Abstract
Severe dyslipidemia and the associated oxidative stress could accelerate the age-related decline in cerebrovascular endothelial function and cerebral blood flow (CBF), leading to neuronal loss and impaired learning abilities. We hypothesized that a chronic treatment with the polyphenol catechin would prevent endothelial dysfunction, maintain CBF responses, and protect learning abilities in atherosclerotic (ATX) mice. We treated ATX (C57Bl/6-LDLR−/− hApoB+/+; 3 mo old) mice with catechin (30 mg·kg−1·day−1) for 3 mo, and C57Bl/6 [wild type (WT), 3 and 6 mo old] mice were used as controls. ACh- and flow-mediated dilations (FMD) were recorded in pressurized cerebral arteries. Basal CBF and increases in CBF induced by whisker stimulation were measured by optical coherence tomography and Doppler, respectively. Learning capacities were evaluated with the Morris water maze test. Compared with 6-mo-old WT mice, cerebral arteries from 6-mo-old ATX mice displayed a higher myogenic tone, lower responses to ACh and FMD, and were insensitive to NOS inhibition (P < 0.05), suggesting endothelial dysfunction. Basal and increases in CBF were lower in 6-mo-old ATX than WT mice (P < 0.05). A decline in the learning capabilities was also observed in ATX mice (P < 0.05). Catechin 1) reduced cerebral superoxide staining (P < 0.05) in ATX mice, 2) restored endothelial function by reducing myogenic tone, improving ACh- and FMD and restoring the sensitivity to nitric oxide synthase inhibition (P < 0.05), 3) increased the changes in CBF during stimulation but not basal CBF, and 4) prevented the decline in learning abilities (P < 0.05). In conclusion, catechin treatment of ATX mice prevents cerebrovascular dysfunctions and the associated decline in learning capacities.
Keywords: endothelial function, pressurized cerebral arteries, cerebral blood flow, morris water maze
One of the primary concerns in neurological sciences is the relationship between cerebral blood flow (CBF) and nerve cell survival (43). The increase in CBF produced by brain activity, or functional hyperemia, is an example of the close interaction between neurons and blood vessels (12). The neurovascular unit including neurons, astrocytes and endothelial cells, physiologically controls CBF locally during neuronal activation (4, 19, 26). Endothelial cells play an important role in CBF regulation by releasing many vasoactive factors such as nitric oxide (NO), prostacyclin, and thromboxane A2 (43). Upon neuronal activation, the rise in CBF is, however, also required upstream in larger arteries to accommodate the local metabolic needs; this is partly an endothelium-dependent dilatory response to the rise in shear stress (8), but vasomotor responses via gap junctions and astrocytic factors may also contribute to the regulation of blood flow in upstream arteries during neuronal activation (4, 19, 26). The release of these endothelium-derived relaxing (EDRF) or constricting (EDCF) factors evolves with aging and diseases such as atherosclerosis (11, 28) and is often characterized by a reduction in EDRF production and a rise in EDCF. An alteration in the integrity of the neurovascular unit but also in the upstream endothelial function would likely alter CBF and perturb the delivery of substrates to brain cells, thus contributing to neuronal degeneration.
Aging and atherosclerosis are major risk factors for stroke and have been associated with a decrease in NO production and an increase in reactive oxygen species (ROS) generation (31), two phenomena that could promote a decrease in CBF (6, 22). Atherosclerotic plaque progression in carotid arteries is associated with ischemic stroke and reduced perfusion to the brain, which are major contributors to cognitive decline as well as a critical and determining factor for dementia. ROS have been thought to attack neurons and induce cognitive decline in rats (35), neurodegenerative diseases such as Alzheimer’s disease and Parkinsonism (35), and neurodegenerative diseases in mice (15). It is not well documented, however, if the cerebral vasculature is also highly sensitive to oxidative stress, leading to endothelial dysfunction and impaired CBF responses to an increase in metabolic demand.
Catechin is a polyphenol antioxidant that has been shown to slow the atherogenic process (9, 23), as well as the decline in the learning ability associated with brain hypoperfusion (44). In a rat model of Parkinson’s disease, resveratrol, another polyphenol, has been shown to protect from neuronal death (25). When green tea is given to rats, polyphenols can cross the blood-brain barrier and display neuroprotective effects, partly by improving the endothelial function via a reduction in lipid peroxidation (16, 17). Likewise, a recent clinical study suggests that the daily consumption of green tea reduces the risk of stroke (30). We therefore hypothesized that a chronic catechin treatment would protect the endothelial cell function in cerebral arteries of atherosclerotic mice and thus prevent an atherosclerosis-associated decline in CBF responses, which could, ultimately, improve their learning capacities.
MATERIALS AND METHODS
Animals and tissue preparation
The procedures and protocols were reviewed and approved by our institutional independent review board and followed the Guide for the Care and Use of Laboratory Animals of Canada. Animals were kept under standard conditions (24°C; 12:12-h light-dark cycle). We used 3- and 6-mo-old C57Bl/6 male mice [wild type (WT), body weight of 29 ± 1 and 37 ± 2 g, respectively, n = 32; Charles River Laboratories, St. Constant, QC, Canada] and atherosclerotic mice (ATX, body weight of 25 ± 1 and 31 ± 2 g, respectively, n = 34); the latter are knockout for the low-density lipoprotein (LDL) receptor but express the human apolipoprotein B-100 gene (LDLR−/−hApoB+/+). The founders of the colony of ATX mice were generously provided by Dr. H. H. Hobbs (University of Texas Southwestern, Dallas, TX). A group of ATX mice received a chronic treatment (from 3 to 6 mo old) with the antioxidant catechin (98% purity; Sigma-Aldrich). These mice received catechin in their drinking water [ATX + CAT; 30 mg·kg−1·day−1 as previously described (9, 10), body weight of 34 ± 2 g; n = 42]. All mice were fed with a normal standard diet (Tecklad 2014S; Harlan Laboratories, Montreal, QC, Canada) since ATX mice develop spontaneous atherosclerotic lesions.
Blood pressure and blood analyses
Mice (3 and 6 mo old) were killed by exsanguination under anesthesia (isoflurane 2.5% in 0.5 l/min O2, 30 – 45 min duration), after monitoring heart rate (HR) and cardiac function (systolic and diastolic blood pressures) using a Millar catheter inserted in the left ventricle via the carotid artery, as previously described (7). The measures of blood pressure and HR in anesthetized mice were taken when a steady state was reached, and represent an average value.
Plasma was collected from a blood sample and frozen at −80°C. Total cholesterol, LDL, and triglycerides were measured in the hospital’s biochemical laboratory (9, 10).
Reactivity studies
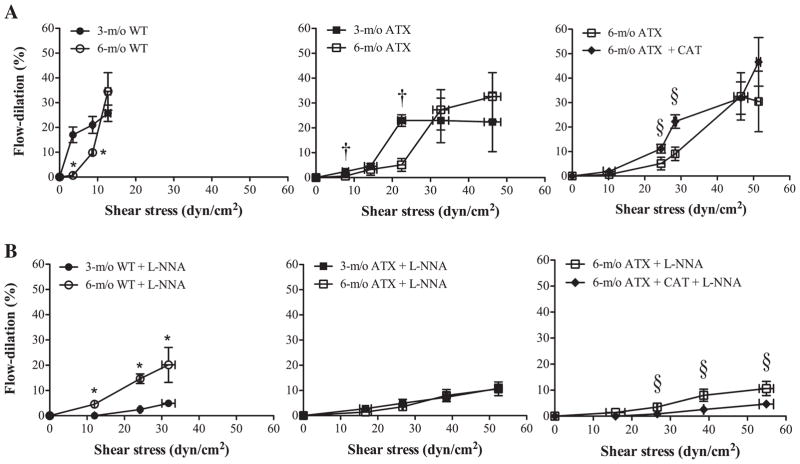
The brain was rapidly removed from the cranial cavity and placed in an ice-cold physiological saline solution (PSS) of the following composition (mmol/l): 130 NaCl, 4.7 KCl, 1.6 CaCl2, 1.17 MgSO4, 14.9 NaHCO3, 1.18 KH2PO4, 0.026 EDTA, and 10 glucose. As previously described (8), cerebral arteries from the circle of Willis were isolated, cannulated at both ends, and pressurized at 60 mmHg (internal diameter of 136 ± 2 μm; n = 15) in PSS oxygenated by a gas mixture containing 12% O2, 5% CO2 and 83% of N2, generating a PaO2 of 150 ± 10 mmHg (n = 5). Briefly, after an equilibration period to allow myogenic tone (MT, the contractile response of resistance arteries to an increase in intraluminal pressure) to develop, the arteries were preconstricted with phenylephrine (PE) using a concentration ranging from 10 to 30 μmol/l. On PE-precontracted arteries, dose-responses curves to ACh (0.1 nM to 30 μM) were constructed. We used Nω-nitro-L-arginine (L-NNA; 10 μmol/l) to investigate the implication of nitric oxide synthase (NOS) in ACh-induced dilations. On different arteries, flow-mediated dilation (FMD) was induced using a flow control peristaltic pump (Living Systems Instrumentation, Burlington, VT) directly connected to the pressure myograph: a single cumulative FMD curve (from 0 to 25 μl/min, 2- or 5-μl step increase at constant internal pressure) was performed on each segment, as previously described (8), in the absence and in the presence of L-NNA. Shear stress was calculated using the following equation: t ± 4ηQ/πr3, where t is the shear stress (dyn/cm2), η the viscosity [0.009 P (8, 26)], Q the flow rate (ml/s) through the lumen, and r the inside radius (cm). FMD are expressed as changes (%) in diameter, normalized to the maximum passive diameter; they were calculated according to the following formula, as previously reported (8): flow dilation = 100 × [(b − a)/(Dmax −a)], where Dmax is the maximal diameter in Ca2+-free solution, b is the diameter reached by the vessel during the steady state of the flow response, and a is the preflow diameter (i.e., the minimal diameter obtained by exposure to PE). FMD are presented in Fig. 1 as the dilation obtained for a given shear stress.
Fig. 1.
Pressurized cerebral arteries isolated from 3- and 6-mo-old wild-type (WT) (n = 10 and 6, respectively) and atherosclerotic (ATX) (n = 6) mice were stimulated with flow, in the absence (A) or in the presence (B) of Nω-nitro-L-arginine (L-NNA, 10 μmol/l). Flow (from 0 to 25 μl/min) induces shear stress (in dyn/cm2) that promotes endothelial-dependent flow dilation (%). Each panel represents the flow dilations obtained at an average given shear stress to compare the endothelial response between groups [WT mice, ATX mice, treated or not with catechin (CAT)]. Data are means ± SE of n mice. Unpaired t-test was performed at each shear stress, in each group of mice: P < 0.05 vs. 3-mo-old WT (*), vs. 3-mo-old ATX (†), and vs. 6-mo-old ATX (§).
Plaque area quantification
The thoracic aorta was removed from mice and carefully dissected from surrounding tissues. The vessel was opened longitudinally and a picture was taken. Plaque area quantification was calculated using Photoshop software and expressed as a percentage of the aortic area (9).
Quantification of superoxide production
The oxidative fluorescent probe dihydroethidium (DHE; Sigma-Aldrich Canada, Oakville, ON, Canada) was used to evaluate in situ superoxide production on aorta sections (9, 29) and on basilar artery sections. DHE is a cell permeable dye that is oxidized by superoxide to ethidium bromide, which subsequently intercalates with DNA and is trapped within cell nuclei. DNA counterstaining was performed using To-Pro-3 (Molecular Probe, Burlington, ON, Canada). Frozen aortic and basilar artery segments were cut into 14-μm-thick sections. Sections were double-stained with a mixture of 5 μmol/l DHE and 2 μmol/l To-Pro-3. Fluorescence was visualized with a scanning confocal microscope LMS 510 (Carl Zeiss, Oberkochen, Germany) with an oil ×40/1.3 Plan-Apochromat objective for aorta sections and an oil ×63/1.4 Apochromat objective for basilar sections (λEx: 490 nm, λEm: 520 nm). The camera’s acquisition settings were identical for all images. Computer-based analysis was performed using Image J software and calculated by the following equation: I= ΣI/(A/N), where I is the fluorescence intensity, ΣI the summation of all nuclei intensity, A the total area of the nuclei, and N the number of nuclei used. Data are expressed as an average fluorescence per nucleus.
Quantification of basal CBF
Blood flow measurements of small brain arteries were performed using a Doppler optical coherence tomography (OCT) System with reconstruction of flow speed using a moving-scatterer-sensitive reconstruction technique adapted from Ref. 39. The system is based on an infrared Super Light Emitting Diode (EXS8710–2411; Exalos, Langhorne, PA) with a center wavelength of 870 nm, a spectral width of 65 nm, and a typical output power of 5 mW providing 2.5 mW of power on the sample. This light source yields a theoretical axial resolution of 7 μm. A custom-built spectrometer was used as the detector using a 1,200 line/mm volume phase holographic grating (Wasatch photonics, Logan, UT) and a high-speed 2048 pixel line camera (Sprint spL2048 −140k; Basler, Exton, PA). The maximum acquisition speed of the camera was 67k A-lines per second, but hard-drive writing limited acquisition to 15k A-lines/s. At this acquisition rate, the maximum detectable Doppler blood flow speed was 3 mm/s. The pixel density corresponded to a wavelength step of Δλ = 0.05 nm/pixel translating into a wave number step of Δk = 415 m−1/pixel. This led to a depth of field of ~3 mm in air. Scanning on the sample was performed using a dual galvanometer system (Thorlabs, Newton, NJ) imaged using a telescope (f1 = 150 mm and f2 = 50 mm) on the back aperture of a ×10 (UMPLFLN 10XW; Olympus, Markham, Ontario) infinity-corrected objective. Figure 2 shows a schematics of the system. Before the in vivo studies, the system was validated for its ability to quantify blood flow in phantoms reproducing absorption and scattering properties of brain tissues in which small capillary tubes were inserted to model small vessels. Doppler measures were consistent with flows generated by a syringe pump over the range −3 to 3 mm/s.
Fig. 2.
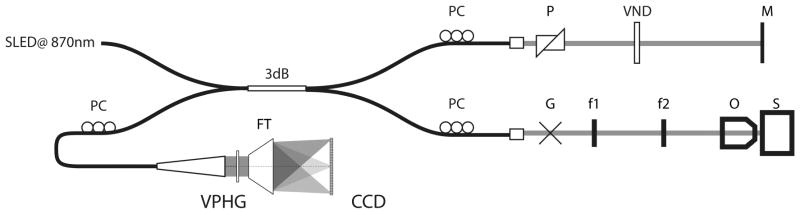
Schematic of optical coherence tomography (OCT) system design. SLED, super luminescent diode; PC, polarization controllers; P, dispersion compension prisms; VND, variable neutral density filter; M, reference mirror; G, dual galvanometer scanners; f, sample arm telescope lenses; O, objective; S, sample; VPHG, volume phase holographic grating; FT, F-theta lens; CCD, charge-coupled device line camera.
The mice were anesthetized with somnotol (65 mg/kg). Skin was removed from the top of the head, and imaging was performed directly through the skull. Series of volume acquisitions were done on each mouse with scan dimensions of 800 × 800 μm and an imaging depth of 1.7 mm. Arteries were found at a distance ranging between 200 and 400 μm from the skull. According to the procedure outlined in Ref. 41, quantitative blood flow (nl/s) was calculated in each mouse on plunging arteries found in each three-dimensional volume data set. An average of three distinct arteries were used per animal. Vessel diameter was estimated as the smallest cross section of the vessel crossing the horizontal imaging plane; arteries with a diameter between 30 and 120 μm were used for the analysis. Blood flow was then estimated by integrating speed over the area of interest (nl·s−1·mm−2) to normalize the data and to compare basal CBF between different animals.
Laser Doppler flowmetry
Laser Doppler flowmetry was used to measure changes in cortical CBF during neuronal stimulation (Transonic Systems, Ithaca, NY), but not basal CBF (34). Anesthetized mice (ketamine, 80 mg/kg ip) were fixed in a stereotaxic frame, and the bone over the barrel cortex was thinned to translucency using a dental drill. Body temperature was maintained at 37°C using a heating pad. The mice undergoing CBF measurement were not ventilated; neither PaO2, PaCO2, nor pH was controlled; however, physiological parameters such as blood pressure, HR, and respiratory rate were very stable during prolonged anesthesia (data not shown). Changes in CBF were recorded before, during, and after whisker stimulation (20 s at 8–10 Hz), with four to six recordings acquired every 30 – 40 s and averaged for each mouse. Cortical CBF changes were expressed as the percentage increase relative to baseline.
Morris water maze
Mice were tested for their learning abilities using a Morris water maze apparatus (160 cm in diameter and 45 cm high) (40). The maze was located in a quiet test room, surrounded by four visual cues on the pool border. All groups were tested for five consecutive days in a circular pool filled with water (19°C, clouded with powdered skim milk). The pool contained a transparent platform (10 cm in diameter) submerged 1.5 cm below the water surface in the center of the northwest quadrant (3, 32). Mice were randomly started from each of the four positions (south, east, west, and north), and they used visuospatial cues to find the hidden platform that remained in the same quadrant throughout testing. Their movements were automatically recorded using HVS 2020 tracker and analyzed with the Water 2020 software (HVS Image, Hampton, UK). Escape latencies, i.e., the time needed to find the platform (maximum 120 s) from the four starting points, were measured daily and individually and used to trace a learning curve. The slope of this learning curve is an index of the learning capacities. On the 6th day, the mice were administered two cue trials (30 s in duration) during which the platform was made visible by lowering the water level 2 cm below the top of the platform. The cue trials were conducted to confirm the absence of physical and visual incapacities.
Statistics
n refers to the number of animals used in each protocol. Continuous variables are expressed as means ± SE. Paired t-test was performed to study the effects of L-NNA on MT. Unpaired t-test was performed to study the effects of L-NNA on ACh-induced dilation and FMD. One-way ANOVA followed by Tukey’s test as post hoc test was used to compare each parameter among the different groups of mice. Performances in the Morris water maze were evaluated by three-way (learning curve) and one-way (cue test) ANOVA followed by Tukey’s test as post hoc test (Sigma Stat 2.0; SPSS, Chicago, IL). The results were considered to be statistically significant when the P value was < 0.05.
RESULTS
Cardiac function, aortic histology, and blood analyses
The HR was stable in WT and ATX mice at 3 and 6 mo old (Table 1). Systolic, diastolic, and mean arterial pressures were increased in 3- and 6-mo-old ATX but not in aged-matched WT mice (Table 1). Catechin treatment had no significant effect on the HR and blood pressure (Table 1), as previously reported (9, 10). However, catechin tended to increase blood pressure in ATX mice by 8–12 mmHg (P > 0.05).
Table 1.
Effect of age and catechin treatment on hemodynamic parameters and cholesterol and triglycerides levels in wild-type and atherosclerotic mice
| WT
|
ATX
|
6-mo-old ATX 3 CAT | |||
|---|---|---|---|---|---|
| 3 mo old | 6 mo old | 3 mo old | 6 mo old | ||
| Hemodynamic parameters | |||||
| HR, beats/min | 377 ± 19 | 330 ± 5 | 371 ± 22 | 349 ± 11 | 354 ± 9 |
| SAP, mmHg | 94 ± 2 | 94 ± 3 | 106 ± 3* | 119 ± 3† | 131 ± 5 |
| DAP, mmHg | 59 ± 3 | 63 ± 3 | 73 ± 2* | 82 ± 2† | 90 ± 4 |
| MAP, mmHg | 71 ± 3 | 67 ± 6 | 84 ± 3* | 94 ± 2† | 103 ± 4 |
| Plasma levels | |||||
| Cholesterol, mmol/l | 2.1 ± 0.1 | 3.1 ± 0.1 | 16.6 ± 2.0* | 20.1 ± 2.2† | 20.1 ± 1.3 |
| LDL, mmol/l | 0.5 ± 0.1 | 1.2 ± 0.1 | 10.3 ± 1.6* | 12.4 ± 1.1† | 12.7 ± 1.0 |
| HDL, mmol/l | 1.3 ± 0.1 | 1.7 ± 0.1 | 1.7 ± 0.1 | 1.9 ± 0.1 | 1.7 ± 0.1 |
| Triglycerides, mmol/l | 0.7 ± 0.1 | 0.7 ± 0.2 | 6.2 ± 0.5* | 7.5 ± 0.8 † | 8.5 ± 0.8 |
Values are means ± SE; n = 8–10 mice in each group. WT, wild type; ATX, atherosclerotic; CAT, catechin; HR, heart rate; SAP, systolic arterial pressure; DAP, diastolic arterial pressure; MAP, mean arterial pressure; HDL, high-density lipoprotein; LDL, low-density lipoprotein. P < 0.05 vs. 3-mo-old WT (*) and vs. 6-mo-old WT (†).
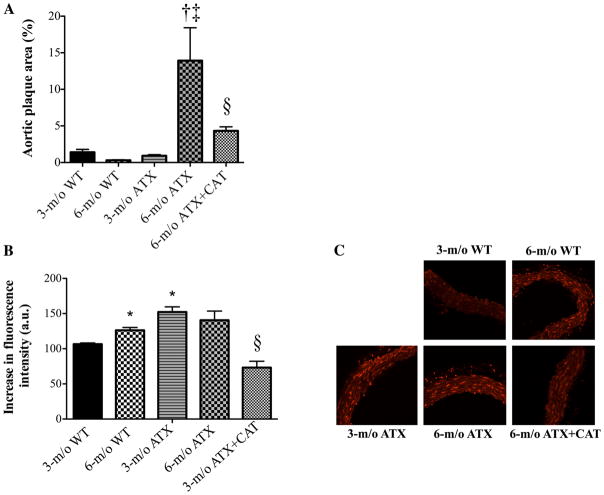
ATX mice had higher cholesterol, LDL, and triglyceride levels at 3 and 6 mo old compared with WT mice (Table 1). High-density lipoprotein (HDL) levels were unchanged. The 3-mo treatment period with catechin did not affect cholesterol, LDL, HDL, and triglyceride levels (Table 1). The aortic plaque area was not significant in WT mice and 3-mo-old ATX mice, whereas it increased significantly in 6-mo-old ATX mice. Catechin limited plaque growth in 6-mo-old ATX mice (Fig. 3A). In ATX mice, atherosclerotic lesions were also present in carotid arteries, but they were not observed in cerebral vessels (data not shown).
Fig. 3.
Effect of a 3-mo treatment with the antioxidant CAT (n = 6) on the atherosclerotic plaque area in the thoracic aorta (A) and the superoxide production quantified by dihydroethidium (DHE) staining (B) in aortic histological sections of 3- and 6-mo-old WT (n = 4 and 6, respectively) and ATX (n = 5 and 6, respectively) mice. C: representative images of DHE staining in the different groups of mice. Data are means ± SE of n mice. One-way ANOVA: P < 0.05 vs. 3-mo-old WT (*), vs. 3-mo-old ATX (†), vs. 6-mo-old WT (‡), and vs. 6-mo-old ATX (§).
Oxidative stress
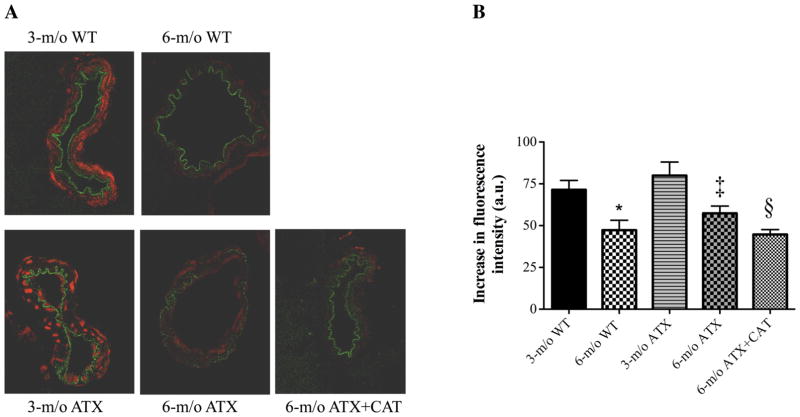
In adult 6-mo-old WT mice, aortic super-oxide production (assessed by DHE staining) was higher than in 3-mo-old mice (Fig. 3B). In contrast, superoxide production was more elevated in 3-mo-old ATX mice and did not further increase at 6 mo of age. Catechin treatment significantly reduced superoxide production in 6-mo-old ATX mice (Fig. 3B). Surprisingly, in basilar arteries, the estimated oxidative stress was higher in arteries from 3-mo-old mice, in either WT or ATX mice, than in vessels from 6-mo-old mice (Fig. 4). Thus oxidative stress did not rise in cerebral arteries between 3 and 6 mo of age and with dyslipidemia, in contrast to what was observed in the aorta. Catechin treatment, however, reduced superoxide production in basilar arteries from 6-mo-old ATX mice, demonstrating its antioxidant effect (Fig. 4).
Fig. 4.
Effect of a 3-mo treatment with the antioxidant CAT (n = 3) on the superoxide production quantified by DHE staining in basilar artery sections of 3-and 6-mo-old WT (n = 3) and ATX (n = 3) mice. The assay (A) was performed on four different sections of the basilar artery per mice, in triplicates. Data are means ± SE of n mice (B). One-way ANOVA: P < 0.05 vs. 3-mo-old WT (*), vs. 6-mo-old ATX (‡), and vs. 6-mo-old ATX (§).
MT
MT was higher in cerebral arteries isolated from 3- and 6-mo-old ATX mice compared with age-matched WT (Fig. 5). In ATX mice, MT was higher in 6- than in 3-mo-old mice. Inhibition of the basal activity of NOS by L-NNA increased MT in 3-mo-old WT and ATX mice but not in arteries isolated from 6-mo-old WT and ATX mice (Fig. 5). Catechin treatment prevented the increase in MT in vessels isolated from 6-mo-old ATX mice and restored the inhibitory effect of L-NNA (Fig. 5).
Fig. 5.
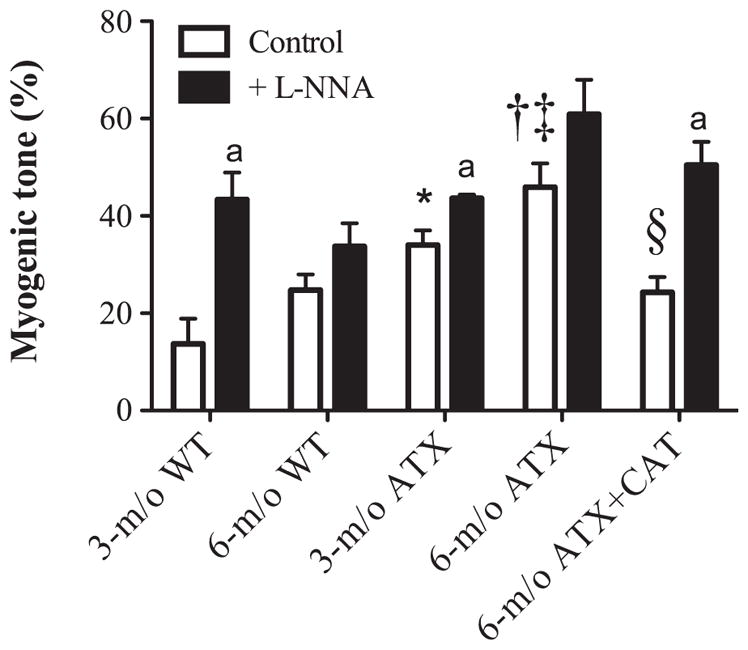
Effect of a 3-mo CAT treatment (n = 6) on cerebral artery myogenic tone induced by an intraluminal pressure of 60 mmHg in cerebral arteries isolated from 3- and 6-mo-old WT (n = 8 and 6, respectively) and ATX (n = 6) mice, before and after acute addition of L-NNA (10 μmol/l). Data are means ± SE of n mice. One-way ANOVA: P < 0.05 vs. 3-mo-old WT (*), vs. 3-mo-old ATX (†), vs. 6-mo-old WT (‡), and vs. 6-mo-old ATX (§). Paired t-test: aP < 0.05 vs. control condition in the absence of L-NNA.
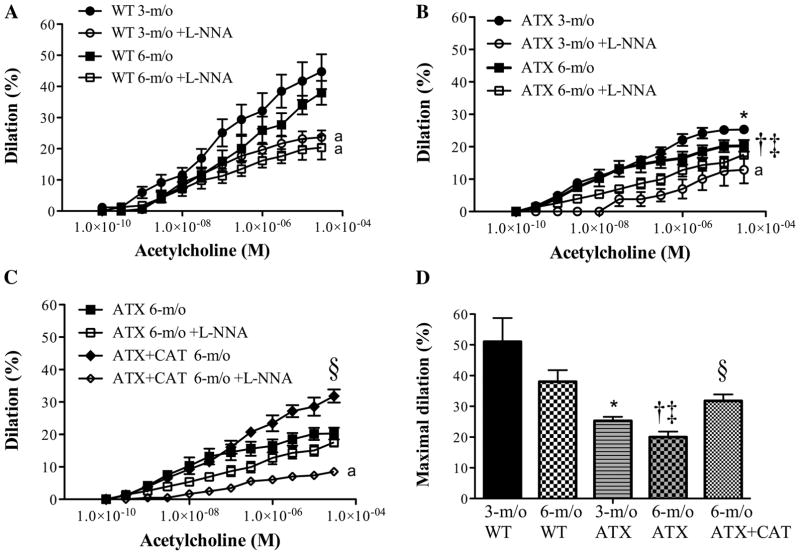
Dose-responses curves to ACh
Dilatory responses to ACh were similar in 3- and 6-mo-old WT mice (Fig. 6, A and D) and were significantly reduced by L-NNA, suggesting a role for endothelial nitric oxide synthase (eNOS) in the dilatory process. However, ACh-induced dilations were significantly lowered in arteries isolated from 3-mo-old ATX mice (Fig. 6, B and D), and even more in arteries isolated from 6-mo-old ATX mice; in the latter, the responses to ACh were unaffected by L-NNA. Treatment with catechin prevented endothelial dysfunction and restored the inhibitory effect of L-NNA (Fig. 6, C and D). Although the sensitivity (EC50) to ACh was similar between groups (data not shown), the efficacy (maximal dilation) of ACh was affected by age and atherosclerosis (Fig. 6D).
Fig. 6.
Dose-response curves to ACh in pressurized cerebral arteries isolated from 3- and 6-mo-old WT (n = 8 and 6, respectively) (A) and ATX (n = 8 and 7, respectively) (B) mice, before and after acute addition of L-NNA (10 μmol/l). C: dose-response curves to ACh in pressurized cerebral arteries isolated from 6-mo-old ATX mice treated (n = 5) or not (n = 7) with CAT. In the dose-response curve to ACh, only dilations obtained at the maximal concentration of ACh (10 μM) were compared between groups (one-way ANOVA). D: efficacy of ACh, or maximal dilation to ACh, in the different groups of mice. Data are means ± SE of n mice. One-way ANOVA: P < 0.05 vs. 3-mo-old WT (*), vs. 3-mo-old ATX (†), vs. 6-mo-old WT (‡), and vs. 6-mo-old ATX (§). Unpaired t-test: aP < 0.05 vs. control condition in the absence of L-NNA.
FMD
There is a direct and positive correlation between the applied flow rate and the induced shear stress (Fig. 7). In an artery of 80–170 μm inner diameter, flow rates from 2 to 25 μl/min induce shear stresses from 1.4 – 6.9 to 13.5–44.1 dyn/cm2, i.e., in the physiological range (36). Figure 1 presents flow dilations obtained at a given shear stress and shows that the sensitivity to shear stress decreases with age in both cerebral arteries from WT and ATX mice (Fig. 1A): at a given shear stress, FMD is lower in 6-mo-old mice. In addition, the sensitivity to shear stress is lower in cerebral arteries from ATX mice than from age-matched WT mice: at a given low shear stress (< 15 dyn/cm2), FMD are lower in ATX mice (Figs. 1A and 7). In addition, the shear stresses induced by applied flow rates are higher in ATX mice. In contrast, catechin treatment improved the amplitude of FMD but not the sensitivity to shear stress (Fig. 1A). Because FMD in cerebral arteries are largely dependent on the activation of the eNOS pathway (8, 21, 27), we tested the effects of L-NNA; in the presence of the NOS inhibitor, shear stress induced by flow significantly increased, particularly in cerebral arteries from WT mice, reflecting the role of eNOS in these mice (Fig. 1B). FMD were significantly reduced by L-NNA in vessels isolated from 3-mo-old WT mice and to a lesser extent in arteries from 6-mo-old WT mice (Fig. 1B), suggesting a premature decline in eNOS activity. In cerebral arteries isolated from ATX mice, NOS inhibition did not further reduce FMD (Fig. 1B). A 3-mo treatment with catechin restored the inhibitory effect of L-NNA (Fig. 1B).
Fig. 7.
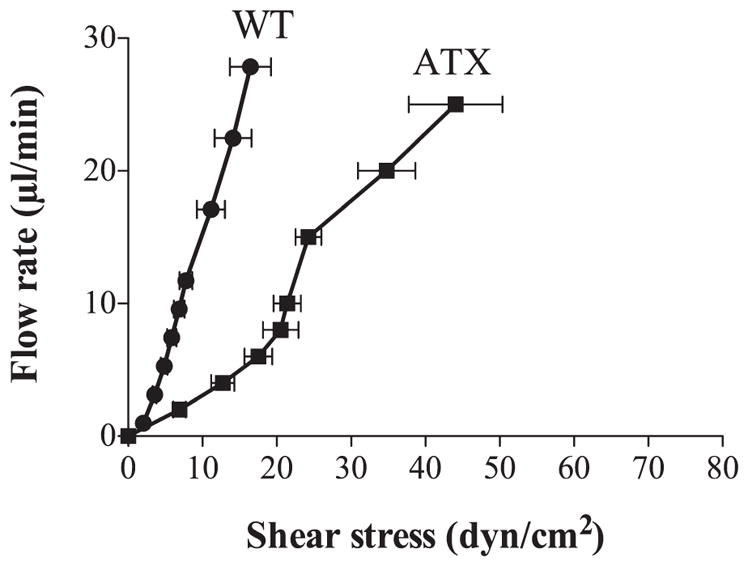
Correlation between the flow rate (from 0 to 25 μl/min) applied to pressurized cerebral arteries isolated from 3-mo-old WT (n = 10) or ATX (n = 6) mice and the shear stress induced (from 0 to 50 dyn/cm2). Data are means ± SE of n mice.
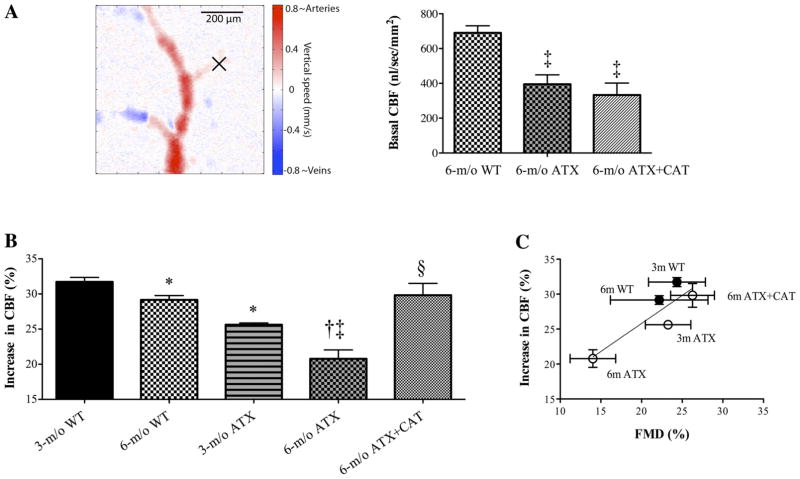
Basal CBF
OCT Doppler-derived CBF is presented in Fig. 8A. Basal CBF was consistently lower in ATX or ATX + CAT mice compared with WT mice (P < 0.001). No significant difference was observed between basal CBF in the treated and untreated groups (Fig. 8A).
Fig. 8.
A: quantification of in vivo basal CBF (nl · s−1 · mm−2) measured in small plunging cerebral arteries (identified by the “X”) using a Doppler OCT system in 6-mo-old WT (n = 4) and ATX mice untreated (n = 4) or treated (n = 3) with CAT. B: effect of a 3-mo CAT (n = 6) treatment on the increase in cerebral blood flow (CBF, %) following whisker stimulation, measured in vivo by laser Doppler, of 3- and 6-mo-old WT (n = 6 and 5, respectively) and ATX (n = 6 and 5, respectively) mice. C: positive correlation (r2 = 0.7675, P = 0.0514) between the average flow-mediated dilations (FMD, %) induced in vitro by a flow rate of 10 μl/min, and the average increases in CBF (%) induced in vivo by neuronal stimulation, in 3- and 6-mo-old WT and ATX mice treated or not with CAT. Data are means ± SE of n mice. One-way ANOVA: P < 0.05 vs. 3-mo-old WT (*), vs. 3-mo-old ATX (†), vs. 6-mo-old WT (‡), and vs. 6-mo-old ATX (§).
Changes in CBF induced by neuronal stimulation
Following whisker stimulation, the increase in cortical CBF in 3-mo-old WT mice (32 ± 1%) was slightly but significantly higher than in 6-mo-old WT (29 ± 1%, P < 0.05) and in 3-mo-old ATX (26 ± 0%, P < 0.05) mice (Fig. 8B). The reduced changes in CBF observed with age in 6-mo-old WT mice (8% reduction) and with atherosclerosis in 3-mo-old ATX mice (19% reduction) were exacerbated (29% reduction) in 6-mo-old ATX (increase in CBF: 21 ± 1%, P < 0.05) (Fig. 8B). Catechin treatment restored the response to a level similar to that measured in 6-mo-old WT mice (increase in CBF: 30 ± 2%, P < 0.05) (Fig. 8B). There was a positive correlation between FMD and increases in CBF (Fig. 8C), suggesting, as expected, that cortical CBF responses are largely dependent on the endothelium-dependent dilatory capacity.
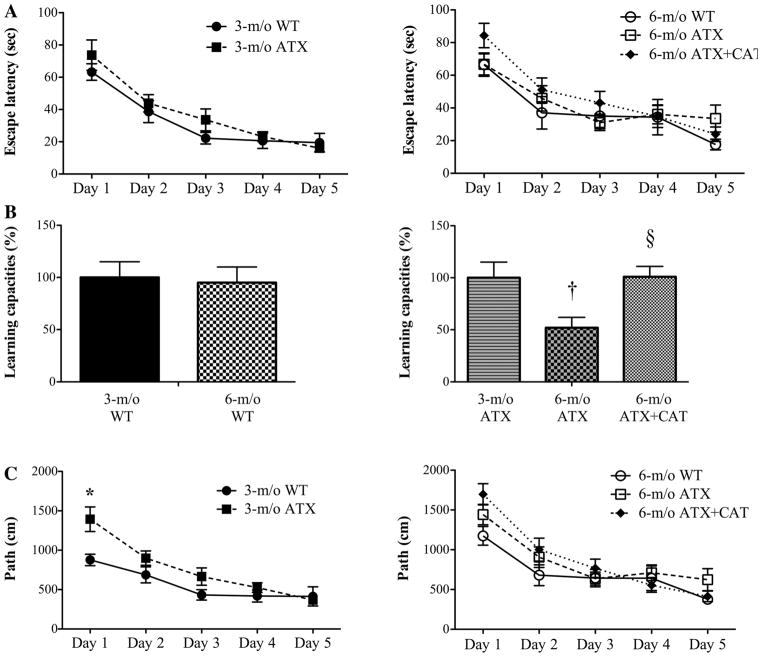
Learning abilities
The duration time for each mouse to find the hidden/submersed platform (escape latency, in s) and the travel distances (path, in cm) decreased throughout the 5 days of the test in both types of mice, at both ages, demonstrating a learning capacity (Fig. 9 and Table 2). The statistical analysis also suggests that the age and the disease status of the mice influence the path but not the escape latency, measured individually at each day of the experiment (Table 2). However, the calculated slopes of the linear regressions of the escape latency curves (as an index of the learning capacities) illustrate a significant decrease in the learning capacity in 6-mo-old ATX compared with 3-mo-old ATX mice (Fig. 9B). In contrast, the learning capacities were not affected in WT mice (Fig. 9B). Catechin treatment improved the learning capacity of 6-mo-old ATX mice, as shown by an increase in the slope of the escape latency curve compared with untreated mice (Fig. 9B). All mice swam at the same speed, and all found the visible platform in the cue trial, demonstrating similar physical or visual capacities to reach the platform (data not shown).
Fig. 9.
Effect of a 3-mo CAT treatment (n = 18) on the learning capacities (escape latency, in s) (A), the calculated (linear regression) slope (%) of the escape latency curves (B), and the path (in cm) (C) of 3- and 6-mo-old WT (n = 11 and 12, respectively) and ATX (n = 12 and 14, respectively) mice. Data are means ± SE of n mice. P < 0.05 vs. 3-mo-old ATX (*), vs. 6-mo-old ATX (†), and vs. 6-mo-old ATX (§).
Table 2.
P values for the 3-way ANOVA main effects and interaction effects obtained with the analysis of the learning capacities of the different groups of mice
| Variable | P Value |
|---|---|
| Escape latency, s | |
| Age | 0.234 |
| Disease | 0.234 |
| Day | <0.0001 |
| Interaction | |
| Age × disease | 0.970 |
| Age × day | 0.515 |
| Disease × day | 0.973 |
| Path, cm | |
| Age | 0.053 |
| Disease | 0.004 |
| Day | <0.0001 |
| Interaction | |
| Age × disease | 0.642 |
| Age × day | 0.642 |
| Disease × day | 0.312 |
The effects of age (3- or 6-mo-old mice), of the disease status (WT or ATX mice), and of the duration (day 1 to day 5) of the Morris water maze test were considered.
DISCUSSION
Our results in ATX mice suggest that oxidative stress could be a potential link between in vitro endothelial function, in vivo CBF responses, and learning abilities: the antioxidant catechin reduces aortic and cerebral DHE fluorescence, a marker of oxidative stress, prevents the decrease in cerebral eNOS activity associated with age and dyslipidemia, and improves the endothelial dilation to a rise in flow. A dysfunctional FMD in response to a neuronal stimulation could limit perfusion, leading to local ischemia and neuronal death and, consequently, promote with time a decline in the learning abilities. Accordingly, catechin restored CBF responses and preserved the learning abilities. Catechin, however, did not normalize the resting flow, suggesting that, at 6 mo of age, the decrease in resting CBF does not explain the cognitive deficit.
Through the basal activity of eNOS, the endothelium is a strong regulator of the vascular tone. In our study, compared with WT mice, MT induced by an intraluminal pressure of 60 mmHg was greater in cerebral arteries isolated from ATX mice and was insensitive to L-NNA, suggesting a dysfunctional resting eNOS activity. In addition, eNOS activity is largely responsible for ACh-induced dilation and for FMD: L-NNA strongly reduced these responses in WT mice, to a lesser extent in 3-mo-old ATX mice, and was inactive in vessels isolated from 6-mo-old ATX mice (Figs. 1 and 6). Altogether, our data on ACh-induced dilation, FMD, and MT point to a decrease in eNOS function as soon as at the age of 3 mo in ATX mice, whereas it is relatively unaffected in WT mice of up to 6 mo of age. This is associated with a reduction in the expression of eNOS proteins in ATX mice (data not shown), although neither ACh-induced dilation nor FMD correlate well with the expression of eNOS protein: while catechin restores both flow and ACh responses, the antioxidant did not increase eNOS expression (data not shown) but improved its activity. We also observed that catechin increased Mn-superoxide dismutase (SOD) expression in the mouse’s cerebral circulation (data not shown), which could also contribute to the beneficial effect of the antioxidant on FMD and ACh-induced dilation and on NOS function in general. This is consistent with a previous study suggesting that SOD applied topically to the cerebral cortex prevented endothelial dysfunction (20).
An increase in flow is one of the most appropriate physiological stimuli to study in vitro endothelial dilatory function. The response to flow is possibly a combination of both dilatory (endothelium-dependent) and contractile (endothelium-independent) responses, with the final level of tone resulting from the interaction between the two (42). The intrinsic and neurohumoral mechanisms that regulate tone vary between arteries from different size and from different regions of the brain. In the present study, flow was applied on pressurized cerebral arteries of the mouse, induced shear stresses in the physiological range, and promoted endothelium-dependent dilations. Flow-induced contractions were never observed, even when flow rate was increased to extraphysiological shear stresses (data not shown). In cerebral arteries, FMD is implicated in various physiological phenomena such as CBF autoregulation and functional hyperemia (12). When neurons increase their metabolic demand, there is an increase in blood flow (14, 38). This small local increase in blood flow, due to the local increase in diameter, promotes dilation in the upstream larger vessels, which sustains the increase in flow required to match the metabolic demand. Because eNOS activity regulates basal tone (MT) and is largely responsible for FMD, the eNOS dysfunction in the upstream vessels likely influences the control of CBF in small arteries of the parenchyma in ATX mice. In the present study, the increase in CBF (which ensures adequate oxygen and glucose supply to activated brain areas) in response to neuronal activity was slightly but significantly reduced with adulthood in WT mice. This reduction in CBF was very significant in 3-mo-old ATX mice and further impaired at 6 mo (Fig. 8B). Because the changes in cortical CBF likely reflect the upstream adaptive responses to the downstream neuronal activation induced by whisker stimulation in our experiments, it is expected that this CBF response is highly dependent on the endothelial function, i.e., FMD, as tested in cortical cerebral arteries in vitro and evidenced by the positive correlation between these two parameters (Fig. 8C). Locally, however, at the site of neuronal activity, other mechanisms than shear stress-mediated responses are involved: it is now clearly established that the neuron-to-astrocyte signaling contributes to the regulation of CBF during neuronal activation (4, 19, 26). Astrocytes can produce and release vasoactive substances such as dilatory NO, cyclooxygenase-derived products, and epoxygenase derivatives (4, 19) and therefore participate in the local dilation of arterioles of the stimulated brain area and thus the local decrease in pressure; this fall in pressure is then responsible for the retrograde propagation of the dilation, as far as the cortical vessels, through shear stress.
Because the brain is totally dependent on a constant blood supply, lower hemodynamic responses in ATX mice are likely to have a physiological impact with time. Is the impaired dilatory function linked to the decrease in learning abilities in ATX mice? It has been suggested that brain hypoperfusion (18) and ROS generation (15) cause ultimately neuronal death. Accordingly, LDLR−/− mice fed a high-fat diet display hippocampal-dependent memory dysfunctions (33). Our data suggest that the loss of learning abilities could be associated with the decline in eNOS activity associated with an abnormal ROS handling. This is supported by the fact that 1) WT mice did not loose their learning capacities within the 3-mo period, while in these mice the reduction of CBF was limited and the maximal eNOS function was maintained and that 2) catechin treatment normalized learning abilities, CBF responses, and eNOS function in 6-mo-old ATX mice. Our data are therefore the first to suggest a potential link between in vitro endothelial function, in vivo CBF responses, and the learning abilities, although we do not demonstrate causal links. Nonetheless, our data clearly show that catechin did not augment the resting CBF; it is possible that catechin only delayed the onset of the cognitive decline, and a longer period of observation should be performed to investigate the evolution of cognition with time in relation to a suboptimal resting CBF and shear stress.
It is striking that, unlike in the aorta, cerebral artery DHE-superoxide production is insensitive to dyslipidemia and even decreases with time. Yet endothelial dysfunction is obvious, and catechin prevents it. One could argue that the beneficial central effects of catechin are due to its peripheral anti-atherosclerotic activity: catechin clearly benefited to the aorta and reduced both oxidative stress and plaque size. Hence, circulating inflammatory factors, microparticles, or oxidative stress-modified proteins may influence the central vasculature. An alternative possibility is that the nature of oxidative stress is different in the cerebral arteries from that measured in peripheral arteries. We used DHE, which detects superoxide anions. One could speculate that hydrogen peroxide is more produced in the brain or that hydroxyl or peroxynitrite radicals are prominent in cerebral arteries in our model. These are speculative and would deserve a multimodal detection of oxidative stress in the brain compared with the periphery.
Mice were treated with catechin at the dose of 30 mg·kg−1·day−1, as previously reported by our group (10). In humans, 50 mg of catechin are equivalent to one cup of black tea (200 ml) plus a small piece of dark chocolate (20 g) or two large apples (2). It is, however, difficult to extrapolate dosage between mice and humans because of likely different pharmacokinetic profiles. To the best of our knowledge, there is no sufficient information available to estimate a reasonable cate-chin dose translation between mice and humans. In humans, catechin intake (from 25 to 124 mg) from tea or from other sources has been shown to be inversely correlated with ischemic heart disease mortality (2) and to reduce (24) or not the risk of stroke (2). These inconsistencies have been reviewed recently (1).
No cerebrovascular atherosclerotic lesions were observed in 6-mo-old ATX mice despite the presence of severe dyslipidemia, atherosclerotic plaque in the aorta and the carotid arteries, and endothelial dysfunction. In humans, atherosclerosis-related strokes are largely associated with peripheral atherosclerosis, including the carotid artery (13). Intracranial artery atherosclerosis is rarely documented, but we know that, with age, cerebral arteries develop atherosclerosis: at bifurcations, arteries from the circle of Willis, isolated from 12-mo-old ATX mice, exhibit macroscopic atherosclerotic lesions, and this is associated with a severe cerebral endothelial dysfunction (unpublished data).
Our study has limitations: our data do not demonstrate the existence of a causal link between changes in CBF, FMD, and the learning abilities. Rather, it suggests a potential link between oxidative stress, the impaired cerebral endothelial function, the lower changes in CBF after neuronal stimulation, and the learning deficit in ATX mice. Our data demonstrate nonetheless that FMD and the change in cortical CBF are well correlated. The long-term effects of an impaired flow response are likely contributing to the decline in the learning capacities, but this needs to be confirmed.
Technically, we did not monitor blood PaO2, PaCO2, pH, cardiac output, or sympathetic nervous activity that may all influence CBF; however, neither HR nor blood pressure changed during the protocol. In addition, catechin can influence cardiac output, sympathetic nerve activity, and oxidative stress, which could in turn influence the changes in CBF and the learning abilities. These possibilities need therefore to be kept in mind during the overall interpretation of our results.
Basal CBF was measured using Doppler OCT, and no significant differences were found between the ATX and the ATX + CAT mice. Doppler OCT measured basal flow in small arterioles over an area of 800 × 800 μm in our experiments. The small area covered may induce spatial sampling errors due to cortical flow heterogeneities. This would be expected to add variability to the data and thus diminish the ability of this technique to assess differences between groups. In addition, basal CBF was measured in the somatosensory cortex, but it is not clear that basal flow in this region correlates with cognitive performance.
We did not test different doses of catechin in the mice but rather a dose that proved to be cardioprotective (9). Likewise, 3-mo-old WT mice were not treated with catechin, because, when given at an early age (from 3 to 6 mo old), catechin prevents the establishment of normal vascular antioxidant defenses, and it negatively affects the vascular endothelial function (unpublished data). In contrast, when catechin is given later to WT mice, from 9 to 12 mo old, for example, the antioxidant reverses age-related vascular dysfunctions (9). It is therefore extremely difficult to compare the impact of an antioxidant treatment between healthy mice with normal free radical pathways and severely dyslipidemic mice with aberrant free radical handling. We tested the effects of a different antioxidant, unrelated to catechin, the N-acetylcysteine (1.0 or 2.0 g · kg−1· day−1, in the drinking water), in mice, but, because of its toxicity revealed by a high mortality rate and a weigh loss, we did not pursue this treatment (unpublished data).
Another limitation of our model is that we cannot differentiate neuronal nitric oxide synthase (nNOS) from eNOS activity in isolated cerebral arteries. Reduced NO bioavailability derived from an increase in oxidative stress, resulting from nNOS dysfunction or ONOO− formation, has been involved in cerebrovascular dysfunction (37). Consequently, severe dyslipidemia may affect NOS functions, impairing both the neurovascular unit communications via nNOS and the vascular response (via eNOS). This hypothesis would have to be tested in eNOS and nNOS knockout mice exposed to severe dyslipidemia.
In conclusion, although the chronic treatment with the polyphenol catechin in ATX mice did not affect dyslipidemia and did not augment resting CBF, it restored the endothelial dilatory function, the changes in CBF during neuronal stimulation, and the learning abilities, whereas it prevented plaque progression; all of these effects were accompanied by a significant reduction in cerebral and aortic oxidative stress. We therefore suggest that an early control of ROS production could be beneficial to prevent cognitive dysfunctions associated with risk factors for cardiovascular diseases such as severe dyslipidemia. If we extrapolate our experimental data to humans, they suggest that a healthy lifestyle could reduce cerebrovascular dysfunction and stroke, in agreement with a recent large epidemiological study (5).
Acknowledgments
GRANTS
This work was supported, in part, by the Foundation of Montreal Heart Institute, the Heart and Stroke Foundation of Quebec, and the Canadian Institutes of Health Research (MOP89733). A. Drouin holds the Frederick Banting and Charles Best Canada Graduate Scholarships-Doctoral Award in association with the Canadian Institute for Health Research.
Footnotes
DISCLOSURES
The authors report no conflict of interest.
References
- 1.Arab L, Liebeskind DS. Tea, flavonoids and stroke in man and mouse. Arch Biochem Biophys. 2010;501:31–36. doi: 10.1016/j.abb.2010.03.015. [DOI] [PubMed] [Google Scholar]
- 2.Arts IC, Hollman PC, Feskens EJ, Bueno de Mesquita HB, Kromhout D. Catechin intake might explain the inverse relation between tea consumption and ischemic heart disease: the Zutphen Elderly Study. Am J Clin Nutr. 2001;74:227–232. doi: 10.1093/ajcn/74.2.227. [DOI] [PubMed] [Google Scholar]
- 3.Brouillette J, Quirion R. The common environmental pollutant diox-in-induced memory deficits by altering estrogen pathways and a major route of retinol transport involving transthyretin. Neurotoxicology. 2008;29:318–327. doi: 10.1016/j.neuro.2007.12.005. [DOI] [PubMed] [Google Scholar]
- 4.Carmignoto G, Gomez-Gonzalo M. The contribution of astrocyte signalling to neurovascular coupling. Brain Res Rev. 2010;63:138–148. doi: 10.1016/j.brainresrev.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 5.Chiuve SE, Rexrode KM, Spiegelman D, Logroscino G, Manson JE, Rimm EB. Primary prevention of stroke by healthy lifestyle. Circulation. 2008;118:947–954. doi: 10.1161/CIRCULATIONAHA.108.781062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Crimi E, Ignarro LJ, Napoli C. Microcirculation and oxidative stress. Free Radic Res. 2007;41:1364–1375. doi: 10.1080/10715760701732830. [DOI] [PubMed] [Google Scholar]
- 7.Drouin A, Gendron ME, Thorin E, Gillis MA, Mahlberg-Gaudin F, Tardif JC. Chronic heart rate reduction by ivabradine prevents endothelial dysfunction in dyslipidaemic mice. Br J Pharmacol. 2008;154:749–757. doi: 10.1038/bjp.2008.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Drouin A, Thorin E. Flow-induced dilation is mediated by Akt-dependent activation of endothelial nitric oxide synthase-derived hydrogen peroxide in mouse cerebral arteries. Stroke. 2009;40:1827–1833. doi: 10.1161/STROKEAHA.108.536805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gendron ME, Theoret JF, Mamarbachi AM, Drouin A, Nguyen A, Bolduc V, Thorin-Trescases N, Merhi Y, Thorin E. Late chronic catechin antioxidant treatment is deleterious to the endothelial function in aging mice with established atherosclerosis. Am J Physiol Heart Circ Physiol. 2010;298:H2062–H2070. doi: 10.1152/ajpheart.00532.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gendron ME, Thorin E. A change in the redox environment and thromboxane A2 production precede endothelial dysfunction in mice. Am J Physiol Heart Circ Physiol. 2007;293:H2508–H2515. doi: 10.1152/ajpheart.00352.2007. [DOI] [PubMed] [Google Scholar]
- 11.Gendron ME, Thorin-Trescases N, Villeneuve L, Thorin E. Aging associated with mild dyslipidemia reveals that COX-2 preserves dilation despite endothelial dysfunction. Am J Physiol Heart Circ Physiol. 2007;292:H451–H458. doi: 10.1152/ajpheart.00551.2006. [DOI] [PubMed] [Google Scholar]
- 12.Girouard H, Iadecola C. Neurovascular coupling in the normal brain and in hypertension, stroke, and Alzheimer disease. J Appl Physiol. 2006;100:328–335. doi: 10.1152/japplphysiol.00966.2005. [DOI] [PubMed] [Google Scholar]
- 13.Halliday A, Harrison M, Hayter E, Kong X, Mansfield A, Marro J, Pan H, Peto R, Potter J, Rahimi K, Rau A, Robertson S, Streifler J, Thomas D. 10-year stroke prevention after successful carotid endarterectomy for asymptomatic stenosis (ACST-1): a multicentre randomised trial. Lancet. 2010;376:1074–1084. doi: 10.1016/S0140-6736(10)61197-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hamel E. Perivascular nerves and the regulation of cerebrovascular tone. J Appl Physiol. 2006;100:1059–1064. doi: 10.1152/japplphysiol.00954.2005. [DOI] [PubMed] [Google Scholar]
- 15.Hamel E, Nicolakakis N, Aboulkassim T, Ongali B, Tong XK. Oxidative stress and cerebrovascular dysfunction in mouse models of Alzheimer’s disease. Exp Physiol. 2008;93:116–120. doi: 10.1113/expphysiol.2007.038729. [DOI] [PubMed] [Google Scholar]
- 16.Haque AM, Hashimoto M, Katakura M, Tanabe Y, Hara Y, Shido O. Long-term administration of green tea catechins improves spatial cognition learning ability in rats. J Nutr. 2006;136:1043–1047. doi: 10.1093/jn/136.4.1043. [DOI] [PubMed] [Google Scholar]
- 17.Hong JT, Ryu SR, Kim HJ, Lee JK, Lee SH, Kim DB, Yun YP, Ryu JH, Lee BM, Kim PY. Neuroprotective effect of green tea extract in experimental ischemia-reperfusion brain injury. Brain Res Bull. 2000;53:743–749. doi: 10.1016/s0361-9230(00)00348-8. [DOI] [PubMed] [Google Scholar]
- 18.Hong JT, Ryu SR, Kim HJ, Lee SH, Lee BM, Kim PY. Involvement of cortical damage in the ischemia/reperfusion-induced memory impairment of Wistar rats. Arch Pharm Res. 2000;23:413–417. doi: 10.1007/BF02975457. [DOI] [PubMed] [Google Scholar]
- 19.Iadecola C, Nedergaard M. Glial regulation of the cerebral microvasculature. Nat Neurosci. 2007;10:1369–1376. doi: 10.1038/nn2003. [DOI] [PubMed] [Google Scholar]
- 20.Iadecola C, Zhang F, Niwa K, Eckman C, Turner SK, Fischer E, Younkin S, Borchelt DR, Hsiao KK, Carlson GA. SOD1 rescues cerebral endothelial dysfunction in mice overexpressing amyloid precursor protein. Nat Neurosci. 1999;2:157–161. doi: 10.1038/5715. [DOI] [PubMed] [Google Scholar]
- 21.Joannides R, Haefeli WE, Linder L, Richard V, Bakkali EH, Thuillez C, Luscher TF. Nitric oxide is responsible for flow-dependent dilatation of human peripheral conduit arteries in vivo. Circulation. 1995;91:1314–1319. doi: 10.1161/01.cir.91.5.1314. [DOI] [PubMed] [Google Scholar]
- 22.Kalaria RN. Cerebral vessels in ageing and Alzheimer’s disease. Pharmacol Ther. 1996;72:193–214. doi: 10.1016/s0163-7258(96)00116-7. [DOI] [PubMed] [Google Scholar]
- 23.Kaliora AC, Dedoussis GV, Schmidt H. Dietary antioxidants in preventing atherogenesis. Atherosclerosis. 2006;187:1–17. doi: 10.1016/j.atherosclerosis.2005.11.001. [DOI] [PubMed] [Google Scholar]
- 24.Keli SO, Hertog MG, Feskens EJ, Kromhout D. Dietary flavonoids, antioxidant vitamins, and incidence of stroke: the Zutphen study. Arch Intern Med. 1996;156:637–642. [PubMed] [Google Scholar]
- 25.Khan MM, Ahmad A, Ishrat T, Khan MB, Hoda MN, Khuwaja G, Raza SS, Khan A, Javed H, Vaibhav K, Islam F. Resveratrol attenuates 6-hydroxydopamine-induced oxidative damage and dopamine depletion in rat model of Parkinson’s disease. Brain Res. 2010;1328:139–151. doi: 10.1016/j.brainres.2010.02.031. [DOI] [PubMed] [Google Scholar]
- 26.Koehler RC, Roman RJ, Harder DR. Astrocytes and the regulation of cerebral blood flow. Trends Neurosci. 2009;32:160–169. doi: 10.1016/j.tins.2008.11.005. [DOI] [PubMed] [Google Scholar]
- 27.Koller A, Sun D, Huang A, Kaley G. Corelease of nitric oxide and prostaglandins mediates flow-dependent dilation of rat gracilis muscle arterioles. Am J Physiol Heart Circ Physiol. 1994;267:H326–H332. doi: 10.1152/ajpheart.1994.267.1.H326. [DOI] [PubMed] [Google Scholar]
- 28.Krummen S, Falck JR, Thorin E. Two distinct pathways account for EDHF-dependent dilatation in the gracilis artery of dyslipidaemic hApoB+/+ mice. Br J Pharmacol. 2005;145:264–270. doi: 10.1038/sj.bjp.0706194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lauzier B, Sicard P, Bouchot O, Delemasure S, Menetrier F, Moreau D, Vergely C, Rochette L. After four hours of cold ischemia and cardioplegic protocol, the heart can still be rescued with postconditioning. Transplantation. 2007;84:1474–1482. doi: 10.1097/01.tp.0000288637.18796.0e. [DOI] [PubMed] [Google Scholar]
- 30.Liang W, Lee AH, Binns CW, Huang R, Hu D, Zhou Q. Tea consumption and ischemic stroke risk: a case-control study in southern China. Stroke. 2009;40:2480–2485. doi: 10.1161/STROKEAHA.109.548586. [DOI] [PubMed] [Google Scholar]
- 31.Miller AA, De Silva TM, Judkins CP, Diep H, Drummond GR, Sobey CG. Augmented superoxide production by Nox2-containing NADPH oxidase causes cerebral artery dysfunction during hypercholesterolemia. Stroke. 2010;41:784–789. doi: 10.1161/STROKEAHA.109.575365. [DOI] [PubMed] [Google Scholar]
- 32.Morris R. Developments of a water-maze procedure for studying spatial learning in the rat. J Neurosci Methods. 1984;11:47–60. doi: 10.1016/0165-0270(84)90007-4. [DOI] [PubMed] [Google Scholar]
- 33.Mulder M, Jansen PJ, Janssen BJ, van de Berg WD, van der Boom H, Havekes LM, de Kloet RE, Ramaekers FC, Blokland A. Low-density lipoprotein receptor-knockout mice display impaired spatial memory associated with a decreased synaptic density in the hippocampus. Neurobiol Dis. 2004;16:212–219. doi: 10.1016/j.nbd.2004.01.015. [DOI] [PubMed] [Google Scholar]
- 34.Nicolakakis N, Aboulkassim T, Ongali B, Lecrux C, Fernandes P, Rosa-Neto P, Tong XK, Hamel E. Complete rescue of cerebrovascular function in aged Alzheimer’s disease transgenic mice by antioxidants and pioglitazone, a peroxisome proliferator-activated receptor gamma agonist. J Neurosci. 2008;28:9287–9296. doi: 10.1523/JNEUROSCI.3348-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ohwada K, Takeda H, Yamazaki M, Isogai H, Nakano M, Shimomura M, Fukui K, Urano S. Pyrroloquinoline Quinone (PQQ) Prevents Cognitive Deficit Caused by Oxidative Stress in Rats. J Clin Biochem Nutr. 2008;42:29–34. doi: 10.3164/jcbn.2008005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Papaioannou TG, Karatzis EN, Vavuranakis M, Lekakis JP, Stefanadis C. Assessment of vascular wall shear stress and implications for atherosclerotic disease. Int J Cardiol. 2006;113:12–18. doi: 10.1016/j.ijcard.2006.03.035. [DOI] [PubMed] [Google Scholar]
- 37.Park L, Anrather J, Zhou P, Frys K, Pitstick R, Younkin S, Carlson GA, Iadecola C. NADPH-oxidase-derived reactive oxygen species mediate the cerebrovascular dysfunction induced by the amyloid beta peptide. J Neurosci. 2005;25:1769–1777. doi: 10.1523/JNEUROSCI.5207-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rancillac A, Rossier J, Guille M, Tong XK, Geoffroy H, Amatore C, Arbault S, Hamel E, Cauli B. Glutamatergic Control of Microvascular Tone by Distinct GABA Neurons in the Cerebellum. J Neurosci. 2006;26:6997–7006. doi: 10.1523/JNEUROSCI.5515-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ren H, Sun T, MacDonald DJ, Cobb MJ, Li X. Real-time in vivo blood-flow imaging by moving-scatterer-sensitive spectral-domain optical Doppler tomography. Opt Lett. 2006;31:927–929. doi: 10.1364/ol.31.000927. [DOI] [PubMed] [Google Scholar]
- 40.Rowe WB, Spreekmeester E, Meaney MJ, Quirion R, Rochford J. Reactivity to novelty in cognitively-impaired and cognitively-unimpaired aged rats and young rats. Neuroscience. 1998;83:669–680. doi: 10.1016/s0306-4522(97)00464-8. [DOI] [PubMed] [Google Scholar]
- 41.Srinivasan VJ, Sakadzic S, Gorczynska I, Ruvinskaya S, Wu W, Fujimoto JG, Boas DA. Quantitative cerebral blood flow with optical coherence tomography. Opt Express. 2010;18:2477–2494. doi: 10.1364/OE.18.002477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Thorin-Trescases N, Bevan JA. High levels of myogenic tone antagonize the dilator response to flow of small rabbit cerebral arteries. Stroke. 1998;29:1194–1200. doi: 10.1161/01.str.29.6.1194. [DOI] [PubMed] [Google Scholar]
- 43.Toda N, Okamura T. Cerebral vasodilators. Jpn J Pharmacol. 1998;76:349–367. doi: 10.1254/jjp.76.349. [DOI] [PubMed] [Google Scholar]
- 44.Xu Y, Zhang JJ, Xiong L, Zhang L, Sun D, Liu H. Green tea polyphenols inhibit cognitive impairment induced by chronic cerebral hypoperfusion via modulating oxidative stress. J Nutr Biochem. 2010;21:741–748. doi: 10.1016/j.jnutbio.2009.05.002. [DOI] [PubMed] [Google Scholar]