SUMMARY
Innate lymphoid cells (ILCs) are an emerging subset of lymphocytes involved in surveillance against virally infected cells. Here we show CD3−CD8high lymphocytes in macaque blood include major subsets of ILCs including NK cells expressing CD16, NKp46 and NKG2A, but also populations of ILCs in mucosal tissues having different properties. One ILC subset secreted IL-17 (ILC17), but these were restricted to mucosal tissues. Some mucosal ILC17 cells expressed classical NK-cell markers, but little NKG2A or NKG2D. Some ILC17 cells secreted IL-22 and TNF-α, but few produced IFN-γ or contained granzyme B. IL-17 production by ILCs was induced by IL-6, TGF-β and IL-23. Further, SIV infection resulted in a significant loss of ILC17 cells, especially in the jejunum, which persisted throughout SIV infection. These findings ILC17 cells may be involved in innate mucosal immune responses, and their loss may contribute to loss of intestinal mucosal integrity and disease progression in HIV/SIV infection.
Keywords: Cytotoxic T lymphocytes, CD8+ T cell, SIV, HIV, intestine, mucosal immunology
INTRODUCTION
Innate Lymphoid Cells (ILCs) represent a novel family of effector lymphocytes representing the first line of defense against virally infected cells and neoplastic cells. ILCs include Natural Killer (NK) cells, lymphoid tissue-inducer (LTi) cells and other subsets that produce interleukin 17 (IL-17), IL-22 (NK-22/ILC22), IL-5 and/or IL-13, which have different functions, but are developmentally related1, 2. Thus, ILCs are a heterogeneous subset of CD3 negative lymphocytes with diverse functions, some of which may overlap with T cell subsets such as Th17, Th22 or Th2 cells1, 2. However, the phenotypical characterization of, and distinctions between NK cells and ILCs, and their relative distribution and function in tissues are not yet clear.
As a major subset of ILCs, NK cells are thought to play an important role in controlling HIV infection and disease progression3–5. Recent studies demonstrate dramatic changes in the phenotype and function of NK cells occur in HIV/SIV infection6–8. Moreover, individuals with “protective” KIR/HLA genotypes and/or robust NK cell responses are over-represented in HIV controllers5, 9–11. This is consistent with studies of SIV-infected macaques in which the magnitude of NK cell responses inversely correlates with viremia and disease progression12–14.
Among NK subsets, expression of distinctive surface molecules are highly polymorphic and vary between species15–17. For example, mouse NK cells express NK1.1 and DX5, whereas human NK cells are generally defined by CD56 expression, which is not expressed by mouse NK cells. In humans, CD56bright NK cells possess higher capacity for proliferation and cytokine production, but CD56dim cells display higher cytotoxicity15, 18, 19. In rhesus macaques (Macaca mulatta), prior studies of NK cells and now ILCs have been complicated by different gating strategies and phenotypic definitions. For example, efforts to identify subsets of NK cells in nonhuman primates have included CD3−CD16+, CD3−CD8αα+, CD3−CD20−HLA-DR+, CD16+/−CD56+/− etc. However, CD56, often used as a primary marker for human NK cells, is also expressed on macaque monocytes, and may influence data interpretation when gating on CD3− populations20. Another NK marker, CD16, also known as FcγRIII, is important in antibody-dependent cell-mediated cytotoxicity, and is among the first of activating receptors expressed on NK cells. However, detection of CD16 with the widely used 3G8 monoclonal antibody clone has been shown to be “masked” by anti-SIV immunoglobulin G immune complexes in SIV-infected rhesus macaques, at least when using whole blood staining techniques21. Therefore, some prior studies may reflect misleading or incomplete definitions of NK cells in SIV-infected macaques. Thus, more detailed phenotyping of NK cells, and now ILCs, needs to be performed, especially in tissues of SIV-infected rhesus macaques to adequately track and characterize innate immune responses.
NK cells have been described in blood and tissues of various species8, 22, yet they are negative for the transcription factor RAR-related orphan γ receptor (RORγt)1. In contrast, the RORγt is required for the generation of LTi cells which are CD117 (c-Kit)high and CD127 (IL-7Rα)high, and other subsets characterized by the production of high amounts of IL-22 including NK-like NKp46+ cells in the intestinal lamina propria which are CD117lowCD127low 23–26. LTi cells are clustered in cryptopatches between crypts of the intestinal lamina propria, where they, together with stromal cells, could direct the formation of isolated lymphoid follicles by recruitment of DCs and B cells, and promote T-helper cell survival and IgA production27, 28. In contrast, IL-22+NKp46+ cells are found within intestinal villi29. In general, NK cells, LTis, ILC22 and other ILC subsets are all characterized by secretion of proinflammatory and cytolytic mediators, and may play critical roles in lentiviral infection30–33. However, little is known about the distribution of IL-17-producing ILC subsets and their responses during SIV infection in rhesus macaques.
To better understand the characteristic of ILCs including NK cells in the peripheral blood and mucosal lymphoid tissues, here we defined ILCs as CD8high lymphocytes, which were lineage negative for B cells (CD20), T cells (CD3), and monocytes (CD14), and examined their anatomical distribution, and changes in tissues after SIV infection. As previously shown for NK cells8, 34, the data suggest that ILCs in peripheral blood are distinct from mucosal-derived ILCs, and IL-17-producing ILCs were mostly restricted to mucosal tissues. Further, although some ILC17 expressed classical NK cell markers, few expressed NKG2A or NKG2D. Finally, there was a significant loss of ILC17 cells in SIV infection, which may contribute to loss of intestinal epithelial mucosal integrity.
RESULTS
CD3−CD8high populations define ILCs subsets in rhesus macaques
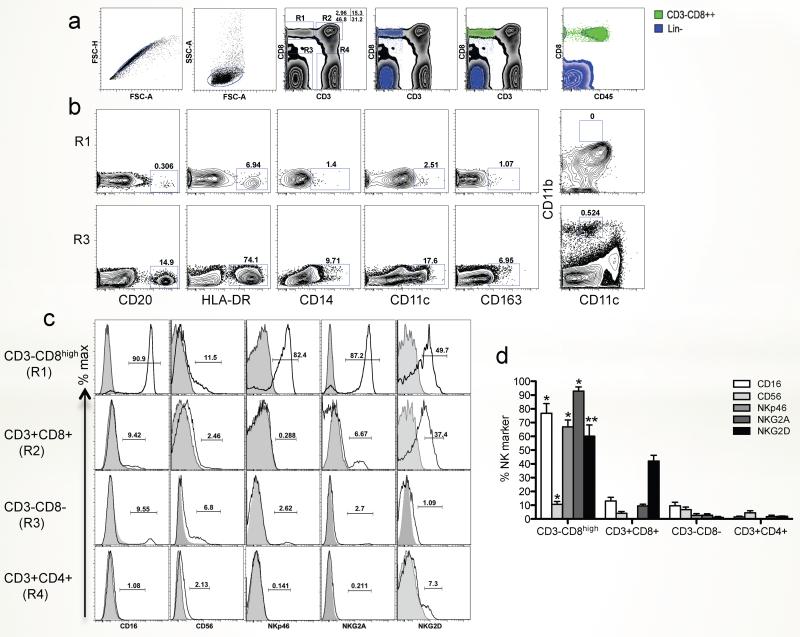
To better define the phenotype of macaque ILCs by flow cytometry, we first gated on CD3−CD8high lymphocytes (Fig. 1a), confirmed they were lymphocytes by CD45 expression, showed they lacked CD20 (B cells), CD14 (monocytes), CD11c (dendritic cells), CD163 and CD11b+CD11cint (macrophage/eosinophil) expression, and then examined their expression of typical NK markers on these Lineage negative (Lin-) CD3−CD8high ILC populations for comparison with CD4+ and CD8+ T cell subsets (Fig. 1b, c). Lin-CD3−CD8high cells were essentially all CD45+ lymphocytes, whereas the Lin−CD8− population was CD45 negative (Fig. 1a). The CD3−CD8high population was thus distinguished from B cells, monocytes, dendritic cells, macrophage and eosinophils, and expressed low levels of HLA-DR (Fig. 1b). Analysis of surface NK markers showed that circulating (blood) CD3−CD8high cells expressed CD16 (90.9%), CD56 (11.5%), NKp46 (82.4%) and NKG2A (87.2%), compared with T cell subsets and/or CD3−CD8− cells, which either lacked, or had much lower expression of these markers (Fig. 2c and d). However, since phenotyping NK cells in rhesus macaques is difficult, and since none of these markers alone could definitively distinguish NK cells, these are referred to here as ILCs.
Figure 1. Phenotypic characterization of innate lymphoid cells in peripheral blood of normal rhesus macaques.
(a) Representative gating strategy to define ILCs in PBMCs. Macaque ILCs were defined as CD3−CD8αhigh gated lymphocytes. (b) Representative dot plot depicting the expression of CD20, HLA-DR, CD14, CD11c, CD163 and CD11b+CD11int on CD3−CD8αhigh (R1) as compared to CD3−CD8αlow/− (R3) subsets. (c) Representative histogram showing expression of classical NK markers, CD16, CD56, NKp46, NKG2A and NKG2D on CD3−CD8αhigh (R1), CD3+CD8+ (R2), CD3-CD8−/intermediate (R3), and CD3+CD4+ (R4) subsets from peripheral blood. (d) Expression of classical NK markers on CD3−CD8αhigh (R1), CD3+CD8+ (R2), CD3-CD8−/intermediate (R3), and CD3+CD4+ (R4) subsets from peripheral blood (n=8). Examples are representative of 8 naive animals. *, p<0.001; **, p<0.05, compared between CD3−CD8high and other three subsets, respectively.
Figure 2. Comparison of anti-CD16 antibody clones for cross-reactivity with rhesus macaques, and distribution of NK cells in lymphoid tissues.
(a) Cross-reactivity and comparison of whole blood (WB) and washed PBMC from the same animals using different anti-CD16 antibody clones in chronically SIV infected rhesus macaques (n=8). Note only the DJ130c clone does not demonstrate differential staining between washed PBMC and WB. (b) Tissue distribution of classical NK cell subsets (CD16+, CD56+, NKG2A+) in naïve rhesus macaques (n=5). *, p<0.05 between using 3G8 and DJ130c clones in PBMC samples (a) or blood compared with other tissues (b); **, p<0.01 between using 3G8 and DJ130c clones in whole blood; #, p<0.01, compared with peripheral blood; ##. p<0.05 between liver and other tissues (except blood), respectively.
Distribution of NK subsets in macaque tissues
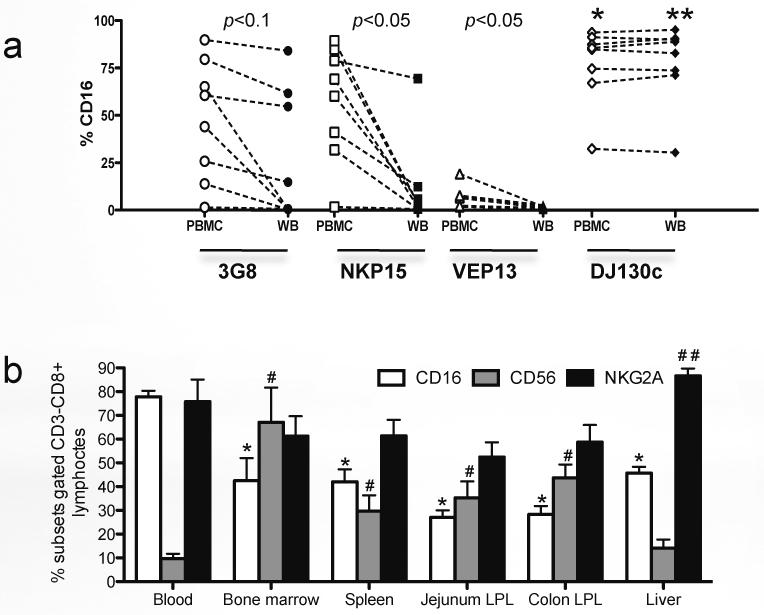
It was previously reported that detection of CD16 on NK cells in whole blood preparations can be “masked” by non-covalent binding of SIV/IgG immune complexes when using the anti-CD16 antibody clone 3G8, which is not observed in fractionated (washed) peripheral blood mononuclear cells21. To identify a better antibody for detecting CD16, we compared 4 different anti-human CD16 antibody clones, which target different CD16 epitopes, which were reported to cross-react with rhesus macaques, and compared percentages of CD16+ ILCs (CD3−CD8high) cells of whole blood and isolated (washed) PBMCs from chronically SIV-infected macaques. Consistent with the previous report, the binding of anti-CD16 antibody was significantly reduced in whole blood when using clones 3G8, NKP15 and VEP13, compared with isolated PBMCs (p<0.05). However, staining with clone DJ130c was not affected, and showed consistent results between whole blood and PBMCs. Moreover, this clone detected even higher levels of CD16 than clone 3G8, even on isolated PBMCs (Fig.2a). Thus, in this study, we used this anti-CD16 clone to detect CD16+ cells.
In macaques, NKG2A+ NK cells are identified as medium-to-large lymphocytes, which display specific functional profiles/properties in SIV infection8. Here, CD16+, CD56+ or NKG2A+ NK or ILC cell subsets were compared in different tissues of normal macaques, and results showed ILC subpopulations were differentially distributed in lymphoid and mucosal tissues. High percentages of CD16+ ILCs were detected in peripheral blood, but proportions of CD56+ ILCs were markedly higher in other tissues including bone marrow, spleen, liver and intestine (Fig. 2b). In contrast, percentages of NKG2A+ ILC/NK cells were variable in different tissues but their highest frequency was in the liver followed by peripheral blood (Fig. 2b). These results indicate ILC/NK subpopulations are differentially distributed in various tissues as heterogeneous lymphocyte populations.
ILCs in blood are distinct from ILC in mucosal lymphoid tissues
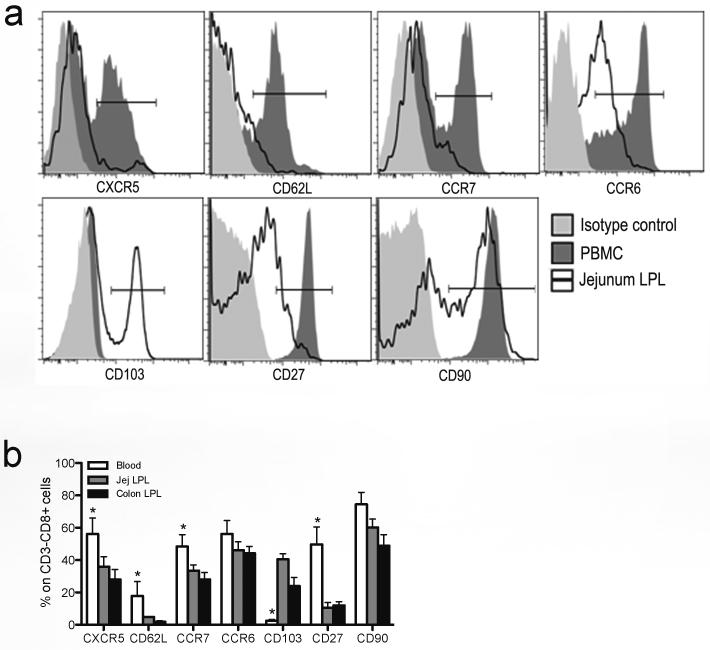
To identify distinct ILCs subpopulations between the peripheral blood and mucosal tissues, we compared the expression of various surface molecules on ILCs. Expression of migration/homing markers including CCR7, CCR6 and CD103 were differentially expressed on ILCs in peripheral and mucosal tissues. Circulating ILCs expressed more CCR7 and CCR6, whereas jejunum-derived ILCs expressed higher levels of CD103, which promotes lymphocyte migration to mucosal epithelium. Further, the expression of CXCR5, CD62L, CD27 and CD90 were also differentially expressed on ILCs in tissues (Fig. 3a). In rhesus macaques, other molecules such as CD27 and CD62L were also differentially expressed on ILCs between peripheral blood and mucosal gut tissues. Mucosal ILCs had higher percentages of CD27low and CD62Low populations than peripheral blood (Fig. 3b). Nonetheless, despite their common CD3−CD8high phenotype, ILCs in different tissues appear to be phenotypically diverse.
Figure 3. Distinct expression of surface molecules on innate lymphoid cells between mucosal tissues and peripheral blood.
(a) Representative histogram displaying different expression of molecules on ILCs from PBMCs and jejunum lamina propria. (b) Statistical comparison of expression between ILCs from PBMCs and jejunum lamina propria in naïve rhesus macaques (n=12). *, p<0.05.
IL-17-producing ILCs are predominantly restricted to mucosal tissues
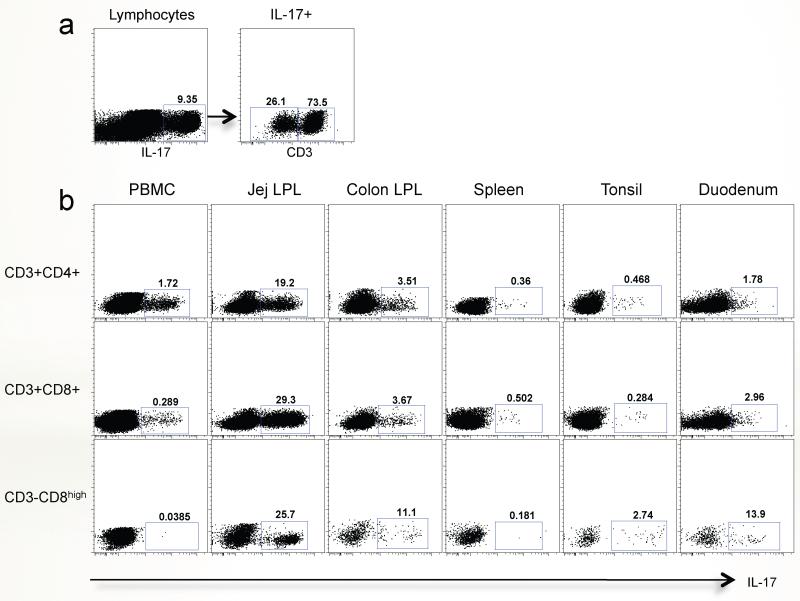
To further investigate and compare ILCs in blood and intestinal tissues, total lymphocyte preparations were stimulated with PMA plus ionomycin. Among the IL-17 producing lymphocytes, there was a substantial population of CD3− lymphocytes in the jejunum as shown in Fig. 4a. Further analyzing these IL-17+CD3− populations in tissues showed they were predominantly CD3−CD8high ILCs, and were essentially restricted to mucosal tissues including the jejunum, colon, tonsil and duodenum, and absent in blood or spleen. Similarly, the vast majority of the Th17 cells (CD3+CD4+-IL-17+) and CD3+CD8+ T cells producing IL-17 were detected in intestinal tissues, but in contrast to the ILCs, there were substantial populations of these in blood (PBMC) as well (Fig. 4b). Moreover, and similar to Th17 and CD8+ T cells, much higher percentages of IL-17-producing ILC cells (defined here as ILC17 cells) were found in the small intestine compared to the colon or any other tissue. A similar distribution of Th17 cells has been previously reported in mice37, but to our knowledge these have not been examined in mucosal tissues of humans. Since the small intestine is a major immune effector site, these data suggest that ILC cells and other IL-17 producing cells are activated, effector cells involved in maintaining mucosal immunity.
Figure 4. Innate lymphoid cells that secrete IL-17 are restricted to mucosal tissues in rhesus macaques.
(a) Jejunum dot plots gated on IL-17-secreting lymphocytes (left) contain both CD3+ and CD3− subsets in jejunum after PMA/Ionomycin stimulation. (b) Comparison of IL-17-producing CD4+ (Th17), CD8+ T cells, and CD3−CD8high (ILCs) in various lymphoid tissues, including peripheral blood, jejunum, colon, spleen, tonsil and duodenum. Noted that IL-17-secreting ILCs are restricted to mucosal tissues, with little to no expression of IL-17 from PBMC or spleen-derived ILCs.
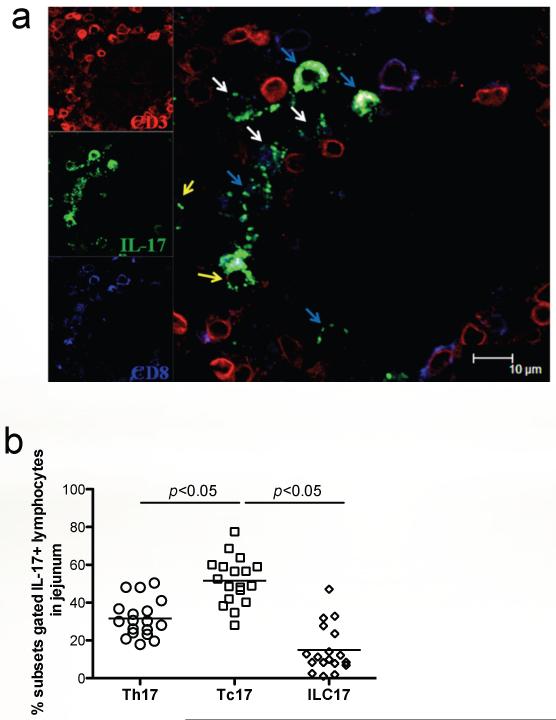
To further identify the distribution of ILC17 in intestinal tissues in macaques, jejunum sections were analyzed by multi-fluorescent immunohistochemistry to determine their location and distribution in situ. By simultaneously labeling tissues with CD3, CD8, and IL-17, we could show ILC17 cells (CD3negCD8+/−IL-17+) were present in the jejunum lamina propria, as well as Tc17 (CD3+CD8+IL-17+) and Th17 (CD3+CD8negIL-17+) cells in normal, uninfected animals (Fig. 5 a and b). After SIV infection, IL-17+ cells were gradually depleted with disease progression, and were practically undetectable in AIDS macaques (data not shown), consistent with previous reports documenting loss of Th17 and Tc17 cells in chronic SIV-infection38, 39.
Figure 5. Detection of ILC17 in jejunum tissues of normal rhesus macaques by confocal microscopy.
(a) Jejunum from a normal macaque showing CD3+ (red), IL-17 (green) and CD8+ (blue) cells. The white arrows show ILC17 cells (CD3−CD8+IL-17+); the yellow arrows demonstrate Th17 cells (CD3+CD8−IL-17+), and the blue arrows show Tc17 cells (CD3+CD8+IL-17+). Scale bar = 10 μm. (b) Relative percentages of Th17 , Tc17, and ILC17 cells gated through total IL-17+ lymphocytes in the jejunum of normal animals as assessed by flow cytometry.
ILC17 cells differentially express classical NK markers and produce an array of cytokines
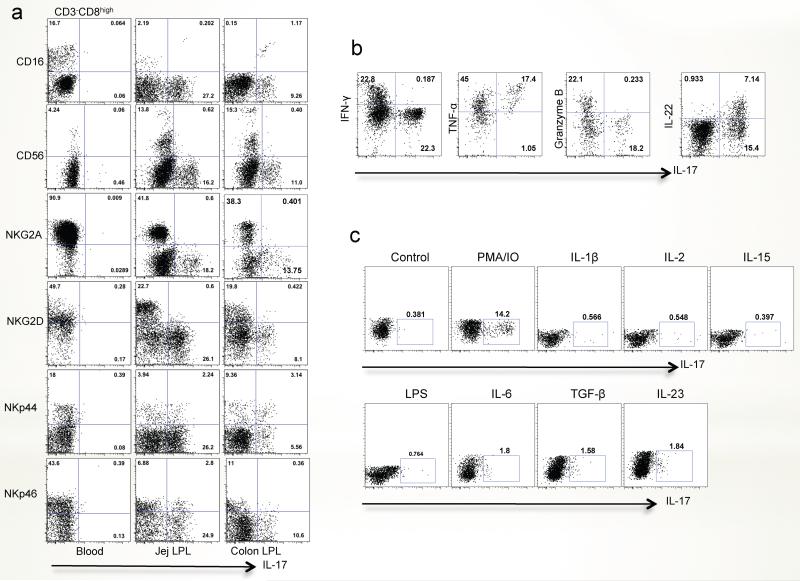
Since we found that ILC17 cells were predominantly distributed in mucosal tissues, we further phenotyped these for expression of classical NK cell markers. As shown in Fig. 6a, ILC17 cells in the jejunum variably expressed NK molecules including CD16, CD56, NKp44 and NKp46 when gating on CD3−CD8high populations. These proportions appear very different than those reported for IL-22-producing NK cells, most of which have been shown to co-express NKp46 (in mice) or NKp44 (in humans). The distribution of ILC17 cells was similar between intestinal tissues. In contrast, little to no production of IL-17 was detected from NKG2A+ or NKG2D+ ILC cells in the intestines or from any peripheral blood-derived ILCs (Fig. 6a).
Figure 6. Phenotyping IL-17-producing ILCs for classical NK cell markers and cytokine secretion in peripheral blood and mucosal tissues of normal rhesus macaques.
(a) Expression of classical NK cell markers on ILC17 cells in the peripheral blood, jejunum and colon. Note there was little to no production of IL-17 from NKG2A+, or NKG2D+ ILCs. (b) Cytokine secretion of ILC17 cells isolated from jejunum after PMA/ionomycin stimulation. Note ILC17 cells also secrete pro-inflammatory (TNF-α), and innate (IL-22) cytokines, but express little to no IFN-γ or Granzyme B. (c) Response of jejunum ILC17 cells to various cytokine and LPS stimulation.
Since NK cells are characterized by their cytotoxicity and/or regulation of inflammatory responses, we examined cytokine secretion of ILC17 cells in response to mitogens or cytokines. Following mitogen stimulation, ILC17 cells also secreted IL-22 and TNF-α. In fact, almost all ILC17 cells co-expressed TNF-α- and approximately 30% co-expressed IL-22 suggesting these were NK-22, cells now believed to play a major role in mucosal defense32, 40, 41. However, little IFN-γ and granzyme B was secreted by ILC17 cells (Fig. 6b). Combined, these data suggest that ILC17 cells might be involved in regulating innate inflammatory responses in gut-associated lymphoid tissues (GALT), rather than having cytotoxic properties.
Although PMA and ionomycin stimulation resulted in IL-17 production, it was not clear if similar results could be elicited by stimulation with more physiologically relevant cytokines. Thus we examined the production of IL-17 from jejunum lamina propria-derived ILC after stimulation with IL-1β (20ng/ml), IL-2 (20U/ml), IL-6 (20ng/ml), IL-15 (20ng/ml), TGF-β (20ng/ml), IL-23 (50ng/ml) and LPS (10ng/ml). To some extent, IL-23, TGF-β and IL-6 all induced IL-17 secretion from ILC17 cells in vitro (Fig. 6c). However, other cytokines or stimuli such as IL-2, IL-15, IL-1β or LPS had little to no effect on IL-17 production from ILC17 cells, compared with PMA/IO stimulation (Fig. 6c). Combined, these data demonstrate that ILC17 cells are a specialized subset localized within mucosal tissues.
Dynamics of CD3−CD8high ILCs in peripheral and mucosal intestinal tissues after SIV infection
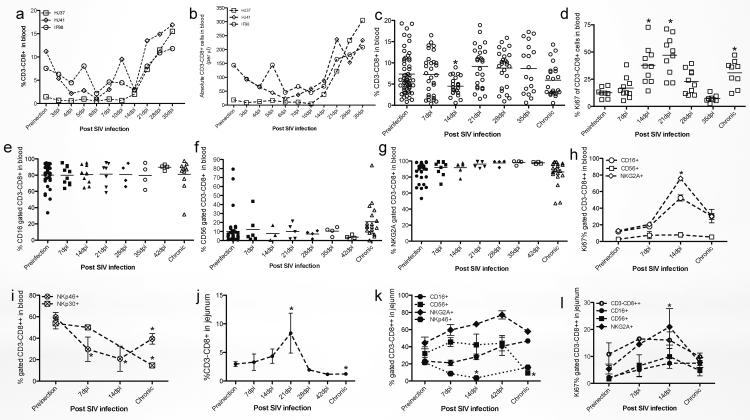
NK cells are reported to play a crucial role in the early defense against many viral infections including HIV/SIV and herpesviruses42–44. Here, we monitored changes in distribution and proliferation of ILCs (CD3−CD8high) after SIV infection in rhesus macaques. In 3 animals followed prospectively in SIV infection, both absolute numbers and percentages of ILCs in blood were reduced in acute infection and then began to increase after 14dpi (Fig. 7a and b). Consistently, when additional animals were examined, circulating ILCs were significantly reduced 7–14 days post SIV infection (compared with healthy macaques, p<0.05), but gradually increased and were sometimes even higher than baseline in chronic infection (Fig. 7c). On average, SIV infection resulted in significant increases in circulating ILCs at 14–21 dpi (Fig. 7d). Due to the disparate phenotypes of ILCs in peripheral blood and mucosal tissues (Fig. 2–4), we also examined mucosal ILCs after SIV infection. To further identify possible subpopulations of ILCs we assessed the expression of various NK cell-associated molecules including CD16, CD56, NKG2A, NKp30 and NKp46 on total ILC populations. Circulating CD16+, CD56+ or NKG2A+ ILCs showed no significant changes during SIV infection (Fig. 7e–g), and displayed similar proliferation profiles (Ki-67+) as total ILCs in blood (Fig. 7D, H). However, percentages of NKp30+ and NKp46+ ILCs were significantly reduced post SIV infection (Fig. 7i), which was not consistent with the dynamics of total circulating ILCs. Thus selective reduction in NKp30+ and NKp46+ ILC subpopulations occurs in acute SIV infection.
Figure 7. Effects of SIV infection on blood and jejunum-derived innate lymphoid cells (CD3−CD8high) or its subpopulations in rhesus macaques.
(a and b) Prospective analysis of changes in percentage (a) and absolute numbers (b) of total CD3−CD8high ILCs in peripheral blood after SIV infection. (c, e–g, and i–k) Dynamic of ILCs and their subpopulation in blood and jejunum ILCs after SIV infection (Naïve, n=55; 7dpi, n=30; 14dpi, n=23; 21dpi, n=26; 28dpi, n=26; 35dpi, n=18; chronic, n=23). (d, h and i) Proliferation (Ki-67+) of circulating or intestinal ILCs after SIV infection (Naïve, n=24; 7dpi, n=5; 14dpi, n=5; 21dpi, n=4; 28dpi, n=3; 42dpi, n=4; chronic, n=14). *, p<0.05, compared with uninfected normal animals.
In contrast to blood, percentages of intestinal ILCs increased in early SIV infection, peaked at day 21, and then gradually decreased. In chronic infection, percentages of ILCs in GALT were significantly reduced compared with normal macaques (Fig. 7j). Further analysis indicated that intestinal CD16+, CD56, or NKG2A+ ILCs increased in response to acute SIV infection, but NKp46+ ILCs significantly decreased after infection. In chronic infection, losses of CD56+ and NKp46+ mucosal ILCs was detected, compared with milder losses of NKG2A+ cells and maintenance of CD16+ populations (Fig. 7k). Similar to circulating ILCs, proliferation rates of total intestinal ILCs and various subpopulations were also markedly increased in acute SIV infection, yet returned to baseline levels in chronically SIV-infected macaques (Fig. 7l). These data demonstrate that intestinal-derived ILCs are indeed distinct from peripheral blood ILCs, and various subpopulations display different dynamics in response to SIV infection. Since larger changes in these populations occur in the intestine compared to blood, we hypothesize this may correlate with the higher levels of CD4+ T cell infection and viral replication in intestinal tissues in early SIV infection45. If ILCs are involved in effector and regulatory functions, dysfunction and/or deletion of these cells may be associated with failure of local viral control during SIV infection.
Loss of mucosal ILC17 cells correlates with disease progression in SIV-infected macaques
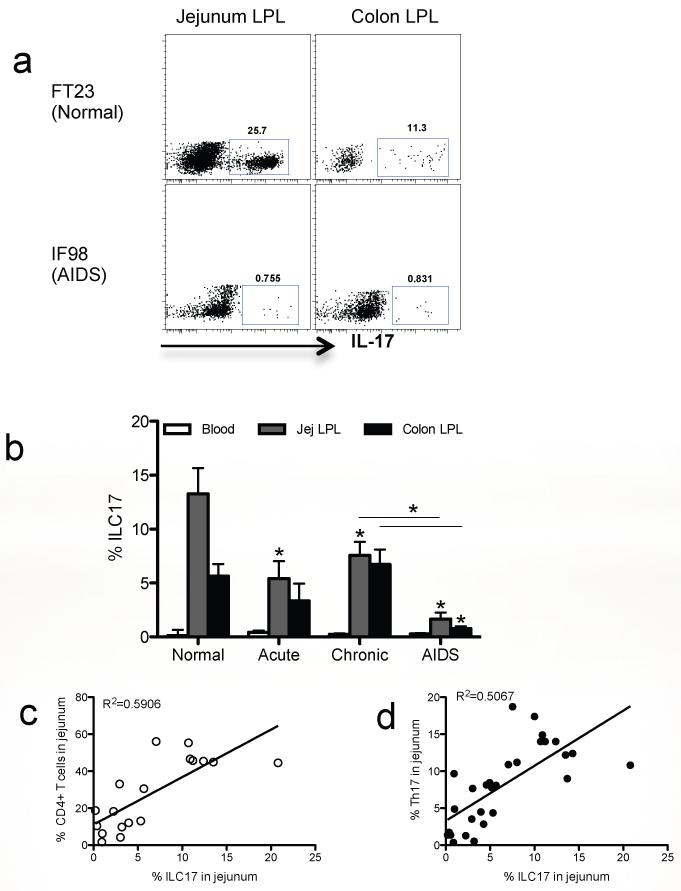
Since ILC17 cells appear to be restricted to mucosal tissues, we further investigated the effects of SIV infection on ILC17 cells in intestinal tissues of rhesus macaques. The baseline percentage of ILC17 cells was ~13.27% ± 2.386 of jejunum lamina propria lymphocytes which was higher than in colon (5.639%% ± 1.124) in SIV naïve/negative animals. However, ILC17 cells in the jejunum were significantly reduced at all stages of SIV infection, and remained at significantly lower levels in animals with symptomatic AIDS (1.654% ± 0.603, p<0.05). Similarly, percentages of ILC17 cell in the colon also showed decreases in acute infection, but there was a slight (but not significant) increase over baseline in chronic infection. However, ILC17 cells were also significantly reduced in the colon in animals with AIDS as compared to chronically SIV-infected animals (Fig. 8a and b).
Figure 8. Reduction of intestinal mucosal ILC17 cells after SIV infection of rhesus macaques.
(a) Dot plot demonstrating ILC17 cells after gating on CD3−CD8high subsets from the jejunum and colon from a representative normal and AIDS macaque. (b) Loss of intestinal ILCs occurs after SIV infection (Normal, n=10; acute, n=6; chronic, 12; AIDS, n=4). *, P<0.05. (c) Correlation between percentages of ILC17 cells and CD4+ T cells or (d) Th17 cells in jejunum during SIV infection (c, n=18; d, n=28).
Due to the marked loss of ILC17 cells in the jejunum after SIV infection, we asked whether levels of ILC17 cells in this tissue correlated with intestinal CD4+ T cell or Th17 cell loss in the jejunum. The data showed that ILC17 cell loss positively correlated with loss of both total CD4+ T cells (R2=0.5906, p=0.0002) and Th17 cells (R2=0.5067, p<0.0001)(Fig. 8c and d). Since HIV/SIV infection results in massive loss of intestinal CD4+ T cells46, including loss of Th17 cells47, 48,, these findings suggest the loss of ILC17 cells may also be involved with the loss of intestinal integrity, and disease progression in AIDS.
DISCUSSION
Innate lymphoid cells (ILCs) are a heterogeneous population of cells thought to play an important role in innate immunity and lentivirus infections. However, more recent evidence suggests they are also involved in regulating adaptive immune responses. Here, we define ILCs as CD3−CD8high lymphocytes that lack CD3, CD20, and CD14 and show they are differentially distributed in blood, lymphoid and nonlymphoid tissues, and display different expression patterns of migration or “memory”-associated molecules in rhesus macaques. Further, we show large proportions of these cells that produce IL-17 upon mitogen stimulation are restricted to mucosal effector sites, and lack features of classical NK cell properties such as Granzyme B. In normal animals, ILCs may be a major source of IL-17, which is known to contribute to the maintenance of intestinal epithelial integrity49. Loss of epithelial integrity is now believed to occur after SIV/HIV infection, resulting in translocation of bacteria and other antigens from the intestinal lumen to the systemic circulation, driving systemic immune activation and SIV/HIV replication50, 51. Therefore, the loss of IL-17 producing cells may be responsible for the loss of mucosal epithelial integrity, driving SIV/HIV replication and persistence.
In addition, mucosal ILC17 have variable expression patterns of NK cell-associated molecules such as CD16, CD56, NKp44 and NKp46, but little to no NKG2A and NKG2D expression. However, mucosal ILC17 cells also appear to encompass all of the intestinal NK-22 cells. Intestinal mucosal ILC17 cells were significantly reduced in the jejunum post SIV infection, and their loss directly correlated with intestinal CD4+ T cell and Th17 cell loss. Since loss of Th17 cells in the intestine has been shown to correlate with disease progression47, 52 these findings suggest that the loss of ILC17 cells may also contribute to disease progression and the pathogenesis of AIDS.
NK cells as a subset of ILCs may phenotypically differ between humans, mice and macaques, yet prior definitions and gating strategies preclude direct comparisons. Although CD56 is regarded as a major marker for NK cells in humans, its expression on monocytes in rhesus macaques20 may interfere with gating strategies simply based on CD3 negative cells, which could include small monocytes in the lymphocyte gate. However, CD8 is a member of the immunoglobulin superfamily found on the majority of thymocytes, T cells, and NK cells (which almost exclusively express CD8a homodimers), and there is little to no expression of CD8 on monocytes. Therefore, CD3−CD8high populations exclude T cells, monocytes and other cells, and Fig. 1 (R1) confirms that circulating CD3−CD8high lymphocytes express low to undetectable levels of CD20, CD14, CD11c, CD163 or CD11b+CD11cint, but highly express classical NK cell markers such as CD16, CD56, NKp46, NKG2A and/or NKG2D. Moreover, detection of CD16 using the anti-CD16 antibody clone DJ130c showed highly consistent results and even better staining than the 3G8 clone which is known to be masked by anti-SIV antibody complexes (Fig. 2a). Thus the anti-CD16 antibody clone DJ130c, appears more suitable for CD16 detection in macaques than antibodies previously used for SIV studies, which could underestimate levels of CD16+ cells in whole blood staining preparations of chronically infected macaques.
ILCs display different distributions and staining characteristics in tissues, especially between peripheral blood and mucosal tissues. Levels of CD56+ ILCs in tissues are significantly higher than in blood (Fig. 2b) and ILCs in GALT are distinct from those in blood in expression of migration or other molecules including CXCR5, CCR7 and CD103, CD27 and CD62L (Fig. 3). In macaques, mucosal ILCs had much higher levels of CD62Llow and CD27low/− subsets than in blood. These data suggest possible diverse functions and immunologic experience of the ILC found in blood and those in tissues.
Previous studies found Th17 cells and NKp44+NK-22 cells could be a major source of IL-17 in tissues32, 43. Within the CD3−CD8high subsets, we found ILCs also produced IL-17 but these were restricted to mucosal tissues such as the jejunum, duodenum and tonsil, compared to blood in which IL-17 was secreted only by CD4+ or CD8+ T cells after PMA/Ionomycin stimulation (Fig. 4). It is well known that Th17 cells are abundant in intestinal tissues, however, we found more Tc17 cells in the jejunum compared to Th17 cells (Fig 5b). We also found more ILC17 cells in the jejunum than the colon, which was not surprising, since the jejunum is purely an effector lymphoid tissue, whereas the colon contains abundant organized lymphoid tissues which consist largely of resting, naïve cells. Further, intestinal IL-17-producing cells partially expressed CD16, CD56, NKp44, NKp46 yet little to no expression of NKG2A and NKG2D (Fig. 6a). Moreover, ILC17 cells predominantly produced TNF-α, but little secretion of cytolytic IFN-γ or granzyme B. In addition, all IL-22+ cells were contained in the IL-17+ subset, yet only about 30% of the IL-17 cells were IL-22+, suggesting ILC17 cells represent a distinct subset from IL-22-producing NK cells in macaques (Fig. 6b). Thus, ILC17 cells may be considered a heterogenous family of ILCs including NK cells, lymphoid tissue inducing cells (LTis), and other cells such as NK-22, which share a common lineage1, 55. Most ILCs including ILC22, and Lymphoid Tissue Inducer cells (LTis) are distinguished by high levels of the transcription factor RAR-related orphan γ receptor (RORγt)30, 56, 57. In rhesus macaques, we tested several anti-human RORγt antibodies, but none reliably cross-reacted with macaques. However, IL-17-producing ILCs in macaques did produce IL-22, and expressed NKp46 and/or NKp44 (Fig. 6a). ILC17 cells also appeared to be lack cytotoxic functions, as they did not express killer inhibitory receptors such as NKG2A, and produced little IFN-γ (Fig.5a and b). Further examination showed mucosal ILC17 could (to some extent) be regulated by TGF-β, IL-6 and IL-23, but not by IL-2, IL-15 or LPS (Fig. 6c). Perhaps robust IL-17 production from ILC17 cells may require costimulatory signals such as toll-like receptor ligands, such as the TLR2 ligand for NK-222. Nonetheless, these data indicate ILC17 cells represent a specific subset restricted to mucosal tissues, and suggest they also play a role in maintaining mucosal integrity and innate immunity.
In blood, percentages of circulating ILCs were reduced in early SIV infection (10–14 days), and then dramatically increased, accompanied by higher proliferative capacity (day 14–21), but no significant differences were detected between naïve and chronically SIV-infected animals (Fig. 7a–d). In contrast to blood, percentages of intestinal ILCs increased and peaked at 21 days post SIV infection, and maintained high proliferation rates through 28 days of infection (Fig.7je and l), but were gradually “depleted” with disease progression. Within the ILCs, the NKp46+ population decreased after SIV infection in both peripheral blood and intestinal mucosal tissues (Fig. 7 i and k), but other subsets expressing classic NK cells markers such as CD16, CD56 (except in mucosal tissues in chronic infection) and NKG2A did not significantly decrease post viral infection (Fig. 7 e–j and k). These results indicate that changes in circulating ILCs differ from those in mucosal tissues. ILCs may also play an important role in innate immunity in mucosal tissues in early viral infection.
SIV infection resulted in reductions of ILC17 cells, especially in the jejunum. In macaques with AIDS, very few ILC17 cells were detectable (Fig. 8a and b). The loss of ILC17 cells directly correlated with the loss of intestinal CD4+ T cells and Th17 cells (Fig.8 c and d), which has been correlated with breakdown of mucosal integrity, resulting in microbial translocation and chronic immune activation39, 50. IL-17, in combination with IL-22, (also derived from most IL-17-producing cells) is important for protection against mucosal bacterial infections and for maintenance of the mucosal barrier by promoting intestinal epithelial integrity38, 39, 52, 58–60. In the intestinal mucosa, ILCs are in close proximity to the epithelial barrier, and may even be intraepithelial or subepitheial32. Although no direct evidence currently shows the loss of intestinal ILC17 cells correlates with breakdown of intestinal mucosal integrity in SIV-infected rhesus macaques, it has been shown that loss of other IL-17 producing cells (CD4+ Th17 cells) correlates with loss of epithelial integrity61. Therefore, general preservation of IL-17 producing cells during HIV infection may prevent the immune activation associated with loss of epithelial integrity and microbial translocation that leads to higher levels of SIV/HIV replication61–63.
EXPERIMENTAL PROCEDURES
Animals and virus
A total of 71 adult Indian rhesus macaques (Macaca mulatta), which were negative for SIV, type D retrovirus, and STLV-1 infection were utilized to examine ILCs cells in tissues and blood. Another 146 macaques were examined for ILC subsets in peripheral blood. All animals were housed at the Tulane National Primate Research Center in accordance with the Association for Assessment and Accreditation of Laboratory Animal Care International standards. All studies were reviewed and approved by the Tulane University Institutional Animal Care and Use Committee. Of the 71 animals, 20 animals were uninfected controls, and others were infected with SIVmac251 and examined in acute (n=23), or chronic infection with either no overt signs of disease (chronic asymptomatic, n=24) or with symptoms of AIDS (n=4). Blood from three animals was prospectively monitored at different time points post SIV infection. To examine cells from tissues such as intestine, spleen, etc., macaques were euthanized for tissue collection as naïve, uninfected controls, or in acute (7–21 days) or chronic infection (SIV infection more than 3 months).
Cell isolation and processing
Mononuclear cells from peripheral blood, spleen, and intestinal tissues were isolated and processed as previously described64. Briefly, total peripheral blood mononuclear cells were isolated from EDTA-treated venous blood by density gradient centrifuge with Lymphocyte Separation Media (MP Biomedicals, LLC, Santa Ana, CA) as per manufacturers instruction. Tissues were collected from the jejunum and colon within minutes of euthanasia and processed immediately for cell suspensions using enzymatic digestion as previously described65.
Phenotyping
Flow cytometry for surface and intracellular staining was performed using standard protocols66. Cells were stained with: CD3 (SP34), Ki67 (B56), CD8α (3B5, Caltag), HLA-DR (Immu-357, Bemckman coulter), TNF-α (MAB11), IFN-γ (B27), CCR6 (11A9), CCR7 (150503, R&D), CD20 (L27), CD14 (M5E2), CD11c (S-HCL-3), CD11b (ICRF44), CD163 (GH/61), CD45 (TU116), CD56 (NCAM16.2), CD16 (3G8 and NKP15, BD biosciences; VEP13, Miltenyi; DJ130c, Dako), NKp30 (Z25, Beckman Coulter), NKp46 (BAB281, Beckman Coulter), NKG2A (Z199, Beckman Coulter), NKG2D (1D11, Biolegend), NKp44 (2.9, Miltenyi), CXCR5 (NIH reagents), CD62L (SK11), CD103 (2G5, Beckman Coulter), CD27 (M-T271), CD90 (5E10), Granzyme B (GB11), IL-17 (eBio64CAP17, eBioscience), and IL-22 (BG/IL22, Biolegend; 142928; R&D), LIVE/DEAD Fixable Aqua Dead Cell Stain Kit (Invitrogen), All antibodies and reagents were purchased from BD Biosciences Pharmingen (San Diego, CA) unless otherwise noted. Samples were resuspended in BD Stabilizing Fixative (BD Biosciences) and acquired on a FACS Calibur or FACSAria flow cytometer (Becton Dickinson). Data was analyzed with Flowjo software (Tree star, Ashland, OR).
Ex vivo tissue culture and multi-color confocal microscopy
Fresh jejunum tissues were obtained from rhesus macaques within 30 min of necropsy and explants prepared and stimulated for detection of IL-17+ cell subsets. Tissues were cut into 1 cm2 sections and cultured in complete RPMI medium (10% heat inactivated fetal calf serum, l-glutamine, penicillin, and streptomycin; Invitrogen) either alone (unstimulated media) or with 100ng/ml phorbol 12-myristate-13-acetate (PMA) plus 0.5mg/ml Calcium Ionophore (Stimulation medium) for 4 hours in the presence of 2uM monensin (Sigma) to block protein transport and release. Tissues were then processed and stained as previously described64. In brief, Tissues were embedded and snap frozen in optimum cold temperature compound (OCT) and 7um frozen sections were stained using unconjugated primary antibodies (CD3, CD8, or IL-17) followed by appropriate secondary antibodies conjugated to Alexa 488 (green), Alexa 568 (red) or Alexa 633 (blue)(Molecular Probes, Eugene, OR). Confocal microscopy was performed using a Leica TCS SP2 confocal microscope equipped with three lasers (Leica Microsystems, Exton, PA). Individual optical slices representing 0.2 um and 32 to 62 optical slices were collected at 512 × 512 pixel resolution. NIH Image (version 1.62) and Adobe Photoshop (version 7.0) were used to assign colors to the channels collected: HNPP/Fast Red, which fluoresces when exposed to a 568-nm wavelength laser, appears red; Alexa 488 (Molecular Probes) appears green; Alexa 633 (Molecular Probes) appears blue (Fig. 5).
Cell stimulation for detection of cytokines
Lymphocytes (106) from jejunum, colon, and tonsil, were stimulated in vitro with 0.1 μM phorbol 12-myristate-13-acetate (PMA) and 0.5 μg/ml ionomycin (Sigma-Aldrich), or 20 U/ml IL-2, 10ng/ml LPS (Sigma), 20ng/ml IL-1β, IL-6, IL-7, IL-15 (R&D), TGF-β or 50ng/ml IL-23 (Biolegend) for 2 hours at 37°C. Cells were cultured for an additional 4 hours in the presence of 5 μg/ml Brefeldin A (Sigma-Aldrich) then stained for cell surface markers and Annexin V, fixed in 2% paraformaldehyde, permeabilized in Cytofix/Cytoperm solution (BD Biosciences), and intracellularly co-stained with fluorochrome-labelled antibodies for the cytokines, and acquired with a FACS Aria cytometer (Becton Dickinson). Data was analyzed with Flowjo software (Tree star, Ashland, OR).
Statistics
Graphical presentation and statistical analysis of the data were performed using GraphPad Prism 4.0 (GraphPad Software, SanDiego, CA). Comparisons between groups were analyzed by a one-way ANOVA and a non-parametric Mann-Whitney T-test. P values <0.05 were considered statistically significant. Correlations between samples were calculated and expressed using the Spearman's coefficient of correlation.
ACKNOWLEDGEMENTS
We thank Julie Bruhn and Calvin Lanclos for flow cytometry support and Megan Gardner, Kelsi Rasmussen, Megan Watkins and Maury Duplantis for technical support. This work was supported by NIH grants AI49080, AI084793, RR000164, and a Faculty Enhancement Grant from Tulane University.
Footnotes
The authors declare no conflict of interests.
REFERENCES
- 1.Spits H, Di Santo JP. The expanding family of innate lymphoid cells: regulators and effectors of immunity and tissue remodeling. Nat Immunol. 2011;12(1):21–27. doi: 10.1038/ni.1962. [DOI] [PubMed] [Google Scholar]
- 2.Crellin NK, Trifari S, Kaplan CD, Satoh-Takayama N, Di Santo JP, Spits H. Regulation of cytokine secretion in human CD127(+) LTi-like innate lymphoid cells by Toll-like receptor 2. Immunity. 2010;33(5):752–764. doi: 10.1016/j.immuni.2010.10.012. [DOI] [PubMed] [Google Scholar]
- 3.Berger CT, Alter G. Natural killer cells in spontaneous control of HIV infection. Curr Opin HIV AIDS. 2011;6(3):208–213. doi: 10.1097/COH.0b013e3283457798. [DOI] [PubMed] [Google Scholar]
- 4.French AR, Yokoyama WM. Natural killer cells and viral infections. Curr Opin Immunol. 2003;15(1):45–51. doi: 10.1016/s095279150200002x. [DOI] [PubMed] [Google Scholar]
- 5.Alter G, Martin MP, Teigen N, Carr WH, Suscovich TJ, Schneidewind A, et al. Differential natural killer cell-mediated inhibition of HIV-1 replication based on distinct KIR/HLA subtypes. J Exp Med. 2007;204(12):3027–3036. doi: 10.1084/jem.20070695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.De Maria A, Fogli M, Costa P, Murdaca G, Puppo F, Mavilio D, et al. The impaired NK cell cytolytic function in viremic HIV-1 infection is associated with a reduced surface expression of natural cytotoxicity receptors (NKp46, NKp30 and NKp44) Eur J Immunol. 2003;33(9):2410–2418. doi: 10.1002/eji.200324141. [DOI] [PubMed] [Google Scholar]
- 7.Mavilio D, Lombardo G, Benjamin J, Kim D, Follman D, Marcenaro E, et al. Characterization of CD56−/CD16+ natural killer (NK) cells: a highly dysfunctional NK subset expanded in HIV-infected viremic individuals. Proc Natl Acad Sci U S A. 2005;102(8):2886–2891. doi: 10.1073/pnas.0409872102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Reeves RK, Gillis J, Wong FE, Yu Y, Connole M, Johnson RP. CD16− natural killer cells: enrichment in mucosal and secondary lymphoid tissues and altered function during chronic SIV infection. Blood. 2010;115(22):4439–4446. doi: 10.1182/blood-2010-01-265595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vieillard V, Fausther-Bovendo H, Samri A, Debre P. Specific phenotypic and functional features of natural killer cells from HIV-infected long-term nonprogressors and HIV controllers. J Acquir Immune Defic Syndr. 2010;53(5):564–573. doi: 10.1097/QAI.0b013e3181d0c5b4. [DOI] [PubMed] [Google Scholar]
- 10.O'Connor GM, Holmes A, Mulcahy F, Gardiner CM. Natural Killer cells from long-term non-progressor HIV patients are characterized by altered phenotype and function. Clin Immunol. 2007;124(3):277–283. doi: 10.1016/j.clim.2007.05.016. [DOI] [PubMed] [Google Scholar]
- 11.Martin MP, Gao X, Lee JH, Nelson GW, Detels R, Goedert JJ, et al. Epistatic interaction between KIR3DS1 and HLA-B delays the progression to AIDS. Nat Genet. 2002;31(4):429–434. doi: 10.1038/ng934. [DOI] [PubMed] [Google Scholar]
- 12.Shieh TM, Carter DL, Blosser RL, Mankowski JL, Zink MC, Clements JE. Functional analyses of natural killer cells in macaques infected with neurovirulent simian immunodeficiency virus. J Neurovirol. 2001;7(1):11–24. doi: 10.1080/135502801300069593. [DOI] [PubMed] [Google Scholar]
- 13.Pereira LE, Johnson RP, Ansari AA. Sooty mangabeys and rhesus macaques exhibit significant divergent natural killer cell responses during both acute and chronic phases of SIV infection. Cell Immunol. 2008;254(1):10–19. doi: 10.1016/j.cellimm.2008.06.006. [DOI] [PubMed] [Google Scholar]
- 14.Bostik P, Kobkitjaroen J, Tang W, Villinger F, Pereira LE, Little DM, et al. Decreased NK cell frequency and function is associated with increased risk of KIR3DL allele polymorphism in simian immunodeficiency virus-infected rhesus macaques with high viral loads. J Immunol. 2009;182(6):3638–3649. doi: 10.4049/jimmunol.0803580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Freud AG, Caligiuri MA. Human natural killer cell development. Immunol Rev. 2006;214:56–72. doi: 10.1111/j.1600-065X.2006.00451.x. [DOI] [PubMed] [Google Scholar]
- 16.Carlyle JR, Mesci A, Fine JH, Chen P, Belanger S, Tai LH, et al. Evolution of the Ly49 and Nkrp1 recognition systems. Semin Immunol. 2008;20(6):321–330. doi: 10.1016/j.smim.2008.05.004. [DOI] [PubMed] [Google Scholar]
- 17.Kulkarni S, Martin MP, Carrington M. The Yin and Yang of HLA and KIR in human disease. Semin Immunol. 2008;20(6):343–352. doi: 10.1016/j.smim.2008.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yokoyama WM, Kim S, French AR. The dynamic life of natural killer cells. Annu Rev Immunol. 2004;22:405–429. doi: 10.1146/annurev.immunol.22.012703.104711. [DOI] [PubMed] [Google Scholar]
- 19.Cooper MA, Fehniger TA, Caligiuri MA. The biology of human natural killer-cell subsets. Trends Immunol. 2001;22(11):633–640. doi: 10.1016/s1471-4906(01)02060-9. [DOI] [PubMed] [Google Scholar]
- 20.Carter DL, Shieh TM, Blosser RL, Chadwick KR, Margolick JB, Hildreth JE, et al. CD56 identifies monocytes and not natural killer cells in rhesus macaques. Cytometry. 1999;37(1):41–50. [PubMed] [Google Scholar]
- 21.Wei Q, Stallworth JW, Vance PJ, Hoxie JA, Fultz PN. Simian immunodeficiency virus (SIV)/immunoglobulin G immune complexes in SIV-infected macaques block detection of CD16 but not cytolytic activity of natural killer cells. Clin Vaccine Immunol. 2006;13(7):768–778. doi: 10.1128/CVI.00042-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chinen H, Matsuoka K, Sato T, Kamada N, Okamoto S, Hisamatsu T, et al. Lamina propria c-kit+ immune precursors reside in human adult intestine and differentiate into natural killer cells. Gastroenterology. 2007;133(2):559–573. doi: 10.1053/j.gastro.2007.05.017. [DOI] [PubMed] [Google Scholar]
- 23.Sawa S, Cherrier M, Lochner M, Satoh-Takayama N, Fehling HJ, Langa F, et al. Lineage relationship analysis of RORgammat+ innate lymphoid cells. Science. 2010;330(6004):665–669. doi: 10.1126/science.1194597. [DOI] [PubMed] [Google Scholar]
- 24.Takatori H, Kanno Y, Watford WT, Tato CM, Weiss G, Ivanov, et al. Lymphoid tissue inducer-like cells are an innate source of IL-17 and IL-22. J Exp Med. 2009;206(1):35–41. doi: 10.1084/jem.20072713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sanos SL, Bui VL, Mortha A, Oberle K, Heners C, Johner C, et al. RORgammat and commensal microflora are required for the differentiation of mucosal interleukin 22-producing NKp46+ cells. Nat Immunol. 2009;10(1):83–91. doi: 10.1038/ni.1684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Luci C, Reynders A, Ivanov, Cognet C, Chiche L, Chasson L, et al. Influence of the transcription factor RORgammat on the development of NKp46+ cell populations in gut and skin. Nat Immunol. 2009;10(1):75–82. doi: 10.1038/ni.1681. [DOI] [PubMed] [Google Scholar]
- 27.Tsuji M, Suzuki K, Kitamura H, Maruya M, Kinoshita K, Ivanov, et al. Requirement for lymphoid tissue-inducer cells in isolated follicle formation and T cell-independent immunoglobulin A generation in the gut. Immunity. 2008;29(2):261–271. doi: 10.1016/j.immuni.2008.05.014. [DOI] [PubMed] [Google Scholar]
- 28.Bouskra D, Brezillon C, Berard M, Werts C, Varona R, Boneca IG, et al. Lymphoid tissue genesis induced by commensals through NOD1 regulates intestinal homeostasis. Nature. 2008;456(7221):507–510. doi: 10.1038/nature07450. [DOI] [PubMed] [Google Scholar]
- 29.Satoh-Takayama N, Vosshenrich CA, Lesjean-Pottier S, Sawa S, Lochner M, Rattis F, et al. Microbial flora drives interleukin 22 production in intestinal NKp46+ cells that provide innate mucosal immune defense. Immunity. 2008;29(6):958–970. doi: 10.1016/j.immuni.2008.11.001. [DOI] [PubMed] [Google Scholar]
- 30.Biron CA, Nguyen KB, Pien GC, Cousens LP, Salazar-Mather TP. Natural killer cells in antiviral defense: function and regulation by innate cytokines. Annu Rev Immunol. 1999;17:189–220. doi: 10.1146/annurev.immunol.17.1.189. [DOI] [PubMed] [Google Scholar]
- 31.Chang YJ, Kim HY, Albacker LA, Baumgarth N, McKenzie AN, Smith DE, et al. Innate lymphoid cells mediate influenza-induced airway hyper-reactivity independently of adaptive immunity. Nat Immunol. 2011;12(7):631–638. doi: 10.1038/ni.2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cella M, Fuchs A, Vermi W, Facchetti F, Otero K, Lennerz JK, et al. A human natural killer cell subset provides an innate source of IL-22 for mucosal immunity. Nature. 2009;457(7230):722–725. doi: 10.1038/nature07537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Crellin NK, Trifari S, Kaplan CD, Cupedo T, Spits H. Human NKp44+IL-22+ cells and LTi-like cells constitute a stable RORC+ lineage distinct from conventional natural killer cells. J Exp Med. 2010;207(2):281–290. doi: 10.1084/jem.20091509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Reeves RK, Rajakumar PA, Evans TI, Connole M, Gillis J, Wong FE, et al. Gut inflammation and indoleamine deoxygenase inhibit IL-17 production and promote cytotoxic potential in NKp44+ mucosal NK cells during SIV infection. Blood. 2011;118(12):3321–3330. doi: 10.1182/blood-2011-04-347260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sun JC, Lopez-Verges S, Kim CC, DeRisi JL, Lanier LL. NK cells and immune “memory”. J Immunol. 2011;186(4):1891–1897. doi: 10.4049/jimmunol.1003035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Paust S, von Andrian UH. Natural killer cell memory. Nat Immunol. 2011;12(6):500–508. doi: 10.1038/ni.2032. [DOI] [PubMed] [Google Scholar]
- 37.Ivanov, Atarashi K, Manel N, Brodie EL, Shima T, Karaoz U, et al. Induction of intestinal Th17 cells by segmented filamentous bacteria. Cell. 2009;139(3):485–498. doi: 10.1016/j.cell.2009.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nigam P, Kwa S, Velu V, Amara RR. Loss of IL-17-producing CD8 T cells during late chronic stage of pathogenic simian immunodeficiency virus infection. J Immunol. 2011;186(2):745–753. doi: 10.4049/jimmunol.1002807. [DOI] [PubMed] [Google Scholar]
- 39.Raffatellu M, Santos RL, Verhoeven DE, George MD, Wilson RP, Winter SE, et al. Simian immunodeficiency virus-induced mucosal interleukin-17 deficiency promotes Salmonella dissemination from the gut. Nat Med. 2008;14(4):421–428. doi: 10.1038/nm1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Aujla SJ, Chan YR, Zheng M, Fei M, Askew DJ, Pociask DA, et al. IL-22 mediates mucosal host defense against Gram-negative bacterial pneumonia. Nat Med. 2008;14(3):275–281. doi: 10.1038/nm1710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zheng Y, Valdez PA, Danilenko DM, Hu Y, Sa SM, Gong Q, et al. Interleukin-22 mediates early host defense against attaching and effacing bacterial pathogens. Nat Med. 2008;14(3):282–289. doi: 10.1038/nm1720. [DOI] [PubMed] [Google Scholar]
- 42.Alter G, Altfeld M. NK cells in HIV-1 infection: evidence for their role in the control of HIV-1 infection. J Intern Med. 2009;265(1):29–42. doi: 10.1111/j.1365-2796.2008.02045.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Reeves RK, Rajakumar PA, Evans TI, Connole M, Gillis J, Wong FE, et al. Gut inflammation and indoleamine deoxygenase inhibit IL-17 production and promote cytotoxic potential in NKp44+ mucosal NK cells during SIV infection. Blood. 2011 doi: 10.1182/blood-2011-04-347260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lee SH, Miyagi T, Biron CA. Keeping NK cells in highly regulated antiviral warfare. Trends Immunol. 2007;28(6):252–259. doi: 10.1016/j.it.2007.04.001. [DOI] [PubMed] [Google Scholar]
- 45.Veazey RS, DeMaria M, Chalifoux LV, Shvetz DE, Pauley DR, Knight HL, et al. Gastrointestinal tract as a major site of CD4+ T cell depletion and viral replication in SIV infection. Science. 1998;280(5362):427–431. doi: 10.1126/science.280.5362.427. [DOI] [PubMed] [Google Scholar]
- 46.Mehandru S, Poles MA, Tenner-Racz K, Horowitz A, Hurley A, Hogan C, et al. Primary HIV-1 infection is associated with preferential depletion of CD4+ T lymphocytes from effector sites in the gastrointestinal tract. J Exp Med. 2004;200(6):761–770. doi: 10.1084/jem.20041196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Favre D, Lederer S, Kanwar B, Ma ZM, Proll S, Kasakow Z, et al. Critical loss of the balance between Th17 and T regulatory cell populations in pathogenic SIV infection. PLoS Pathog. 2009;5(2):e1000295. doi: 10.1371/journal.ppat.1000295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Brenchley JM, Paiardini M, Knox KS, Asher AI, Cervasi B, Asher TE, et al. Differential Th17 CD4 T cell depletion in pathogenic and nonpathogenic lentiviral infections. Blood. 2008 doi: 10.1182/blood-2008-05-159301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hartigan-O'connor DJ, Hirao LA, McCune JM, Dandekar S. Th17 cells and regulatory T cells in elite control over HIV and SIV. Curr Opin HIV AIDS. 2011;6(3):221–227. doi: 10.1097/COH.0b013e32834577b3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Brenchley JM, Price DA, Schacker TW, Asher TE, Silvestri G, Rao S, et al. Microbial translocation is a cause of systemic immune activation in chronic HIV infection. Nat Med. 2006;12(12):1365–1371. doi: 10.1038/nm1511. [DOI] [PubMed] [Google Scholar]
- 51.Estes JD, Harris LD, Klatt NR, Tabb B, Pittaluga S, Paiardini M, et al. Damaged intestinal epithelial integrity linked to microbial translocation in pathogenic simian immunodeficiency virus infections. PLoS Pathog. 2010;6(8):e1001052. doi: 10.1371/journal.ppat.1001052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Klatt NR, Harris LD, Vinton CL, Sung H, Briant JA, Tabb B, et al. Compromised gastrointestinal integrity in pigtail macaques is associated with increased microbial translocation, immune activation, and IL-17 production in the absence of SIV infection. Mucosal Immunol. 2010;3(4):387–398. doi: 10.1038/mi.2010.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Paust S, Gill HS, Wang BZ, Flynn MP, Moseman EA, Senman B, et al. Critical role for the chemokine receptor CXCR6 in NK cell-mediated antigen-specific memory of haptens and viruses. Nat Immunol. 2010;11(12):1127–1135. doi: 10.1038/ni.1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Vivier E, Raulet DH, Moretta A, Caligiuri MA, Zitvogel L, Lanier LL, et al. Innate or adaptive immunity? The example of natural killer cells. Science. 2011;331(6013):44–49. doi: 10.1126/science.1198687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cua DJ, Tato CM. Innate IL-17-producing cells: the sentinels of the immune system. Nat Rev Immunol. 2010;10(7):479–489. doi: 10.1038/nri2800. [DOI] [PubMed] [Google Scholar]
- 56.Eberl G, Marmon S, Sunshine MJ, Rennert PD, Choi Y, Littman DR. An essential function for the nuclear receptor RORgamma(t) in the generation of fetal lymphoid tissue inducer cells. Nat Immunol. 2004;5(1):64–73. doi: 10.1038/ni1022. [DOI] [PubMed] [Google Scholar]
- 57.Ouyang W, Kolls JK, Zheng Y. The biological functions of T helper 17 cell effector cytokines in inflammation. Immunity. 2008;28(4):454–467. doi: 10.1016/j.immuni.2008.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ota N, Wong K, Valdez PA, Zheng Y, Crellin NK, Diehl L, et al. IL-22 bridges the lymphotoxin pathway with the maintenance of colonic lymphoid structures during infection with Citrobacter rodentium. Nat Immunol. 2011;12(10):941–948. doi: 10.1038/ni.2089. [DOI] [PubMed] [Google Scholar]
- 59.Zaph C, Du Y, Saenz SA, Nair MG, Perrigoue JG, Taylor BC, et al. Commensal-dependent expression of IL-25 regulates the IL-23-IL-17 axis in the intestine. J Exp Med. 2008;205(10):2191–2198. doi: 10.1084/jem.20080720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Klatt NR, Brenchley JM. Th17 cell dynamics in HIV infection. Curr Opin HIV AIDS. 2010;5(2):135–140. doi: 10.1097/COH.0b013e3283364846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Douek D. HIV disease progression: immune activation, microbes, and a leaky gut. Top HIV Med. 2007;15(4):114–117. [PubMed] [Google Scholar]
- 62.Kanwar B, Favre D, McCune JM. Th17 and regulatory T cells: implications for AIDS pathogenesis. Curr Opin HIV AIDS. 2010;5(2):151–157. doi: 10.1097/COH.0b013e328335c0c1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rice L, Orlow D, Ceonzo K, Stahl GL, Tzianabos AO, Wada H, et al. CpG oligodeoxynucleotide protection in polymicrobial sepsis is dependent on interleukin-17. J Infect Dis. 2005;191(8):1368–1376. doi: 10.1086/428452. [DOI] [PubMed] [Google Scholar]
- 64.Xu H, Wang X, Pahar B, Moroney-Rasmussen T, Alvarez X, Lackner AA, et al. Increased B7-H1 expression on dendritic cells correlates with programmed death 1 expression on T cells in simian immunodeficiency virus-infected macaques and may contribute to T cell dysfunction and disease progression. J Immunol. 2010;185(12):7340–7348. doi: 10.4049/jimmunol.1001642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wang X, Xu H, Gill AF, Pahar B, Kempf D, Rasmussen T, et al. Monitoring alpha4beta7 integrin expression on circulating CD4+ T cells as a surrogate marker for tracking intestinal CD4+ T-cell loss in SIV infection. Mucosal Immunol. 2009;2(6):518–526. doi: 10.1038/mi.2009.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wang X, Xu H, Pahar B, Alvarez X, Green LC, Dufour J, et al. Simian immunodeficiency virus selectively infects proliferating CD4+ T cells in neonatal rhesus macaques. Blood. 2010;116(20):4168–4174. doi: 10.1182/blood-2010-03-273482. [DOI] [PMC free article] [PubMed] [Google Scholar]