Abstract
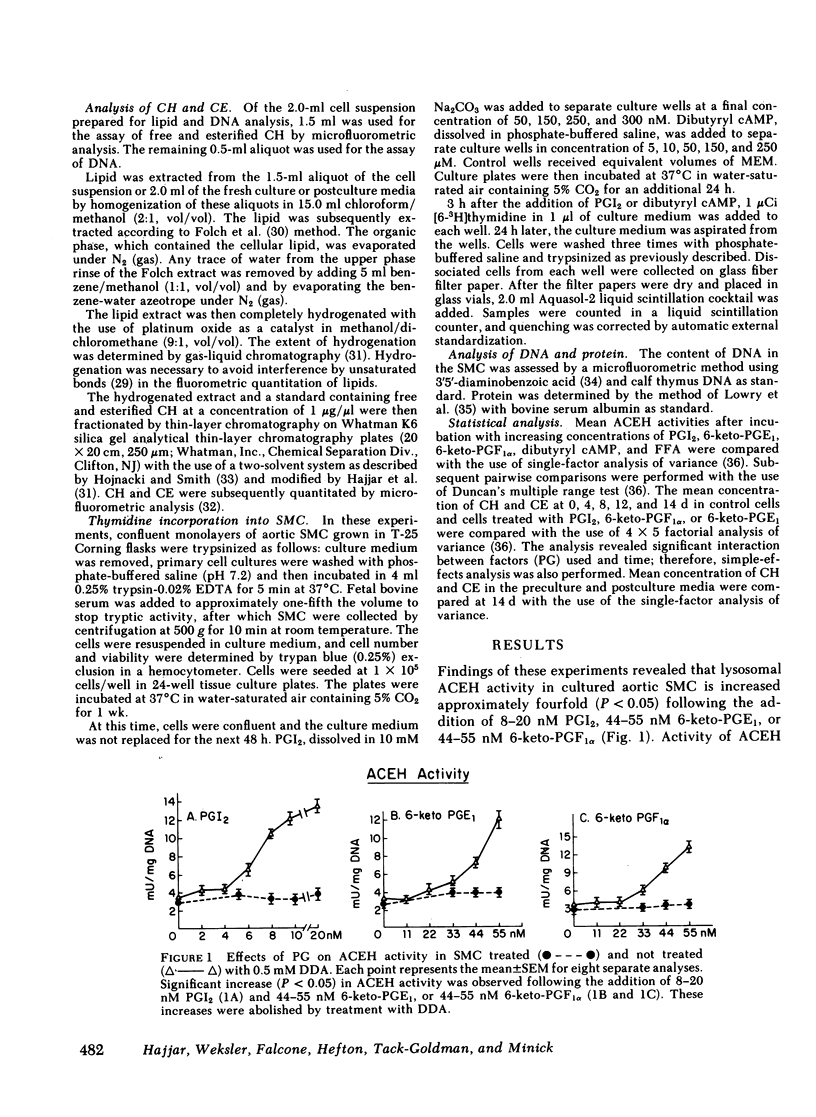
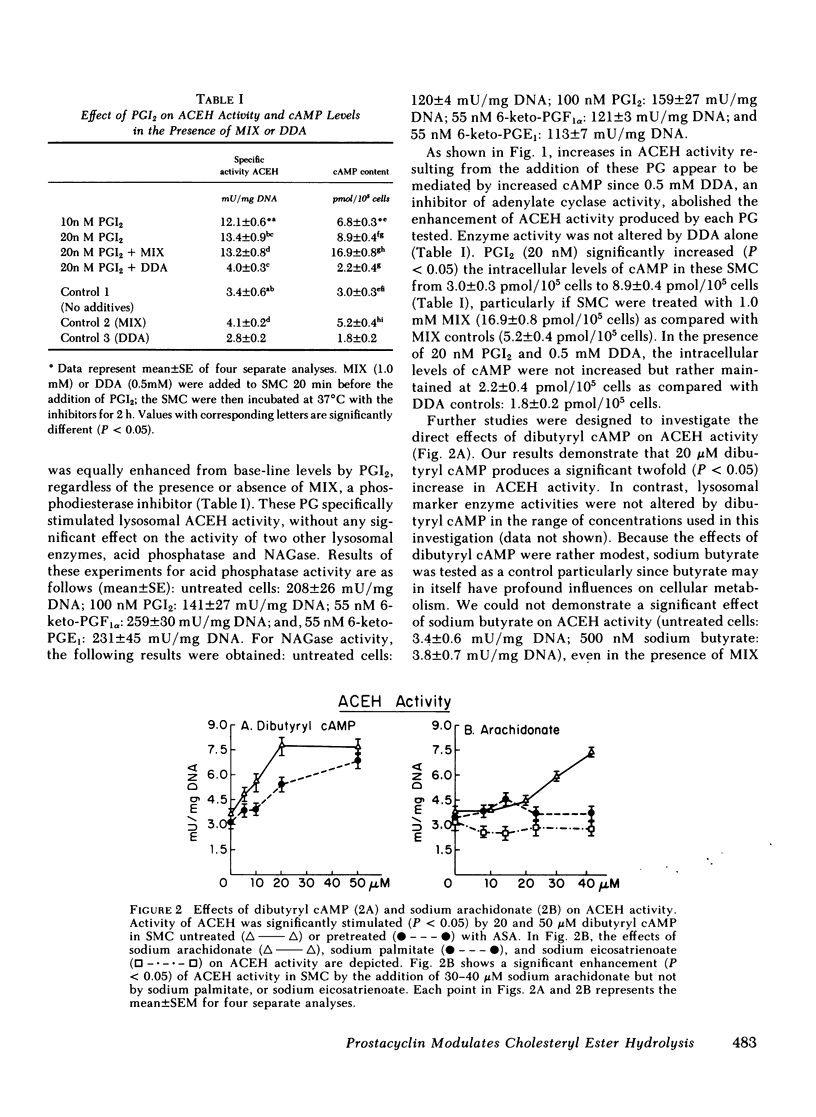
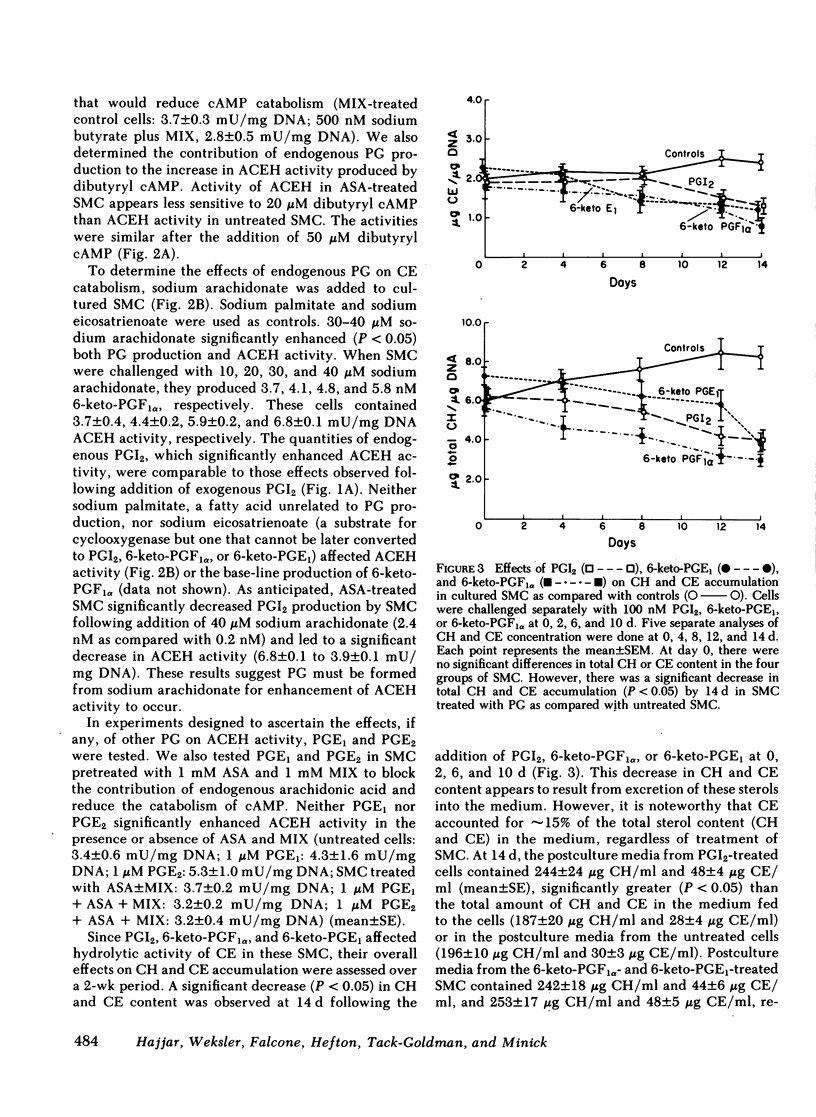
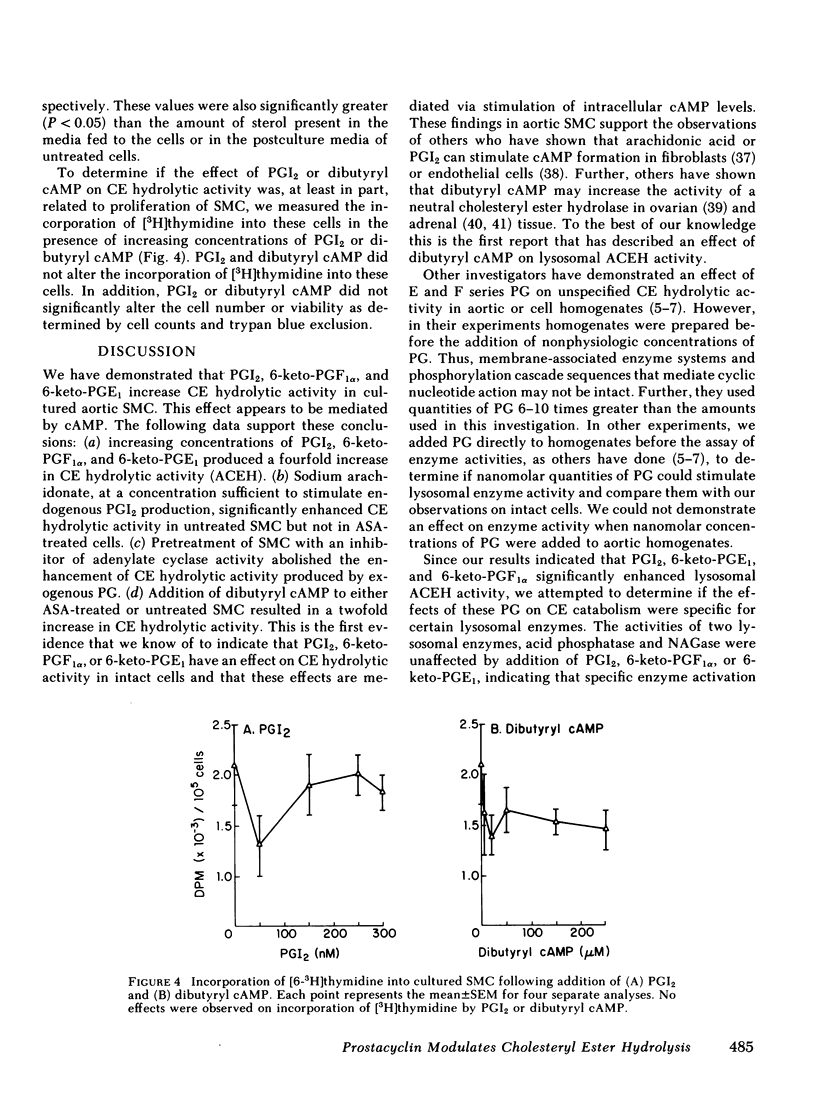
We tested the hypothesis that prostacyclin (PGI2), 6-keto-prostaglandinF1 alpha(6-keto-PGF1 alpha), and several E series prostaglandins (PG) may affect the activity of cholesteryl ester (CE) hydrolase since our previous experiments indicated that smooth muscle cells (SMC) in neointima of injured rabbit aorta (a) acquire the capacity to produce PGI2 and (b) have increased lysosomal CE hydrolytic (acid cholesteryl ester hydrolase [ACEH])activity. Using cultured SMC from rabbit thoracic aorta, we demonstrated that PGI2, 6-keto-PGF1 alpha, and 6-keto-PGE1 enhanced ACEH activity fourfold. No significant effects on ACEH activity were observed with PGE1 or PGE2. Preincubation of SMC with an inhibitor of adenylate cyclase activity (dideoxyadenosine) abolished the effect of these PG on CE hydrolytic activity. Addition of dibutyryl cAMP to these SMC significantly increased ACEH activity. Although concentrations of PGI2 used significantly increased cAMP levels, proliferation of these SMC was not observed. In related experiments, we determined if the addition of PGI2, 6-keto-PGF1 alpha, or 6-keto-PGE1 to cultured aortic SMC would enhance the egress of unesterified cholesterol and CE from these SMC. A significant loss of total cholesterol from PG-treated SMC was observed at the end of 14 d. Results suggest that increased synthesis of PGI2 by neointimal SMC in the injured aortic wall may, at least in part, explain the changes in CE catabolism and accumulation following injury. These PG may also be important in CE metabolism and accumulation in human arteries.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Balasubramaniam S., Goldstein J. L., Faust J. R., Brunschede G. Y., Brown M. S. Lipoprotein-mediated regulation of 3-hydroxy-3-methylglutaryl coenzyme A reductase activity and cholesteryl ester metabolism in the adrenal gland of the rat. J Biol Chem. 1977 Mar 10;252(5):1771–1779. [PubMed] [Google Scholar]
- Bates S. R., Rothblat G. H. Regulation of cellular sterol flux and synthesis by human serum lipoproteins. Biochim Biophys Acta. 1974 Jul 26;360(1):38–55. doi: 10.1016/0005-2760(74)90178-7. [DOI] [PubMed] [Google Scholar]
- Berberian P. A., Ziboh V. A., Hsia S. L. Inhibition of cholesterol esterification in rabbit aorta by prostaglandin E2. Atherosclerosis. 1977 Jun;27(2):213–220. doi: 10.1016/0021-9150(77)90058-2. [DOI] [PubMed] [Google Scholar]
- Brecher P., Pyun H. Y., Chobanian A. V. Effect of atherosclerosis on lysosomal cholesterol esterase activity in rabbit aorta. J Lipid Res. 1977 Mar;18(2):154–163. [PubMed] [Google Scholar]
- Brown M. S., Ho Y. K., Goldstein J. L. The cholesteryl ester cycle in macrophage foam cells. Continual hydrolysis and re-esterification of cytoplasmic cholesteryl esters. J Biol Chem. 1980 Oct 10;255(19):9344–9352. [PubMed] [Google Scholar]
- Bunting S., Gryglewski R., Moncada S., Vane J. R. Arterial walls generate from prostaglandin endoperoxides a substance (prostaglandin X) which relaxes strips of mesenteric and coeliac ateries and inhibits platelet aggregation. Prostaglandins. 1976 Dec;12(6):897–913. doi: 10.1016/0090-6980(76)90125-8. [DOI] [PubMed] [Google Scholar]
- Claesson H. E. Prostaglandin I2 synthesis and elevation of cyclic AMP levels in 3T3 fibroblasts. Biochim Biophys Acta. 1980 Jun 23;618(3):399–406. doi: 10.1016/0005-2760(80)90258-1. [DOI] [PubMed] [Google Scholar]
- Coffey R. G., Hadden E. M., Hadden J. W. Phytohemagglutinin stimulation of guanylate cyclase in human lymphocytes. J Biol Chem. 1981 May 10;256(9):4418–4424. [PubMed] [Google Scholar]
- Dusting G. J., Moncada S., Vane J. R. Prostacyclin (PGX) is the endogenous metabolite responsible for relaxation of coronary arteries induced by arachindonic acid. Prostaglandins. 1977 Jan;13(1):3–15. doi: 10.1016/0090-6980(77)90037-5. [DOI] [PubMed] [Google Scholar]
- Edwards P. A., Lemongello D., Fogelman A. M. The effect of glucagon, norepinephrine, and dibutyryl cyclic AMP on cholesterol efflux and on the activity of 3-hydroxy-3-methylglutaryl CoA reductase in rat hepatocytes. J Lipid Res. 1979 Jan;20(1):2–7. [PubMed] [Google Scholar]
- Eldor A., Falcone D. J., Hajjar D. P., Minick C. R., Weksler B. B. Diet-induced hypercholesterolemia inhibits the recovery of prostacyclin production by injured rabbit aorta. Am J Pathol. 1982 May;107(2):186–190. [PMC free article] [PubMed] [Google Scholar]
- Eldor A., Falcone D. J., Hajjar D. P., Minick C. R., Weksler B. B. Recovery of prostacyclin production by de-endothelialized rabbit aorta. Critical role of neointimal smooth muscle cells. J Clin Invest. 1981 Mar;67(3):735–741. doi: 10.1172/JCI110090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FOLCH J., LEES M., SLOANE STANLEY G. H. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957 May;226(1):497–509. [PubMed] [Google Scholar]
- Goldstein J. L., Brown M. S. The low-density lipoprotein pathway and its relation to atherosclerosis. Annu Rev Biochem. 1977;46:897–930. doi: 10.1146/annurev.bi.46.070177.004341. [DOI] [PubMed] [Google Scholar]
- Goldstein J. L., Brown M. S. The low-density lipoprotein pathway and its relation to atherosclerosis. Annu Rev Biochem. 1977;46:897–930. doi: 10.1146/annurev.bi.46.070177.004341. [DOI] [PubMed] [Google Scholar]
- Gryglewski R. J., Bunting S., Moncada S., Flower R. J., Vane J. R. Arterial walls are protected against deposition of platelet thrombi by a substance (prostaglandin X) which they make from prostaglandin endoperoxides. Prostaglandins. 1976 Nov;12(5):685–713. doi: 10.1016/0090-6980(76)90047-2. [DOI] [PubMed] [Google Scholar]
- Hajjar D. P., Falcone D. J., Fowler S., Minick C. R. Endothelium modifies the altered metabolism of the injured aortic wall. Am J Pathol. 1981 Jan;102(1):28–39. [PMC free article] [PubMed] [Google Scholar]
- Hajjar D. P., Wight T. N., Smith S. C. Lipid accumulation and ultrastructural change within the aortic wall during early spontaneous atherogenesis. Am J Pathol. 1980 Sep;100(3):683–705. [PMC free article] [PubMed] [Google Scholar]
- Haley N. J., Fowler S., de Duve C. Lysosomal acid cholesteryl esterase activity in normal and lipid-laden aortic cells. J Lipid Res. 1980 Nov;21(8):961–969. [PubMed] [Google Scholar]
- Haslam R. J., Davidson M. M., Desjardins J. V. Inhibition of adenylate cyclase by adenosine analogues in preparations of broken and intact human platelets. Evidence for the unidirectional control of platelet function by cyclic AMP. Biochem J. 1978 Oct 15;176(1):83–95. doi: 10.1042/bj1760083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haslam R. J., Davidson M. M., Fox J. E., Lynham J. A. Cyclic nucleotides in platelet function. Thromb Haemost. 1978 Oct 31;40(2):232–240. [PubMed] [Google Scholar]
- Hojnacki J. L., Smith S. C. Separation of six lipid classes on one thin-layer chromatogram. J Chromatogr. 1974 Apr 10;90(2):364–367. doi: 10.1016/s0021-9673(00)92542-1. [DOI] [PubMed] [Google Scholar]
- Hopkins N. K., Gorman R. R. Regulation of endothelial cell cyclic nucleotide metabolism by prostacyclin. J Clin Invest. 1981 Feb;67(2):540–546. doi: 10.1172/JCI110064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ignarro L. J. Regulation of lysosomal enzyme release by prostaglandins, autonomic neurohormones and cyclic nucleotides. Front Biol. 1975;43(4):481–523. [PubMed] [Google Scholar]
- Itaya K., Ui M. A new micromethod for the colorimetric determination of inorganic phosphate. Clin Chim Acta. 1966 Sep;14(3):361–366. doi: 10.1016/0009-8981(66)90114-8. [DOI] [PubMed] [Google Scholar]
- Jaffe E. A., Nachman R. L., Becker C. G., Minick C. R. Culture of human endothelial cells derived from umbilical veins. Identification by morphologic and immunologic criteria. J Clin Invest. 1973 Nov;52(11):2745–2756. doi: 10.1172/JCI107470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KISSANE J. M., ROBINS E. The fluorometric measurement of deoxyribonucleic acid in animal tissues with special reference to the central nervous system. J Biol Chem. 1958 Jul;233(1):184–188. [PubMed] [Google Scholar]
- Kuehl F. A., Jr Prostaglandins, cyclic nucleotides and cell function. Prostaglandins. 1974 Feb 25;5(4):325–340. doi: 10.1016/s0090-6980(74)80116-4. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lakshmanan M. R., Nepokroeff C. M., Ness G. C., Dugan R. E., Porter J. W. Stimulation by insulin of rat liver -hydroxy- -methylglutaryl coenzyme A reductase and cholesterol-synthesizing activities. Biochem Biophys Res Commun. 1973 Feb 5;50(3):704–710. doi: 10.1016/0006-291x(73)91301-6. [DOI] [PubMed] [Google Scholar]
- Levin R. I., Jaffe E. A., Weksler B. B., Tack-Goldman K. Nitroglycerin stimulates synthesis of prostacyclin by cultured human endothelial cells. J Clin Invest. 1981 Mar;67(3):762–769. doi: 10.1172/JCI110093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh J. M. The role of cyclic AMP in gonadal steroidogenesis. Biol Reprod. 1976 Feb;14(1):30–53. doi: 10.1095/biolreprod14.1.30. [DOI] [PubMed] [Google Scholar]
- Miller O. V., Aiken J. W., Shebuski R. J., Gorman R. R. 6-keto-prostaglandin E1 is not equipotent to prostacyclin (PGI2) as an antiaggregatory agent. Prostaglandins. 1980 Aug;20(2):391–400. doi: 10.1016/s0090-6980(80)80056-6. [DOI] [PubMed] [Google Scholar]
- Moncada S., Gryglewski R., Bunting S., Vane J. R. An enzyme isolated from arteries transforms prostaglandin endoperoxides to an unstable substance that inhibits platelet aggregation. Nature. 1976 Oct 21;263(5579):663–665. doi: 10.1038/263663a0. [DOI] [PubMed] [Google Scholar]
- Naghshineh S., Treadwell C. R., Gallo L., Vahouny G. V. Activation of adrenal sterol ester hydrolase by dibutyryl cAMP and protein kinase. Biochem Biophys Res Commun. 1974 Dec 11;61(3):1076–1082. doi: 10.1016/0006-291x(74)90265-4. [DOI] [PubMed] [Google Scholar]
- Nepokroeff C. M., Lakshmanan M. R., Ness G. C., Dugan R. E., Porter J. W. Regulation of the diurnal rhythm of rat liver beta-hydroxy-beta-methylglutaryl coenzmye A reductase activity by insulin, glucagon, cyclic AMP and hydrocortisone. Arch Biochem Biophys. 1974 Feb;160(2):387–396. doi: 10.1016/0003-9861(74)90412-3. [DOI] [PubMed] [Google Scholar]
- Nicolosi R. J., Smith S. C., Santerre R. F. Simultaneous fluorometric analysis of five lipid classes on thin-layer chromatograms. J Chromatogr. 1971 Aug 5;60(1):111–117. [PubMed] [Google Scholar]
- Peters T. J., Müller M., De Duve C. Lysosomes of the arterial wall. I. Isolation and subcellular fractionation of cells from normal rabbit aorta. J Exp Med. 1972 Nov 1;136(5):1117–1139. doi: 10.1084/jem.136.5.1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapp N. S., Zenser T. V., Davis B. B. 6-Ketoprostaglandin E1 stimulation of rat and rabbit renal adenylate cyclase-cyclic AMP systems. Biochim Biophys Acta. 1981 Mar 5;673(2):163–169. [PubMed] [Google Scholar]
- Ross R. The smooth muscle cell. II. Growth of smooth muscle in culture and formation of elastic fibers. J Cell Biol. 1971 Jul;50(1):172–186. doi: 10.1083/jcb.50.1.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shio H., Haley N. J., Fowler S. Characterization of lipid-laden aortic cells from cholesterol-fed rabbits. II. Morphometric analysis of lipid-filled lysosomes and lipid droplets in aortic cell populations. Lab Invest. 1978 Oct;39(4):390–397. [PubMed] [Google Scholar]
- Spiegelman B. M., Green H. Cyclic AMP-mediated control of lipogenic enzyme synthesis during adipose differentiation of 3T3 cells. Cell. 1981 May;24(2):503–510. doi: 10.1016/0092-8674(81)90341-x. [DOI] [PubMed] [Google Scholar]
- Subbiah M. T., Dicke B. A. Effect of prostaglandins E1 and F1alpha on the activities of cholesteryl ester synthetase and cholesteryl ester hydrolases of pigeon aorta in vitro. Atherosclerosis. 1977 May;27(1):107–111. doi: 10.1016/0021-9150(77)90030-2. [DOI] [PubMed] [Google Scholar]
- Subbiah M. T. Prostaglandin E2 biosynthesis and effect in pigeon aorta. Possible role in atherogenesis. Atherosclerosis. 1978 Apr;29(4):487–495. doi: 10.1016/0021-9150(78)90177-6. [DOI] [PubMed] [Google Scholar]
- Szego C. M. The role of cyclic AMP in lysosome mobilization and their nucleotropic translocation in steroid hormonal target cells. Adv Cyclic Nucleotide Res. 1972;1:541–564. [PubMed] [Google Scholar]
- Takano T., Black W. J., Peters T. J., de Duve C. Assay, kinetics, and lysosomal localization of an acid cholesteryl esterase in rabbit aortic smooth muscle cells. J Biol Chem. 1974 Nov 10;249(21):6732–6737. [PubMed] [Google Scholar]
- Takatori T., Yamaoka A. Effects of prostaglandins on the metabolism of cholesteryl ester in rat testes: changes in the synthesis and hydrolysis of cholesteryl ester and the activity of cholesterol side-chain cleavage enzyme. J Endocrinol. 1978 Oct;79(1):41–48. doi: 10.1677/joe.0.0790041. [DOI] [PubMed] [Google Scholar]
- Tateson J. E., Moncada S., Vane J. R. Effects of prostacyclin (PGX) on cyclic AMP concentrations in human platelets. Prostaglandins. 1977 Mar;13(3):389–397. doi: 10.1016/0090-6980(77)90019-3. [DOI] [PubMed] [Google Scholar]
- Trzeciak W. H., Boyd G. S. Activation of cholesteryl esterase in bovine adrenal cortex. Eur J Biochem. 1974 Jul 1;46(1):201–207. doi: 10.1111/j.1432-1033.1974.tb03612.x. [DOI] [PubMed] [Google Scholar]
- Vahouny G. V., Chanderbhan R., Hinds R., Hodges V. A., Treadwell C. R. ACTH-induced hydrolysis of cholesteryl esters in rat adrenal cells. J Lipid Res. 1978 Jul;19(5):570–577. [PubMed] [Google Scholar]
- Williams M. T., Clark M. R., Ling W. Y., LeMaire W. J., Caron M. G., Marsh J. M. Role of cyclic AMP in the actions of luteinizing hormone on steroidogenesis in the corpus luteum. Adv Cyclic Nucleotide Res. 1978;9:573–582. [PubMed] [Google Scholar]
- Wong P. Y., Lee W. H., Quilley C. P., McGiff J. C. Metabolism of prostacyclin: formation of an active metabolite in the liver. Fed Proc. 1981 May 15;40(7):2001–2004. [PubMed] [Google Scholar]
- Wong P. Y., Malik K. U., Desiderio D. M., McGiff J. C., Sun F. F. Hepatic metabolism of prostacyclin (PGI2) in the rabbit: formation of a potent novel inhibitor of platelet aggregation. Biochem Biophys Res Commun. 1980 Mar 28;93(2):486–494. doi: 10.1016/0006-291x(80)91103-1. [DOI] [PubMed] [Google Scholar]



