Abstract
Self-management programs that include cognitive behavioral strategies have been shown to improve gastrointestinal symptoms, psychological distress, and quality of life in persons with irritable bowel syndrome (IBS). However, less is known about the physiological impact of such a change. As part of a randomized controlled trial using a Comprehensive Self-Management (CSM) intervention (n = 126) compared to Usual Care (UC) (n = 62), cortisol levels were measured in four weekly first morning urine samples at baseline and at 3, 6, and 12 month follow-up. In addition, diary (28 days) ratings of stress were recorded at baseline, 3, 6, and 12 months. The omnibus test of all three outcome times showed no differences in urine cortisol levels between CSM and UC groups (p = .400); however, at 3 months the CSM group had significantly higher cortisol levels than the UC group (p = .012). The CSM group reported lower daily stress levels (p = .046 from omnibus test of all three time points) than UC, with the effect getting stronger over time. Despite marked improvements in reported stress and previously reported gastrointestinal and psychological distress symptoms at later follow-ups, the CSM program did not reduce urine cortisol levels in adults with IBS. These results suggest that the first void urine cortisol levels are not reflective of self reported daily stress in this patient population.
Keywords: irritable bowel syndrome, self-management, cortisol, stress, adult
Irritable bowel syndrome (IBS) is a functional gastrointestinal (GI) disorder that is defined by recurring abdominal pain/discomfort that is relieved by a bowel movement or associated with changes in stool frequency or consistency (Drossman et al., 2006). IBS affects approximately 5–15% of the population worldwide (Chang & Lu, 2007; Drossman, Corazziari, Talley, Thompson, & Whitehead, 2000; Gwee, Wee, Wong, & Png, 2004; Hungin, Whorwell, Tack, & Mearin, 2003) and twice as many women are diagnosed with IBS than men (Chang, Toner et al., 2006; Voci & Cramer, 2009). High health care utilization is found in patients with IBS. As a result, IBS has a significant impact on healthcare costs in terms of both diagnosis and treatment as well as individual patient’s quality of life (QoL) (Brun-Strang, Dapoigny, Lafuma, Wainsten, & Fagnani, 2007; Nyrop et al., 2007).
Alterations in centrally processing of afferent input from the gastrointestinal tract may contribute to the pathophysiology of IBS (Ballenger et al., 2001; Mulak & Bonaz, 2004). Several studies support the conjecture that patients with IBS have increased levels of psychological distress (e.g., anxiety, depression); moreover, psychological distress and feeling stressed have been associated with the onset or symptom exacerbation (Drossman et al., 1999; Hertig, Cain, Jarrett, Burr, & Heitkemper, 2007; Mayer, Craske, & Naliboff, 2001). Similar to previous studies by Levy et al (1997) and Suls et al (1994), Blanchard et al (2008) found no significant difference between IBS and non-IBS patients on retrospective report of major life events.
Gender Differences in IBS
It has been suggested that gender differences in coping styles and resources may explain the higher occurrence of IBS symptoms and prevalence of this syndrome in women as compared to men (Adeyemo, Spiegel, & Chang, 2010; Lund & Lundeberg, 2008; Rao, 2009). How sex (e.g., ovarian hormones) and/or gender (e.g., social role) contribute to the female predominance in IBS remains to be determined. Several potential physiologic factors, e.g., visceral sensitivity, GI motility and autonomic nervous system homeostasis, may contribute to gender differences in IBS symptoms (Chang, Mayer et al., 2006; Heitkemper & Jarrett, 2008; Lund & Lundeberg, 2008; Schmulson et al., 2010). Women with IBS report greater psychological distress, as well as lower health-related QoL than men with IBS (Chang, Toner et al., 2006). Additional factors such as gender role may contribute to gender differences in QoL and psychological adjustment to a chronic, intermittent health condition (Farnam, Somi, Sarami, & Farhang, 2008; Voci & Cramer, 2009).
Cortisol and Psychological Stress
Psychological, cognitive, and emotional stressors activate the hypothalamic-pituitary-adrenal (HPA) axis in the central nervous system (Pruessner et al., 2010) and result in the release of corticotropin releasing factor (CRF) from the hypothalamus. CRF stimulates the release of adrenocorticotropin hormone (ACTH) from the anterior pituitary, and ultimately, cortisol from the adrenal cortex. Cortisol level has been used as a physiologic indicator of neuroendocrine and psychological responses to stress in groups of patients with chronic disorders such as IBS (Burr, Jarrett, Cain, Jun, & Heitkemper, 2009; Chang et al., 2009; Ehlert, Nater, & Bohmelt, 2005; FitzGerald, Kehoe, & Sinha, 2009), chronic fatigue syndrome (Roberts, Papadopoulos, Wessely, Chalder, & Cleare, 2009) and posttraumatic stress disorder (PTSD) (Yehuda et al., 2009). In addition, cortisol has been used as an objective marker to confirm data reported in questionnaires (Pruessner, Hellhammer, Pruessner, & Lupien, 2003) and improvement following stress reduction programs in various chronic medical conditions (Matousek, Dobkin, & Pruessner, 2010). Pruessner et al (2003) reported a positive association between free salivary cortisol response after awakening and self-report severity of depression and stress levels in young male college students. A recent review in patients with various chronic illnesses by Matousek and colleagues (2010) noted that by using rigorous methodology such as repeated measurement of salivary cortisol levels after awakening, cortisol is a good candidate to assess the effectiveness of mindfulness meditation practice.
A number of studies have demonstrated that acute or chronic physical and psychological stressors modulate GI motor and sensory functions in patients with IBS (Dickhaus et al., 2003; Monnikes et al., 2001; Murray et al., 2004; Posserud et al., 2004). The role of the HPA axis in IBS is less well described. HPA axis dysregulation occurs in some IBS patients (Bohmelt, Nater, Franke, Hellhammer, & Ehlert, 2005; Chang et al., 2009; Dinan et al., 2006; FitzGerald et al., 2009). For example, women with IBS had higher unstimulated 24-hour plasma and first morning void urine cortisol levels than healthy control and IBS non-patients (Chang et al., 2009; Heitkemper et al., 1996). However, others have failed to show IBS versus control differences under stimulated stress (e.g., public speaking and mental stress tests) conditions (Elsenbruch et al., 2006; Posserud et al., 2004). The discrepancy in cortisol levels may due to type of specimen collection (i.e., blood, urine, or saliva). In plasma cortisol is 95% protein-bound and 5% free (active) forms. Only the free form of cortisol is found in urine and saliva. Blood draw may be stressful for subjects thus limiting it use as a repeated measure of stress. While 24-hour urine collection may provide useful information on total cortisol secretion, it can be challenging for subjects to comply with collection procedures. Another approach is to examine overnight urine cortisol (first voided urine) levels. Other investigators have used overnight cortisol in studies of stress and symptoms in a sample of women during the transition from premenopause to postmenopause (Woods & Mitchell, 2010). We utilized this approach in a prior sample of menstruating women and found that women with IBS had higher overnight urine cortisol levels as compared to IBS-nonpatients and controls (Heitkemper et al., 1996).
Interventions for IBS
Currently several pharmacologic and non-pharmacologic treatments for IBS have been developed and tested (Drossman et al., 2003; Lackner et al., 2008; Lackner, Mesmer, Morley, Dowzer, & Hamilton, 2004). Nevertheless, more than two- thirds of IBS patients using a large (n = 1966) internet survey reported dissatisfaction with the medications they currently use to treat their IBS symptoms (Drossman et al., 2009). Currently several pharmacologic and non-pharmacologic treatments are undergoing development and preliminary testing. Over the last 2 decades, several nonpharmacological intervention studies have been found to improve GI symptoms, psychological distress and QoL in patients with IBS (Drossman et al., 2003; Heitkemper et al., 2004; Jarrett et al., 2009; Lackner et al., 2010; Whitehead et al., 2004). A comprehensive self-management (CSM) program developed by our team also demonstrated effectiveness in decreasing psychological distress, GI symptoms and increasing QoL in patients with IBS (Heitkemper et al., 2004; Jarrett et al., 2009) as well as a trend of towards decreased urine cortisol levels in young women with IBS as compared with the usual care group (Heitkemper et al., 2004). However, no studies could be found which examined the impact of a behavioral intervention on indicators of HPA axis activity in concert with the report of daily stress in women and men with IBS.
The aims of these analyses were: 1) To test the effect of a CSM intervention on urine cortisol and daily stress levels at 3, 6, and 12 month follow-ups compared to a usual care (UC) group in adults with IBS, 2) to explore gender differences in urine cortisol levels and daily stress reports at baseline assessment, and 3) to test whether the intervention effectiveness differed in women versus men. We hypothesized that 1) participants in the CSM group would demonstrate greater reduction on cortisol and daily stress levels at 3, 6, and 12 month follow-ups as compared with the UC group and 2) the intervention would have a stronger effect on women than on men.
Materials and Methods
Design
This is a secondary data analysis of data collected during a randomized controlled trial study, Nursing Management of Irritable Bowel Syndrome: Improving Outcomes, conducted between 2004 and 2008 (Jarrett et al., 2009). A three-arm design was used to test the efficacy of a CSM intervention delivered entirely in person (CSM-IP) or predominantly by telephone (CSM-T/IP) as compared with UC. All three groups completed interviews, questionnaires, and kept daily health diaries at baseline and each of three follow-up assessment periods (three, six, and twelve months post-randomization). Four weekly first morning urine samples were collected to determine cortisol and creatinine levels at baseline and follow-up. Subjects were instructed to collect urine first thing upon arising prior to drinking or eating. To control for urine concentration, cortisol levels were divided by urine creatinine levels. A more detail description of study design has been published previously (Jarrett et al., 2009).
Sample
The majority of participants were recruited through community advertisements. The remainder came from letters mailed to patients attending a university-based gastroenterology clinic. To be eligible for the study, participants had to be 18–70 years of age, have a medical diagnosis of IBS, and reported symptoms consistent with the Rome II criteria (Drossman et al., 2000). Individuals were excluded if they had a coexistent history of GI, renal, or gynecological pathology or surgery that might mimic IBS-type symptoms, or who were frequently taking specific medications for IBS (e.g., antidiarrheals, laxatives, or antispasmodics) that had common GI side effects or other medications that would alter cortisol levels. However, women on oral contraceptive pills (OCPs) or hormone replacement therapy (HRT) were not excluded. In addition, participants with comorbid conditions such as severe fibromyalgia, type I or II diabetes mellitus, infectious diseases, or symptoms of dementia were also excluded. Human Subjects’ approval was obtained through the University of Washington Institutional Review Boards (IRB) prior to the recruitment (Jarrett et al., 2009).
Measures
Demographics
Participants self-reported their age (in years), gender (women and men), marital status (single or married), formal education level (less than or at least a baccalaureate degree), employment status (full-time, part-time, unemployed or retired) and predominant bowel pattern (constipation or diarrhea or alternating).
Daily Stress Level
A single item, ‘stressed’, of 26 items in the daily health diary was used. The subjects were asked to ‘mark the highest severity that you experienced with each symptom over the past 24 hours’ on a 5-point Likert scale from 0 = not present, 1 = mild, 2 = moderate, 3 = severe, and 4 = very severe. The overall stress level was computed by collapsing the 28 days of diary for each subject to determine the percent of days with moderate-to-very severe stress.
Urine Cortisol Levels
Cortisol levels were quantified by radioimmunoassay with Coat-A-Count kit developed by Diagnostic Products Corporation (Los Angeles, CA) involving dichloromethane extraction of steroids. Aliquots of sample were added to tubes coated with antibodies against cortisol, exposed to 125-iodinated cortisol and counted in a Packard gamma counter (Cobra II, Downers Grove, IL). Assays were performed on duplicate samples and standards. The cortisol detection limit was 0.3 μg/dl and there was low cross-reactivity with other steroids. The intra- and inter-assay variations were 4.8% and 8.8%, respectively.
Urine Creatinine Levels
Creatinine levels were measured relying on Jaffe’s reaction, wherein a yellow/orange color forms when the metabolite is treated with alkaline picrate. The color derived from creatinine present in the urine was then destroyed at acidic pH. The differences in color before and after acidification at 500 nm were detected by a spectrophotometer/plate reader (Molecular Devices Corporation, Sunnyvale, CA). The creatinine concentration was determined using a creatinine standard curve. The assays were performed on duplicate samples and standards. The intra- and inter-assay variations were 1.9% and 8.5%, respectively.
Statistical Analysis
For the purpose of this study, the data of CSM-IP and CSM-T/IP were combined to one CSM group based on prior comparisons that showed no difference in the outcomes (Jarrett et al., 2009). The primary aim of this study was to test whether participants in the CSM intervention had lower urine cortisol levels and reported lower stress levels than those in the UC group. The urine cortisol and percent of days with moderate-to-very severe stress levels were the primary outcome measures. The cortisol level was expressed in ng per mg creatinine. Log10 transformation was used to reduce the skewness of the cortisol levels. The intraclass correlation coefficient (ICC) was computed for the reliability across the four baseline urine cortisol levels within-person. For all samples combined the ICC were 0.23 for untransformed and 0.34 for log10 transformed cortisol levels. Further, we dropped urine samples with creatinine levels less than 100 mg/dl because a low creatinine level indicates diluted urine (Arndt, 2009) which may mean that urine did not represent the full night urine or first morning void. The ICC was improved to 0.39 for untransformed and 0.36 for log10 transformed cortisol levels. Change scores from baseline to all follow-up times were computed.
The primary outcome analyses were calculated using mixed model analysis of covariance (ANCOVA). Each model contains intervention group (CSM versus UC) and three follow-up occasions (3, 6, and 12 months post-randomization) as fixed factors, subject number as random factor, and baseline values of the outcome measure, age, and gender as covariates. The primary outcomes are changes from baseline to all three follow-up periods on cortisol levels and percent of days with moderate-to-very severe stress levels. The coefficient on intervention group measures how much the CSM group differs from the UC group at the three follow-up times, on average, and the corresponding p-value tests whether this difference is statistically significant. In addition to this omnibus test, ANCOVA was used in separate analyses for each follow-up time point (3, 6, and 12 month post randomization). With this sample size, the power analysis showed that at each time point there is 80% power for detecting a difference of 0.15 on log10 of cortisol levels and eleven point differences on stress level scale.
The second aim was to explore gender differences on urine cortisol and self-reported daily stress levels at baseline assessment. ANOVA was used to analyze cortisol and percent of days with moderate-to-very severe stress levels in women and men with IBS at baseline. A p value of < .05 was accepted as the level of significance.
The third aim was to test whether intervention effectiveness differed between women and men. A mixed model ANCOVA was used to analyze the change in cortisol and percent of days with moderate-to-very severe stress levels from baseline to all three follow-up periods. The coefficient for the gender by group interaction measures how much the treatment effect differs between women and men at the three follow-up times, on average, and the corresponding p-value tests whether this difference is statistically significant at the p-value < .05. In addition, ANCOVA was used in separate analyses on each follow-up time point (3, 6, and 12 month post-randomization).
Results
Sample Description
Subjects were randomized to the CSM groups (n = 126) and the UC group (n = 62). The demographics are described elsewhere (Jarrett et al., 2009). Mean age of the patients were 44 ± 14 years. The majority of the patients were women, Caucasian, and relatively well educated. The demographic data between CSM and UC groups were not significantly different.
At baseline, 143 women and 31 men provided urine for cortisol/creatinine data and 161 women and 26 men provided self-reported percent of days with moderate-to-very severe stress levels. The baseline sample sizes apply to Aim 2 analyses. Results for Aims 1 and 3 are based on subjects who provided outcome data on at least one of the follow-up occasions (three, six, and twelve months post randomization). Post intervention 90 women and 15 men provided the data for urine cortisol/creatinine levels and 140 women and 20 men provided data for percent of days with moderate-to-very severe stress levels. Twenty-seven women were taking OCPs or receiving HRT.
Intervention Effect on Cortisol and Stress Levels
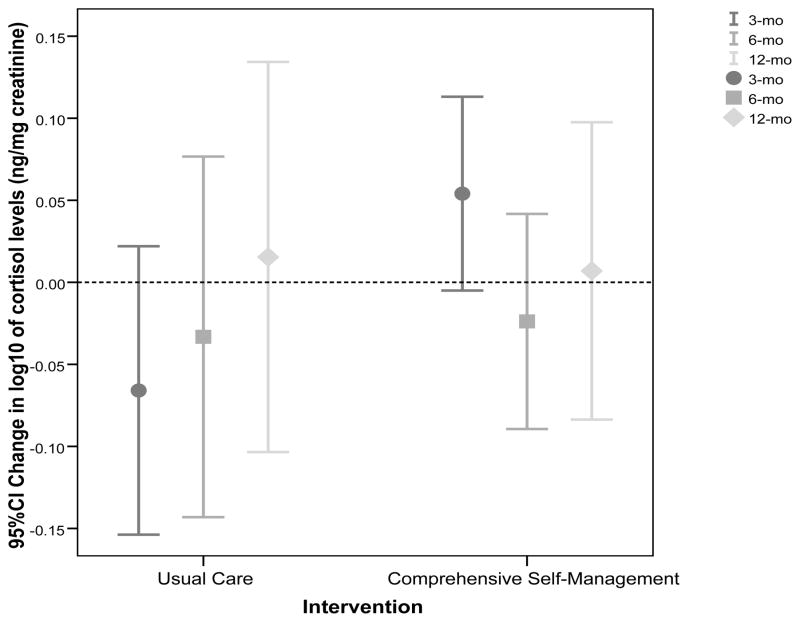
Table 1 shows that log10 of urine cortisol levels are not significantly different at baseline between CSM and UC groups. The mixed model ANCOVA demonstrated a non-significant main effect of CSM on log10 of urine cortisol level changes from baseline to each follow-up period, as well as no interaction effect between CSM and time (p = .400 and .287). Further examination of each follow-up time point showed log10 of urine cortisol levels decreased from baseline to three-month follow up in the UC group but increased in the CSM group, p = .012 after controlling for covariates. However, there were no group differences at the six- and twelve-month follow-up. These results are further illustrated in Figure 1. The graph shows an increasing trend on cortisol levels in the UC group toward the end of follow-up period as compared to the CSM group.
Table 1.
Mean (SD) of Baseline and Change Scores in Urine Cortisol and Percent of Days with Moderate-to-Very Severe Stress Levels by Intervention Group.
| Variable | Mean (SD)
|
Mean (SD) change score from baseline
|
P valuea | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Baseline | n | 3 Months | n | 6 Months | n | 12 Months | n | ||
| Log10 of cortisol levels (ng/mg creatinine) | |||||||||
| UC | 1.43 (0.20) | 35 | −0.07 (0.23) | 29 | −0.03 (0.28) | 28 | 0.02 (0.27) | 22 | .400/.287 |
| CSM | 1.43 (0.20) | 70 | 0.05 (0.22) | 54 | −0.02 (0.24) | 53 | 0.01 (0.32) | 50 | |
| P valueb | .957 | .012 | .821 | .531 | |||||
|
| |||||||||
| Percent of days with moderate-to-very severe stress levels | |||||||||
| UC | 24.6 (22.6) | 53 | −1.5 (19.5) | 45 | 3.8 (22.0) | 46 | 4.3 (25.1) | 48 | .046/.034 |
| CSM | 23.3 (22.5) | 107 | −1.4 (20.8) | 100 | −3.2 (19.1) | 96 | −4.4 (17.9) | 96 | |
| P valueb | .749 | .939 | .056 | .009 | |||||
UC, usual care; CSM, comprehensive self-management.
P values are from mixed model analysis of covariance, using all three follow-up periods to test the effect of CSM controlling for baseline of the outcome measure, age, and gender, first p value illustrates the main effect of CSM across three time points and second p value represents the interaction effect between CSM and follow-up time point.
P values are from analysis of covariance that test the effect of CSM at each follow-up time point (3, 6, and 12 months) controlling for baseline of the outcome measure, age, and gender.
Table includes all subjects who provided outcome data for at least one of the follow-ups.
Figure 1.
Mean (95% CI) of change in log10 of cortisol levels (ng/mg creatinine) in urine from baseline across three follow-up assessments in the comprehensive self-management and the usual care groups. The dotted line at zero represents no change from baseline cortisol levels.
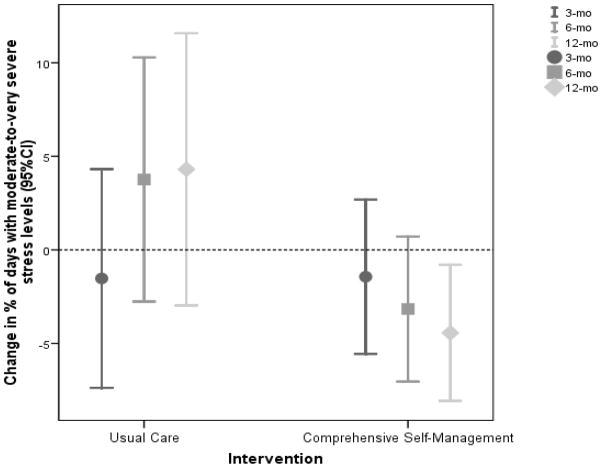
The mixed model ANCOVA demonstrated significant self-reported daily stress changes from baseline, as well as an interaction effect between CSM and time (p = .046 and .034). The percent of days with moderate-to-very severe stress at 6 and 12 months decreased more in the CSM group than the UC group, p = .056 and p = .009 respectively. These results are further depicted in Figure 2. The graph shows a significantly greater reduction in daily stress levels in the CSM group as compared to the UC group at follow up points.
Figure 2.
Mean (95% CI) of change in percent of days with moderate-to-very severe stress levels from baseline across three follow-up assessments in the comprehensive self-management and the usual care groups by daily self report. The dotted line at zero represents no change from baseline percent of days with moderate-to-very severe stress levels.
Excluding women who were using OCPs or HRT did not substantially change the results of urine cortisol and stress levels.
Gender Differences in Cortisol and Stress Levels
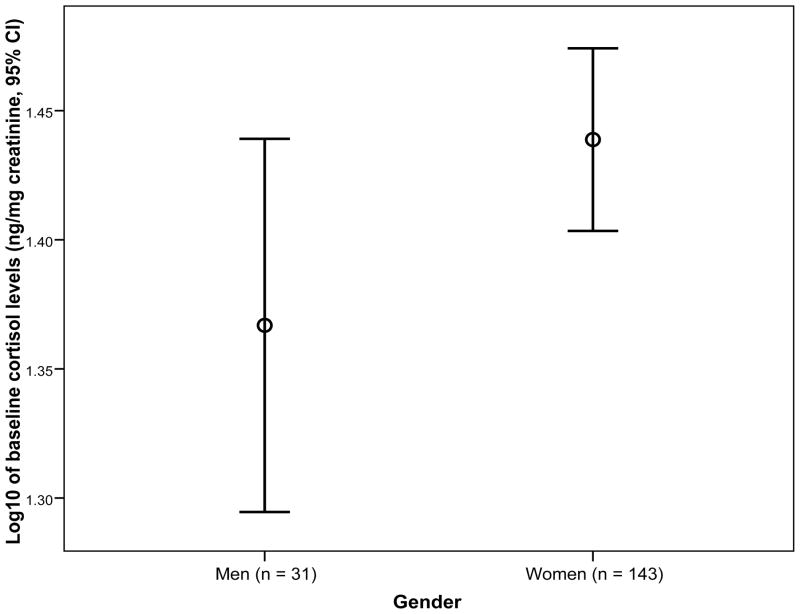
Figure 3 illustrates the mean on log10 of urine cortisol levels at baseline in women and men with IBS. In this analysis, women with IBS had higher (albeit a trend toward significant) mean cortisol levels than men prior to the intervention (M = 1.44, SD = 0.21 vs. M = 1.37, SD = 0.20 ng/mg creatinine, p = .087). After controlling for creatinine and age, there was no significant gender difference on cortisol levels at baseline (p = .275). When the percent of days with moderate-to-very severe stress levels at baseline were compared, women had higher but not significantly different stress levels (M = 25.6, SD = 23.4) compared to men (M = 22.0, SD = 27.3), p = .463.
Figure 3.
Mean (95% CI) on log10 of cortisol levels (ng/mg creatinine) at baseline in women and men. The number of subjects is in the parentheses next to each gender. No significant difference was found after controlling for baseline creatinine and age.
Table 2 demonstrates means (SDs) of baseline and change scores in log10 of urine cortisol and percent of days with moderate-to-very severe stress levels in women and men with IBS by intervention. The mixed model analysis across three time follow up points showed non-significant interactions between intervention and group for both cortisol (p = .175) and stress (p = .277). Analyses of individual time points showed a marginally significant interaction for stress at 3 months follow-up (p = .043).
Table 2.
Mean (SD) of Baseline and Change Scores in Urine Cortisol and Percent of Days with Moderate-to-Very Severe Stress Levels by Gender and Intervention Group
| Variable | Mean (SD)
|
Mean (SD) change score from baseline
|
P valuea | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Baseline | n | 3 Months | n | 6 Months | n | 12 Months | n | ||
| Log10 of cortisol levels (ng/mg creatinine) | |||||||||
| Women | |||||||||
| UC | 1.45 (0.21) | 30 | −0.07 (0.25) | 24 | −0.05 (0.31) | 23 | 0.02 (0.30) | 17 | .175 |
| CSM | 1.42 (0.19) | 60 | 0.05 (0.20) | 46 | −0.00 (0.22) | 45 | 0.01 (0.33) | 42 | |
| Men | |||||||||
| UC | 1.30 (0.07) | 5 | −0.07 (0.17) | 5 | 0.03 (0.15) | 5 | 0.00 (0.13) | 5 | |
| CSM | 1.44 (0.26) | 10 | 0.07 (0.33) | 8 | −0.14 (0.32) | 8 | −0.00 (0.29) | 8 | |
| P valueb | .357 | .353 | .815 | .218 | |||||
|
| |||||||||
| Percent of days with moderate-to-very severe stress levels | |||||||||
| Women | |||||||||
| UC | 26.6 (22.8) | 46 | −1.8 (18.7) | 38 | 3.0 (20.2) | 39 | 2.1 (24.5) | 41 | .277 |
| CSM | 24.2 (22.3) | 94 | −3.6 (20.5) | 88 | −4.3 (18.6) | 83 | −6.0 (17.3) | 83 | |
| Men | |||||||||
| UC | 11.2 (17.3) | 7 | 0.0 (24.6) | 7 | 8.2 (32.0) | 7 | 17.4 (26.2) | 7 | |
| CSM | 17.4 (24.0) | 13 | 14.5 (15.5) | 12 | 4.0 (21.6) | 13 | 5.8 (19.3) | 13 | |
| P valueb | .384 | .043 | .519 | .976 | |||||
UC, usual care; CSM, comprehensive self-management.
P values are from mixed model analysis of covariance, using all three follow-up periods to test the interaction effect between gender and the intervention controlling for baseline of the outcome measure and age.
P values are from analysis of covariance that test the interaction effect between gender and the intervention at each follow-up time point (3, 6, and 12 months) controlling for baseline of the outcome measure and age.
Table includes all subjects who provided outcome data for at least one of the follow-ups
Discussion
The purpose of this study was to determine the effects of a CSM intervention on both urine cortisol levels and self report of daily stress in patients with IBS. The findings of this study indicate that despite improvement in self report of daily stress, the intervention did not lead to a reduction in first void urine cortisol levels.
In the current study stress was quantified with the use of a single daily diary item ‘stressed’. In an early study, we reported that 23 women with GI symptoms typical of IBS but without a diagnosis of IBS had significantly higher daily stress levels (M = 1.9, SD = 0.7) than 26 healthy women (M = 1.4, SD = 0.6), p = .030 (Levy et al., 1997). Women with diagnosed IBS also had higher levels of daily stress (M = 1.8, SD = 0.7) but not significantly different than the healthy comparison group. In a subsequent comparison in another population, we found that women with IBS had significantly higher levels of daily stress when compared to women without IBS. In that study, IBS women reported stress due to parental role, relationships with others, and work or school (Hertig et al., 2007). Similarly, Whitehead et al (1992) reported that the 39 women with IBS had significantly more stressful events and more severe ratings of those events than 232 normal comparison women and 108 women with other functional bowel diseases when assessed every three months over a one-year period.
Other studies of cognitive behavioral therapy (CBT) in IBS patients have not examined daily self-reported stress as an outcome measure. Although GI symptoms decreased at 3 and 6 months (Jarrett et al., 2009), self reported stress only began to decrease at 6 months and persisted to 12 months suggesting that the link between stress and abdominal symptoms in IBS may not be as straightforward as originally thought. It may be the case that patients with IBS receiving CBT deal better with their stress and modify their lifestyle accordingly over time.
The positive effect on self report of stress following a cognitively focused self management CSM intervention without a change in cortisol levels is comparable to a study in 24 men with coronary artery disease (CAD) (Ryden, Hedback, & Jonasson, 2009). Ryden and colleagues using a pre-test post-test design found that stress management behavioral therapy delivered in 20 sessions over a 1-year period failed to produce decreases in salivary cortisol despite decreases in everyday self-reported stress. The use of biobehavioral indices of improvement in patients with IBS has received little attention. Heymann-Monnikes et al (2000) tested a 10-week CBT intervention on QoL and laboratory rectal visceral sensitivity as measured with the barostat. Despite improvements in QoL as measured at 3 and 6 month follow-ups, there were no differences in rectal sensitivity indicating improvements in subjective but not a physiologic measure. Such findings, albeit limited with regard to IBS patients, suggest that current biomarkers of visceral sensitivity and overnight cortisol levels may not be sensitive enough to reflect self report of stress and QoL.
Other investigators using different patient populations have utilized cortisol as a marker of stress in conjunction with self report of stress levels following a variety of stress-reducing interventions (Galvin, Benson, Deckro, Fricchione, & Dusek, 2006; Matousek et al., 2010; Walton, Pugh, Gelderloos, & Macrae, 1995; Yehuda et al., 2009). Again, despite consistent improvements in self-reported stress or QoL with these interventions, there is less certainty as to whether there is a concomitant impact on the HPA axis. Regardless of the duration, stress-related reduction programs do elicit similar positive psychological effects but with inconsistent changes in physiological measures (Cummins & Gevirtz, 1993; Hansen, Garde, Skovgaard, & Christensen, 2001).
This analysis began with the conjecture that the stress of IBS would lead to increased cortisol in IBS patients, and that CSM would lead to stress reduction and hence lowered cortisol. However, the literature relating to this conjecture is conflicting. Four studies have measured basal cortisol levels in patients with IBS. Chang et al (2009) found significantly lower basal plasma ACTH levels and slightly higher basal plasma cortisol levels in young women with IBS as compared with healthy control women. Additionally, the investigators reported a significant positive correlation of plasma cortisol levels with anxiety symptoms rather than IBS symptoms (e.g., abdominal pain and bowel pattern) prior to a visceral stimulus. Similarly, in our prior study with a separate sample (Heitkemper et al., 1996) higher basal afternoon urine cortisol levels were found in women with IBS as compared to those IBS non-patients (symptoms of IBS but no diagnosis) and healthy controls. On the contrary, Bohmelt et al (2005) found significantly lower salivary cortisol levels after awakening in patients with IBS as compared to controls. Our recent publication (Heitkemper, Cain, Burr, Jun, & Jarrett, In Press), using a separate sample from that reported here, found lower first morning urine cortisol in women with IBS compared to women without IBS. There has been also conflicting evidence on the response to an acute stressor in persons with IBS compared to healthy controls. A blunted cortisol response has been shown in IBS patients exposed to CRH (Bohmelt et al., 2005) and lumbar puncture (FitzGerald et al., 2009), however Chang et al (2009) found an increased cortisol response to sigmoidoscopy among persons with IBS. Inconsistent observations may be due to different subject populations (e.g., length of time since diagnosis), environments, specimen collection (e.g., plasma, urine, or saliva; spot or serial), time of specimen collection, and study designs.
We found significantly higher cortisol levels at 3 months post randomization in the CSM group as compared to the UC group. This could be a spurious result due to the multiple comparisons that were performed, or it could be a real effect, with an initial low cortisol and the increase in cortisol at 3 months representing improved regulation of the HPA axis that is increased to normal. Given the lack of data on the normal range for first morning urine cortisol among healthy people, it is not possible to tell whether the subjects with IBS had baseline urine cortisol levels that were higher or lower than normal. Other investigators have found increased cortisol in response to psychological interventions. For example, a longitudinal psychotherapeutic intervention study in 28 PTSD survivors of the World Trade Center attacks found increased 24-hour urine cortisol levels in those who significantly improved their PTSD and depression severity as compared with those who did not improve, at both post-treatment and at the 3-month follow-up (Yehuda et al., 2009). Another study, Roberts and co-investigators (2009) reported significantly increased (p < .05) daily salivary cortisol levels following a 15-session CBT in patients with chronic fatigue syndrome. However, in that study, salivary cortisol levels did not differ between those who responded and those who did not respond to the therapy (Roberts et al., 2009). Thus, the increase in overnight urine cortisol levels in our study at 3 months is somewhat consistent with that found in other populations following a cognitive intervention.
To our knowledge, this is the first self-management intervention study of gender differences and cortisol levels in concert with self report of daily stress levels in patients with IBS. Women with IBS are more likely to have other co-existing health problems (fatigue, sleep disturbances) (Cain et al., 2009), as well as psychological distress (depression) and poorer QoL than men (Chang, Toner et al., 2006). We found slightly higher urine cortisol and self-reported stress levels in women with IBS relative to men at baseline. However, this was not significant after controlling for creatinine and age.
As compared to men, women with IBS in both the CSM and UC groups were more likely to report a greater reduction in daily stress post intervention. In addition, women in the CSM group had greater change scores in self-reported stress levels than those in the UC group. In another study, women with IBS were more likely to change dietary habits following a dietary intervention as compared to men (Faresjo, Johansson, Faresjo, Roos, & Hallert, 2010). In IBS, male versus female differences may be due to differences in coping style, higher levels of somatic and psychological symptoms, and health seeking patterns (Hungin, Chang, Locke, Dennis, & Barghout, 2005). Gender-related differences in IBS have been addressed in a number of published reviews (Adeyemo et al., 2010; Chang, Toner et al., 2006; Farnam et al., 2008). One study of 200 women and 50 men with IBS in Great Britain reported that women had higher response rate and overall improvement post hypnotherapy relative to men, and men demonstrated a lower long-term improvement in IBS symptoms following the treatment than women (Gonsalkorale, Miller, Afzal, & Whorwell, 2003).
The current study has several limitations. First, this study used an existing data set that had a relatively small number of men. Second, there was no healthy non-IBS control group to compare the overnight cortisol levels in first voided urine with the intervention groups. A onetime urine collection after awakening may not adequately reflect HPA axis activity. Urine collection may be a burden for the participants. Third, we were unable to verify subject compliance with the desired protocol for urine collection. Although subjects were instructed on the importance of following the study protocol, subjects were not asked to report the collection time. In spite of those attempts, it is possible that the samples were not the first morning voided urine. Furthermore, we eliminated samples in which the urine creatinine values were less than 100 mg/dl because of concerns that samples may not have been representative of overnight values (Arndt, 2009).
Salivary cortisol levels have been used in many studies related to HPA axis activity (Bohmelt et al., 2005; Ehlert et al., 2005), self-report stress response (Almeida, McGonagle, & King, 2009; Pruessner et al., 2003), and intervention effect (Galvin et al., 2006; Matousek et al., 2010; Roberts et al., 2009; Ryden et al., 2009). The collection of saliva for cortisol levels is less burdensome for the subjects as compared to blood or urine. Therefore, the utility of using repeated measures of salivary cortisol remains to be explored in intervention trials in patients with IBS.
In conclusion, the comprehensive self-management program shows effectiveness in self report of daily stress reduction but no effect on reducing morning urine cortisol level as compared with the usual care over time. There is no evidence of a differential effect of treatment on women compared to men; however, the sample size of men is small meaning the power of this comparison is low.
Acknowledgments
Supported by grants from the NINR, NIH (R01 NR004142) and the Center for Women’s Health and Gender Research (P30 NR004001). We would like to thank our research nurses, Pam Barney and Pam Weisman, who conducted the intervention sessions; Ernie Tolentino and Joyce Tsuji, who conducted the laboratory assay; and to the patients who volunteered to participate.
Contributor Information
Wimon Deechakawan, Email: wimond@u.washington.edu.
Kevin C. Cain, Email: cain@u.washington.edu.
Monica E. Jarrett, Email: jarrett@u.washington.edu.
Robert L. Burr, Email: bobburr@u.washington.edu.
Margaret M. Heitkemper, Email: heit@u.washington.edu.
References
- Adeyemo MA, Spiegel BM, Chang L. Meta-analysis: do irritable bowel syndrome symptoms vary between men and women? Alimentary Pharmacology and Therapeutics. 2010;32:738–755. doi: 10.1111/j.1365-2036.2010.04409.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almeida DM, McGonagle K, King H. Assessing daily stress processes in social surveys by combining stressor exposure and salivary cortisol. Biodemography and Social Biology. 2009;55:219–237. doi: 10.1080/19485560903382338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arndt T. Urine-creatinine concentration as a marker of urine dilution: reflections using a cohort of 45,000 samples. Forensic Science International. 2009;186:48–51. doi: 10.1016/j.forsciint.2009.01.010. [DOI] [PubMed] [Google Scholar]
- Ballenger JC, Davidson JR, Lecrubier Y, Nutt DJ, Lydiard RB, Mayer EA. Consensus statement on depression, anxiety, and functional gastrointestinal disorders. Journal of Clinical Psychiatry. 2001;62(Suppl 8):48–51. [PubMed] [Google Scholar]
- Blanchard EB, Lackner JM, Jaccard J, Rowell D, Carosella AM, Powell C, Kuhn E. The role of stress in symptom exacerbation among IBS patients. Journal of Psychosomatic Research. 2008;64:119–128. doi: 10.1016/j.jpsychores.2007.10.010. [DOI] [PubMed] [Google Scholar]
- Bohmelt AH, Nater UM, Franke S, Hellhammer DH, Ehlert U. Basal and stimulated hypothalamic-pituitary-adrenal axis activity in patients with functional gastrointestinal disorders and healthy controls. Psychosomatic Medicine. 2005;67:288–294. doi: 10.1097/01.psy.0000157064.72831.ba. [DOI] [PubMed] [Google Scholar]
- Brun-Strang C, Dapoigny M, Lafuma A, Wainsten JP, Fagnani F. Irritable bowel syndrome in France: quality of life, medical management, and costs: the Encoli study. European Journal of Gastroenterology & Hepatology. 2007;19:1097–1103. doi: 10.1097/MEG.0b013e3282f1621b. [DOI] [PubMed] [Google Scholar]
- Burr RL, Jarrett ME, Cain KC, Jun SE, Heitkemper MM. Catecholamine and cortisol levels during sleep in women with irritable bowel syndrome. Neurogastroenterology and Motility. 2009;21:1148–e1197. doi: 10.1111/j.1365-2982.2009.01351.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cain KC, Jarrett ME, Burr RL, Rosen S, Hertig VL, Heitkemper MM. Gender differences in gastrointestinal, psychological, and somatic symptoms in irritable bowel syndrome. Digestive Diseases and Sciences. 2009;54:1542–1549. doi: 10.1007/s10620-008-0516-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang FY, Lu CL. Irritable bowel syndrome in the 21st century: perspectives from Asia or South-east Asia. Journal of Gastroenterology and Hepatology. 2007;22:4–12. doi: 10.1111/j.1440-1746.2006.04672.x. [DOI] [PubMed] [Google Scholar]
- Chang L, Mayer EA, Labus JS, Schmulson M, Lee OY, Olivas TI, Naliboff BD. Effect of sex on perception of rectosigmoid stimuli in irritable bowel syndrome. American Journal of Physiology, Regulatory, Integrative and Comparative Physiology. 2006;291:R277–284. doi: 10.1152/ajpregu.00729.2005. [DOI] [PubMed] [Google Scholar]
- Chang L, Sundaresh S, Elliott J, Anton PA, Baldi P, Licudine A, Mayer EA. Dysregulation of the hypothalamic-pituitary-adrenal (HPA) axis in irritable bowel syndrome. Neurogastroenterology and Motility. 2009;21:149–159. doi: 10.1111/j.1365-2982.2008.01171.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang L, Toner BB, Fukudo S, Guthrie E, Locke GR, Norton NJ, Sperber AD. Gender, age, society, culture, and the patient’s perspective in the functional gastrointestinal disorders. Gastroenterology. 2006;130:1435–1446. doi: 10.1053/j.gastro.2005.09.071. [DOI] [PubMed] [Google Scholar]
- Cummins SE, Gevirtz RN. The relationship between daily stress and urinary cortisol in a normal population: an emphasis on individual differences. Behavioral Medicine. 1993;19:129–134. doi: 10.1080/08964289.1993.9935182. [DOI] [PubMed] [Google Scholar]
- Dickhaus B, Mayer EA, Firooz N, Stains J, Conde F, Olivas TI, Naliboff BD. Irritable bowel syndrome patients show enhanced modulation of visceral perception by auditory stress. American Journal of Gastroenterology. 2003;98:135–143. doi: 10.1111/j.1572-0241.2003.07156.x. [DOI] [PubMed] [Google Scholar]
- Dinan TG, Quigley EM, Ahmed SM, Scully P, O’Brien S, O’Mahony L, Keeling PW. Hypothalamic-pituitary-gut axis dysregulation in irritable bowel syndrome: plasma cytokines as a potential biomarker? Gastroenterology. 2006;130:304–311. doi: 10.1053/j.gastro.2005.11.033. [DOI] [PubMed] [Google Scholar]
- Drossman DA, Corazziari E, Delvaux M, Spiller RC, Talley NJ, Thompson WG, Whitehead WE. Rome III The functional gastrointestinal disorders. 3. McLean, VA: Degnon Associates, Inc; 2006. [Google Scholar]
- Drossman DA, Corazziari E, Talley NJ, Thompson WG, Whitehead WE. Rome II: The functional gastrointestinal disorders. 2. Lawrence, KS: Allen Press, Inc; 2000. [Google Scholar]
- Drossman DA, Creed FH, Olden KW, Svedlund J, Toner BB, Whitehead WE. Psychosocial aspects of the functional gastrointestinal disorders. Gut. 1999;45(Suppl 2):II25–30. doi: 10.1136/gut.45.2008.ii25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drossman DA, Morris CB, Schneck S, Hu YJ, Norton NJ, Norton WF, Bangdiwala SI. International survey of patients with IBS: symptom features and their severity, health status, treatments, and risk taking to achieve clinical benefit. Journal of Clinical Gastroenterology. 2009;43:541–550. doi: 10.1097/MCG.0b013e318189a7f9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drossman DA, Toner BB, Whitehead WE, Diamant NE, Dalton CB, Duncan S, Bangdiwala SI. Cognitive-behavioral therapy versus education and desipramine versus placebo for moderate to severe functional bowel disorders. Gastroenterology. 2003;125:19–31. doi: 10.1016/s0016-5085(03)00669-3. [DOI] [PubMed] [Google Scholar]
- Ehlert U, Nater UM, Bohmelt A. High and low unstimulated salivary cortisol levels correspond to different symptoms of functional gastrointestinal disorders. Journal of Psychosomatic Research. 2005;59:7–10. doi: 10.1016/j.jpsychores.2005.03.005. [DOI] [PubMed] [Google Scholar]
- Elsenbruch S, Lucas A, Holtmann G, Haag S, Gerken G, Riemenschneider N, Schedlowski M. Public speaking stress-induced neuroendocrine responses and circulating immune cell redistribution in irritable bowel syndrome. American Journal of Gastroenterology. 2006;101:2300–2307. doi: 10.1111/j.1572-0241.2006.00837.x. [DOI] [PubMed] [Google Scholar]
- Faresjo A, Johansson S, Faresjo T, Roos S, Hallert C. Sex differences in dietary coping with gastrointestinal symptoms. European Journal of Gastroenterology and Hepatology. 2010;22:327–333. doi: 10.1097/MEG.0b013e32832b9c53. [DOI] [PubMed] [Google Scholar]
- Farnam A, Somi MH, Sarami F, Farhang S. Five personality dimensions in patients with irritable bowel syndrome. Neuropsychiatric Disease and Treatment. 2008;4:959–962. doi: 10.2147/ndt.s3836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FitzGerald LZ, Kehoe P, Sinha K. Hypothalamic-pituitary-adrenal axis dysregulation in women with irritable bowel syndrome in response to acute physical stress. Western Journal of Nursing Research. 2009;31:818–836. doi: 10.1177/0193945909339320. [DOI] [PubMed] [Google Scholar]
- Galvin JA, Benson H, Deckro GR, Fricchione GL, Dusek JA. The relaxation response: reducing stress and improving cognition in healthy aging adults. Complementary Therapies in Clinical Practice. 2006;12:186–191. doi: 10.1016/j.ctcp.2006.02.004. [DOI] [PubMed] [Google Scholar]
- Gonsalkorale WM, Miller V, Afzal A, Whorwell PJ. Long term benefits of hypnotherapy for irritable bowel syndrome. Gut. 2003;52:1623–1629. doi: 10.1136/gut.52.11.1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gwee KA, Wee S, Wong ML, Png DJ. The prevalence, symptom characteristics, and impact of irritable bowel syndrome in an asian urban community. American Journal of Gastroenterology. 2004;99:924–931. doi: 10.1111/j.1572-0241.2004.04161.x. [DOI] [PubMed] [Google Scholar]
- Hansen AM, Garde AH, Skovgaard LT, Christensen JM. Seasonal and biological variation of urinary epinephrine, norepinephrine, and cortisol in healthy women. Clinica Chimica Acta. 2001;309:25–35. doi: 10.1016/s0009-8981(01)00493-4. [DOI] [PubMed] [Google Scholar]
- Heitkemper M, Jarrett M, Cain K, Shaver J, Bond E, Woods NF, Walker E. Increased urine catecholamines and cortisol in women with irritable bowel syndrome. American Journal of Gastroenterology. 1996;91:906–913. [PubMed] [Google Scholar]
- Heitkemper MM, Cain KC, Burr RL, Jun S-E, Jarrett ME. Is childhood abuse or neglect associated with symptom reports and physiological measures in women with irritable bowel syndrome? Biological Research for Nursing. doi: 10.1177/1099800410393274. (In Press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heitkemper MM, Jarrett ME. Update on irritable bowel syndrome and gender differences. Nutrition in Clinical Practice. 2008;23:275–283. doi: 10.1177/0884533608318672. [DOI] [PubMed] [Google Scholar]
- Heitkemper MM, Jarrett ME, Levy RL, Cain KC, Burr RL, Feld A, Weisman P. Self-management for women with irritable bowel syndrome. Clinical Gastroenterology and Hepatology. 2004;2:585–596. doi: 10.1016/s1542-3565(04)00242-3. [DOI] [PubMed] [Google Scholar]
- Hertig VL, Cain KC, Jarrett ME, Burr RL, Heitkemper MM. Daily stress and gastrointestinal symptoms in women with irritable bowel syndrome. Nursing Research. 2007;56:399–406. doi: 10.1097/01.NNR.0000299855.60053.88. [DOI] [PubMed] [Google Scholar]
- Heymann-Monnikes I, Arnold R, Florin I, Herda C, Melfsen S, Monnikes H. The combination of medical treatment plus multicomponent behavioral therapy is superior to medical treatment alone in the therapy of irritable bowel syndrome. American Journal of Gastroenterology. 2000;95:981–994. doi: 10.1111/j.1572-0241.2000.01937.x. [DOI] [PubMed] [Google Scholar]
- Hungin AP, Chang L, Locke GR, Dennis EH, Barghout V. Irritable bowel syndrome in the United States: prevalence, symptom patterns and impact. Alimentary Pharmacology & Therapeutics. 2005;21:1365–1375. doi: 10.1111/j.1365-2036.2005.02463.x. [DOI] [PubMed] [Google Scholar]
- Hungin AP, Whorwell PJ, Tack J, Mearin F. The prevalence, patterns and impact of irritable bowel syndrome: an international survey of 40,000 subjects. Alimentary Pharmacology & Therapeutics. 2003;17:643–650. doi: 10.1046/j.1365-2036.2003.01456.x. [DOI] [PubMed] [Google Scholar]
- Jarrett ME, Cain KC, Burr RL, Hertig VL, Rosen SN, Heitkemper MM. Comprehensive self-management for irritable bowel syndrome: randomized trial of in-person vs. combined in-person and telephone sessions. Am J Gastroenterol. 2009;104:3004–3014. doi: 10.1038/ajg.2009.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lackner JM, Gudleski GD, Keefer L, Krasner SS, Powell C, Katz LA. Rapid response to cognitive behavior therapy predicts treatment outcome in patients with irritable bowel syndrome. Clinical Gastroenterology and Hepatology. 2010;8:426–432. doi: 10.1016/j.cgh.2010.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lackner JM, Jaccard J, Krasner SS, Katz LA, Gudleski GD, Holroyd K. Self-administered cognitive behavior therapy for moderate to severe irritable bowel syndrome: clinical efficacy, tolerability, feasibility. Clinical Gastroenterology and Hepatology. 2008;6:899–906. doi: 10.1016/j.cgh.2008.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lackner JM, Mesmer C, Morley S, Dowzer C, Hamilton S. Psychological treatments for irritable bowel syndrome: a systematic review and meta-analysis. Journal of Consulting and Clinical Psychology. 2004;72:1100–1113. doi: 10.1037/0022-006X.72.6.1100. [DOI] [PubMed] [Google Scholar]
- Levy RL, Cain KC, Jarrett M, Heitkemper MM. The relationship between daily life stress and gastrointestinal symptoms in women with irritable bowel syndrome. Journal of Behavioral Medicine. 1997;20:177–193. doi: 10.1023/a:1025582728271. [DOI] [PubMed] [Google Scholar]
- Lund I, Lundeberg T. Is it all about sex? Acupuncture for the treatment of pain from a biological and gender perspective. Acupuncture in Medicine. 2008;26:33–45. doi: 10.1136/aim.26.1.33. [DOI] [PubMed] [Google Scholar]
- Matousek RH, Dobkin PL, Pruessner J. Cortisol as a marker for improvement in mindfulness-based stress reduction. Complementary Therapies in Clinical Practice. 2010;16:13–19. doi: 10.1016/j.ctcp.2009.06.004. [DOI] [PubMed] [Google Scholar]
- Mayer EA, Craske M, Naliboff BD. Depression, anxiety, and the gastrointestinal system. Journal of Clinical Psychiatry. 2001;62(Suppl 8):28–36. discussion 37. [PubMed] [Google Scholar]
- Monnikes H, Tebbe JJ, Hildebrandt M, Arck P, Osmanoglou E, Rose M, Heymann-Monnikes I. Role of stress in functional gastrointestinal disorders. Evidence for stress-induced alterations in gastrointestinal motility and sensitivity. Digestive Diseases. 2001;19:201–211. doi: 10.1159/000050681. [DOI] [PubMed] [Google Scholar]
- Mulak A, Bonaz B. Irritable bowel syndrome: a model of the brain-gut interactions. Medical Science Monitor. 2004;10:RA55–62. [PubMed] [Google Scholar]
- Murray CD, Flynn J, Ratcliffe L, Jacyna MR, Kamm MA, Emmanuel AV. Effect of acute physical and psychological stress on gut autonomic innervation in irritable bowel syndrome. Gastroenterology. 2004;127:1695–1703. doi: 10.1053/j.gastro.2004.08.057. [DOI] [PubMed] [Google Scholar]
- Nyrop KA, Palsson OS, Levy RL, Korff MV, Feld AD, Turner MJ, Whitehead WE. Costs of health care for irritable bowel syndrome, chronic constipation, functional diarrhoea and functional abdominal pain. Alimentary Pharmacology & Therapeutics. 2007;26:237–248. doi: 10.1111/j.1365-2036.2007.03370.x. [DOI] [PubMed] [Google Scholar]
- Posserud I, Agerforz P, Ekman R, Bjornsson ES, Abrahamsson H, Simren M. Altered visceral perceptual and neuroendocrine response in patients with irritable bowel syndrome during mental stress. Gut. 2004;53:1102–1108. doi: 10.1136/gut.2003.017962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruessner JC, Dedovic K, Pruessner M, Lord C, Buss C, Collins L, Lupien SJ. Stress regulation in the central nervous system: evidence from structural and functional neuroimaging studies in human populations - 2008 Curt Richter Award Winner. Psychoneuroendocrinology. 2010;35:179–191. doi: 10.1016/j.psyneuen.2009.02.016. [DOI] [PubMed] [Google Scholar]
- Pruessner M, Hellhammer DH, Pruessner JC, Lupien SJ. Self-reported depressive symptoms and stress levels in healthy young men: associations with the cortisol response to awakening. Psychosomatic Medicine. 2003;65:92–99. doi: 10.1097/01.psy.0000040950.22044.10. [DOI] [PubMed] [Google Scholar]
- Rao K. Recent research in stress, coping and women’s health. Current Opinion in Psychiatry. 2009;22:188–193. doi: 10.1097/YCO.0b013e328320794a. [DOI] [PubMed] [Google Scholar]
- Roberts AD, Papadopoulos AS, Wessely S, Chalder T, Cleare AJ. Salivary cortisol output before and after cognitive behavioural therapy for chronic fatigue syndrome. Journal of Affective Disorders. 2009;115:280–286. doi: 10.1016/j.jad.2008.09.013. [DOI] [PubMed] [Google Scholar]
- Ryden M, Hedback B, Jonasson L. Does stress reduction change the levels of cortisol secretion in patients with coronary artery disease? Journal of Cardiopulmonary Rehabilitation and Prevention. 2009;29:314–317. doi: 10.1097/HCR.0b013e3181ac785f. [DOI] [PubMed] [Google Scholar]
- Schmulson M, Adeyemo M, Gutierrez-Reyes G, Charua-Guindic L, Farfan-Labonne B, Ostrosky-Solis F, Chang L. Differences in gastrointestinal symptoms according to gender in Rome II positive IBS and dyspepsia in a Latin American population. American Journal of Gastroenterology. 2010;105:925–932. doi: 10.1038/ajg.2010.58. [DOI] [PubMed] [Google Scholar]
- Suls J, Wan CK, Blanchard EB. A multilevel data-analytic approach for evaluation of relationships between daily life stressors and symptomatology: patients with irritable bowel syndrome. Health Psychology. 1994;13:103–113. doi: 10.1037//0278-6133.13.2.103. [DOI] [PubMed] [Google Scholar]
- Voci SC, Cramer KM. Gender-related traits, quality of life, and psychological adjustment among women with irritable bowel syndrome. Quality of Life Research. 2009;18:1169–1176. doi: 10.1007/s11136-009-9532-9. [DOI] [PubMed] [Google Scholar]
- Walton KG, Pugh ND, Gelderloos P, Macrae P. Stress reduction and preventing hypertension: preliminary support for a psychoneuroendocrine mechanism. Journal of Alternative and Complementary Medicine. 1995;1:263–283. doi: 10.1089/acm.1995.1.263. [DOI] [PubMed] [Google Scholar]
- Whitehead WE, Crowell MD, Robinson JC, Heller BR, Schuster MM. Effects of stressful life events on bowel symptoms: subjects with irritable bowel syndrome compared with subjects without bowel dysfunction. Gut. 1992;33:825–830. doi: 10.1136/gut.33.6.825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitehead WE, Levy RL, Von Korff M, Feld AD, Palsson OS, Turner M, Drossman DA. The usual medical care for irritable bowel syndrome. Alimentary Pharmacology & Therapeutics. 2004;20:1305–1315. doi: 10.1111/j.1365-2036.2004.02256.x. [DOI] [PubMed] [Google Scholar]
- Woods NF, Mitchell ES. Sleep symptoms during the menopausal transition and early postmenopause: observations from the Seattle Midlife Women’s Health Study. Sleep. 2010;33:539–549. doi: 10.1093/sleep/33.4.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yehuda R, Bierer LM, Sarapas C, Makotkine I, Andrew R, Seckl JR. Cortisol metabolic predictors of response to psychotherapy for symptoms of PTSD in survivors of the World Trade Center attacks on September 11, 2001. Psychoneuroendocrinology. 2009;34:1304–1313. doi: 10.1016/j.psyneuen.2009.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]