physiologic pH.
Figure 4. pH-dependent microsphere release of model protein FITC-BSA.
Cumulative, pH-dependent release of FITC-BSA from microspheres composed of (A) P4 (B) P5 and (C) P6. (D) FITC-BSA release from P6 microspheres in an environment modeling a recovering ischemic tissue. (E) Visual demonstration of the dissolution of P6 microspheres upon transition from pH 6.2 to 6.8 and 7.4.
To further characterize the mechanism of protein release from the microspheres, all data were fit with the Weibull model (Supplementary Info S-3), which can be used to determine whether release kinetics are diffusion-controlled [49]. This analysis indicated that protein release from P6 microspheres was diffusion-controlled, or dictated by the diffusivity of the protein through the polymer microsphere matrix, in the ischemic pH ranges (< 7.0). Conversely, the results of this analysis indicated that all other microspheres and experimental conditions did not produce diffusion-controlled release. The different mechanism of release for other formulations was likely caused by contribution of other factors such as loss of particle integrity due to conditions being at or below the polymer LCST.
In order to mimic physiologic ischemic recovery, release kinetics were measured from P6 microspheres as the pH was increased from 6.2, to 6.8, and 7.4 (Fig. 4D). The release rate (%release/day) under each pH condition was quantified by the slope and was found to be 0.71% in pH 6.2, 1.8% in pH 6.8, and 27.1% in pH 7.4. It was evident that the release rate was highly dependent on the environmental pH, and that microspheres rapidly dissolved at pH 7.4. In order to assess the morphologic change of the microspheres based on pH change from ischemic to physiologic conditions, samples of P6 microspheres were taken from solutions of pH 5.5, 6.5, and 7.4 and prepared for SEM. Representative SEM images shown in Fig. 4E demonstrate pH-dependent dissolution of the microspheres.
3.4 Bioactivity of FGF Encapsulated within P6 Microspheres
Fibroblasts transfected to constitutively express luciferase (LR-3T3s) were treated with free FGF-2, control microspheres containing albumin, or microspheres loaded with FGF-2. As anticipated, free FGF-2 triggered a dose-dependent increase in the rate of proliferation of the LR3T3s (Supplemental Information S-5). Control microspheres showed no significant increase in fibroblast proliferation over time, and a small but significant reduction (35% lower cell number at 72h relative to no treatment samples at 72h, p<0.05) in cell number was observed at the 72 h time point at the highest microparticle dose (Fig. 5A). The FGF-2 microspheres produced a dose dependent increase in cell number at 24, 48, and 72 h as shown in Fig. 5B. At the highest dose of 9 ng/mL FGF-2, the microspheres induced an 11 fold increase in fibroblast number relative to the NT control after 72 h of treatment, indicating that FGF-2 bioactivity was robustly maintained following encapsulation into microspheres. Compared with the effect of free FGF-2 on proliferation of LR-3T3s (Supplemental Information S-5), there was a delayed response to FGF-2 delivered via microspheres. This delay is logically explained by the time required for the FGF-2 to be released from the microspheres in order to become bioavailable. This explanation is further supported by the finding that the relative number of LR-3T3s at the 72h endpoint was similar for free versus P6 microsphere-based delivery of FGF-2.
Figure 5. Confirmation of bioactivity and cytocompatibility of P6 microsphere-based growth factor delivery in vitro.
(A) BSA-loaded P6 microspheres showed little toxicity to LR3T3s with a small but significant reduction at 72 h at the highest dose. (B) FGF-loaded P6 microspheres mediated a time- and dose-dependent increase in the proliferation of LR3T3s.
3.5 In Vivo Sustained Release and Histocompatibility of Microspheres
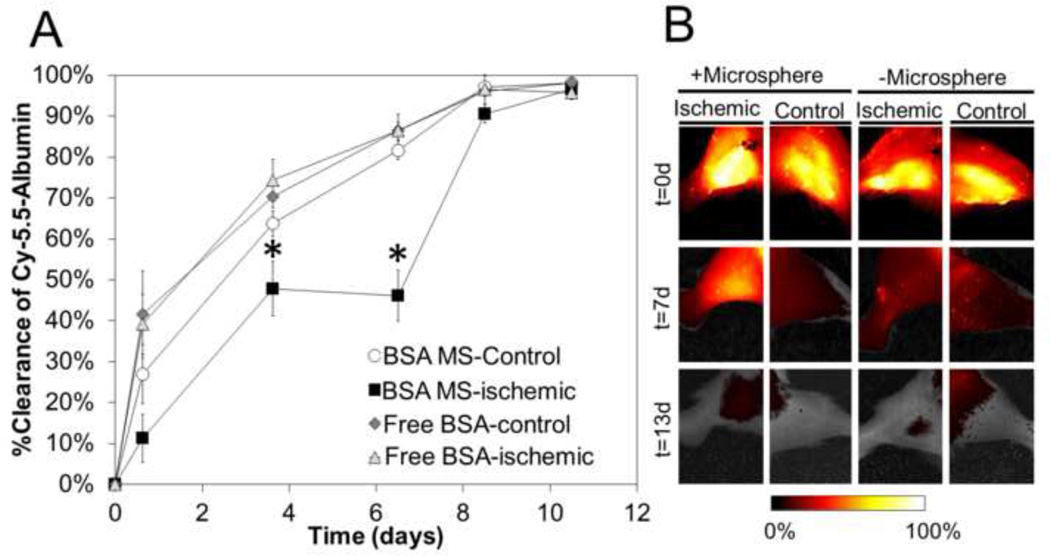
Cy5.5-labeled albumin was injected free in solution or encapsulated within P6 microspheres into the ischemic and contralateral control gastrocnemii of mice 4 h following induction of ischemia. As shown in Fig. 6, delivery via P6 microspheres resulted in more sustained presence of the model protein in the ischemic tissue of the surgery limb, with significantly more model protein retained locally at the injection site at days 3.5 and 7 relative to all other control conditions. On the other hand, free protein was rapidly cleared from both ischemic and control limbs, and protein delivered to control tissue via P6 microspheres was also rapidly cleared. These data indicate that reduced pH in the ischemic tissue resulted in slower P6 microsphere dissolution and more sustained delivery of protein cargo. However, by day 9, the clearance of the protein delivered via P6 microspheres matched the other controls.
Figure 6. P6 microspheres increase persistence of a model protein in ischemic tissues in mice.
(A) In vivo fluorescence imaging of Cy5.5-albumin indicated significantly higher (p < 0.05) persistence of protein in the ischemic gastrocnemius at day 3.5 and 7 following delivery via P6 microspheres. (B) Representative fluorescence intensity images of each treatment visually confirmed that P6 microsphere protein delivery to the ischemic limb prolonged local protein retention at day 7 and that the model protein was cleared in all groups by day 13.
The rapid clearance that was evident from the P6 microspheres in the ischemic limb between days 7–9 likely correlated to restoration of physiological pH in the ischemic muscle tissue. In our own unpublished observations gathered using hyperspectral imaging, we have found that the A/J mouse strain recovers the hemoglobin oxygen saturation in the paw of the ischemic limb to 80% of that seen in the contralateral control by day 7. Others have reported similar results suggesting that mice can recover from hind limb ischemia in as little as 7 days. For example, blood flow in a ligated paw had recovered to 70% by 7 days in C57BL/6 mice [43] and deep tissue diffuse correlation spectroscopy measurements on SV-129/C57-B16 mice indicated nearly 70% recovery by day 7 [50]. Similarly in a spinotrapezious ischemic model in balb/c mice, by day 7, there was a complete disappearance of ischemic regions and perfusion was completely restored [51]. This suggests that restoration of normal physiologic conditions, including pH, likely triggered microsphere dissolution and clearance of the residual protein between days 7 and 9. For example, in vitro experimental conditions mimicking tissue recovery by adjusting the pH from 6.2 for 11 d, to 6.8 for 11 d, to 7.4 showed rapid release and microsphere dissolution upon exposure to pH 7.4 (Fig. 4D), which is in agreement with our in vivo data in Fig. 6.
H&E staining of histological sections from the gastrocnemius demonstrated that no morphological changes were microscopically apparent in tissues treated with P6 microspheres. In healthy tissues, a homogenous population of striated muscle cells was present, and the tissues treated with P6 microspheres showed no indication of increased inflammation or fibrosis (Fig. 7). Histological analysis of the ischemic limb revealed the presence of mononuclear cells indicative of inflammatory response and signs of fibrosis and adipose cells present within the striated muscle, which are consistent with ischemic muscle damage. However, this tissue response was not apparently exacerbated in any way by the local injection of P6 microspheres (Fig. 7). A more thorough sampling of histology is including in the Supplementary Information S6 and S7. Previous studies using similar polymers in an injectable hydrogel approach for protein delivery to the ischemic heart noted macrophage infiltration and a few foreign body giant cells at 28 days near the polymer injection site [37]. However, in this study, in order to achieve in situ hydrogel formation, a greater than 20 fold higher overall polymer concentration was used compared to the current in vivo experiments. This injectable hydrogel approach was also applied in a different tissue site and animal species, but this higher polymer concentration may account for the increased host response observed. Using the microsphere approach, the polymer dose may be decreased further by increasing the amount of protein loaded into each microsphere until the loading capacity is reached (an additional 20 fold decrease in polymer concentration is available).
Figure 7. P6 microspheres do not elicit a negative host response in vivo.
At day 14, H&E of the mouse gastrocnemius reveals no overt, deleterious host response to the microspheres in the healthy or ischemic tissue. There is a clear difference between the control and ischemic mouse limbs, with the latter showing significant ischemic tissue damage.
4.0 Conclusion
Growth factor therapies have had limited impact in clinical practice due to poor bioavailability, rapid clearance, and short half-lives due to degradation. Thus, persistent growth factor activity localized to pathological sites is a major limitation to protein therapy for regenerative medicine. The p(NIPAAm-co-PAA-co-BA) microsphere platform presented here enables sustained, pH-dependent release of a bioactive growth factor in vitro and can increase the persistence following injectable delivery to an ischemic site in vivo. Furthermore, these microspheres “intelligently” respond to signs of tissue ischemic recovery and are rapidly cleared upon restoration of physiologic pH. This novel, injectable delivery system has tremendous potential for sustained local activation of reparative processes stimulated by FGF-2 or other therapeutic proteins within ischemic tissues damaged by PAD, trauma, or other disease processes.
Supplementary Material
Acknowledgments
This research was supported by NIH 1 R21 HL110056 and Vanderbilt University School of Engineering. 1H NMR was conducted at the Small Molecule NMR Facility Core. UV-VIS and SEM were conducted through the use of the core facilities of the Vanderbilt Institute of Nanoscale Science and Engineering (VINSE). In vivo imaging was conducted through the Vanderbilt University Institute for Imaging Science (VUIIS). The Vanderbilt Transitional Pathology Shared Resource assisted in the preparation of histological specimens.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hirsch AT, Criqui MH, Treat-Jacobson D, Regensteiner JG, Creager MA, Olin JW, Krook SH, Hunninghake DB, Comerota AJ, Walsh ME, McDermott MM, Hiatt WR. Peripheral arterial disease detection, awareness, and treatment in primary care. JAMA. 2001;286:1317–1324. doi: 10.1001/jama.286.11.1317. [DOI] [PubMed] [Google Scholar]
- 2.Ostchega Y, Paulose-Ram R, Dillon CF, Gu Q, Hughes JP. Prevalence of peripheral arterial disease and risk factors in persons aged 60 and older: data from the National Health and Nutrition Examination Survey 1999-2004. J Am Geriatr Soc. 2007;55:583–589. doi: 10.1111/j.1532-5415.2007.01123.x. [DOI] [PubMed] [Google Scholar]
- 3.Olin JW, Sealove BA. Peripheral Artery Disease: Current Insight Into the Disease and Its Diagnosis and Management. Mayo Clin Proc. 2010;85:678–692. doi: 10.4065/mcp.2010.0133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McDermott MM, Greenland P, Liu K, Guralnik JM, Celic L, Criqui MH, Chan C, Martin GJ, Schneider J, Pearce WH, Taylor LM, Clark E. The ankle brachial index is associated with leg function and physical activity: the Walking and Leg Circulation Study. Ann Intern Med. 2002;136:873–883. doi: 10.7326/0003-4819-136-12-200206180-00008. [DOI] [PubMed] [Google Scholar]
- 5.McDermott MM, Liu K, Greenland P, Guralnik JM, Criqui MH, Chan C, Pearce WH, Schneider JR, Ferrucci L, Celic L, Taylor LM, Vonesh E, Martin GJ, Clark E. Functional decline in peripheral arterial disease: associations with the ankle brachial index and leg symptoms. JAMA. 2004;292:453–461. doi: 10.1001/jama.292.4.453. [DOI] [PubMed] [Google Scholar]
- 6.Kannel WB, McGee DL. Update on some epidemiologic features of intermittent claudication: the Framingham Study. J Am Geriatr Soc. 1985;33:13–18. doi: 10.1111/j.1532-5415.1985.tb02853.x. [DOI] [PubMed] [Google Scholar]
- 7.Bhatt DL, Steg PG, Ohman EM, Hirsch AT, Ikeda Y, Mas JL, Goto S, Liau CS, Richard AJ, Rother J, Wilson PW. International prevalence, recognition, and treatment of cardiovascular risk factors in outpatients with atherothrombosis. JAMA. 2006;295:180–189. doi: 10.1001/jama.295.2.180. [DOI] [PubMed] [Google Scholar]
- 8.Jones WS, Annex BH. Growth factors for therapeutic angiogenesis in peripheral arterial disease. Curr Opin Cardiol. 2007;22:458–463. doi: 10.1097/HCO.0b013e328236741b. [DOI] [PubMed] [Google Scholar]
- 9.van Royen N, Piek JJ, Buschmann I, Hoefer I, Voskuil M, Schaper W. Stimulation of arteriogenesis; a new concept for the treatment of arterial occlusive disease. Cardiovasc Res. 2001;49:543–553. doi: 10.1016/s0008-6363(00)00206-6. [DOI] [PubMed] [Google Scholar]
- 10.Beenken A, Mohammadi M. The FGF family: biology, pathophysiology and therapy. Nat Rev Drug Discov. 2009;8:235–253. doi: 10.1038/nrd2792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee K, Silva EA, Mooney DJ. Growth factor delivery-based tissue engineering: general approaches and a review of recent developments. J R Soc Interface. 2011;8:153–170. doi: 10.1098/rsif.2010.0223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huynh DP, Nguyen MK, Pi BS, Kim MS, Chae SY, Lee KC, Kim BS, Kim SW, Lee DS. Functionalized injectable hydrogels for controlled insulin delivery. Biomaterials. 2008;29:2527–2534. doi: 10.1016/j.biomaterials.2008.02.016. [DOI] [PubMed] [Google Scholar]
- 13.Phelps EA, Landazuri N, Thule PM, Taylor WR, Garcia AJ. Bioartificial matrices for therapeutic vascularization. P Natl Acad Sci USA. 2010;107:3323–3328. doi: 10.1073/pnas.0905447107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Golub JS, Kim YT, Duvall CL, Bellamkonda RV, Gupta D, Lin AS, Weiss D, Taylor WR, Guldberg RE. Sustained VEGF delivery via PLGA nanoparticles promotes vascular growth. Am J Physiol-Heart C. 2010;298:H1959–H1965. doi: 10.1152/ajpheart.00199.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fundueanu G, Constantin M, Ascenzi P, Simionescu BC. An intelligent multicompartmental system based on thermo-sensitive starch microspheres for temperature-controlled release of drugs. Biomedical Microdevices. 2010;12:693–704. doi: 10.1007/s10544-010-9422-5. [DOI] [PubMed] [Google Scholar]
- 16.Zou GK, Song YL, Zhou W, Yu M, Liang LH, Sun DC, Li DH, Deng ZX, Zhu WZ. Effects of local delivery of bFGF from PLGA microspheres on osseointegration around implants in diabetic rats. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2012 doi: 10.1016/j.tripleo.2011.07.006. [DOI] [PubMed] [Google Scholar]
- 17.Leo E, Ruozi B, Tosi G, Vandelli MA. PLA-microparticles formulated by means a thermoreversible gel able to modify protein encapsulation and release without being co-encapsulated. Int J Pharm. 2006;323:131–138. doi: 10.1016/j.ijpharm.2006.05.047. [DOI] [PubMed] [Google Scholar]
- 18.Zhu GZ, Mallery SR, Schwendeman SP. Stabilization of proteins encapsulated in injectable poly (lactide-coglycolide) Nature Biotechnology. 2000;18:52–57. doi: 10.1038/71916. [DOI] [PubMed] [Google Scholar]
- 19.Vert M, Li S, Garreau H. More About the Degradation of La/Ga-Derived Matrices in Aqueous-Media. Journal of Controlled Release. 1991;16:15–26. [Google Scholar]
- 20.Neyret S, Vincent B. The properties of polyampholyte microgel particles prepared by microemulsion polymerization. Polymer. 1997;38:6129–6134. [Google Scholar]
- 21.Sawai T, Yamazaki S, Ikariyama Y, Aizawa M. Ph-Responsive Swelling of the Ultrafine Microsphere. Macromolecules. 1991;24:2117–2118. [Google Scholar]
- 22.Rodriguez BE, Wolfe MS, Fryd M. Nonuniform Swelling of Alkali Swellable Microgels. Macromolecules. 1994;27:6642–6647. [Google Scholar]
- 23.Saunders BR, Crowther HM, Vincent B. Poly[(methyl methacrylate)-co-(methacrylic acid)] microgel particles: Swelling control using pH, cononsolvency, and osmotic deswelling. Macromolecules. 1997;30:482–487. [Google Scholar]
- 24.Park TG, Hoffman AS. Estimation of Temperature-Dependent Pore-Size in Poly(N-Isopropylacrylamide) Hydrogel Beads. Biotechnol Progr. 1994;10:82–86. doi: 10.1021/bp00025a010. [DOI] [PubMed] [Google Scholar]
- 25.Chu LY, Park SH, Yamaguchi T, Nakao S. Preparation of micron-sized monodispersed thermoresponsive coreshell microcapsules. Langmuir. 2002;18:1856–1864. [Google Scholar]
- 26.Chu LY, Park SH, Yamaguchi T, Nakao S. Preparation of thermo-responsive core-shell microcapsules with a porous membrane and poly(N-isopropylacrylamide) gates. J Membrane Sci. 2001;192:27–39. [Google Scholar]
- 27.Gupta MK, Meyer TA, Nelson CE, Duvall CL. Poly(PS-b-DMA) micelles for reactive oxygen species triggered drug release. J Control Release. 2012;162:591–598. doi: 10.1016/j.jconrel.2012.07.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mahmoud EA, Sankaranarayanan J, Morachis JM, Kim G, Almutairi A. Inflammation responsive logic gate nanoparticles for the delivery of proteins. Bioconjug Chem. 2011;22:1416–1421. doi: 10.1021/bc200141h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ugelstad J, Berge A, Ellingsen T, Aune O, Kilaas L, Nilsen TN, Schmid R, Stenstad P, Funderud S, Kvalheim G, Nustad K, Lea T, Vartdal F, Danielsen H. Monosized Magnetic Particles and Their Use in Selective Cell-Separation. Makromol Chem-M Symp. 1988;17:177–211. [Google Scholar]
- 30.Deng Y, Wang C, Shen X, Yang W, Jin L, Gao H, Fu S. Preparation, characterization, and application of multistimuli-responsive microspheres with fluorescence-labeled magnetic cores and thermoresponsive shells. Chemistry. 2005;11:6006–6013. doi: 10.1002/chem.200500605. [DOI] [PubMed] [Google Scholar]
- 31.Bartlett RL, Panitch A. Thermosensitive Nanoparticles with pH-Triggered Degradation and Release of Antiinflammatory Cell-Penetrating Peptides. Biomacromolecules. 2012;13:2578–2584. doi: 10.1021/bm300826v. [DOI] [PubMed] [Google Scholar]
- 32.Helisch A, Wagner S, Khan N, Drinane M, Wolfram S, Heil M, Ziegelhoeffer T, Brandt U, Pearlman JD, Swartz HM, Schaper W. Impact of mouse strain differences in innate hindlimb collateral vasculature. Arterioscl Throm Vas. 2006;26:520–526. doi: 10.1161/01.ATV.0000202677.55012.a0. [DOI] [PubMed] [Google Scholar]
- 33.Hagberg H. Intracellular Ph during Ischemia in Skeletal-Muscle - Relationship to Membrane-Potential, Extracellular Ph, Tissue Lactic-Acid and Atp. Pflug Arch Eur J Phy. 1985;404:342–347. doi: 10.1007/BF00585346. [DOI] [PubMed] [Google Scholar]
- 34.Marcinek DJ, Kushmerick MJ, Conley KE. Lactic acidosis in vivo: testing the link between lactate generation and H+ accumulation in ischemic mouse muscle. Journal of Applied Physiology. 2010;108:1479–1486. doi: 10.1152/japplphysiol.01189.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Filler RM, Das JB, Haase GM, Donahoe PK. Muscle Surface Ph as a Monitor of Tissue Perfusion and Acid-Base Status. J Pediatr Surg. 1971;6:535. doi: 10.1016/0022-3468(71)90375-7. &. [DOI] [PubMed] [Google Scholar]
- 36.Garbern JC, Hoffman AS, Stayton PS. Injectable pH- and temperature-responsive poly(N-isopropylacrylamide-co-propylacrylic acid) copolymers for delivery of angiogenic growth factors. Biomacromolecules. 2010;11:1833–1839. doi: 10.1021/bm100318z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Garbern JC, Minami E, Stayton PS, Murry CE. Delivery of basic fibroblast growth factor with a pH-responsive, injectable hydrogel to improve angiogenesis in infarcted myocardium. Biomaterials. 2011;32:2407–2416. doi: 10.1016/j.biomaterials.2010.11.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ferrito M, Tirrell DA. Poly(2-ethylacrylic acid) Macromol. Synth. 1992;11:59–62. [Google Scholar]
- 39.Fundueanu G, Constantin M, Stanciu C, Theodoridis G, Ascenzi P. pH- and temperature-sensitive polymeric microspheres for drug delivery: the dissolution of copolymers modulates drug release. J Mater Sci-Mater M. 2009;20:2465–2475. doi: 10.1007/s10856-009-3807-0. [DOI] [PubMed] [Google Scholar]
- 40.Yang HJ, Park IS, Na K. Biocompatible microspheres based on acetylated polysaccharide prepared from water-in-oil-in-water (W-1/O/W-2) double-emulsion method for delivery of type II diabetic drug (exenatide) Colloid Surface A. 2009;340:115–120. [Google Scholar]
- 41.Couffinhal T, Silver M, Zheng LP, Kearney M, Witzenbichler B, Isner JM. Mouse model of angiogenesis. Am J Pathol. 1998;152:1667–1679. [PMC free article] [PubMed] [Google Scholar]
- 42.Duvall CL, Taylor WR, Weiss D, Guldberg RE. Quantitative microcomputed tomography analysis of collateral vessel development after ischemic injury. Am J Physiol-Heart C. 2004;287:H302–H310. doi: 10.1152/ajpheart.00928.2003. [DOI] [PubMed] [Google Scholar]
- 43.Duvall CL, Weiss D, Robinson ST, Alameddine FMF, Guldberg RE, Taylor WR. The role of osteopontin in recovery from hind limb ischemia. Arterioscl Throm Vas. 2008;28:290–295. doi: 10.1161/ATVBAHA.107.158485. [DOI] [PubMed] [Google Scholar]
- 44.Taylor LD, Cerankowski LD. Preparation of Films Exhibiting a Balanced Temperature-Dependence to Permeation by Aqueous-Solutions - Study of Lower Consolute Behavior. J Polym Sci Pol Chem. 1975;13:2551–2570. [Google Scholar]
- 45.Murthy N, Robichaud JR, Tirrell DA, Stayton PS, Hoffman AS. The design and synthesis of polymers for eukaryotic membrane disruption. J Control Release. 1999;61:137–143. doi: 10.1016/s0168-3659(99)00114-5. [DOI] [PubMed] [Google Scholar]
- 46.Feldermann A, Toy AA, Phan H, Stenzel MH, Davis TP, Barner-Kowollik C. Reversible addition fragmentation chain transfer copolymerization: influence of the RAFT process on the copolymer composition. Polymer. 2004;45:3997–4007. [Google Scholar]
- 47.Ye ML, Kim S, Park K. Issues in long-term protein delivery using biodegradable microparticles. Journal of Controlled Release. 2010;146:241–260. doi: 10.1016/j.jconrel.2010.05.011. [DOI] [PubMed] [Google Scholar]
- 48.de Gracia Lux C, Joshi-Barr S, Nguyen T, Mahmoud E, Schopf E, Fomina N, Almutairi A. Biocompatible polymeric nanoparticles degrade and release cargo in response to biologically relevant levels of hydrogen peroxide. J Am Chem Soc. 2012;134:15758–15764. doi: 10.1021/ja303372u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Papadopoulou V, Kosmidis K, Vlachou M, Macheras P. On the use of the Weibull function for the discernment of drug release mechanisms. International Journal of Pharmaceutics. 2006;309:44–50. doi: 10.1016/j.ijpharm.2005.10.044. [DOI] [PubMed] [Google Scholar]
- 50.Mesquita RC, Skuli N, Kim MN, Liang JM, Schenkel S, Majmundar AJ, Simon MC, Yodh AG. Hemodynamic and metabolic diffuse optical monitoring in a mouse model of hindlimb ischemia. Biomed Opt Express. 2010;1:1173–1187. doi: 10.1364/BOE.1.001173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mac Gabhann F, Peirce SM. Collateral Capillary Arterialization following Arteriolar Ligation in Murine Skeletal Muscle. Microcirculation. 2010;17:333–347. doi: 10.1111/j.1549-8719.2010.00034.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.