Abstract
Neisseria gonorrhoeae isolated from patients with disseminated infection (DGI) often resist complement (C′)-dependent killing by normal human serum (NHS) and less commonly by convalescent DGI serum. 7 of 10 NHS specimens completely inhibited killing of serum-resistant (serr) gonococci by convalescent or immune DGI serum. Immunoglobulin G (IgG) purified from NHS was shown to be the blocking agent. In addition, IgM (plus C′) purified from NHS was shown to be fivefold more effective (wt/wt) in killing serum-sensitive (sers) gonococci than equivalent amounts of IgM tested in the presence of IgG (whole serum). Although inhibition of NHS killing of sers gonococci required a 640% excess of IgG, only a 40% excess was required to block immune serum killing of serr gonococci. F(ab′)2 prepared from IgG also blocked killing of serr gonococci by immune serum indicating antigenic specificity of blocking IgG.
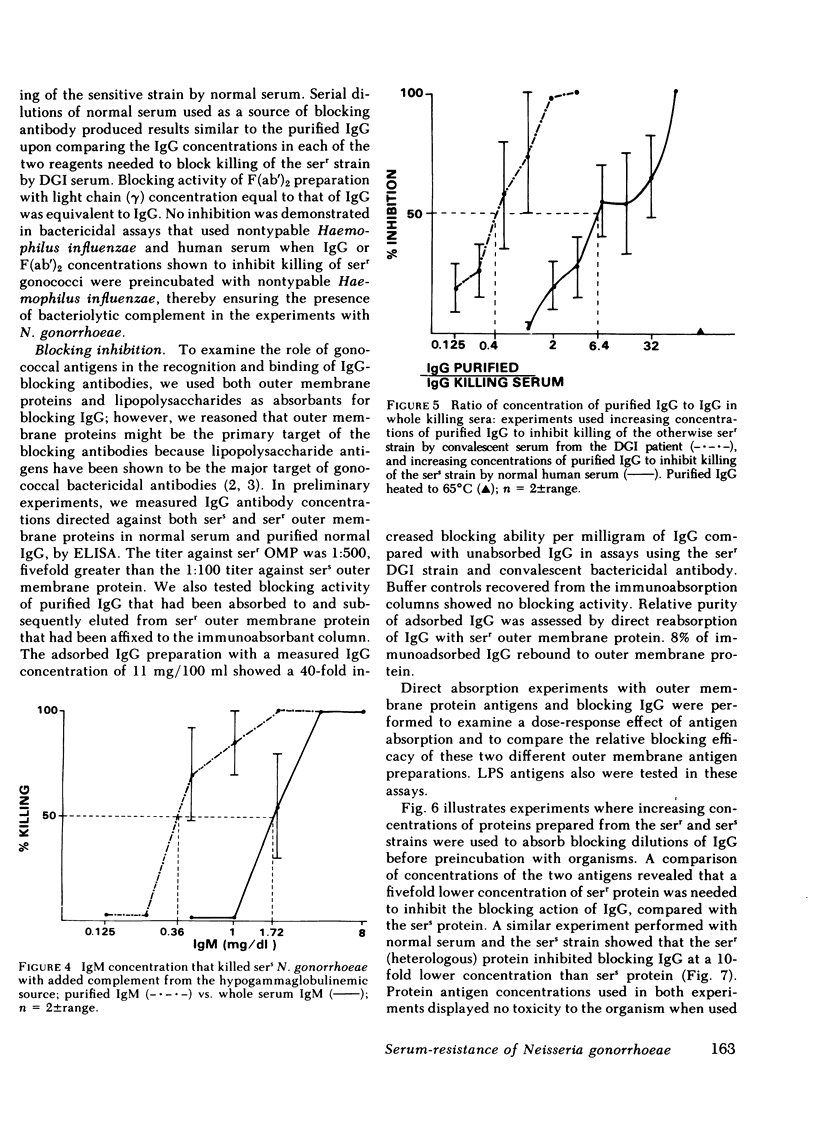
IgG immunoconcentrated against outer membrane protein (OMP) derived from serr gonococci showed 40-fold increased blocking activity over normal IgG (wt/wt) and lacked antibody activity directed against gonococcal lipopolysaccharide by ELISA. Using direct immunoabsorption of IgG with purified gonococcal OMP; serr-OMP was found sixfold more effective than sers-OMP in neutralizing the blocking of immune serum killing of serr gonococci, and 10-fold more effective in systems that used excess blocking IgG, NHS, and sers gonococci. Blocking IgG preabsorbed with whole serr gonococci lost 75% of its ability to block immune serum killing compared with no loss in this system using a similar absorption with sers gonococci. IgG purified from NHS contained fivefold higher titers of antibody against serr-OMP than sers-OMP by ELISA.
Full text
PDF









Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blake M. S., Gotschlich E. C., Swanson J. Effects of proteolytic enzymes on the outer membrane proteins of Neisseria gonorrhoeae. Infect Immun. 1981 Jul;33(1):212–222. doi: 10.1128/iai.33.1.212-222.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchanan T. M., Hildebrandt J. F. Antigen-specific serotyping of Neisseria gonorrhoeae: characterization based upon principal outer membrane protein. Infect Immun. 1981 Jun;32(3):985–994. doi: 10.1128/iai.32.3.985-994.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bürgin-Wolff A., Hernandez R., Just M. Separation of rubella IgM, IgA, and IgG antibodies by gel filtration on agarose. Lancet. 1971 Dec 11;2(7737):1278–1280. doi: 10.1016/s0140-6736(71)90600-3. [DOI] [PubMed] [Google Scholar]
- Ely K. R., Colman P. M., Abola E. E., Hess A. C., Peabody D. S., Parr D. M., Connell G. E., Laschinger C. A., Edmundson A. B. Mobile Fc region in the Zie IgG2 cryoglobulin: comparison of crystals of the F(ab')2 fragment and the intact immunoglobulin. Biochemistry. 1978 Mar 7;17(5):820–823. doi: 10.1021/bi00598a011. [DOI] [PubMed] [Google Scholar]
- Engvall E., Perlmann P. Enzyme-linked immunosorbent assay, Elisa. 3. Quantitation of specific antibodies by enzyme-labeled anti-immunoglobulin in antigen-coated tubes. J Immunol. 1972 Jul;109(1):129–135. [PubMed] [Google Scholar]
- Galanos C., Lüderitz O. Electrodialysis of lipopolysaccharides and their conversion to uniform salt forms. Eur J Biochem. 1975 Jun;54(2):603–610. doi: 10.1111/j.1432-1033.1975.tb04172.x. [DOI] [PubMed] [Google Scholar]
- Goldschneider I., Gotschlich E. C., Artenstein M. S. Human immunity to the meningococcus. I. The role of humoral antibodies. J Exp Med. 1969 Jun 1;129(6):1307–1326. doi: 10.1084/jem.129.6.1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldschneider I., Gotschlich E. C., Artenstein M. S. Human immunity to the meningococcus. II. Development of natural immunity. J Exp Med. 1969 Jun 1;129(6):1327–1348. doi: 10.1084/jem.129.6.1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiss J. M. Bactericidal activity of meningococcal antisera. Blocking by IgA of lytic antibody in human convalescent sera. J Immunol. 1975 Jun;114(6):1779–1784. [PubMed] [Google Scholar]
- Griffiss J. M., Bertram M. A. Immunoepidemiology of meningococcal disease in military recruits. II. Blocking of serum bactericidal activity by circulating IgA early in the course of invasive disease. J Infect Dis. 1977 Dec;136(6):733–739. doi: 10.1093/infdis/136.6.733. [DOI] [PubMed] [Google Scholar]
- Guttman R. M., Waisbren B. A. Bacterial blocking activity of specific IgG in chronic Pseudomonas aeruginosa infection. Clin Exp Immunol. 1975 Jan;19(1):121–130. [PMC free article] [PubMed] [Google Scholar]
- HESS E. V., HUNTER D. K., ZIFF M. GONOCOCCAL ANTIBODIES IN ACUTE ARTHRITIS. JAMA. 1965 Feb 15;191:531–534. doi: 10.1001/jama.1965.03080070015004. [DOI] [PubMed] [Google Scholar]
- Hall W. H., Manion R. E., Zinneman H. H. Blocking serum lysis of Brucella abortus by hyperimmune rabbit immunoglubulin A. J Immunol. 1971 Jul;107(1):41–46. [PubMed] [Google Scholar]
- Heckels J. E. The surface of Neisseria gonorrhoeae: isolation of the major components of the outer membrane. J Gen Microbiol. 1977 Apr;99(2):333–341. doi: 10.1099/00221287-99-2-333. [DOI] [PubMed] [Google Scholar]
- Heckels J. E. The surface properties of Neisseria gonorrhoeae: topographical distribution of the outer membrane protein antigens. J Gen Microbiol. 1978 Oct;108(2):213–219. doi: 10.1099/00221287-108-2-213. [DOI] [PubMed] [Google Scholar]
- Hildebrandt J. F., Mayer L. W., Wang S. P., Buchanan T. M. Neisseria gonorrhoeae acquire a new principal outer-membrane protein when transformed to resistance to serum bactericidal activity. Infect Immun. 1978 Apr;20(1):267–272. doi: 10.1128/iai.20.1.267-272.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James J. F., Swanson J. Studies on gonococcus infection. XIII. Occurrence of color/opacity colonial variants in clinical cultures. Infect Immun. 1978 Jan;19(1):332–340. doi: 10.1128/iai.19.1.332-340.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston K. H. Antigenic diversity of the serotype antigen complex of Neisseria gonorrhoeae: analysis by an indirect enzyme-linked immunoassay. Infect Immun. 1980 Apr;28(1):101–110. doi: 10.1128/iai.28.1.101-110.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasper D. L., Rice P. A., McCormick W. M. Bactericidal antibody in genital infection due to Neisseria gonorrhoeae. J Infect Dis. 1977 Feb;135(2):243–251. doi: 10.1093/infdis/135.2.243. [DOI] [PubMed] [Google Scholar]
- Kramer N., Perez H. D., Goldstein I. M. An immunoglobulin (IgG) inhibitor of polymorphonuclear leukocyte motility in a patient with recurrent infection. N Engl J Med. 1980 Nov 27;303(22):1253–1258. doi: 10.1056/NEJM198011273032202. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lambden P. R., Heckels J. E., James L. T., Watt P. J. Variations in surface protein composition associated with virulence properties in opacity types of Neisseria gonorrhoeae. J Gen Microbiol. 1979 Oct;114(2):305–312. doi: 10.1099/00221287-114-2-305. [DOI] [PubMed] [Google Scholar]
- McCutchan J. A., Katzenstein D., Norquist D., Chikami G., Wunderlich A., Braude A. I. Role of blocking antibody in disseminated gonococcal infection. J Immunol. 1978 Nov;121(5):1884–1888. [PubMed] [Google Scholar]
- McDade R. L., Jr, Johnston K. H. Characterization of serologically dominant outer membrane proteins of Neisseria gonorrhoeae. J Bacteriol. 1980 Mar;141(3):1183–1191. doi: 10.1128/jb.141.3.1183-1191.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munford R. S., Hall C. L. Radioimmunoassay for Gram-negative bacterial lipopolysaccharide O antigens: influence of antigen solubility. Infect Immun. 1979 Oct;26(1):42–48. doi: 10.1128/iai.26.1.42-48.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- OSBORN M. J. STUDIES ON THE GRAM-NEGATIVE CELL WALL. I. EVIDENCE FOR THE ROLE OF 2-KETO- 3-DEOXYOCTONATE IN THE LIPOPOLYSACCHARIDE OF SALMONELLA TYPHIMURIUM. Proc Natl Acad Sci U S A. 1963 Sep;50:499–506. doi: 10.1073/pnas.50.3.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice P. A., Goldenberg D. L. Clinical manifestations of disseminated infection caused by Neisseria gonorrhoeae are linked to differences in bactericidal reactivity of infecting strains. Ann Intern Med. 1981 Aug;95(2):175–178. doi: 10.7326/0003-4819-95-2-175. [DOI] [PubMed] [Google Scholar]
- Rice P. A., Kasper D. L. Characterization of gonococcal antigens responsible for induction of bactericidal antibody in disseminated infection. J Clin Invest. 1977 Nov;60(5):1149–1158. doi: 10.1172/JCI108867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice P. A., McCormack W. M., Kasper D. L. Natural serum bactericidal activity against Neisseria gonorrhoeae isolates from disseminated, locally invasive, and uncomplicated disease. J Immunol. 1980 May;124(5):2105–2109. [PubMed] [Google Scholar]
- Schoolnik G. K., Buchanan T. M., Holmes K. K. Gonococci causing disseminated gonococcal infection are resistant to the bactericidal action of normal human sera. J Clin Invest. 1976 Nov;58(5):1163–1173. doi: 10.1172/JCI108569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoolnik G. K., Ochs H. D., Buchanan T. M. Immunoglobulin class responsible for gonococcal bactericidal activity of normal human sera. J Immunol. 1979 May;122(5):1771–1779. [PubMed] [Google Scholar]
- Siber G. R., Schur P. H., Aisenberg A. C., Weitzman S. A., Schiffman G. Correlation between serum IgG-2 concentrations and the antibody response to bacterial polysaccharide antigens. N Engl J Med. 1980 Jul 24;303(4):178–182. doi: 10.1056/NEJM198007243030402. [DOI] [PubMed] [Google Scholar]
- Swanson J. 125I-labeled peptide mapping of some heat-modifiable proteins of the gonococcal outer membrane. Infect Immun. 1980 Apr;28(1):54–64. doi: 10.1128/iai.28.1.54-64.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson J. Studies on gonococcus infection. XIV. Cell wall protein differences among color/opacity colony variants of Neisseria gonorrhoeae. Infect Immun. 1978 Jul;21(1):292–302. doi: 10.1128/iai.21.1.292-302.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tramont E. C., Sadoff J. C., Wilson C. Variability of the lytic susceptibility of Neisseria gonorrhoeae to human sera. J Immunol. 1977 May;118(5):1843–1851. [PubMed] [Google Scholar]
- WEISSBACH A., HURWITZ J. The formation of 2-keto-3-deoxyheptonic acid in extracts of Escherichia coli B. I. Identification. J Biol Chem. 1959 Apr;234(4):705–709. [PubMed] [Google Scholar]
- Waisbren B. A., Brown I. A factor in the serum of patients with persisting infection that inhibits the bactericidal activity of normal serum against the organism that is causing the infection. J Immunol. 1966 Sep;97(3):431–437. [PubMed] [Google Scholar]
- Winkelhake J. L., Kasper D. L. Affinity chromatography of anti-meningococcal antiserum. J Immunol. 1972 Oct;109(4):824–833. [PubMed] [Google Scholar]
- Yount W. J., Dorner M. M., Kunkel H. G., Kabat E. A. Studies on human antibodies. VI. Selective variations in subgroup composition and genetic markers. J Exp Med. 1968 Mar 1;127(3):633–646. doi: 10.1084/jem.127.3.633. [DOI] [PMC free article] [PubMed] [Google Scholar]