Abstract
The healthy human prostate accumulates the highest level of zinc of any soft tissue in the body. This unique property is retained in BPH, but is lost in prostatic malignancy, which implicates changes in zinc and its transporters in carcinogenesis. Indeed, zinc concentrations diminish early in the course of prostate carcinogenesis, preceding histopathological changes, and continue to decline during progression toward castration-resistant disease. Numerous studies suggest that increased zinc intake might protect against progression of prostatic malignancy. Despite increased dietary intake, zinc accumulation might be limited by the diminished expression of zinc uptake transporters, resulting in decreased intratumoural zinc levels. This finding can explain the conflicting results of various epidemiological studies evaluating the role of zinc supplementation on primary and secondary prostate cancer prevention. Overall, more research into the mechanisms of zinc homeostasis are needed to fully understand its impact on prostate carcinogenesis. Only then can the potential of zinc and zinc transport proteins be harnessed in the diagnosis and treatment of men with prostate cancer.
Introduction
Zinc is essential for life. This metal ion is second only to iron in terms of its concentration in the body and is a co-factor for more than 300 enzymes, with three major biological roles: structural, regulatory and as catalyst (Fig. 1).1, 2 Oysters contain more zinc per serving than any other food, but red meat and poultry provide the majority of zinc in the Western diet. Other food sources rich in zinc include beans, nuts, whole grains and dairy products.3 The importance of zinc is perhaps best illustrated by the fact that failure to accumulate zinc is a sine qua non of prostatic carcinogenesis. Numerous experimental studies have provided compelling evidence that zinc has a protective effect against prostate carcinogenesis both in vitro and in vivo.4–11 However, epidemiological investigations evaluating the role of zinc supplementation in prostate cancer development and progression have yielded conflicting results.12–18
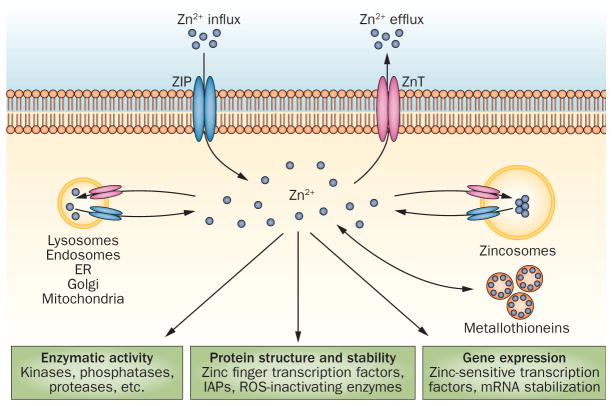
Figure 1.
The biological roles of zinc. Second only to iron in terms of its concentration in the body, zinc is a co-factor for more than 300 enzymes, with three major biological roles: structural, regulatory and catalytic. Zinc stabilizes the structure of a number of proteins, DNA and RNA as well as ribosomes. Zinc is a component of >3,000 transcription factors, and a zinc ion is located at the active site of a various enzymes, where it participates directly in the catalytic reaction. Abbreviations: ER, endoplasmic reticulum; IAP, inhibitor of apoptosis protein; ZIP, Zrt-like, Irt-like protein; ZnT, zinc transporter.
In this Review, we summarize the current data regarding the relationship between zinc and prostate carcinogenesis, focusing on the mechanistic role of zinc and its transporters in the disease. We also outline the potential opportunities for harnessing zinc for the diagnosis and treatment of prostate malignancy.
Zinc levels and prostatic malignancy
The normal human prostate accumulates the highest content of zinc of all soft tissues in the body, with a typical zinc content of 1,018±124 μg/g dry tissue.19 The high cellular accumulation of zinc results in increased levels of mitochondrial zinc that inhibit m-aconitase activity and citrate oxidation 5. Although this unique capability is retained in the hyperplastic tissue of BPH, which has a zinc content of 1,142±77 μg per gram of dry tissue, prostate carcinogenesis involves a dramatic reduction in zinc accumulation (to 146±10 μg/g dry tissue).19, 20, 21 A significant decrease in plasma zinc levels is also observed in patients with prostate carcinoma compared with healthy controls and patients with BPH.22
The reduction in zinc concentration occurs early in the course of prostate cancer development. This trend continues during tumour progression toward castration-resistant disease in prostate cancer patients.5, 7, 19, 20 Failure to accumulate zinc is an exemplary feature of prostatic malignancy, a phenomenon that is observed when comparing long-term cultured normal with malignant prostate cell lines.10 Initial experimental support for this concept was provided by studies of the malignant cell lines LNCaP and PC3. LNCaP cells demonstrate low tumorigenicity compared with the highly aggressive tumorigenic PC3 cells. Correspondingly, LNCaP cells possess a higher endogenous zinc level (630 ng/mg protein) than PC3 cells (190 ng/mg protein).23 In addition, the tumorigenic KRAS-transformed prostate epithelial cell line, RWPE-2, accumulate less intracellular zinc compared with the nontumorigenic parental RWPE-1 cells.24 These results strongly implicate zinc concentration as a defining feature in prostate cancer development and progression.
Bypassing zinc as a tumour suppressor
The functional role of zinc accumulation in the prostate is to inhibit citrate oxidation by the highly specialized secretory epithelial cells. 5 Blockade of this enzymatic pathway permits the production and secretion of extremely high levels of citrate, which is a major constituent of the prostatic fluid.5 In addition to its physiological role, zinc has associated effects and consequences that could be inhibitory to malignant cells (Fig. 2). Accordingly, the inability to accumulate zinc could potentially result in the loss of these inhibitory effects, leading to the development and progression of prostate cancer.
Figure 2.
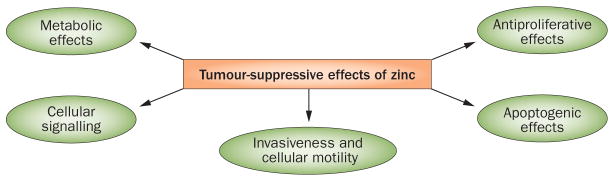
The roles of zinc in tumour suppression. In addition to its physiological role, zinc has inhibitory effects on malignant cells. Increased zinc uptake reduces the malignant potential and growth of prostate cancer cells through multiple mechanisms, whereas zinc deficiency augments tumorigenic properties of prostate cancer cells, at least in part, via activation of NF-κB-regulated pathways.
Metabolic and bioenergetic effects
Intramitochondrial accumulation of high levels of zinc inhibits mitochondrial aconitase activity, which, in turn, inhibits citrate oxidation.25 This inhibition essentially truncates the Krebs cycle and markedly decreases cellular energy production. By undergoing a metabolic transformation to zinc-deficient citrate-oxidizing cells with a functional Krebs cycle, the energy requirements for malignancy can be met.23, 26
Antiproliferative effects
Zinc seems to have a role in regulating cell division. Indeed, the intracellular accumulation of zinc inhibits proliferation of prostate cancer cells by arresting cells at the G2/M cell cycle check point.27 Liang et al. demonstrate that the mechanism of the zinc-mediated inhibition of prostatic cell growth involves up-regulation of p21 gene expression. In addition, the authors suggest that the inhibitory effect of zinc may be mediated by the interaction of zinc with the p13suc1 subunit of Cdc2 kinase.27 Further, studies by Han et al. reveal that nuclear p21 levels are depressed by zinc deficiency in LNCaP prostate cancer cells.28 Reflecting the tumorigenicity of the different cell lines, sevenfold higher zinc levels were necessary to inhibit the growth of PC3 cells compared with LNCaP cells.27
Effects on intracellular signalling pathways
Treatment of PC-3 and DU-145 prostate cancer cell lines with physiological levels of zinc inhibits the activity of transcription factor NF-κB. Consequently, the expression of NF-κB-regulated proangiogenic and prometastatic molecules is reduced in these cell lines, including vascular endothelial growth factor (VEGF), IL-6, IL-8, and matrix metalloproteinase 9 (MMP-9) in prostate cancer cells.11, 29 By contrast, depletion of intracellular zinc increases expression of protumorigenic cytokines in prostate cancer cell lines via a NF-κB-dependent pathway.10 Zinc deficiency also results in hyperphosphorylation of protein kinase B (Akt) and E3 ubiquitin-protein ligase (Mdm2), as well as reduction in nuclear p53 accumulation, in human normal prostate epithelial PrEC cells.28 A high level of Akt phosphorylation and low nuclear level of p21 coincides with high G1-to-S ratio in zinc-deficient LNCaP prostate cancer cells.28 In addition, zinc downregulates both induced and constitutive levels of hypoxia inducible factor-1α (HIF-1α) in human prostate cancer cells in vitro.30 Zinc-mediated down-regulation of HIF-1α coincides with reduced invasiveness of C27 Human prostate cancer cells.30
Apoptogenic effects
Zinc treatment results in the translocation of cytochrome c from the mitochondria to the cytosol, which triggers the activation of the caspase-9 and caspase-3, the cleavage of nuclear poly(ADP)-ribose polymerase (PARP), and, ultimately, apoptosis (Fig. 3).31, 32 Zinc-mediated release of cytochrome c can be potentially attributed to its effect on the mitochondrial level of the pro-apoptotic Bax protein. Indeed, treatment of PC-3 cells with zinc increases the mitochondrial and cellular levels of Bax protein and the cellular Bax/Bcl-2 ratio.33 The increase in cellular Bax level appears to involve zinc induction of Bax gene expression.33 The apoptogenic effect of elevated zinc concentrations in prostate cancer cells in vitro could also be attributable to the downregulation of antiapoptotic Bcl-2 and survivin proteins.34
Figure 3.

A zinc-mediated shift in the Bax to Bcl-2 ratio promotes the translocation of cytochrome c from the mitochondria to the cytosol, which triggers the activation of caspase 9 and caspase 3, cleavage of nuclear PARP and, ultimately, apoptosis. NF-κB activation is a major pathway contributing to prostate cancer development and progression. NF-κB regulates the susceptibility of malignant cells to apoptosis through transcriptional control of antiapoptotic genes, which act on different levels of the apoptotic pathway. The central mechanisms of IAP apoptotic suppression seem to be through direct caspase and pro-caspase inhibition. Abbreviations: IAP inhibitor of apoptosis protein; PARP, poly(ADP)-ribose polymerase.
Invasive and migratory effects
Zinc suppresses the expression of ICAM-1, an intercellular adhesion molecule playing an important role in cell-cell and cell-extracellular matrix interactions, which coincides with reduced invasiveness and adhesion of castration-resistant PC3 prostate cancer cells.29 Other studies have also demonstrated that the ability of androgen-dependent LNCaP prostate cancer cells to invade Matrigel is strongly suppressed in the presence of zinc at the concentration range of 150–250 μM.35 Furthermore, the invasive potential of these cells is associated with the ability of zinc to irreversibly inhibit aminopeptidase N.36 Aminopeptidase N (APN/CD13) is a 150 kDa membrane-bound ubiquitously expressed protease, which modulates cell motility and adhesion to extracellular matrix and therefore, plays a critical role in tumor proliferation, invasiveness and angiogenesis.37
Zinc transport
Although low plasma zinc concentrations have been reported in patients with prostate carcinoma (0.6+/−0.03 μg/ml in patients with prostatic malignancies vs. 0.95+/−0.1 μg/ml in healthy controls),38 this is not the likely culprit for a decreased zinc accumulation in malignant prostate cells. Instead, prostate cancer modulates the specialized mechanisms that are required for both zinc uptake and release.39 Zinc transporters are largely assigned to the two metal-transporter families: the ZIP (Zrt-like, Irt-like proteins) family, which imports zinc from extracellular fluid and the ZnT (zinc transporter) family, which functions in exporting zinc or redistributing it intracellularly in such organelles as mitochondria and endosome/lysosome compartment.40, 41
Among the 10 human ZnT members identified to date, ZnT-1 is the only zinc transport protein that is localized to the plasma membrane.41, 42, 43 Expression of ZnT-1 is induced by high zinc concentrations in LNCaP and PC-3 human prostate cancer cells,44 consistent with its putative role as a zinc export protein. Indeed, one in vitro study demonstrated that induction of ZNT1 gene transcription is mediated by the binding of the metal-specific transcription factor MTF-1 to two metal response elements (MRE) in the ZnT-1 promoter.45 High-level expression of ZnT-1 and ZnT-3 and low levels of metallothionein (an endogenous zinc chelator) have been demonstrated in androgen-independent cells that were derived from the initially androgen-dependent LNCaP cells.46 In fact, this newly derived cell line demonstrated much lower zinc levels than the parent LNCaP cell line.46 Functional implications of these findings are not well delineated in the literature. Furthermore, lack of expression of the ZNT7 gene, a zinc transporter localized on the Golgi membrane as well as the ZnT7-positive vesicles, has been implicated in prostatic carcinogenesis in a mouse model.47 This study clearly demonstrate that a null-mutation of the Znt7 gene accelerates prostate tumor formation in a transgenic adenocarcinoma of the mouse prostate model (TRAMP) mice.47
To date, 14 mammalian ZIP members have been identified;43 however, only ZIP1, ZIP2, ZIP3, and ZIP4 have been shown to be localized to the plasma membrane and therefore, participate in importing zinc from extracellular fluid.41, 48–53 ZIP1 is important for the extraction of zinc from circulation as the primary source of cellular zinc, whereas ZIP2 and ZIP3 seem to be important for the retention of zinc in the cellular compartment.54 All these transporters are markedly downregulated in human adenocarcinomatous prostate glands, as compared with normal peripheral zone glandular epithelium and benign hyperplastic glands, demonstrated by immunohistochemical staining of clinical tissue specimens.33, 34, 55 In addition, men in high-risk patient populations, such as African American men, seem to have altered prostatic zinc homeostasis, resulting in a decreased intracellular zinc accumulation that coincides with downregulation of hZIP1 and hZIP2.56
The ZIP1 has been characterized as the major zinc uptake transporter, regulating cellular zinc accumulation in normal and transformed prostate cells.24, 57, 58 Overexpression of ZIP1 in transfected RWPE-2 and PC-3 cells results in a novel zinc uptake activity not found in nontransfected cells.24, 58 Over-expression of ZIP1 in RWPE-2 cells also results in suppression of cell growth due to increased apoptosis.24 By contrast, antisense inhibition of hZIP1 expression results in a significant decrease of zinc uptake in PC3 cells.58 Our recent studies demonstrate that overexpression of hZIP1 reduces the tumorigenic potential and growth of PC3 prostate cancer cells both in vitro and in vivo.9 Other studies by Chen et al.55 demonstrated that overexpression of hZIP4 also inhibits proliferation and aggressive behaviour of DU145 prostate cancer cells. By contrast, silencing hZIP4 is associated with increased cell proliferation and invasiveness in 22RV1-shRNA prostate cancer cells.55 Thus, in prostate cells, zinc uptake transporters can behave as tumour suppressors. Importantly, reduced expression of zinc uptake transporters can result in decreased intraprostatic zinc levels, regardless of the degree of supplemental zinc intake.
To escape the anti-tumor effects of intracellular zinc, malignant prostate cells could potentially silence the expression of zinc uptake transporters, an approach that may be achieved through multiple cellular mechanisms.
Epigenetic silencing
Makhov et al.59 demonstrated that demethylation of the promoter region of the activator protein (AP)-2α protein—a critical transcription factor for SLC39A1 and SLC39A3 gene expression—in DU-145 and LNCap cell resulted in increased hZIP1 and hZIP3 transporter expression and augmented zinc uptake. Increased zinc uptake coincided with reduced proliferation of both DU145 and LNCap cells (Makhov P., personal communication). Interestingly, an analysis of the promoter regions of the SLC39A1 and SLC39A3 genes did not reveal hypermethylation in either cell line.59 These findings suggest that hypermethylation of the SLC39A1 and SLC39A3 promoters is not the primary mechanism responsible for the downregulation of these genes in prostate cancer cells. Moreover, methylation patterns of the (AP)-2α promoter region from patients’ tissues with Gleason score 6 prostate cancer were found to be markedly increased in comparison with the adjacent normal prostatic tissue.59 Gleason score 6 prostate cancer is considered to be low-risk, well-differentiated and most commonly presents as localized disease. These data suggest that the methylation of AP-2alpha promoter region in prostate cancer cells is an early event in neoplastic development.
MicroRNA
Array profiling of tumour tissue specimens have shown that several microRNAs are present in higher levels than in normal prostate tissue; namely, microRNA-183, microRNA-96, and microRNA-182. These putative zinc-regulating microRNAs were further investigated in vitro using human primary prostate cultures and established prostate cancer cell lines. Transient transfection of microRNA-182 decreased hZIP1 protein in both normal and malignant prostate epithelial cell lines.60 Overexpression of the entire microRNA-183-96-182 cluster suppressed five additional zinc transporters (hZIP1, hZIP3, hZIP7, hZIP9, hZnT1, and hZnT7), diminished labile zinc pools and reduced zinc uptake, demonstrating this microRNA cluster is indeed a regulator of zinc homeostasis.60
Overexpression of RREB-1
RREB-1 is a downstream effector of the Ras-Raf-MEK-ERK pathway.61 Previous studies have demonstrated that the transfection of PC3 prostate cancer cells with constitutively active Ras inhibited hZIP1 promoter activity, whereas the transfection with dominantly negative Ras increased its promoter activity.61 These effects were shown to be mediated by RREB-1 as they were attenuated when the RREB-1 binding site in the hZIP1 promoter was mutated.61 Notably, the hZIP1 promoter contains a strong inhibitory cis-acting element to which the RREB-1 transcription factor binds, which represses the reporter activity.61 Overexpression of RREB-1 results in a decrease in the abundance of hZIP1 in the plasma membrane of PC3 prostate cancer cells, whereas RREB-1 knockdown with specific siRNA significantly (P<0.05) increases hZIP1 expression.62 Indeed, studies employing prostate tissue microarrays and tissue sections confirmed an inverse relationship between RREB-1 and hZIP1 staining.62
The process whereby a normal prostate epithelial cell transforms into a cancerous one involves loss of the ability to accumulate intracellular zinc. The decrease in cellular zinc content coincides with a dramatic reduction in expression of the zinc uptake transporters in malignant cells in situ. Given that zinc levels diminish early in the course of prostate cancer, the profile of zinc transport genes may serve as a potential tumor biomarker for early diagnosis and risk assessment for prostate cancer.
Experimental data versus epidemiology
Given the wealth of cellular data on the role of zinc and zinc transporters in prostate cancer, it is perhaps unsurprising that zinc supplementation has been investigated in the prevention and treatment of prostate cancer. Although numerous in vitro and in vivo experiments suggest that increasing zinc intake might be protective against prostate carcinogenesis,4–11, 63–66 the results remain controversial.
Feng et al. demonstrated that zinc administration by an osmotic pump inhibited the growth of PC3 xenogafts in nude mice.6 The inhibitory effect of zinc seems to result from apoptosis, mediated by the mitochondrial membrane permeability-related Bax and Bcl-2 proteins.6 Experiments using the transgenic adenocarcinoma of the mouse prostate (TRAMP) model also suggest that an ‘optimal’ level of zinc in animal diet (30 ppm) is protective against prostate carcinogenesis.67 Findings by Banudevi et al.68, 69 document the chemopreventative effects of zinc on prostate carcinogenesis induced by N-methyl-N-nitrosourea and testosterone in the adult male Sprague–Dawley rats. Our own studies provide evidence that hZIP1 transporter overexpression induces regression of prostate tumor growth in a PC3 xenograft mouse model.9 Overall, the experimental data strongly support a protective role of zinc against prostate carcinogenesis.
However, epidemiological studies evaluating the role of zinc supplementation on primary and secondary prostate cancer prevention have yielded conflicting results. Some studies reported an adverse effect with zinc supplementation,13, 17, 18 whereas others have reported either a protective effect with borderline significance15, 70 or no effect at all.12, 14, 16 Epstein et al.71 suggest that that high dietary intake of zinc is associated with a lower prostate cancer-specific mortality after diagnosis, particularly in men with localized disease. Furthermore, Platz et al.70 suggest that the risk of prostate cancer is slightly lower among men with moderate and high zinc intake. Similarly, Gonzales et al.72 reported that “average intake of supplemental zinc was not associated with a reduced prostate cancer risk overall; however, risk of advanced prostate cancer decreased with greater intake of supplemental zinc”. It is critical to note that the recommended dietary allowance of zinc for adult males is 11 mg/day.73 A thorough study by Leitzmann et al.17 detected no statistically significant association between supplemental zinc intake of 100 mg/day and increased prostate cancer risk. Moreover, this study reported that zinc ingestion of 100 mg/day was actually associated with a significantly increased risk of advanced prostate cancer.17 Several potential explanations for this association between a high zinc intake and prostate carcinogenesis can be considered. One possibility is that ingestion of excessively high supplemental zinc has undesirable metabolic effects, such as immune dysfunction74 and impaired antioxidant defense,75 which indirectly promote prostate cancer progression. Moreover, ingestion of high levels of zinc decreases its intestinal absorption and assimilation, which paradoxically could lower circulating and tissue levels of zinc.76 Park et al.77 assessed levels of serum zinc and risk of developing prostate cancer in a multiethnic cohort of men. In this work, the investigators failed to demonstrate a relationship between serum zinc levels and prostate cancer. However, as the authors acknowledge, no evidence supports that a serum zinc level measured at random intervals reflects zinc concentration in prostatic tissues. Our studies demonstrate that failure to accumulate zinc because of a diminished expression of zinc uptake transporters might result in decreased intratumoural zinc levels despite adequate dietary zinc intake.9 These findings could potentially explain the conflicting results of recent epidemiological studies evaluating the role of zinc supplementation on primary and secondary prostate cancer prevention.
Another confounding factor complicating the interpretation of epidemiological studies is the likely possibility that the increased risk of advanced prostate cancer observed in men who take zinc supplements could be caused by these individuals taking additional unspecified supplements. Lawson et al.78 reported that among men taking a zinc supplement, multivitamin use was associated with an increased risk of fatal prostate cancer, whereas no association with multivitamins was observed for men not taking a zinc supplement. We have previously demonstrated that 50% of men at high risk of developing prostate cancer took one or more supplements to prevent prostate cancer, and more than 25% took three or more agents concomitantly.79 This study evaluated a population of high-risk patients in the USA, including black men, men with at least one first-degree relative or two or more second-degree relatives with prostate cancer, or men who tested positively for the BRCA1 gene mutation. Our data are in concordance with Leitzmann et al.17 and demonstrated that, compared with nonusers, men who consumed supplemental zinc also consumed more multivitamins, as well as supplemental calcium, copper, iron, vitamin E, lycopene, and folate.79
Even the individuals solely intent on consuming commercially available zinc are at risk because of the presence of nonessential, potentially harmful trace elements contained in these supplements,80 which might further influence study results. For example, some publications suggest that cadmium contaminants in zinc supplements could be associated with prostate carcinogenesis.80 Studies performed in New Zealand by Krone et al. revealed that cadmium, a known carcinogen, was detected in all seven tested zinc-containing dietary supplements, in ratios ranging from 0.039 μg to 1.46 μg cadmium per 15 mg zinc.81 Thus, consumption of approximately 140 mg/day of zinc (the daily level of zinc supplement intake among the high-intake group studied by Leitzmann et al. 17) could also result in simultaneous intake of as much as 13.6 μg cadmium per day. Total mean daily exposure to cadmium from foods, as estimated in the U.S. Food and Drug Administration Total Diet Study is 10 μg cadmium/person/day.80 In addition, the bioavailability of zinc is controlled by metallothioneins, which are upregulated by the presence of heavy metals, including cadmium. Thus, as suggested by Platz et al.,70 high concentrations of one metal might influence the bioavailability of another. Humans accumulate cadmium with age. It has been suggested that even small repeated low doses of cadmium could accumulate in the body and have adverse effects on prostate health associated with cadmium intake.82
Therapeutic applications of zinc
Multiple studies have revealed that a decrease in the intraprostatic zinc concentration and a concomitant reduction in expression levels of zinc uptake transporters (hZIP1, hZIP2, and hZIP3) represent early steps in the development of prostate malignancy.2,3,33,34 Moreover, zinc concentrations diminish prior to any discernible histopathological changes, and continue to decline during progression towards hormone-independent growth.57,83 One cannot overemphasize the importance of early detection of prostate cancer in that it could have life-saving implications for those patients with potentially aggressive disease. Indeed, studies by Ghosh et al.84 demonstrate that the progression of prostate cancer could be observed in vivo by monitoring zinc content in the prostates of TRAMP mice using a novel fluorescent sensor for mobile zinc. Thus, intraprostatic zinc levels, as well as the expression status of zinc transport genes, might serve as potential tumour biomarkers for the early diagnosis and risk stratification of men with prostate cancer.
Further clinical utility of zinc status on diagnostic measures in human subjects was evaluated by Cortesi et al.85 They demonstrate that zinc concentration in prostate tissue segments inversely correlates with the aggressiveness of prostatic malignancy, i.e. the higher the Gleason score the greater the local zinc depletion.85 Cortesi et al. suggest that local zinc concentration mapping may improve patient selection for biopsy, localize biopsy site and stratify between localized and focal therapy (e.g., Cryotherapy, Brachytherapy).85
Overall, the described studies clearly demonstrate the value of zinc-based prostate cancer diagnostics. Thus, zinc status could serve as an adjunctive measure to PSA screening, as it provides more-specific information than elevated PSA alone. Although elevated PSA could signify the presence of multiple prostatic abnormalities (for example, carcinoma, BPH, and inflammation), reduced zinc and its uptake transporters levels offer a unique specificity for prostatic malignancy.
Development of new therapeutic strategies for advanced prostate cancer represent an urgent need in today’s clinical landscape. In addition to its potential role as a diagnostic tool, zinc might underlie the development of novel therapeutic agents for the treatment of prostate cancer. Studies by Liguori et al.86 demonstrated potent antitumour activity of novel heteroleptic zinc(II) complexes in vitro. Unfortunately, the antitumour effect of zinc could be compromised in ZIP-deficient malignant cells, which are incapable of accumulating zinc. Indeed, our recent studies demonstrate that failure to accumulate zinc because of a reduced expression of zinc uptake transporters might result in diminished intratumoural zinc levels despite adequate zinc intake.9 However, use of zinc ionophores, such as clioquinol, could potentially facilitate the process of zinc uptake in ZIP-deficient cells.87 Clioquinol treatment of ZIP-deficient prostate cancer cells in the presence of physiological levels of zinc resulted in 80% inhibition of cell proliferation.88 Our prior work showed that the treatment of PC3 and DU145 prostate cancer cells with pyrithione (an alternate zinc ionophore) under similar experimental conditions inhibited activation of NF-κB and sensitized tumour cells to tumour necrosis factor (TNF)-α and paclitaxel-mediated cell death.11 Intriguingly, the ability of paclitaxel to cause apoptosis in LNCaP prostate cancer cells was significantly reduced when cells were cultured under zinc-deficient conditions.89 Given the prevalence of zinc deficiency, determining how chemotherapeutic action is modulated by zinc adequacy may have clinical importance. Water-soluble analogues of the zinc ionophore 1-hydroxypyridine-2-thione (ZnHPT) have been found to increase the intracellular zinc levels and suppress growth of malignant cells, both in vitro and in vivo.90 However, systemic administration of zinc ionophores will likely increase the cellular levels of zinc in multiple bodily tissues due to the lack of target specificity for tumor tissue and might, therefore, induce unforeseen toxicities. Interestingly, Shah et al.91 discussed the feasibility of the direct intratumoural injection of zinc acetate in a xenograft mouse model of prostate cancer. This approach halted prostate cancer growth and substantially extended the survival durations of the experimental animals, without any detectable toxicity in other tissues. In addition, our own results demonstrated that the administration of physiological levels of zinc can prevent docetaxel-mediated mitotic arrest, thereby protecting zinc-accumulating myeloid progenitor TF-1 cells from docetaxel-mediated toxicity. Importantly, such treatment had no effect on docetaxel-mediated apoptosis in PC3 and DU145 prostate cancer cells with compromised zinc uptake.92 Thus, zinc status can modulate antitumour efficacy of certain chemotherapeutic agents.
Another potential approach to target prostate cancer is to use zinc-chelating agents rather than zinc ionophores. Zinc levels are markedly decreased in malignant prostate tissues,19, 20,21 and in this zinc-deficient state, a normal prostate cell can compensate for decreased levels via upregulation of zinc uptake transporters. In this scenario, administration of a zinc chelator would have minimal, if any, effect on the overall intracellular zinc level. However, the situation is dramatically different in a malignant prostate cancer cell. In addition to having lower baseline zinc levels, malignant cells are also severely limited in their ability to respond to zinc chelation because of a compromised expression of zinc uptake transporters. Findings from our laboratory indicate that treatment of prostate cancer cells with the zinc specific chelator, N,N,N′,N′-tetrakis(2-pyridylmethyl) ethylenediamine (TPEN) induces rapid and selective depletion of the antiapoptic protein E3 ubiquitin-protein ligase XIAP (XIAP) at post-translational level.93 Inhibition or downregulation of XIAP in cancer cells lowers the apoptotic threshold, thereby inducing cell death and enhancing the cytotoxic action of chemotherapeutic agents.94, 95 Indeed, TPEN-mediated XIAP depletion coincides with increased activation of the apoptosis-mediating caspase 3 and caspase 9, as well as increased sensitization of apoptosis-resistant tumour cells to subsequent treatment with TNF-related apoptosis-inducing ligand (TRAIL).93 These findings were also supported by Zuo et al.,96 who demonstrated that novel polypyridyl chelators deplete cellular zinc and destabilize XIAP in human prostate and breast cancer cells. In light of the fact that prostate cancer cells are unable to counteract zinc chelation, a new potential therapeutic avenue opens for exploration.
Conclusions
Failure to accumulate zinc is a sine qua non of prostate carcinogenesis. Whereas epidemiological studies have shown mixed results, experimental data strongly suggest a protective role of zinc against prostatic carcinogenesis. Multiple in vitro and in vivo experimental studies support the concept that zinc uptake transporters might function as tumour suppressors in prostate cancer. Failure to accumulate zinc owing to a diminished expression of zinc uptake transporters might result in decreased intratumoural zinc levels, despite adequate dietary zinc. This issue was not addressed by previous epidemiologic studies. Although an interest regarding zinc’s role in prostate carcinogenesis is increasingly apparent, more studies on the effects of zinc on prostate biology are necessary. Focusing on this area of research should clarify the mechanism(s) of zinc homeostasis in malignant prostate cells and provide new strategies for the screening, diagnosis, and treatment of men with prostate cancer.
Key Points.
In vitro and in vivo experimental studies have revealed a strong association between prostate cancer and the downregulation of intracellular zinc content
To escape the antitumour effects of intracellular zinc, malignant prostate cells can reduce expression of zinc uptake transporters through multiple mechanisms
Failure to accumulate zinc via the diminished expression of zinc uptake transporters can, in turn, result in decreased intratumoural zinc levels despite adequate dietary zinc intake
This finding can explain the conflicting results of various epidemiological studies evaluating the role of zinc supplementation on primary and secondary prostate cancer prevention
The importance of changes in zinc accumulation in prostate carcinogenesis emphasizes the potential roles of zinc and zinc transport proteins for the development of novel diagnostic and therapeutic modalities
Review criteria.
A Pubmed and Web of Knowledge database searches was performed for abstracts and articles in English language. The following key words were utilized: Prostate Cancer, Zinc, Transporters, Prevention, Treatment, Epidemiology, Clinical Trials, Dietary Supplements. Relevant abstracts up to 2012 were included and relevant articles and their bibliographies were reviewed.
Acknowledgments
This work was supported in part by the National Institutes of Health Grants (RO1 CA134463, RO3 CA167671, CCSG, P30 CA006927) to VMK; the American Institute for Cancer Research Grant (09A023) to RGU; and the Department of Defense Physician Research Training Award (W81XWH-10-1-0187) and Bucks County Board of Associates Award to AK.
Footnotes
Competing interests
The authors declare no competing interests.
Author contributions V. Kolenko and A. Kutikov researched the data for the article. A. Kutikov and R. Uzzo discussed the article’s content. V. Kolenko and E. Teper wrote the manuscript, after which V. Kolenko and R. Uzzo edited the manuscript before submission.
References
- 1.Chasapis CT, Loutsidou AC, Spiliopoulou CA, Stefanidou ME. Zinc and human health: an update. Arch Toxicol. 2012;86:521–34. doi: 10.1007/s00204-011-0775-1. [DOI] [PubMed] [Google Scholar]
- 2.Rink L, Gabriel P. Zinc and the immune system. Proc Nutr Soc. 2000;59:541–52. doi: 10.1017/s0029665100000781. [DOI] [PubMed] [Google Scholar]
- 3.Trumbo P, Yates AA, Schlicker S, Poos M. Dietary reference intakes: vitamin A, vitamin K, arsenic, boron, chromium, copper, iodine, iron, manganese, molybdenum, nickel, silicon, vanadium, and zinc. J Am Diet Assoc. 2001;101:294–301. doi: 10.1016/S0002-8223(01)00078-5. [DOI] [PubMed] [Google Scholar]
- 4.Costello LC, Feng P, Milon B, Tan M, Franklin RB. Role of zinc in the pathogenesis and treatment of prostate cancer: critical issues to resolve. Prostate Cancer Prostatic Dis. 2004;7:111–7. doi: 10.1038/sj.pcan.4500712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Costello LC, Franklin RB. The clinical relevance of the metabolism of prostate cancer; zinc and tumor suppression: connecting the dots. Mol Cancer. 2006;5:17. doi: 10.1186/1476-4598-5-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Feng P, Li TL, Guan ZX, Franklin RB, Costello LC. Effect of zinc on prostatic tumorigenicity in nude mice. Ann N Y Acad Sci. 2003;1010:316–20. doi: 10.1196/annals.1299.056. [DOI] [PubMed] [Google Scholar]
- 7.Franklin RB, Costello LC. Zinc as an anti-tumor agent in prostate cancer and in other cancers. Arch Biochem Biophys. 2007;463:211–7. doi: 10.1016/j.abb.2007.02.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Franklin RB, Milon B, Feng P, Costello LC. Zinc and zinc transporters in normal prostate and the pathogenesis of prostate cancer. Front Biosci. 2005;10:2230–9. doi: 10.2741/1692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Golovine K, et al. Overexpression of the zinc uptake transporter hZIP1 inhibits nuclear factor-kappaB and reduces the malignant potential of prostate cancer cells in vitro and in vivo. Clin Cancer Res. 2008;14:5376–84. doi: 10.1158/1078-0432.CCR-08-0455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Golovine K, et al. Depletion of intracellular zinc increases expression of tumorigenic cytokines VEGF, IL-6 and IL-8 in prostate cancer cells via NF-kappaB-dependent pathway. Prostate. 2008;68:1443–9. doi: 10.1002/pros.20810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Uzzo RG, et al. Zinc Inhibits Nuclear Factor-kappaB Activation and Sensitizes Prostate Cancer Cells to Cytotoxic Agents. Clin Cancer Res. 2002;8:3579–83. [PubMed] [Google Scholar]
- 12.Chang ET, Hedelin M, Adami HO, Gronberg H, Balter KA. Re: Zinc supplement use and risk of prostate cancer. J Natl Cancer Inst. 2004;96:1108. doi: 10.1093/jnci/djh206. author reply 1108–9. [DOI] [PubMed] [Google Scholar]
- 13.Gallus S, et al. Dietary zinc and prostate cancer risk: a case-control study from Italy. Eur Urol. 2007;52:1052–6. doi: 10.1016/j.eururo.2007.01.094. [DOI] [PubMed] [Google Scholar]
- 14.Key TJ, Silcocks PB, Davey GK, Appleby PN, Bishop DT. A case-control study of diet and prostate cancer. Br J Cancer. 1997;76:678–87. doi: 10.1038/bjc.1997.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kristal AR, Stanford JL, Cohen JH, Wicklund K, Patterson RE. Vitamin and mineral supplement use is associated with reduced risk of prostate cancer. Cancer Epidemiol Biomarkers Prev. 1999;8:887–92. [PubMed] [Google Scholar]
- 16.Lee MM, et al. Case-control study of diet and prostate cancer in China. Cancer Causes Control. 1998;9:545–52. doi: 10.1023/a:1008840105531. [DOI] [PubMed] [Google Scholar]
- 17.Leitzmann MF, et al. Zinc supplement use and risk of prostate cancer. J Natl Cancer Inst. 2003;95:1004–7. doi: 10.1093/jnci/95.13.1004. [DOI] [PubMed] [Google Scholar]
- 18.Zhang Y, Coogan P, Palmer JR, Strom BL, Rosenberg L. Vitamin and mineral use and risk of prostate cancer: the case-control surveillance study. Cancer Causes Control. 2008 doi: 10.1007/s10552-008-9282-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zaichick V, Sviridova TV, Zaichick SV. Zinc in the human prostate gland: normal, hyperplastic and cancerous. Int Urol Nephrol. 1997;29:565–74. doi: 10.1007/BF02552202. [DOI] [PubMed] [Google Scholar]
- 20.Ogunlewe JO, Osegbe DN. Zinc and cadmium concentrations in indigenous blacks with normal, hypertrophic, and malignant prostate. Cancer. 1989;63:1388–92. doi: 10.1002/1097-0142(19890401)63:7<1388::aid-cncr2820630725>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 21.Costello LC, Franklin RB. Zinc is decreased in prostate cancer: an established relationship of prostate cancer! J Biol Inorg Chem. 2011;16:3–8. doi: 10.1007/s00775-010-0736-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Christudoss P, Selvakumar R, Fleming JJ, Gopalakrishnan G. Zinc status of patients with benign prostatic hyperplasia and prostate carcinoma. Indian J Urol. 2011;27:14–8. doi: 10.4103/0970-1591.78405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Costello LC, Franklin RB. Novel role of zinc in the regulation of prostate citrate metabolism and its implications in prostate cancer. Prostate. 1998;35:285–96. doi: 10.1002/(sici)1097-0045(19980601)35:4<285::aid-pros8>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 24.Huang L, Kirschke CP, Zhang Y. Decreased intracellular zinc in human tumorigenic prostate epithelial cells: a possible role in prostate cancer progression. Cancer Cell Int. 2006;6:10. doi: 10.1186/1475-2867-6-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Singh KK, Desouki MM, Franklin RB, Costello LC. Mitochondrial aconitase and citrate metabolism in malignant and nonmalignant human prostate tissues. Mol Cancer. 2006;5:14. doi: 10.1186/1476-4598-5-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Costello LC, Franklin RB. The intermediary metabolism of the prostate: a key to understanding the pathogenesis and progression of prostate malignancy. Oncology. 2000;59:269–82. doi: 10.1159/000012183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liang JY, et al. Inhibitory effect of zinc on human prostatic carcinoma cell growth. Prostate. 1999;40:200–7. doi: 10.1002/(sici)1097-0045(19990801)40:3<200::aid-pros8>3.0.co;2-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Han CT, Schoene NW, Lei KY. Influence of zinc deficiency on Akt-Mdm2-p53 and Akt-p21 signaling axes in normal and malignant human prostate cells. Am J Physiol Cell Physiol. 2009;297:C1188–99. doi: 10.1152/ajpcell.00042.2009. [DOI] [PubMed] [Google Scholar]
- 29.Uzzo RG, et al. Diverse effects of zinc on NF-kappaB and AP-1 transcription factors: implications for prostate cancer progression. Carcinogenesis. 2006;27:1980–90. doi: 10.1093/carcin/bgl034. [DOI] [PubMed] [Google Scholar]
- 30.Nardinocchi L, et al. Zinc downregulates HIF-1alpha and inhibits its activity in tumor cells in vitro and in vivo. PLoS One. 2010;5:e15048. doi: 10.1371/journal.pone.0015048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Feng P, Li TL, Guan ZX, Franklin RB, Costello LC. Direct effect of zinc on mitochondrial apoptogenesis in prostate cells. Prostate. 2002;52:311–8. doi: 10.1002/pros.10128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Feng P, et al. Zinc induces mitochondria apoptogenesis in prostate cells. Mol Urol. 2000;4:31–6. [PubMed] [Google Scholar]
- 33.Feng P, Li T, Guan Z, Franklin RB, Costello LC. The involvement of Bax in zinc-induced mitochondrial apoptogenesis in malignant prostate cells. Mol Cancer. 2008;7:25. doi: 10.1186/1476-4598-7-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ku JH, Seo SY, Kwak C, Kim HH. The role of survivin and Bcl-2 in zinc-induced apoptosis in prostate cancer cells. Urol Oncol. 2012;30:562–8. doi: 10.1016/j.urolonc.2010.06.001. [DOI] [PubMed] [Google Scholar]
- 35.Ishii K, et al. Evidence that the prostate-specific antigen (PSA)/Zn2+ axis may play a role in human prostate cancer cell invasion. Cancer Lett. 2004;207:79–87. doi: 10.1016/j.canlet.2003.09.029. [DOI] [PubMed] [Google Scholar]
- 36.Ishii K, et al. Aminopeptidase N regulated by zinc in human prostate participates in tumor cell invasion. Int J Cancer. 2001;92:49–54. [PubMed] [Google Scholar]
- 37.Wickstrom M, Larsson R, Nygren P, Gullbo J. Aminopeptidase N (CD13) as a target for cancer chemotherapy. Cancer Sci. 2011;102:501–8. doi: 10.1111/j.1349-7006.2010.01826.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Goel T, Sankhwar SN. Comparative study of zinc levels in benign and malignant lesions of the prostate. Scand J Urol Nephrol. 2006;40:108–12. doi: 10.1080/00365590500368922. [DOI] [PubMed] [Google Scholar]
- 39.McMahon RJ, Cousins RJ. Regulation of the zinc transporter ZnT-1 by dietary zinc. Proc Natl Acad Sci U S A. 1998;95:4841–6. doi: 10.1073/pnas.95.9.4841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cousins RJ, Liuzzi JP, Lichten LA. Mammalian zinc transport, trafficking, and signals. J Biol Chem. 2006;281:24085–9. doi: 10.1074/jbc.R600011200. [DOI] [PubMed] [Google Scholar]
- 41.Kambe T, Yamaguchi-Iwai Y, Sasaki R, Nagao M. Overview of mammalian zinc transporters. Cell Mol Life Sci. 2004;61:49–68. doi: 10.1007/s00018-003-3148-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liuzzi JP, Cousins RJ. Mammalian zinc transporters. Annu Rev Nutr. 2004;24:151–72. doi: 10.1146/annurev.nutr.24.012003.132402. [DOI] [PubMed] [Google Scholar]
- 43.Foster M, Hancock D, Petocz P, Samman S. Zinc transporter genes are coordinately expressed in men and women independently of dietary or plasma zinc. J Nutr. 2011;141:1195–201. doi: 10.3945/jn.111.140053. [DOI] [PubMed] [Google Scholar]
- 44.Hasumi M, et al. Regulation of metallothionein and zinc transporter expression in human prostate cancer cells and tissues. Cancer Lett. 2003;200:187–95. doi: 10.1016/s0304-3835(03)00441-5. [DOI] [PubMed] [Google Scholar]
- 45.Majumder S, et al. Chromium(VI) down-regulates heavy metal-induced metallothionein gene transcription by modifying transactivation potential of the key transcription factor, metal-responsive transcription factor 1. J Biol Chem. 2003;278:26216–26. doi: 10.1074/jbc.M302887200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Iguchi K, et al. Zinc and metallothionein levels and expression of zinc transporters in androgen-independent subline of LNCaP cells. J Androl. 2004;25:154–61. doi: 10.1002/j.1939-4640.2004.tb02771.x. [DOI] [PubMed] [Google Scholar]
- 47.Tepaamorndech S, Huang L, Kirschke CP. A null-mutation in the Znt7 gene accelerates prostate tumor formation in a transgenic adenocarcinoma mouse prostate model. Cancer Lett. 2011;308:33–42. doi: 10.1016/j.canlet.2011.04.011. [DOI] [PubMed] [Google Scholar]
- 48.Gaither LA, Eide DJ. The human ZIP1 transporter mediates zinc uptake in human K562 erythroleukemia cells. J Biol Chem. 2001;276:22258–64. doi: 10.1074/jbc.M101772200. [DOI] [PubMed] [Google Scholar]
- 49.Gaither LA, Eide DJ. Functional expression of the human hZIP2 zinc transporter. J Biol Chem. 2000;275:5560–4. doi: 10.1074/jbc.275.8.5560. [DOI] [PubMed] [Google Scholar]
- 50.Costello LC, Liu Y, Zou J, Franklin RB. Evidence for a zinc uptake transporter in human prostate cancer cells which is regulated by prolactin and testosterone. J Biol Chem. 1999;274:17499–504. doi: 10.1074/jbc.274.25.17499. [DOI] [PubMed] [Google Scholar]
- 51.Eide DJ. The SLC39 family of metal ion transporters. Pflugers Arch. 2004;447:796–800. doi: 10.1007/s00424-003-1074-3. [DOI] [PubMed] [Google Scholar]
- 52.Dufner-Beattie J, Huang ZL, Geiser J, Xu W, Andrews GK. Mouse ZIP1 and ZIP3 genes together are essential for adaptation to dietary zinc deficiency during pregnancy. Genesis. 2006;44:239–51. doi: 10.1002/dvg.20211. [DOI] [PubMed] [Google Scholar]
- 53.Kim BE, et al. Zn2+-stimulated endocytosis of the mZIP4 zinc transporter regulates its location at the plasma membrane. J Biol Chem. 2004;279:4523–30. doi: 10.1074/jbc.M310799200. [DOI] [PubMed] [Google Scholar]
- 54.Desouki MM, Geradts J, Milon B, Franklin RB, Costello LC. hZip2 and hZip3 zinc transporters are down regulated in human prostate adenocarcinomatous glands. Mol Cancer. 2007;6:37. doi: 10.1186/1476-4598-6-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chen QG, et al. The role of zinc transporter ZIP4 in prostate carcinoma. Urol Oncol. 2011 doi: 10.1016/j.urolonc.2010.11.010. [DOI] [PubMed] [Google Scholar]
- 56.Rishi I, et al. Prostate cancer in African American men is associated with downregulation of zinc transporters. Appl Immunohistochem Mol Morphol. 2003;11:253–60. doi: 10.1097/00129039-200309000-00009. [DOI] [PubMed] [Google Scholar]
- 57.Franklin RB, et al. hZIP1 zinc uptake transporter down regulation and zinc depletion in prostate cancer. Mol Cancer. 2005;4:32. doi: 10.1186/1476-4598-4-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Franklin RB, et al. Human ZIP1 is a major zinc uptake transporter for the accumulation of zinc in prostate cells. J Inorg Biochem. 2003;96:435–42. doi: 10.1016/s0162-0134(03)00249-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Makhov PB, et al. Reversal of epigenetic silencing of AP-2alpha results in increased zinc uptake in DU-145 and LNCaP prostate cancer cells. Carcinogenesis. 2011;32:1773–81. doi: 10.1093/carcin/bgr212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mihelich BL, et al. miR-183-96-182 cluster is overexpressed in prostate tissue and regulates zinc homeostasis in prostate cells. J Biol Chem. 2011;286:44503–11. doi: 10.1074/jbc.M111.262915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Milon BC, Agyapong A, Bautista R, Costello LC, Franklin RB. Ras responsive element binding protein-1 (RREB-1) down-regulates hZIP1 expression in prostate cancer cells. Prostate. 2010;70:288–96. doi: 10.1002/pros.21063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zou J, Milon BC, Desouki MM, Costello LC, Franklin RB. hZIP1 zinc transporter down-regulation in prostate cancer involves the overexpression of ras responsive element binding protein-1 (RREB-1) Prostate. 2011 doi: 10.1002/pros.21368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Costello LC, Franklin RB. Re: Dietary zinc and prostate cancer risk: a case-control study from Italy. Eur urol 2007;52:1052–7. Eur Urol. 2007;52:1262–3. doi: 10.1016/j.eururo.2007.04.022. author reply 1263–4. [DOI] [PubMed] [Google Scholar]
- 64.Costello LC, Franklin RB, Feng P, Tan M. Re: Zinc supplement use and risk of prostate cancer. J Natl Cancer Inst. 2004;96:239–40. doi: 10.1093/jnci/djh045. author reply 240–1. [DOI] [PubMed] [Google Scholar]
- 65.Costello LC, Franklin RB, Feng P, Tan M, Bagasra O. Zinc and prostate cancer: a critical scientific, medical, and public interest issue (United States) Cancer Causes Control. 2005;16:901–15. doi: 10.1007/s10552-005-2367-y. [DOI] [PubMed] [Google Scholar]
- 66.Franklin RB, Costello LC. Re: Vitamin and mineral use and risk of prostate cancer: the case-control surveillance: Zhang et al. Cancer Causes Control. 2008 Dec 18 [Epub ahead of print] Cancer Causes Control. 2009;20:1529–31. doi: 10.1007/s10552-009-9363-6. author reply 1533. [DOI] [PubMed] [Google Scholar]
- 67.Prasad AS, et al. Dietary zinc and prostate cancer in the TRAMP mouse model. J Med Food. 2010;13:70–6. doi: 10.1089/jmf.2009.0042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Banudevi S, et al. Chemopreventive effects of zinc on prostate carcinogenesis induced by N-methyl-N-nitrosourea and testosterone in adult male Sprague-Dawley rats. J Cancer Res Clin Oncol. 2011;137:677–86. doi: 10.1007/s00432-010-0926-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Banudevi S, et al. Protective effect of zinc on N-methyl-N-nitrosourea and testosterone-induced prostatic intraepithelial neoplasia in the dorsolateral prostate of Sprague Dawley rats. Exp Biol Med (Maywood) 2011;236:1012–21. doi: 10.1258/ebm.2011.010392. [DOI] [PubMed] [Google Scholar]
- 70.Platz EA, et al. Prediagnostic toenail cadmium and zinc and subsequent prostate cancer risk. Prostate. 2002;52:288–96. doi: 10.1002/pros.10115. [DOI] [PubMed] [Google Scholar]
- 71.Epstein MM, et al. Dietary zinc and prostate cancer survival in a Swedish cohort. Am J Clin Nutr. 2011;93:586–93. doi: 10.3945/ajcn.110.004804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Gonzalez A, Peters U, Lampe JW, White E. Zinc intake from supplements and diet and prostate cancer. Nutr Cancer. 2009;61:206–15. doi: 10.1080/01635580802419749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.(http://www.nal.usda.gov/fnic/DRI//DRI_Vitamin_A/442-501_150.pdf).
- 74.Chandra RK. Excessive intake of zinc impairs immune responses. Jama. 1984;252:1443–6. [PubMed] [Google Scholar]
- 75.Samman S, Roberts DC. The effect of zinc supplements on lipoproteins and copper status. Atherosclerosis. 1988;70:247–52. doi: 10.1016/0021-9150(88)90175-x. [DOI] [PubMed] [Google Scholar]
- 76.King JC, Shames DM, Woodhouse LR. Zinc homeostasis in humans. J Nutr. 2000;130:1360S–6S. doi: 10.1093/jn/130.5.1360S. [DOI] [PubMed] [Google Scholar]
- 77.Park SY, Wilkens LR, Morris JS, Henderson BE, Kolonel LN. Serum zinc and prostate cancer risk in a nested case-control study: The multiethnic cohort. Prostate. 2012 doi: 10.1002/pros.22565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lawson KA, et al. Multivitamin use and risk of prostate cancer in the National Institutes of Health-AARP Diet and Health Study. J Natl Cancer Inst. 2007;99:754–64. doi: 10.1093/jnci/djk177. [DOI] [PubMed] [Google Scholar]
- 79.Uzzo RG, et al. Prevalence and patterns of self-initiated nutritional supplementation in men at high risk of prostate cancer. BJU Int. 2004;93:955–60. doi: 10.1111/j.1464-410X.2004.04759.x. [DOI] [PubMed] [Google Scholar]
- 80.Krone CA, Harms LC. Re: Zinc supplement use and risk of prostate cancer. J Natl Cancer Inst. 2003;95:1556. doi: 10.1093/jnci/djg088. author reply 1556–7. [DOI] [PubMed] [Google Scholar]
- 81.Krone CA, Wyse EJ, Ely JT. Cadmium in zinc-containing mineral supplements. Int J Food Sci Nutr. 2001;52:379–82. doi: 10.1080/09637480120057602. [DOI] [PubMed] [Google Scholar]
- 82.Achanzar WE, et al. Cadmium-induced malignant transformation of human prostate epithelial cells. Cancer Res. 2001;61:455–8. [PubMed] [Google Scholar]
- 83.Johnson LA, Kanak MA, Kajdacsy-Balla A, Pestaner JP, Bagasra O. Differential zinc accumulation and expression of human zinc transporter 1 (hZIP1) in prostate glands. Methods. 2010;52:316–21. doi: 10.1016/j.ymeth.2010.08.004. [DOI] [PubMed] [Google Scholar]
- 84.Ghosh SK, et al. A novel imaging approach for early detection of prostate cancer based on endogenous zinc sensing. Cancer Res. 2010;70:6119–27. doi: 10.1158/0008-5472.CAN-10-1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Cortesi M, et al. Clinical assessment of the cancer diagnostic value of prostatic zinc: a comprehensive needle-biopsy study. Prostate. 2008;68:994–1006. doi: 10.1002/pros.20766. [DOI] [PubMed] [Google Scholar]
- 86.Liguori PF, et al. Non-classical anticancer agents: synthesis and biological evaluation of zinc(II) heteroleptic complexes. Dalton Trans. 2010;39:4205–12. doi: 10.1039/b922101h. [DOI] [PubMed] [Google Scholar]
- 87.Ding WQ, Lind SE. Metal ionophores - an emerging class of anticancer drugs. IUBMB Life. 2009;61:1013–8. doi: 10.1002/iub.253. [DOI] [PubMed] [Google Scholar]
- 88.Costello LC, Franklin RB. Cytotoxic/tumor suppressor role of zinc for the treatment of cancer: an enigma and an opportunity. Expert Rev Anticancer Ther. 2012;12:121–8. doi: 10.1586/era.11.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Killilea AN, Downing KH, Killilea DW. Zinc deficiency reduces paclitaxel efficacy in LNCaP prostate cancer cells. Cancer Lett. 2007;258:70–9. doi: 10.1016/j.canlet.2007.08.010. [DOI] [PubMed] [Google Scholar]
- 90.Magda D, et al. Synthesis and anticancer properties of water-soluble zinc ionophores. Cancer Res. 2008;68:5318–25. doi: 10.1158/0008-5472.CAN-08-0601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Shah MR, et al. Direct intra-tumoral injection of zinc-acetate halts tumor growth in a xenograft model of prostate cancer. J Exp Clin Cancer Res. 2009;28:84. doi: 10.1186/1756-9966-28-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Makhov P, et al. Docetaxel-mediated apoptosis in myeloid progenitor TF-1 cells is mitigated by zinc: Potential implication for prostate cancer therapy. Prostate. 2011 doi: 10.1002/pros.21357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Makhov P, et al. Zinc chelation induces rapid depletion of the X-linked inhibitor of apoptosis and sensitizes prostate cancer cells to TRAIL-mediated apoptosis. Cell Death Differ. 2008;15:1745–51. doi: 10.1038/cdd.2008.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Schimmer AD, Dalili S, Batey RA, Riedl SJ. Targeting XIAP for the treatment of malignancy. Cell Death Differ. 2006;13:179–88. doi: 10.1038/sj.cdd.4401826. [DOI] [PubMed] [Google Scholar]
- 95.Schimmer AD, et al. Small-molecule antagonists of apoptosis suppressor XIAP exhibit broad antitumor activity. Cancer Cell. 2004;5:25–35. doi: 10.1016/s1535-6108(03)00332-5. [DOI] [PubMed] [Google Scholar]
- 96.Zuo J, et al. Novel polypyridyl chelators deplete cellular zinc and destabilize the X-linked inhibitor of apoptosis protein (XIAP) prior to induction of apoptosis in human prostate and breast cancer cells. J Cell Biochem. 2012 doi: 10.1002/jcb.24132. [DOI] [PMC free article] [PubMed] [Google Scholar]