Abstract
Dendritic cell (DC) maturation is characterized by upregulation cell surface MHC class II (MHCII) and costimulatory molecules and production of a variety of cytokines that can shape both innate and adaptive immunity. Paradoxically, transcription of the MHCII genes as well as its activator, CIITA, is rapidly silenced during DC maturation. The mechanisms that control CIITA/MHCII expression and silencing have been not fully understood. We report here that the tumor suppressor TSC1 is a critical regulator of DC function for both innate and adaptive immunity. Its deficiency in DCs results in increased mTOR complex 1 (mTORC1) but decreased mTORC2 signaling, altered cytokine production, impaired CIITA/MHCII expression, and defective antigen presentation to CD4 T cells following TLR4 stimulation. We demonstrate further that IRF4 can directly bind to CIITA promoters and decreased IRF4 expression is partially responsible for decreased CIITA/MHC-II expression in TSC1 deficient DCs. Moreover, we identify that CIITA/MHCII silencing during DC maturation requires mTORC1 activity. Together, our data reveal unexpected roles of TSC1/mTOR that control multifaceted functions of DCs.
INTRODUCTION
Dendritic cells (DCs) are professional antigen presentation cells (APC) specialized for the initiation of immune responses and provide the key link between the innate and adaptive immune systems. They are divided into two major subsets: conventional DCs (cDCs) and plasmacytoid DCs (pDCs). cDCs exist in two functionally and phenotypically distinct states, immature and mature. The immature cDCs are highly active at all forms of endocytosis; express low levels of MHC class II (MHCII) and costimulatory molecules such as CD40, CD80, and CD86 at the cell surface (1, 2). Inflammatory stimuli such as exposure to pathogens trigger an irreversible DC maturation process that is accompanied by increased production of cytokines and expression of costimulatory molecules and MHCII on the cell surface. DC maturation ensures effective induction of adaptive immune responses through presenting antigens by MHC molecules and providing costimulation to T cells (3–5).
MHCII expression in DCs is dynamically regulated by multiple mechanisms. Among those, the MHCII transactivator (CIITA) is essential for MHCII transcription by formation of a multiple component transcription activation complex (6–11). In immature cDCs, CIITA is actively transcribed, leading to high levels of MHCII mRNA expression. However, immature DCs maintain low levels of MHCII protein at the cell surface in the steady state due to ubiquitination of the MHCII β chain, which leads to rapid internalization of the MHCII protein to the endosomal compartment. Upon DC maturation following TLR stimulation, MHCII ubiquitination is rapidly decreased (12–15), allowing translocation of MHCII to cell surface for antigen presentation. Concurrently, during DC maturation, CIITA is rapidly silenced at the transcription level (16). The downregulation of CIITA ensures silencing of new MHCII transcription in mature DCs (17). Silencing of MHCII gene in mature DCs has been proposed to allow temporal ‘fix’ of microbial peptide-MHCII complexes expressed on DC surface to promote specific anti-microbial T cell responses. CIITA repression in mature DCs is known to involve changes in histone acetylation across the gene locus and specific binding of PRDM1 to the promoters (16, 17). However, the mechanisms that trigger CIITA-MHCII silencing during DC maturation is unknown. Recent studies have revealed that the mammalian target of rapamycin (mTOR), a serine-threonine kinase that acts as a central regulator for protein synthesis and cell growth, plays important roles in innate immunity (18). mTOR forms two signaling complexes, mTORC1 and mTORC2, with distinct signaling properties. The mTOR complex 1 (mTORC1) consists of mTOR, raptor, and mLST8; while the mTOR complex 2 (mTORC2) contains mTOR, rictor, and mLST8. mTORC1 phosphorylates pS6K1 and 4E-BP1 to promote cell growth and proliferation and is sensitive to rapamycin inhibition. mTORC2 phosphorylates Akt on serine 473, PKCα, and PKCθ to regulate cell survival, actin polymerization, and Th2 immune response, respectively (19, 20). Although studies of mTOR deficiency in the innate immune cells have not been reported, inhibition of mTORC1 by rapamycin can influence cytokine production following TLR stimulation (21, 22). Furthermore, defects of effector molecules downstream of mTOR have profound impacts on innate immunity. Deficiency of pS6K1/2 substantially decreases TLR- or virus-induced IFN-α production by pDCs (23). In contrast, deficiency of 4E-BP1/2, which suppress translation initiation, causes enhanced IFNα and β production and resistance to viral infection in vitro and in vivo due to increased IRF-7 translation (24).
The tuberous sclerosis complex 1 (TSC1) is a tumor suppressor that associates with TSC2 to form a heterodimer. TSC1 stabilizes TSC2 by preventing ubiquitin-mediated degradation (25). TSC1/2 complex inhibits RheB, a small GTPase protein that promotes to mTORC1 activation (26). Although emerging evidence indicates that TSC1 is a critical regulator in multiple cell lineages within the immune system (27–36), its role in DCs to control adaptive immune responses is unclear. In this study, we demonstrate that mTORC1 is critical for MHCII silencing during DC maturation. TSC1 inhibits mTORC1 activation in DCs to ensuring MHCII expression on DCs, which is required for antigen presentation and CD4 T cell activation. We further reveal that TSC1 through mTORC1 promotes expression of IRF4, which directly binds to the CIITA promoters in DCs resulting in CIITA induction and subsequent MHCII expression.
MATERIALS AND METHODS
Mice and Reagents
TSC1f/f-ERCre mice were previously described (31, 37, 38). CD11cCre mice were purchased from the Jackson Laboratory (39). Mice were intraperitoneally (i.p.) injected with 200 µl 10 mg/ml tamoxifen on day 1, 2 and 5, followed by bone marrow (BM) harvesting on day 8. OTII TCR transgenic mice and C57BL/6 mice were purchased from the Jackson Laboratory. All animals were housed in specific-pathogen free conditions. Experiments described were approved by the Institutional Animal Care and Use Committee of Duke University. Lipopolysaccharides (LPS) from Escherichia coli 0127:B8, rapamycin, and GM-CSF were purchased from Sigma-Aldrich (St. Louis, MO), EMD Biosciences (San Diego, CA), and PeproTech (Rocky Hill, NJ), respectively.
Generation of BMDC
BM cells from femurs and tibias were flushed and plated into Petri dishes containing RPMI 1640 supplemented with 10% FBS, 10mM HEPES-pH7.0, 100 U/ml penicillin, 1,000 U/ml streptomycin, and 20 mM L-glutamine, and 20ng/mL GM-CSF. After 3 days of culture at 37°C in CO2 incubator, half of the medium was replaced by fresh medium with 40 ng/ml GM-CSF. CD11C+ cDC were purified with the Mouse CD11c Positive Selection Kit (STEMCELL Technologies, Vancouver, Canada) on day 7 – 9 according to the manufacturer’s protocol. The purity of CD11c+ cDC was usually greater than 90%.
Flow Cytometry
Cells were stained in PBS containing 2% FBS at 4°C. Antibodies included FITC-conjugate anti-CD11c and -CD4, PE-conjugated anti-CD69, Allophycocyanin-conjugated anti-CD25, a-CD11c, and -CD80, PE/Cy5-conjugated anti-CD86, PE/Cy7-conjugated anti-IA/IE (MHCII), and biotin-conjugated anti-MHC-I. Stained samples were collected using a FACSCanto II Flow Cytometer (BD Bioscience, San Jose, CA) and data were analyzed using FlowJo software Version 9.0. Intracellular staining was performed using BD cytofix/Cytoperm™ Kit.
In vitro and in vivo DC-mediated T cell activation
For in vitro T cell proliferation, 2 ×105 TSC1KO and WT BMDCs per well/48 well-plate were treated with LPS at 100 ng/ml or LPS plus ovalbumin peptide 323–339 (OVA323–339, 2 µg/ml) or OVA257–264 (SIINFEKL, 2 µg/ml) overnight. T cells were purified from OTII TCR or OTI TCR transgenic mice using the negative selection LD column (Miltenyi Biotec, Germany) and labeled with CFSE (Molecular Probes, Eugene, OR) using a previously described protocol (40). 4 × 105 CFSE labeled OTII or OTI T cells were added into each well containing OVA323–339 or OVA257–264 treated TSC1KO and WT BMDC, and then co-cultured for 24 or 72 hours. After 24 and 72 hours, T cells were harvested and stained with the indicated antibodies to assess upregulation of activation markers and proliferation respectively using flow cytometry. For in vivo T cell activation, 1.5 × 106 CFSE-labeled Thy1.1+Thy1.2+ OTII T cells were i.v. injected into Thy1.1+ C57BL/6 mice on day 0. The recipient mice were i.v. injected with 5 × 106 LPS- or LPS + OVA323–339-treated WT or TSC1KO BMDCs on day 1. Splenocytes and LN cells in the recipients were stained and analyzed on day 4.
ELISA
Right after 200,000 purified CD11c+ BMDCs were plated into each well of 24 well-plates (Becton Dickinson Labware, Franklin Lakes, NJ), both TSC1 WT and KO BMDCs were treated with LPS at 10 ng/mL for 0, 3, 6 and 9 hours. The culture supernatants were harvested to detect TNFα, IL12p40, and IL6 cytokine levels determined using commercial ELISA kit for (Biolegend, San Diego, CA) according to the manufacturer’s instruction. The cells were used to isolate total RNA isolation for real-time RT-qPCR.
Realtime RT-qPCR
The total RNA isolation, reverse transcription, and real-time RT-qPCR were performed as previously described (31). Values for qPCR analysis were normalized by the level of β-actin gene, are expressed as fold changes over WT DCs without LPS stimulation or over MIGR1 infected cells, which was arbitrarily considered as 1. The primer pairs are used as follows: β-actin, forward 5’-TGTCCACCTTCCAGCAGATGT-3’ and reverse 5’- AGCTCAGTAACAGTCCGCCTAGA-3’; TNFα, forward 5’-CCCCAAAGGGATGAGAAGTT-3’ and reverse 5’-CACTTGGTGGTTTGCTACGA-3’; IL12p40, forward 5’-TCTGAGCCACTCACATCTGC-3’ and reverse 5’-TTGGTGCTTCACACTTCAGG-3’; IL6, forward 5’-TTCCATCCAGTTGCCTTCTTG-3’ and reverse 5’-TTGGGAGTGGTATCCTCTGTGA-3’; IRF4, forward 5’-GCAGCTCACTTTGGATGACA-3’ and reverse 5’-CCAAACGTCACAGGACATTG-3’; IRF8, forward 5’-GATCGAACAGATCGACAGCA-3’ and reverse 5’-GCTGGTTCAGCTTTGTCTCC-3’; MHCI,forward 5’-GGTGCTGCAGAGCATTACAA-3’ and reverse 5’-GGTCTCCACAAGCTCCATGT-3’; MHCIIβ, forward 5’-TCATCCGTCACAGGAGTCAG-3’ and reverse 5’-TCACAAGAGCTGAGGTGGTG-3’; MHCIIα, forward 5’-GAGTCACACCCTGGAAAGGA-3’ and reverse 5’-ACAGCCTCAGGGTCAAGAGA-3; CIITA, forward 5’-CAGAGGAAGGCTTTGGTGAG-3’ and reverse 5’-GCTTCCTGTGCTTTGAGTCC-3’; CIITA promoters, mCIITA-RT-P1, forward 5’-AGCTGGGTCTGCAACAGTCT-3’, mCIITA-RT-P3F, forward 5’-CTGGCGGTCGGCATCAC-3’, mCIITA-RT-P4F, forward 5’-AGGCAGCACTCAGAAGCACG-3’, and CIITA-RT- Ex2R, reverse 5’-GGTCGGCATCACTGTTAAGG-3’.
Western blot assay
BMDCs, seeded in 6 well-plates in medium containing 5 ng/mL GM-CSF overnight, were rested in serum-free medium without GM-CSF for 5–6 hours, and then treated with LPS (10 ng/mL) for 0, 15, 30, and 45 minutes. Cells were washed once in cold PBS and lysed in 1% Triton-100 lysis buffer (1% Triton-100, 150 mM NaCl, 50 mM Tris, pH 7.4, 1mM EDTA) with protease and phosphatase inhibitor cocktails (Sigma). Cell lysates were subjected to immunoblotting analysis as previously described (41). Rabbit anti-TSC1, TSC2, and IRF8 were obtained from Cell Signaling Technology. Anti-CIITA and goat anti-IRF4 antibodies were from Abcam (Cambridge, MA) and Santa Cruz biotechnology, respectively.
Retroviral transduction
pcDNA3-myc-CIITA, pcDNA3-cFlag-RelB, shRNA for Raptor and shRNA control were purchased from Addgene (Cambridge, MA). Both CIITA and RelB cDNA fragments were cloned into the MIGR1 retroviral vector to generate MIGR-CIITA and MIGR-RelB. IRF4 was a gift from Dr. Kovats (Oklahoma Medical Research Foundation). Retroviruses were generated using the Phoenix-Eco package cell line using the calcium-mediated transfection method. For infection, 2 × 105 BMDC cells in 1 ml culture medium on day4 were mixed with 0.5 ml of viral supernatant in a 6-well plate along with 5 µg/mL of Polybrene. Cells were centrifuged at 2500g for 1.5 hours at room temperature. Medium was changed with fresh DC culture medium 7 hours after infection. Infected cells were used for experiments 48 hours after infection. For some experiments indicated in Figures, GFP+ cells were sorted for isolating total RNA. For shRNA studies, 24 hours after lentivirus infection, cells were treated with puromycin at 2 µg/ml for 4 days.
Chromatin Immunoprecipitation (ChIP)
ChIP was done essentially as described previously (17, 42). Briefly, BMDCs were initially treated with 1% formaldehyde for 10 min to ensure cross-linkage followed by cell and nuclear lysis (50mM Tris, pH 8.1, 10mM EDTA, 1% SDS, 0.5mM PMSF) and shearing. Immunoprecipitated chromatin was collected and washed sequentially with TSE buffer (20 mM Tris, pH 8.1, 50 mM NaCl, 2 mM EDTA, 0.1% SDS, 1.0% Triton X-100) and LiCl buffer (100mM Tris, pH 8.1, 50mM LiCl, 1% Nonidet P-40,1% sodium deoxycholic acid, 1 mM EDTA). DNA was then eluted with 50 mM NaHCO3 containing 1% SDS from the protein A/G beads (Santa Cruz) and reverse-cross-linked at 65 °C overnight followed by proteinase K treatment. DNA was then purified via phenol/chloroform extraction and ethanol precipitation. For each amplification, 3 µl of DNA was analyzed by real-time qPCR. The amplification primers used are: mCIITA p1 forward 5’-CTG CAC CGG AAT GAG GAA AC-3’; mCIITA p1 reverse 5’-TGG AGT CGC CTC TCA TCC A-3’; mCIITA p3 forward, 5’-AGA GTC AGT GTT GCC TAC CA-3’; mCIITA p3 reverse 5’-GAG TTT CAC CCA GAG TGT TG-3’.
Statistical analysis
P values were calculated with Student’s t-test. P values of less than 0.05 were considered significant or very significant.
RESULTS
TSC1 is not essential for DC development
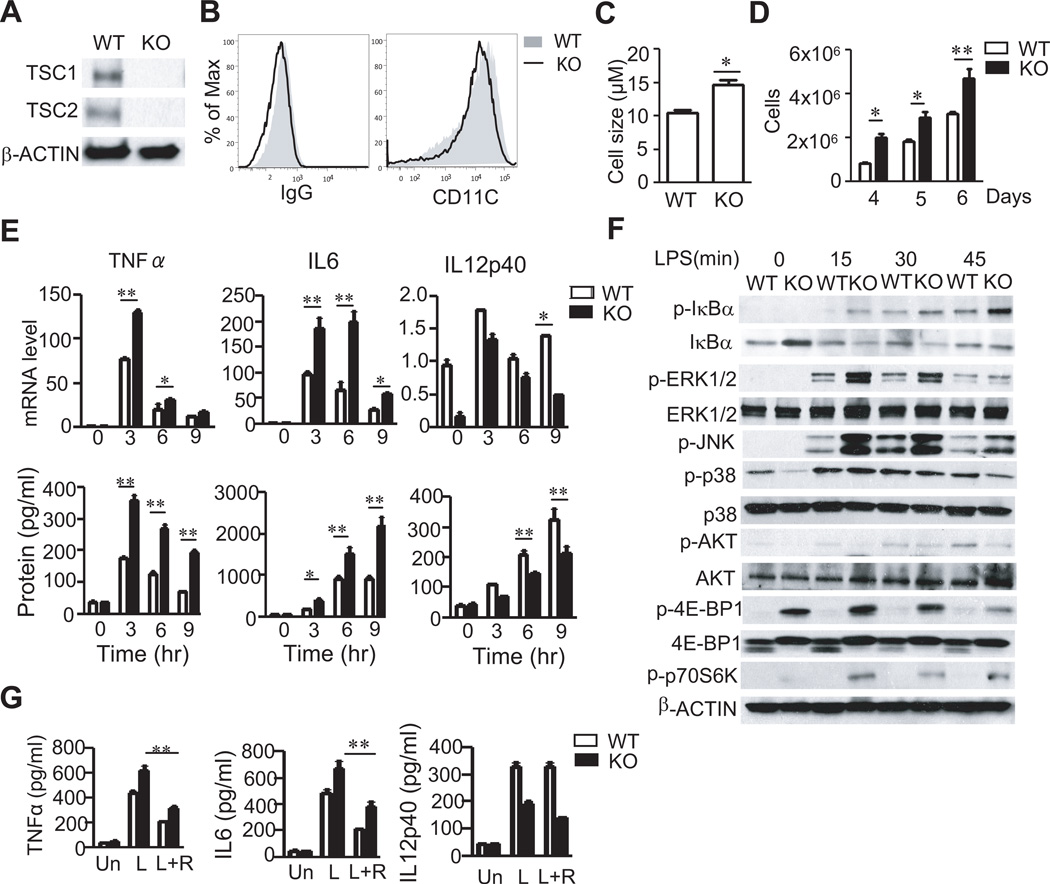
To investigate whether TSC1 plays a role in bone marrow derived DC (BMDC) maturation, we generated TSC1 conditional knockout mice carrying the ER-Cre transgene (TSC1f/f-ERCre, TSC1KO) (31). TSC1 was efficiently deleted in BMDCs following tamoxifen treatment (Figure 1A). Furthermore, TSC2 protein was severely decreased in TSC1KO BMDCs, which is consistent with the reported role of TSC1 on TSC2 stability in other cells (27, 31, 32). Almost all in vitro differentiated TSC1f/f (WT) and TSC1KO BMDC were CD11c+ and TSC1KO BMDCs expressed similar levels of CD11c to WT BMDCs (Figure 1B), suggesting that in vitro GM-CSF mediated DC differentiation is not prevented by TSC1 deficiency. However, TSC1KO BMDCs expanded faster and were bigger in size than WT BMDCs during in vitro differentiation (Figure 1C and 1D). Together, these data demonstrated that TSC1 is not essential for DC differentiation but controls DC growth and expansion in vitro.
Figure 1. TSC1 deficiency promotes BMDC growth and expansion.
(A) Detection of TSC1 and TSC2 proteins in WT and TSC1 KO BMDCs by western blot with indicated antibodies. (B) CD11c expression on GM-CSF-induced DCs. Overlaid histograms showed CD11c staining in WT and TSC1KO BMDCs with IgG isotype as a control. (C) Enlarged cell size of TSC1KO DCs. Cell sizes of BMDCs cultured on day 7 were determined by Cellmeter Auto T4. (D) Enhanced expansion of TSC1KO DCs. Data in C and D were mean ± SD from three experiments. (E) Cytokine mRNA and protein induced in WT and TSC1KO BMDCs following LPS stimulation for the indicated times. Bar graphs indicate mean ± SD. Data are representative of four independent experiments. (F) LPS induced signaling in WT and TSC1KO BMDCs. BMDCs were starved in serum-free medium without GM-CSF for 6 hours, and then treated with LPS at 10ng/ml for 0, 15, 30 and 45 min. Cell lysates were subjected to immunoblotting analysis with indicated antibodies. (G) Effects of rapamycin on LPS-induced cytokine production. WT and TSC1KO BMDCs were untreated or treated with rapamycin (20 ng/ml) 30 min before LPS stimulation for 6 hours. Un, unstimulated; L, LPS; R, Rapamycin. *, p<0.05; **, p<0.01 determined by unpaired Student t-test.
Increased TNFα and IL6 but decreased IL12p40 production by TSC1 deficient BMDCs
To examine how absence of TSC1 may affect TLR-mediated innate immune responses in BMDCs, we first measured TLR-induced cytokine production. Following LPS stimulation, TSC1KO BMDCs produced more TNFα and IL6 at both the protein and mRNA levels than WT BMDCs. In contrast, IL12p40 production was reduced in TSC1KO BMDCs (Figure 1E). To explore the mechanisms how TSC1 regulates TLR induced cytokine production, we compared TLR signaling between WT and TSC1KO BMDCs. LPS-induced phosphorylation of IκBα, JNK1/2, and Erk1/2 was increased in TSC1KO BMDCs as compared with WT BMDCs. However, p38 phosphorylation was similar between WT and TSC1KO BMDCs (Figure 1F).
Phosphorylation of 4E-BP1 and p70S6K, an mTORC1 dependent event, was substantially increased in TSC1KO BMDCs. However, phosphorylation of Akt at serine 473, an mTORC2-mediated event, was decreased in TSC1KO BMDCs (Figure 1F). Thus TSC1 deficiency leads to increased mTORC1 but decreased mTORC2 signaling in BMDCs. Treatment with rapamycin reduced TNFα and IL6 production by both of WT and TSC1KO BMDCs (Figure 1G), suggesting that mTORC1 promotes production of these cytokines. However, rapamycin exerted minimal effect on IL12p40 production in WT BMDCs and was not able to restore IL12 production in TSC1KO BMDCs. It has been reported that rapamycin treatment increases IL12p40 expression (21, 22, 43–45). The inability of rapamycin to restore IL12p40 expression in TSC1KO BMDCs suggests that TSC1 may also promote IL12p40 expression via mTORC1-independent mechanisms.
Selective defect of MHCII expression in TSC1KO BMDCs during maturation
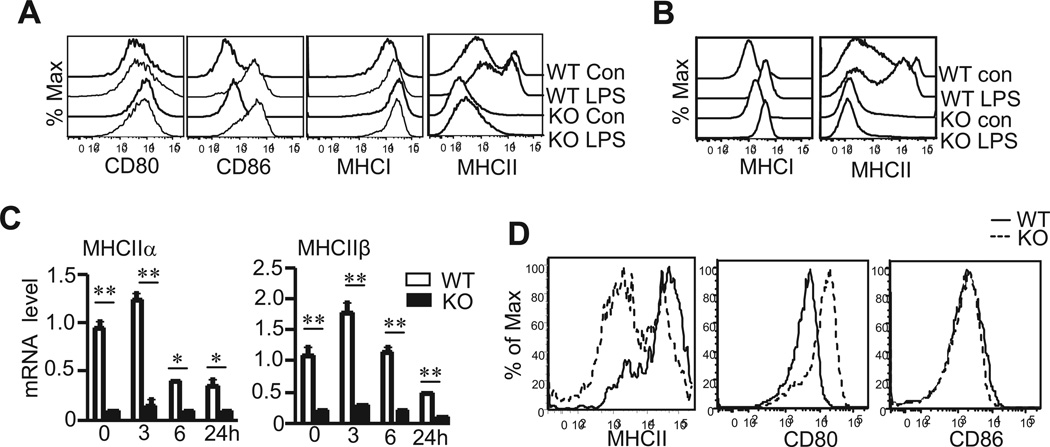
An important function of DCs is to shape adaptive immunity through antigen presentation and costimulation. During DC maturation following recognition of pathogen associated pattern molecules (PAMPs), DCs upregulate MHCI and II for effective presentation of foreign antigens to T cells as well as CD80 and CD86 for providing costimulation to ensure full T cell activation. TSC1KO BMDCs expressed similar levels of CD80, CD86, and MHCI compared with WT BMDCs before and after LPS stimulation. Surprisingly, cell surface MHCII expression on TSC1KO BMDCs was greatly decreased before and after LPS stimulation as compared with WT BMDCs (Figure 2A). Furthermore, intracellular MHCII but not MHCI levels in TSC1KO BMDCs were similarly decreased, indicating an overall decrease of MHCII expression in TSC1KO BMDCs that is not due to impaired translocation of MHCII to the cell surface (Figure 2B). In WT BMDCs, MHCIIα and β chain mRNAs were expressed at high levels before LPS stimulation, further upregulated 3 hours after LPS stimulation, and then down regulated following prolonged LPS stimulation. In TSC1KO BMDCs, both mRNAs were expressed at much lower levels than WT BMDCs. Thus, TSC1 deficiency causes decreased MHCII expression at least at the transcriptional level (Figure 2C). To further establish the relevance of TSC1 and MHCII expression in DCs in vivo, we generated and analyzed TSC1f/f-CD11cCre mice. As shown in Figure 2D, MHCII but not CD80 or CD86 expression in CD11c+ DCs from TSC1f/f-CD11cCre mice was also reduced.
Figure 2. Decreased MHCII expression in TSC1KO BMDCs.
In vitro differentiated WT and TSC1KO BMDCs were unstimulated or treated with LPS at 10ng/ml overnight or for the indicated times. Cells were then used for cell surface or intracellular staining or for making RNA. (A) Cell surface CD80, CD86, MHCI, and MHCII expression on gated CD11C+ DCs. (B) Intracellular staining of MHCI and MHCII in gated CD11c+ DCs. (C) MHCIIα and β mRNA levels determined by real-time RT-qPCR. Data shown represent three experiments with the SD. (D) Decreased MHCII expression in TSC1 deficient DCs in vivo. Overlaid histograms show MHCII, CD80, and CD86 staining on gated CD11c+ DCs from WT and TSC1f/f-CD11cCre mice. *, p<0.05; **, p<0.01 determined by unpaired Student t-test.
In addition to DCs, B cells and macrophages also express MHCII and can present antigens to activate T cells. Neither TSC1 deficient B cells isolated from tamoxifen-treated TSC1f/f-ERCre mice nor TSC1KO BM derived macrophages (BMMCs) displayed decreased MHCII mRNA or cell surface protein expression. Moreover, IRF4 and CIITA mRNA levels in TSC1KO B cells and macrophages were not decreased as compared to WT controls (Supplemental Figure 1). Together, these data suggest that TSC1 is selectively required for MHCII expression in DCs.
Impaired capacity of TSC1KO BMDCs to activate CD4+ T cells
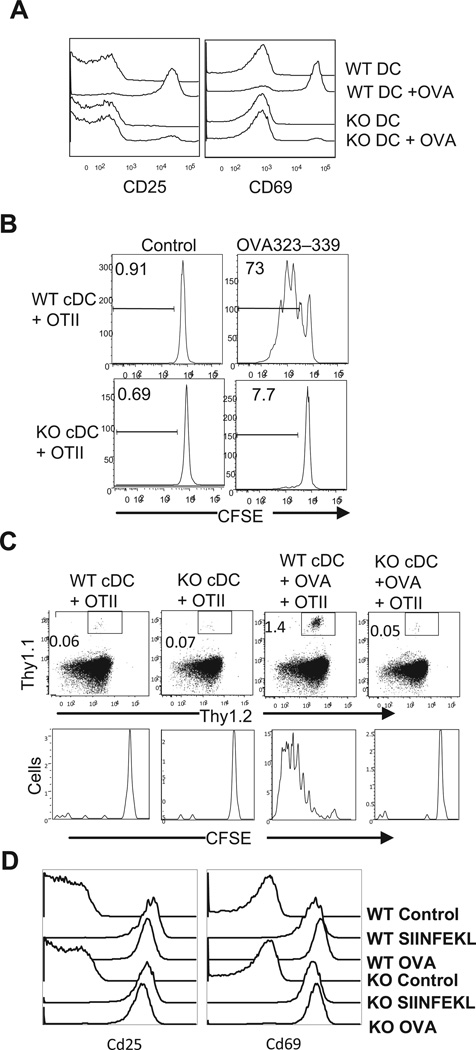
TSC1 deficient BMDCs express increased costimulatory molecules but decreased MHCII, prompting us to explore how TSC1 deficiency may affect DC function as APCs to activate T cells. In vitro, TSC1KO DCs, pretreated with LPS plus OVA323–339 peptide overnight, failed to induce MHCII-restricted OVA323–339 specific CD4+ OTII T cells to upregulate CD69 or CD25, markers for early T cell activation (Figure 3A). In contrast, WT BMDCs potently induced CD69 and CD25 upregulation in OTII T cells in an OVA323–339-dependent manner. Consistent with a defect in inducing early T cell activation, TSC1KO BMDCs loaded with OVA323–339 were unable to induce OTII T cell proliferation in a CFSE dilution assay (Figure 3B).
Figure 3. Defective in presentation of MHCII restricted peptide to CD4+ T cells by TSC1 deficient BMDCs.
(A) TSC1KO DCs are impaired to induce CD4+ T cell to express activation markers. WT and TSC1KO BMDCs were treated with LPS or LPS plus OVA323–339 and then either cocultured with CFSE-labeled purified OTII T cells for 24 hours (A) or 72 hours (B) or used for immunization in vivo (C). (A) CD25 and CD69 expression on gated CD4+Vα2+ cells. (B) CFSE-dilution in gated CD4+Vα2+ cells. (C) CFSE-labeled OTII cells (Thy1.1+Thy1.2+) were i.v. injected into Thy1.1+ congenic mice. After 24 hours, recipient mice were injected i.v. OVA/LPS treated DCs. After an additional 72 hours, splenocytes and lymph node cells from recipient mice were stained for Thy1.1, Thy1.2 and CD4 antibodies, followed by FASC analysis. Top panels, Thy1.1 and Thy1.2 expression on gated CD4+ T cells. Bottom panels, CFSE dilution of gated Thy1.1+Thy1.2+ OTII T cells. (D) Efficient presentation of MHCI-restricted antigen to CD8 T cells by TSC1KO DCs. Purified OTI CD8 T cells were mixed with WT and TSC1KO DCs treated with LPS, LPS plus SIIFEKL, or LPS plus OVA. Following overnight culture, expression of CD25 and CD69 on OTI cells was examined. Data shown are representative of two independent experiments.
To determine whether TSC1KO BMDCs are also compromised to activate T cells in vivo, we transferred CFSE-labeled Thy1.1+ Thy1.2+ OTII T cells into congenic Thy1.1 mice. Twenty-four hours later, recipient mice were immunized intravenously with WT and TSC1KO BMDCs pretreated with LPS or LPS plus OVA323–339. Seventy-two hours after DC immunization, Thy1.1+1.2+ OTII T cells in the recipients immunized with WT but not TSC1KO DCs loaded with OVA (Figure 3C) proliferated vigorously. Different from presenting antigens to CD4 T cells, the ability of TSC1KO BMDCs to present OVA257–267 (SIIFEKL) to and to activate MHCI restricted OTI T cells was not compromised (Figure 3D). Moreover, the decreased MHCII expression did not occur in TSC1f/f-ERCre BMDCs without tamoxifen treatment (Supplemental Figure 2), further supporting that the phenotypes in TSC1KO BMDCs is dependent on deletion of TSC1. Together, these data demonstrated that TSC1 expression in DCs is critical for DCs to fulfill its function to trigger CD4 T cell activation.
Critical role of TSC1 for CIITA transcription to promote MHCII expression in BMDCs
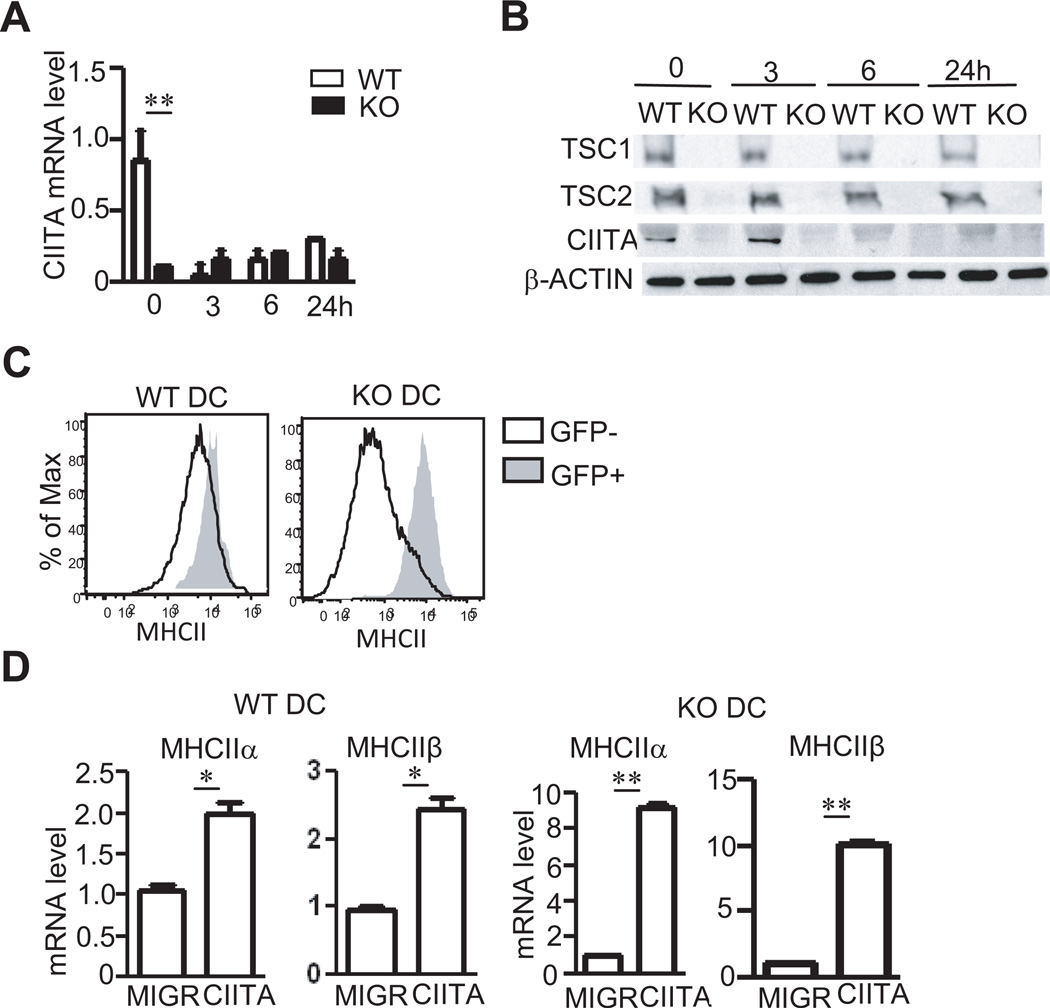
To explore mechanisms by which TSC1 promotes MHCII expression in BMDCs, we first examined CIITA expression due to its essential role in MHCII transcription (46, 47). In WT BMDCs, CIITA mRNA and protein levels were drastically decreased 3 and 6 hours after LPS stimulation, respectively. In TSC1KO BMDCs, both CIITA mRNA and protein levels were substantially decreased in as compared with WT controls before LPS stimulation (Figure 4A and 4B). Retroviral transduction of CIITA into BMDCs not only increased MHCII expression in WT BMDCs but also restored MHCII expression in TSC1KO BMDCs to WT levels for both protein and mRNA (Figure 4C and 4D). Together, these data suggest that decreased CIITA transcription contributes to defective MHCII expression in TSC1KO BMDCs.
Figure 4. Decreased CIITA expression in TSC1 KO BMDCs.
(A,B) CIITA mRNA (A) and protein (B) levels in WT and TSC1KO BMDCs before and after LPS (10 ng/ml) stimulation for the indicated times. (C,D) Restoration of MHCII expression in TSC1KO BMDCs by CIITA overexpression. At day 4 of in vitro differentiation, WT and TSC1KO BMDCs were transduced with control MIGR vector or MIGR-CIITA retrovirus and cultured in the presence of GM-CSF for additional 2–3 days. Cells were then stained for CD11c and MHCII or sorted for CD11c+GFP+ cells to make RNA. (C) Overlaid histograms show MHCII expression on GFP and GFP+ CD11c+ DCs. (D) MHCIIα and β mRNA levels in sorted GFP+ WT and TSC1KO BMDCs determined by real-time RT-qPCR. *, p<0.05; **, p<0.01 determined by unpaired Student t-test.
TSC1 promotes CIITA-MHCII expression through IRF4
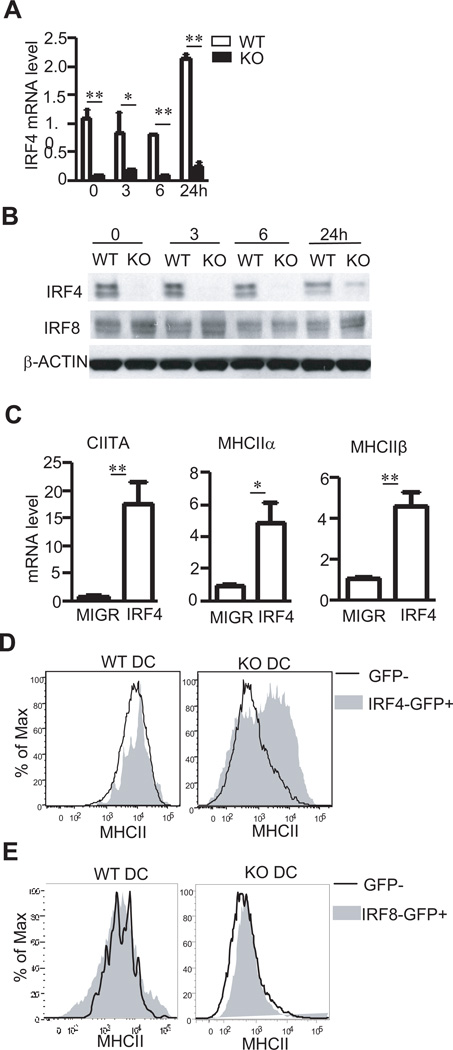
A recent study has revealed that IRF4 and IRF8 are important for MHCII expression in splenic and BM derived DCs (48) and can associate with the CIITA promoter (17). However, a direct role of IRF4 in activating CIITA/MHCII in DCs has not demonstrated. Both IRF4 mRNA transcript and protein were severely reduced in TSC1KO BMDCs (Figure 5A and 5B). Retroviral transduction of IRF4 into TSC1KO BMDCs considerably increased CIITA mRNA levels as well as MHCIIα and β mRNA levels (Figure 5C). Moreover, IRF4 transduced (GFP+) TSC1KO BMDCs expressed much higher levels of MHCII protein on cell surface than untransduced GFP− cells (Figure 5D). Different from IRF4, IRF8 protein levels were not decreased in TSC1KO BMDCs (Figure 5B). Furthermore, retroviral transduction of IRF8 into TSC1KO BMDCs failed to rescue MHCII expression in these cells (Figure 5E). Together, these data suggest that TSC1 promotes the expression of IRF4, which in turn plays a critical role for CIITA and MHCII expression.
Figure 5. Contribution of decreased IRF4 expression to defective CIITA/MHCII expression in TSC1 KO BMDCs.
(A,B) IRF4 mRNA (A) and protein (B) levels in WT and TSC1KO BMDCs before and after LPS (10 ng/ml) stimulation for the indicated times. (C) upregulation of CIITA, MHCIIα, and MHCIIβ mRNA in TSC1KO BMDCs by IRF4 overexpression. TSC1KO BMDCs were similarly transduced with control MIGR vector or MIGR-IRF4 retrovirus as in figure 4C. CIITA, MHCIIα, and MHCIIβ mRNA levels in sorted TSC1KO GFP+ CD11c+ BMDCs were determined by real-time RT-qPCR. (D) Overlaid histograms show MHCII expression on GFP and GFP+ CD11c+ WT and TSC1KO DCs following transduction with IRF4 expressing retrovirus. (E) Overexpressed IRF8 couldn’t rescue MHCII expression in TSC1KO BMDCs. WT and TSC1KO BMDCs were similarly transduced with retrovirus expression IRF8 and analyzed for MHCII expression as in (C). **, p<0.01 determined by unpaired Student t-test.
IRF4 binds to CIITA promoters to control CIITA expression
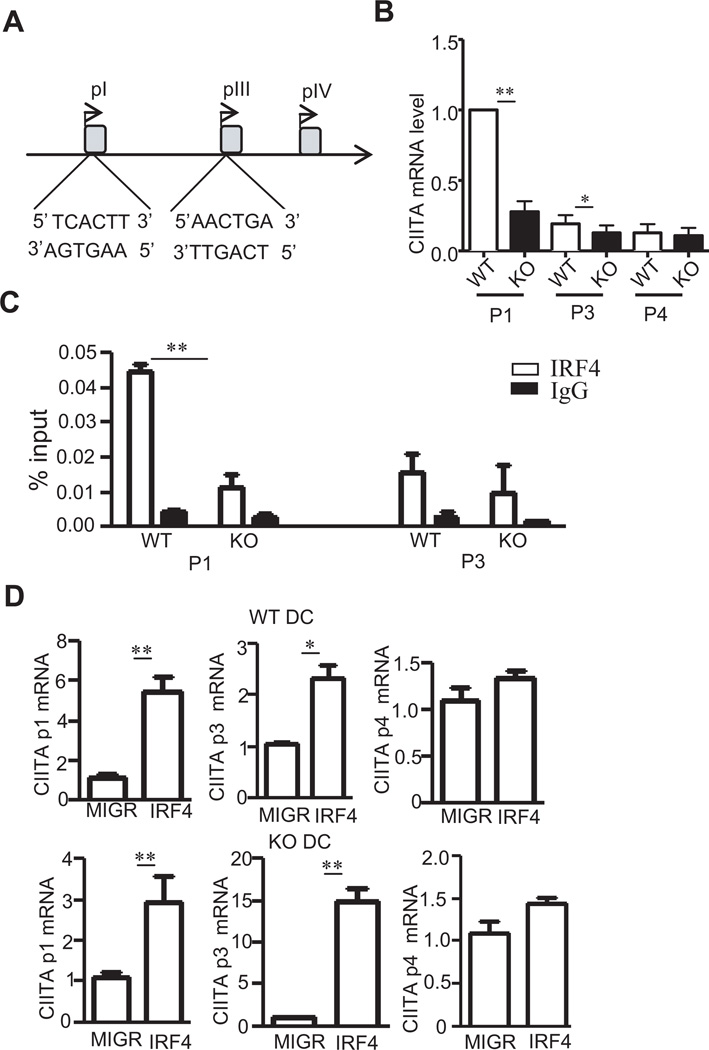
Since expression of IRF4 increased both CIITA and MHCII expression in TSC1KO DCs, we sought to determine whether IRF4 directly activates CIITA transcription. In mouse, CIITA transcription is controlled by several promoters, pI, pIII, and pIV. DCs mainly utilize pI, and to a lesser degree, pIII (49) (Figure 6A). Measurement of CIITA transcripts derived from these promoters by real-time RT-qPCR showed a dramatic decrease of pI specific CIITA transcript and a slight decrease of pIII specific CIITA transcript in TSC1KO BMDCs compared to WT BMDCs (Figure 6B). Using chromatin immunoprecipitation (ChIP) with anti-IRF4 antibody, we were able to detect association of IRF4 to both CIITA pI and pIII promoters in WT BMDCs. However, such association was reduced in TSC1KO BMDCs (Figure 6C), which is consistent with the drastic decrease of IRF4 protein in these cells. Transduction of WT BMDCs with IRF4 resulted in 5 and 2 fold increases of pI and pIII transcripts, respectively. In TSC1KO BMDCs, both transcripts were also increased following IRF4 transduction (Figure 6D). However, IRF4 did not increase transcription from pIV in either WT or TSC1KO BMDCs. These results, together with those in Figure 5, demonstrate that IRF4 binds to both pI and pIII of CIITA to promote CIITA transcription and that TSC1 regulates CIITA-MHCII expression at least partially via maintaining IRF4 expression in DCs.
Figure 6. IRF4 binds to CIITA promoter1 and III.
(A) Schematic drawing of CIITA promoters. Potential IRF4 binding sites are shown. (B) CIITA pI, pIII, and pIV specific mRNA levels in WT and TSC1KO BMDCs determined by real-time qPCR. (C) Direct binding of IRF4 to pI and pIII of CIITA determined by ChIP and real time qPCR. WT and TSC1KO BMDCs were subjected to ChIP with anti-IRF4 or a control IgG, followed by real-time qPCR. The results are presented as immunoprecipitated pI and pIII relative to the total input DNA. Data were generated from three independent experiments. (D) IRF4 promotes pI and pIII but not pIV activity of CIITA in both WT and TSC1KO BMDCs. pI, pIII, and pIV specific CIITA mRNA in WT and TSC1KO BMDCs transduced with either GFP or GFP + IRF4 as described in figure 5C were quantified by real-time RT-qPCR. *, p<0.05; **, p<0.01 determined by unpaired Student t-test.
Critical role of mTORC1 signaling for CIITA-MHCII silencing during DC maturation
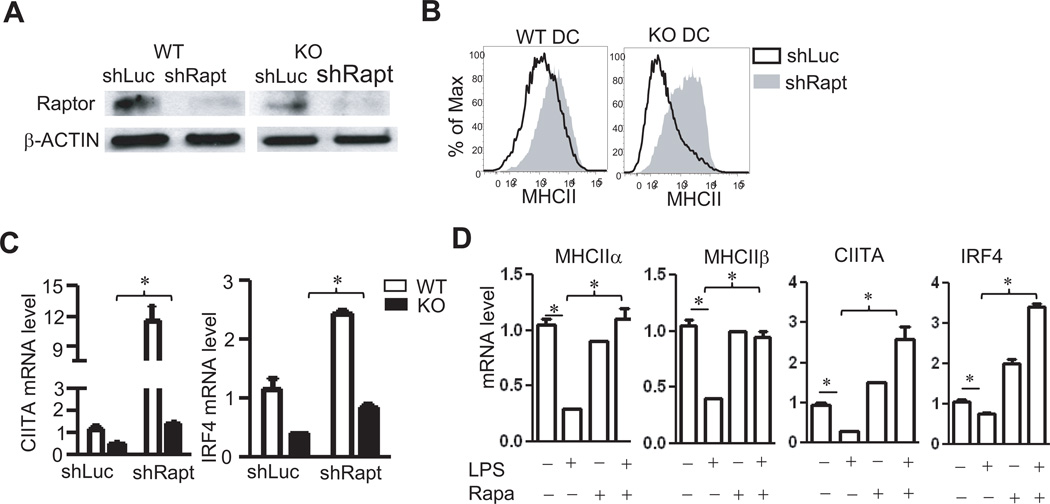
As shown in Figure 1, mTORC1 signaling was enhanced but mTORC2 signaling was decreased in TSC1KO DCs. To determine whether enhanced mTORC1 activity leads to defective CIITA-MHCII expression in TSC1KO BMDCs, we used shRNA to knock down raptor, an essential component of the mTORC1 complex, in both WT and TSC1KO BMDCs. Western blot analysis showed substantial reduction of raptor protein in WT and TSC1KO BMDCs expressing shRaptor but not a scrambled shRNA control (Figure 7A). In WT BMDCs with shRaptor, cell surface MHCII expression was increased; the increase of MHCII expression in TSC1KO BMDCs with shRaptor was much more drastic than in WT BMDCs (Figure 7B). Compared to scrambled control shRNA, shRaptor increased IRF4 mRNA about 2 fold and CIITA mRNA about 10 fold in WT BMDCs. It also increased IRF4 and CIITA expression in TSC1KO BMDCs to levels close to WT BMDCs with the control shRNA (Figure 7C). These observations indicate that TSC1 promotes IRF4-CIITA-MHCII expression by inhibiting mTORC1.
Figure 7. Activated mTORC1 contributes to loss of MHCII expression in TSC1 KO BMDCs.
WT and TSC1KO BMDCs were transduced with lentivirus expressing either a control shLuc or a Raptor shRNA on day 3 followed by puromycin selection. DCs were examined on day 7 or day 8. (A) Assessment of raptor protein levels in by immunoblotting analysis. (B) Increased MHCII expression in Raptor-knockdown WT and TSC1KO BMDCs. (C) Decreased Raptor protein increased CIITA and IRF4 mRNA levels in both WT and TSC1KO BMDCs determined by real-time RT-qPCR. (D) Rapamycin treatment prevented LPS induced CIITA/MHCII silencing. WT BMDCs were treated with LPS or LPS plus rapamycin for 6 hours. MHCIIα, MHCIIβ, CIITA and IRF4 mRNA transcripts from treated cells were determined by real-time RT-qPCR. Data are representative of three independent experiments. *, p<0.05; **, p<0.01 determined by unpaired Student t-test.
TLR-induced DC maturation is accompanied by rapid silencing of CIITA and MHCII transcription. To test whether mTORC1 plays a role in CIITA and MHCII silencing, we treated WT BMDCs with LPS in the presence or absence of rapamycin. In immature DCs, MHCIIα and β as well as CIITA and IRF4 are actively transcribed. Six hours after LPS stimulation, CIITA, MHCα, and MHCβ mRNA were drastically decreased, accompanied by a modest decrease of IRF4 mRNA (Figure 7D). Rapamycin treatment by itself did not obviously alter MHCIIα and β mRNA levels but increased CIITA and IRF4 mRNA levels. Addition of rapamycin during LPS stimulation resulted in 2 – 3 fold increase of CIITA and IRF4 mRNA and, importantly, MHCIIα and β mRNA remained at high levels similar to unstimulated immature DCs (Figure 7D). These data suggest that mTORC1 activity is critical for TLR-induced silencing of CIITA and MHCII during DC maturation.
Discussion
Following recognition of pathogen pattern molecules, immature DCs undergo an irreversible maturation process that is characterized by upregulation of cell surface MHCII and costimulatory molecules, and production of a variety of cytokines in order to induce effective and appropriate adaptive immune responses to control infection (50). Paradoxically, MHCII and CIITA transcription is rapidly silenced during DC maturation and involves loss of histone acetylation across the gene locus and specific binding of PRDM1 to the promoters (16, 17). However, the signaling mechanisms that lead to CIITA/MHCII silencing have been unclear. In this study, we have revealed a novel function of mTORC1 in DCs for CIITA/MHCII silencing during DC maturation and demonstrated that TSC1 is a critical regulator that controls multiple DC functions via modulating mTOR signaling.
In TSC1 deficient DCs, LPS induced mTORC1 signaling is enhanced but mTORC2 signaling is decreased, indicating the TSC1 differentially controls mTORC1 and mTORC2 signaling in DCs. These observations are consistent with the role of TSC1 in macrophages as well as several other immune cell lineages such as T cells and mast cells (27–29, 31, 32). In addition to controlling mTOR signaling, TSC1 also regulates several other signaling events in DCs. TLR4-induced Erk1/2, JNK1/2, and IKK-NFκB activation is enhanced in TSC1 deficient DCs. Since Erk1/2, JNK1/2, and IKKs are activated through different signal cascades downstream of TLR4, the data suggest that TSC1 may be involved in negative control of proximal TLR signaling. It has been reported that FoxO1 promotes TLR4 signaling and its phosphorylation by Akt following TLR4 stimulation represents a negative feedback mechanisms +to prevent uncontrolled inflammatory responses (51). A potential mechanism is that the decreased mTORC2/Akt activity in TSC1KO DCs may result in elevated FoxO1 activity, leading to enhanced TLR4 signaling and activation of these downstream enzymes as well as increased cytokine production. The ability of TSC1 to control multiple signaling events is consistent with our data showing that inhibition of mTORC1 with rapamycin is not able to fully restore normal cytokine production in TSC1 deficient BMDCs, suggesting that either mTORC2 or mTOR independent mechanisms are involved. Further studies are needed to illustrate the mechanisms by which TSC1 regulates these canonical TLR signaling events.
One of the striking findings in our study is that TSC1/mTOR control CIITA/MHCII expression. We have revealed that TSC1 deficient DCs express low levels of CIITA and MHCII without obvious defects in upregulation of CD80/86 and MHCI. The impaired CIITA/MHCII expression in TSC1KO DCs is at least partially caused by enhanced mTORC1 signaling in these cells as knocking down raptor expression in TSC1KO DCs almost restores CIITA/MHCII expression to WT levels. The inhibitory effects of mTORC1 for CIITA/MHCII expression can also be observed in WT DCs as raptor knockdown in these cells upregulates CIITA/MHCII expression. Furthermore, acute treatment of WT DCs with rapamycin prevents TLR4-induced CIITA/MHCII silencing during DC maturation. Since rapamycin treatment only slightly increased IRF4 expression, it suggests that mTORC1 may also suppress CIITA/MHCII expression through IRF4-independent mechanisms or that rapamycin-resistant functions of mTORC1 may regulate IRF4 expression in DCs. Of note, Erk1/2 have been previously found to be involved in CIITA/MHCII silencing (52). In addition, Erk1/2 promotes mTOR signaling in T cells (41). The elevated Erk1/2 activity in TSC1KO DCs may also contribute to downregulation of MHCII expression in these cells.
CIITA expression is controlled by different promoters in different cell lineages. In cDCs and macrophages, CIITA is transcribed primarily from pI and to a less extent from pIII. In B cells and pDCs, CIITA is mainly transcribed from pII and critical. pIV is mainly responsible for IFNγ induced CIITA transcription in non-hematopoietic cells (16, 49). PU.1, IRF8, NFκB, and Sp1 bind to pI to promote CIITA expression, while Blimp1 inhibits CIITA transcription (17). It has also been noted that IRF4 deficient DCs expressed low levels of MHCII and both pI and pIII CIITA transcripts are undetectable in these cells (53). However, how IRF4 promotes MHCII expression has been unclear. Our data indicate that IRF4 binds to pI and pIII but not pIV of CIITA and overexpression of IRF4 upregulates pI and pIII specific CIITA transcription in WT DCs, indicating that IRF4 directly activates CIITA transcription via pI and pIII promoters. Furthermore, our study identified TSC1 as a regulator critical for IRF4 expression in DCs. Future studies should determine how TSC1 promotes IRF4 expression and how decreased IRF4 expression in TSC1 deficient DCs may contribute to other phenotypic and functional abnormalities in these cells.
In addition to regulating DC maturation, we have recently demonstrated that TSC1 also plays critical roles in macrophages (31). In both macrophages and DCs, TSC1 inhibits TLR-induced IL-6 and TNFα production, promoting mTORC1 but inhibiting mTORC2, and negatively controls JNK1/2 activation. However, TSC1 also plays differential roles in macrophages and DCs. TSC1 inhibits Erk1/2 and NFκB activation but is critical for IRF4-CIITA-MHC expression in DCs but not in macrophages. Additionally, TSC1 plays both positive and negative roles in TLR4-induced IL-12p40 expression in DCs and macrophages, respectively. While the mechanisms underlying differential roles of TSC1 between these two types of closely related innate immune cell lineages are unclear at present, our data suggest cell type specific or context dependent function of TSC1-mTOR signaling, which may explain the differences regarding the role of mTOR in innate immunity among different reports (22, 23, 43, 45, 54, 55). Moreover, the different roles of TSC1 among difference cell types also raise concern over targeting mTOR signaling for therapeutic purposes. Additional in depth understanding of mTOR signaling in different cell lineages are needed before manipulating mTOR signaling in the innate immune cells for treatment of immune related diseases.
In summary, our data suggest that TSC1 performs both positive and negative roles during DC maturation. On one hand, it inhibits the production of certain inflammatory cytokines such as IL-6 and IFNα and the upregulation of costimulatory molecules. On the other hand, it promotes IL-12 production and is essential for activation of IRF4-CIITA-MHCII transcription. Our data also reveal that mTORC1 is critical for CIITA/MHCII silencing during DC maturation and that TSC1 modulates DCs function at least partially by inhibiting mTORC1 function.
Supplementary Material
ACKNOWLEDGEMENT
We thank Dr. Mari Shinohara for advices, the Duke Cancer Center Flow Cytometry Core Facility for excellent service, and the people in the lab for helpful discussions.
This study is supported by funding from the National Institutes of Health (AI076357, AI079088, and AI101206), and the American Cancer Society (RSG-08-186-01-LIB) to X-P.Z, and from NIH (CA114504, CA080990) to K.L.W.
Abbreviations used in this article
- mTOR
mammalian target of rapamycin
- TSC
tuberous sclerosis complex
- KO
knockout
- MHCII
MHC class II
- DC
dendritic cells
- pDC
plasmacytoid DC
- CIITA
MHCII transactivator
- IRF
IFN regulatory factor
Footnotes
AUTHOR CONTRIBUTIONS
H.P., T.F.O., G.W., J.Y., and J.S. designed and performed experiments, and analyzed data. K.W. designed experiments and analyzed data. X-P.Z. conceived the project, designed experiments and analyzed data. H.P. and X-P.Z. wrote the manuscript.
CONFLICT OF INTEREST DISCLOSURES
The authors declare no competing financial interests.
References
- 1.Mellman I, Steinman RM. Dendritic cells: specialized and regulated antigen processing machines. Cell. 2001;106:255–258. doi: 10.1016/s0092-8674(01)00449-4. [DOI] [PubMed] [Google Scholar]
- 2.Leon B, Ardavin C. Monocyte-derived dendritic cells in innate and adaptive immunity. Immunol Cell Biol. 2008;86:320–324. doi: 10.1038/icb.2008.14. [DOI] [PubMed] [Google Scholar]
- 3.Inaba K, Witmerpack M, Inaba M, Hathcock KS, Sakuta H, Azuma M, Yagita H, Okumura K, Linsley PS, Ikehara S, Muramatsu S, Hodes RJ, Steinman RM. The Tissue Distribution of the B7-2 Costimulator in Mice - Abundant Expression on Dendritic Cells in-Situ and during Maturation in-Vitro. J Exp Med. 1994;180:1849–1860. doi: 10.1084/jem.180.5.1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Delamarre L, Mellman I. Harnessing dendritic cells for immunotherapy. Sem Immunol. 2011;23:2–11. doi: 10.1016/j.smim.2011.02.001. [DOI] [PubMed] [Google Scholar]
- 5.Sousa CR. Essay - Dendritic cells in a mature age. Nat Rev Immunol. 2006;6:476–483. doi: 10.1038/nri1845. [DOI] [PubMed] [Google Scholar]
- 6.Chin KC, Mao C, Skinner C, Riley JL, Wright KL, Moreno CS, Stark GR, Boss JM, Ting JP. Molecular analysis of G1B and G3A IFN gamma mutants reveals that defects in CIITA or RFX result in defective class II MHC and Ii gene induction. Immunity. 1994;1:687–697. doi: 10.1016/1074-7613(94)90039-6. [DOI] [PubMed] [Google Scholar]
- 7.Ting JP, Trowsdale J. Genetic control of MHC class II expression. Cell. 2002;109(Suppl):S21–S33. doi: 10.1016/s0092-8674(02)00696-7. [DOI] [PubMed] [Google Scholar]
- 8.Riley JL, Westerheide SD, Price JA, Brown JA, Boss JM. Activation of class II MHC genes requires both the X box region and the class II transactivator (CIITA) Immunity. 1995;2:533–543. doi: 10.1016/1074-7613(95)90033-0. [DOI] [PubMed] [Google Scholar]
- 9.Beresford GW, Boss JM. CIITA coordinates multiple histone acetylation modifications at the HLA-DRA promoter. Nat Immunol. 2001;2:652–657. doi: 10.1038/89810. [DOI] [PubMed] [Google Scholar]
- 10.Chang CH, Guerder S, Hong SC, van Ewijk W, Flavell RA. Mice lacking the MHC class II transactivator (CIITA) show tissue-specific impairment of MHC class II expression. Immunity. 1996;4:167–178. doi: 10.1016/s1074-7613(00)80681-0. [DOI] [PubMed] [Google Scholar]
- 11.Mach B, Steimle V, Martinez-Soria E, Reith W. Regulation of MHC class II genes: lessons from a disease. Annu Rev Immunol. 1996;14:301–331. doi: 10.1146/annurev.immunol.14.1.301. [DOI] [PubMed] [Google Scholar]
- 12.Shin JS, Ebersold M, Pypaert M, Delamarre L, Hartley A, Mellman I. Surface expression of MHC class II in dendritic cells is controlled by regulated ubiquitination. Nature. 2006;444:115–118. doi: 10.1038/nature05261. [DOI] [PubMed] [Google Scholar]
- 13.van Niel G, Wubbolts R, ten Broeke T, Buschow SI, Ossendorp FA, Melief CJ, Raposo G, van Balkom BW, Stoorvogel W. Dendritic Cells Regulate Exposure of MHC Class II at Their Plasma Membrane by Oligoubiquitination. Immunity. 2006;25:885–894. doi: 10.1016/j.immuni.2006.11.001. [DOI] [PubMed] [Google Scholar]
- 14.Ohmura-Hoshino M, Matsuki Y, Aoki M, Goto E, Mito M, Uematsu M, Kakiuchi T, Hotta H, Ishido S. Inhibition of MHC class II expression and immune responses by c-MIR. J Immunol. 2006;177:341–354. doi: 10.4049/jimmunol.177.1.341. [DOI] [PubMed] [Google Scholar]
- 15.Ohmura-Hoshino M, Matsuki Y, Mito-Yoshida M, Goto E, Aoki-Kawasumi M, Nakayama M, Ohara O, Ishido S. Cutting edge: requirement of MARCH-I-mediated MHC II ubiquitination for the maintenance of conventional dendritic cells. J Immunol. 2009;183:6893–6897. doi: 10.4049/jimmunol.0902178. [DOI] [PubMed] [Google Scholar]
- 16.Landmann S, Muhlethaler-Mottet A, Bernasconi L, Suter T, Waldburger JM, Masternak K, Arrighi JF, Hauser C, Fontana A, Reith W. Maturation of dendritic cells is accompanied by rapid transcriptional silencing of class II transactivator (CIITA) expression. J Exp Med. 2001;194:379–391. doi: 10.1084/jem.194.4.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Smith MA, Wright G, Wu J, Tailor P, Ozato K, Chen X, Wei S, Piskurich JF, Ting JP, Wright KL. Positive regulatory domain I (PRDM1) and IRF8/PU.1 counter-regulate MHC class II transactivator (CIITA) expression during dendritic cell maturation. J Biol Chem. 2011;286:7893–7904. doi: 10.1074/jbc.M110.165431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Thomson AW, Turnquist HR, Raimondi G. Immunoregulatory functions of mTOR inhibition. Nat Rev Immunol. 2009;9:324–337. doi: 10.1038/nri2546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zoncu R, Efeyan A, Sabatini DM. mTOR: from growth signal integration to cancer, diabetes and ageing. Nat Rev Mol Cell Biol. 2011;12:21–35. doi: 10.1038/nrm3025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee K, Gudapati P, Dragovic S, Spencer C, Joyce S, Killeen N, Magnuson MA, Boothby M. Mammalian target of rapamycin protein complex 2 regulates differentiation of Th1 and Th2 cell subsets via distinct signaling pathways. Immunity. 2010;32:743–753. doi: 10.1016/j.immuni.2010.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schmitz F, Heit A, Dreher S, Eisenächer K, Mages J, Haas T, Krug A, Janssen K-P, Kirschning CJ, Wagner H. Mammalian target of rapamycin (mTOR) orchestrates the defense program of innate immune cells. Eur J Immunol. 2008;38:2981–2992. doi: 10.1002/eji.200838761. [DOI] [PubMed] [Google Scholar]
- 22.Haidinger M, Poglitsch M, Geyeregger R, Kasturi S, Zeyda M, Zlabinger GJ, Pulendran B, Horl WH, Saemann MD, Weichhart T. A versatile role of mammalian target of rapamycin in human dendritic cell function and differentiation. J Immunol. 2010;185:3919–3931. doi: 10.4049/jimmunol.1000296. [DOI] [PubMed] [Google Scholar]
- 23.Cao WP, Manicassamy S, Tang H, Kasturi SP, Pirani A, Murthy N, Pulendran B. Toll-like receptor-mediated induction of type I interferon in plasmacytoid dendritic cells requires the rapamycin-sensitive PI(3) K-mTOR-p70S6K pathway. Nat Immunol. 2008;9:1157–1164. doi: 10.1038/ni.1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Colina R, Costa-Mattioli M, Dowling RJ, Jaramillo M, Tai LH, Breitbach CJ, Martineau Y, Larsson O, Rong L, Svitkin YV, Makrigiannis AP, Bell JC, Sonenberg N. Translational control of the innate immune response through IRF-7. Nature. 2008;452:323–328. doi: 10.1038/nature06730. [DOI] [PubMed] [Google Scholar]
- 25.Huang J, Manning BD. The TSC1-TSC2 complex: a molecular switchboard controlling cell growth. Biochem J. 2008;412:179–190. doi: 10.1042/BJ20080281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Inoki K, Li Y, Xu T, Guan KL. Rheb GTPase is a direct target of TSC2 GAP activity and regulates mTOR signaling. Genes Develop. 2003;17:1829–1834. doi: 10.1101/gad.1110003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.O'Brien TF, Gorentla BK, Xie D, Srivatsan S, McLeod IX, He YW, Zhong XP. Regulation of T-cell survival and mitochondrial homeostasis by TSC1. Eur J Iummunol. 2011;41:3361–3370. doi: 10.1002/eji.201141411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yang K, Neale G, Green DR, He W, Chi H. The tumor suppressor Tsc1 enforces quiescence of naive T cells to promote immune homeostasis and function. Nat Immunol. 2011;12:888–897. doi: 10.1038/ni.2068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wu Q, Liu Y, Chen C, Ikenoue T, Qiao Y, Li CS, Li W, Guan KL, Zheng P. The tuberous sclerosis complex-mammalian target of rapamycin pathway maintains the quiescence and survival of naive T cells. J Immunol. 2011;187:1106–1112. doi: 10.4049/jimmunol.1003968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhong XP, Shin J, Gorentla BK, O’Brien T, Srivatsan S, Xu L, Chen Y, Xie D, Pan H. Receptor signaling in immune cell development and function. Immunol Res. 2011;49:109–123. doi: 10.1007/s12026-010-8175-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pan H, O’Brien TF, Zhang P, Zhong XP. The Role of TSC1 in Regulating Innate Immunity. J Immunol. 2012;188:3658–3666. doi: 10.4049/jimmunol.1102187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shin J, Pan H, Zhong XP. Regulation of mast cell survival and function by tuberous sclerosis complex 1. Blood. 2012;119:3306–3314. doi: 10.1182/blood-2011-05-353342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhong XP. An expanded role of the tumor suppressor TSC1 in T cell tolerance. Cell Cycle. 2012;11:3909–3910. doi: 10.4161/cc.22235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Xie DL, Wu J, Lou YL, Zhong XP. Tumor suppressor TSC1 is critical for T-cell anergy. Proc Natl Acad Sci USA. 2012;109:14152–14157. doi: 10.1073/pnas.1119744109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.O'Brien TF, Gorentla BK, Xie D, Srivatsan S, McLeod IX, He YW, Zhong XP. Regulation of T-cell survival and mitochondrial homeostasis by TSC1. Eur J Immunol. 2011;41:3361–3370. doi: 10.1002/eji.201141411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang L, Zhang H, Li L, Xiao Y, Rao E, Miao Z, Chen H, Sun L, Li H, Liu G, Zhao Y. TSC1/2 signaling complex is essential for peripheral naive CD8+ T cell survival and homeostasis in mice. PloS one. 2012;7:e30592. doi: 10.1371/journal.pone.0030592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kwiatkowski DJ, Zhang HB, Bandura JL, Heiberger KM, Glogauer M, el-Hashemite N, Onda H. A mouse model of TSC1 reveals sex-dependent lethality from liver hemangiomas, and up-regulation of p70S6 kinase activity in Tsc1 null cells. Human Mol Genet. 2002;11:525–534. doi: 10.1093/hmg/11.5.525. [DOI] [PubMed] [Google Scholar]
- 38.Shapiro-Shelef M, Lin KI, Savitsky D, Liao J, Calame K. Blimp-1 is required for maintenance of long-lived plasma cells in the bone marrow. J Exp Med. 2005;202:1471–1476. doi: 10.1084/jem.20051611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stranges PB, Watson J, Cooper CJ, Choisy-Rossi CM, Stonebraker AC, Beighton RA, Hartig H, Sundberg JP, Servick S, Kaufmann G, Fink PJ, Chervonsky AV. Elimination of antigen-presenting cells and autoreactive T cells by Fas contributes to prevention of autoimmunity. Immunity. 2007;26:629–641. doi: 10.1016/j.immuni.2007.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhong XP, Hainey EA, Olenchock BA, Jordan MS, Maltzman JS, Nichols KE, Shen H, Koretzky GA. Enhanced T cell responses due to diacylglycerol kinase zeta deficiency. Nat Immunol. 2003;4:882–890. doi: 10.1038/ni958. [DOI] [PubMed] [Google Scholar]
- 41.Gorentla BK, Wan CK, Zhong XP. Negative regulation of mTOR activation by diacylglycerol kinases. Blood. 2011;117:4022–4031. doi: 10.1182/blood-2010-08-300731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gyory I, Wu J, Fejer G, Seto E, Wright KL. PRDI-BF1 recruits the histone H3 methyltransferase G9a in transcriptional silencing. Nat Immunol. 2004;5:299–308. doi: 10.1038/ni1046. [DOI] [PubMed] [Google Scholar]
- 43.Weichhart T, Costantino G, Poglitsch M, Rosner M, Zeyda M, Stuhlmeier KM, Kolbe T, Stulnig TM, Horl WH, Hengstschlager M, Muller M, Saemann MD. The TSC-mTOR signaling pathway regulates the innate inflammatory response. Immunity. 2008;29:565–577. doi: 10.1016/j.immuni.2008.08.012. [DOI] [PubMed] [Google Scholar]
- 44.Ohtani M, Nagai S, Kondo S, Mizuno S, Nakamura K, Tanabe M, Takeuchi T, Matsuda S, Koyasu S. Mammalian target of rapamycin and glycogen synthase kinase 3 differentially regulate lipopolysaccharide-induced interleukin-12 production in dendritic cells. Blood. 2008;112:635–643. doi: 10.1182/blood-2008-02-137430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Turnquist HR, Cardinal J, Macedo C, Rosborough BR, Sumpter TL, Geller DA, Metes D, Thomson AW. mTOR and GSK-3 shape the CD4+ T-cell stimulatory and differentiation capacity of myeloid DCs after exposure to LPS. Blood. 2010;115:4758–4769. doi: 10.1182/blood-2009-10-251488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Boss JM, Choi NM, Majumder P. Regulation of major histocompatibility complex class II genes. Cur Opin Immunol. 2011;23:81–87. doi: 10.1016/j.coi.2010.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.LeibundGut-Landmann S, Waldburger JM, Krawczyk M, Otten LA, Suter T, Fontana A, Acha-Orbea H, Reith W. Specificity and expression of CIITA, the master regulator of MHC class II genes. Eur J Immunol. 2004;34:1513–1525. doi: 10.1002/eji.200424964. [DOI] [PubMed] [Google Scholar]
- 48.Tamura T, Tailor P, Yamaoka K, Kong HJ, Tsujimura H, O'Shea JJ, Singh H, Ozato K. IFN regulatory factor-4 and-8 govern dendritic cell subset development and their functional diversity. J Immunol. 2005;174:2573–2581. doi: 10.4049/jimmunol.174.5.2573. [DOI] [PubMed] [Google Scholar]
- 49.MuhlethalerMOttet A, Otten LA, Steimle V, Mach B. Expression of MHC class II molecules in different cellular and functional compartments is controlled by differential usage of multiple promoters of the transactivator CIITA. Embo J. 1997;16:2851–2860. doi: 10.1093/emboj/16.10.2851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Guermonprez P, Valladeau J, Zitvogel L, Thery C, Amigorena S. Antigen presentation and T cell stimulation by dendritic cells. Annu Rev Immunol. 2002;20:621–667. doi: 10.1146/annurev.immunol.20.100301.064828. [DOI] [PubMed] [Google Scholar]
- 51.Fan WS, Morinaga H, Kim JJ, Bae E, Spann NJ, Heinz S, Glass CK, Olefsky JM. FoxO1 regulates Tlr4 inflammatory pathway signalling in macrophages. Embo J. 2010;29:4223–4236. doi: 10.1038/emboj.2010.268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yao Y, Xu Q, Kwon MJ, Matta R, Liu Y, Hong SC, Chang CH. ERK and p38 MAPK signaling pathways negatively regulate CIITA gene expression in dendritic cells and macrophages. J Immunol. 2006;177:70–76. doi: 10.4049/jimmunol.177.1.70. [DOI] [PubMed] [Google Scholar]
- 53.Suzuki S, Honma K, Matsuyama T, Suzuki K, Toriyama K, Akitoyo I, Yamamoto K, Suematsu T, Nakamura M, Yui K, Kumatori A. Critical roles of interferon regulatory factor 4 in CD11bhighCD8alpha- dendritic cell development. Proc Natl Acad Sci USA. 2004;101:8981–8986. doi: 10.1073/pnas.0402139101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hackstein H, Taner T, Zahorchak AF, Morelli AE, Logar AJ, Gessner A, Thomson AW. Rapamycin inhibits IL-4-induced dendritic cell maturation in vitro and dendritic cell mobilization and function in vivo. Blood. 2003;101:4457–4463. doi: 10.1182/blood-2002-11-3370. [DOI] [PubMed] [Google Scholar]
- 55.Zhao J, Benakanakere MR, Hosur KB, Galicia JC, Martin M, Kinane DF. Mammalian target of rapamycin (mTOR) regulates TLR3 induced cytokines in human oral keratinocytes. Mol Immunol. 2010;48:294–304. doi: 10.1016/j.molimm.2010.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.