Abstract
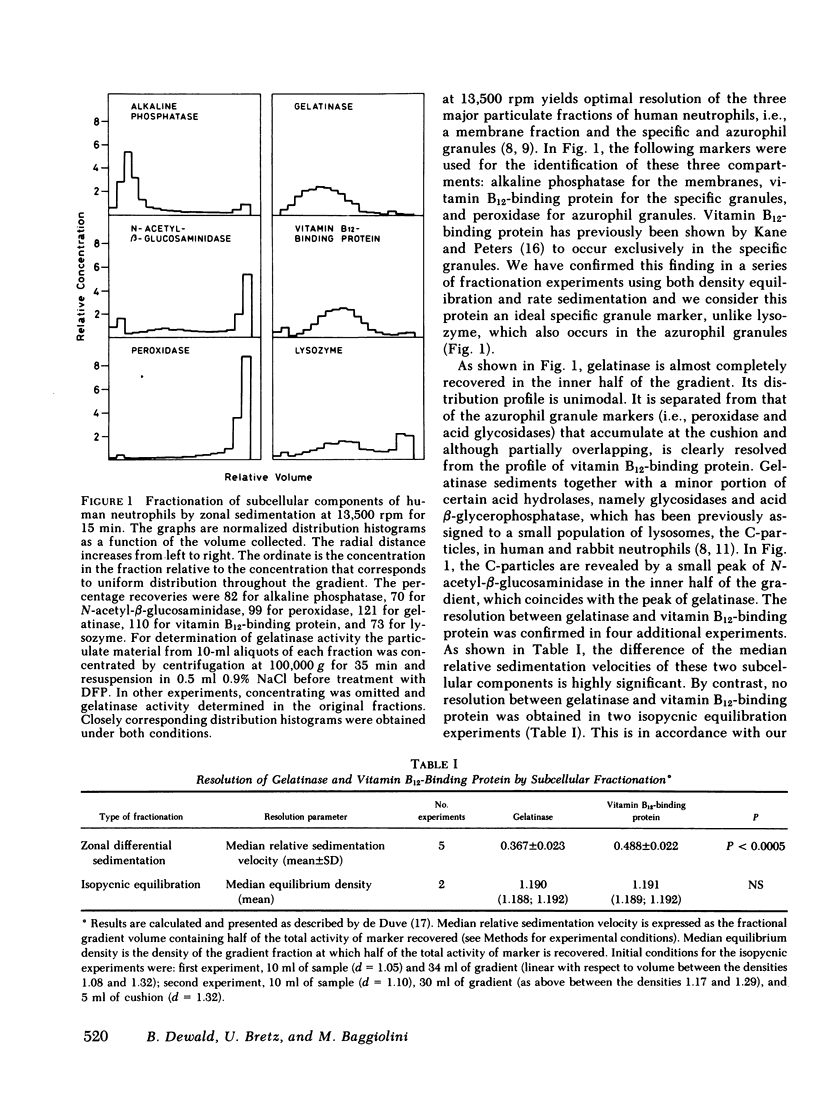
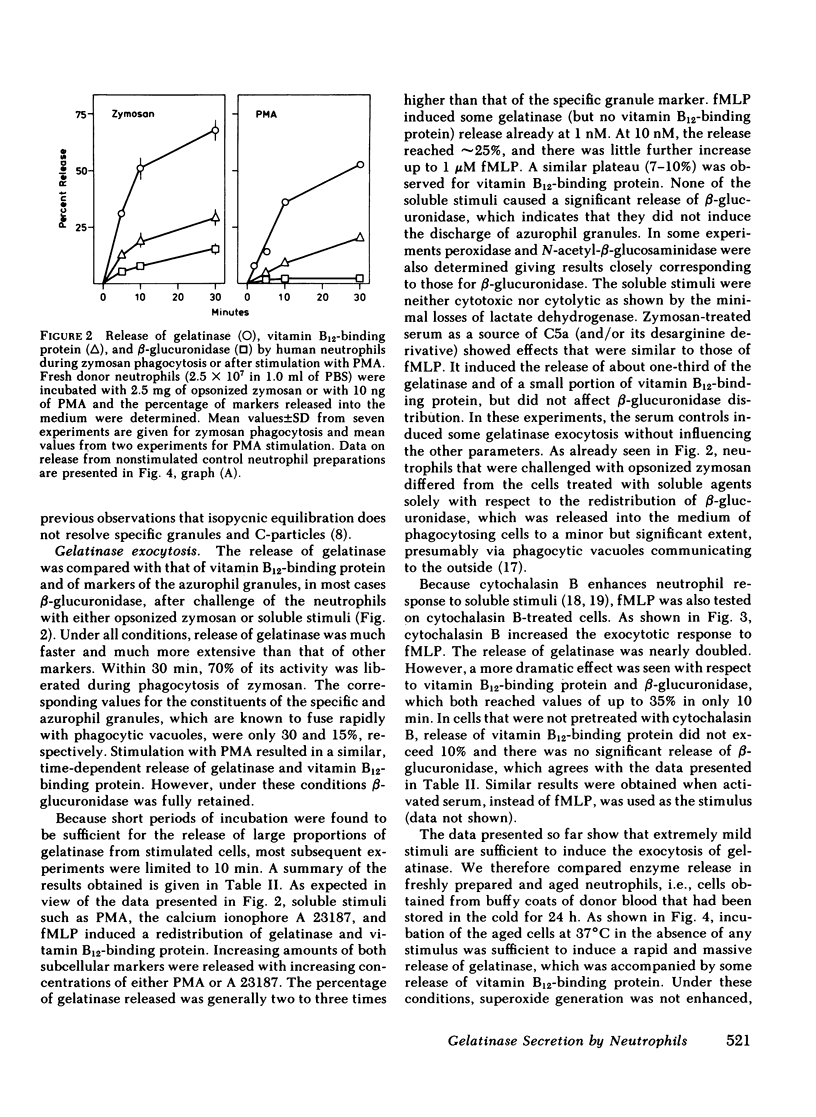
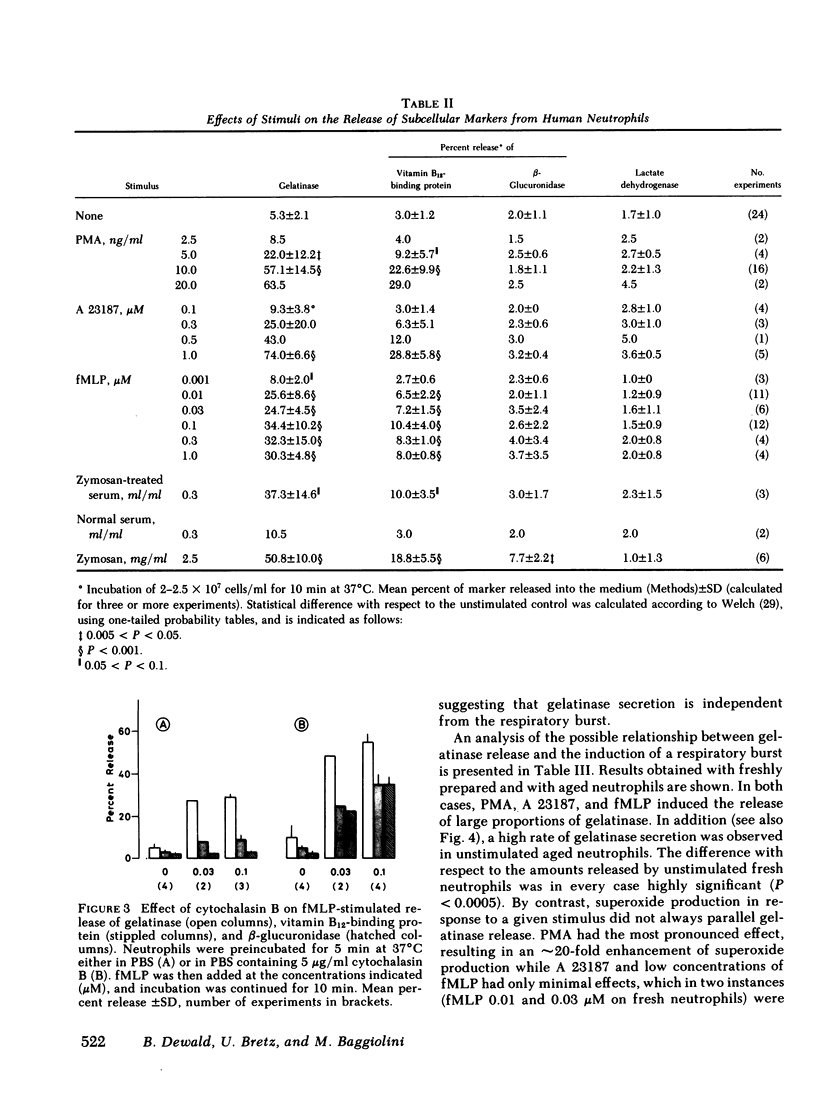
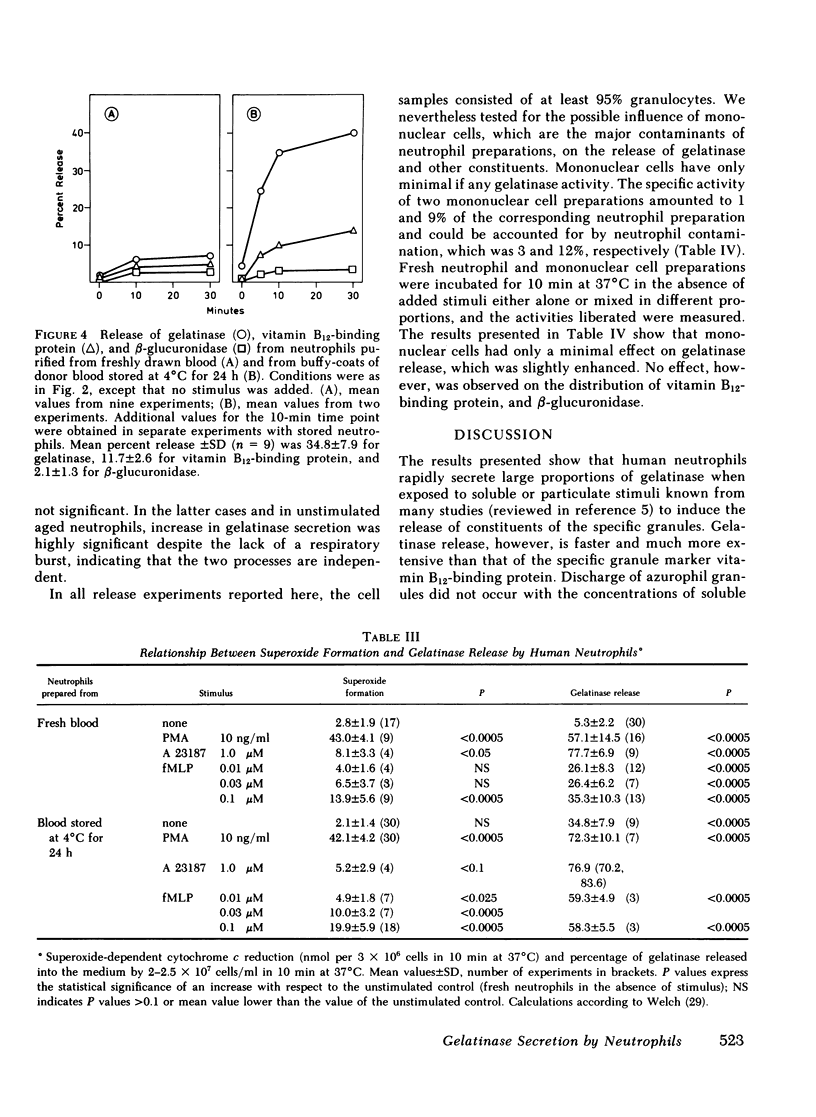
Gelatinase is a metallo-proteinase that acts specifically on denatured collagen. In human neutrophils, this enzyme is localized in small, morphologically still unidentified storage organelles that are resolved from the specific and the azurophil granules upon subcellular fractionation by differential sedimentation. When neutrophils isolated from freshly drawn blood are exposed to soluble stimuli such as N-formyl-methionyl-leucyl-phenylalanine, zymosan-activated serum, phorbol myristate acetate, or the calcium ionophore A 23187, or are induced to phagocytose opsonized zymosan, they rapidly release gelatinase in large amounts (30-70% of the cellular content in 10 min). When neutrophils from donor blood, which had been stored for 24 h at 4°C are used, extensive release even occurs without added stimuli by simply warming to 37°C.
Gelatinase release appears to occur by secretion because it is not dependent on phagocytosis. It is paralelled by the release of specific granule contents (vitamin B12-binding protein), but is more rapid and much more extensive. It is, however, dissociated from the discharge of azurophil granules (as assessed by β-glucuronidase). In addition, it was found that gelatinase release does not depend on the activation of the respiratory burst, although the two responses are often observed in parallel. Release is not due to cell damage as the cytoplasmic enzyme lactate dehydrogenase is fully retained.
The distinct subcellular distribution and kinetics of release of gelatinase reported in this paper uncover a novel, truly secretory compartment of human neutrophils, which is highly responsive to stimulation. Gelatinase and possibly other enzymes stored in this secretory organelle may be involved in the early events of neutrophil mobilization, the response to chemotactic signals and diapedesis.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baggiolini M., Hirsch J. G., De Duve C. Further biochemical and morphological studies of granule fractions from rabbit heterophil leukocytes. J Cell Biol. 1970 Jun;45(3):586–597. doi: 10.1083/jcb.45.3.586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bainton D. F. Sequential degranulation of the two types of polymorphonuclear leukocyte granules during phagocytosis of microorganisms. J Cell Biol. 1973 Aug;58(2):249–264. doi: 10.1083/jcb.58.2.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker E. L. A multifunctional receptor on the neutrophil for synthetic chemotactic oligopeptides. J Reticuloendothel Soc. 1979 Dec;26(Suppl):701–709. [PubMed] [Google Scholar]
- Bentwood B. J., Henson P. M. The sequential release of granule constitutents from human neutrophils. J Immunol. 1980 Feb;124(2):855–862. [PubMed] [Google Scholar]
- Bretz U., Baggiolini M. Association of the alkaline phosphatase of rabbit polymorphonuclear leukocytes with the membrane of the specific granules. J Cell Biol. 1973 Dec;59(3):696–707. doi: 10.1083/jcb.59.3.696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bretz U., Baggiolini M. Biochemical and morphological characterization of azurophil and specific granules of human neutrophilic polymorphonuclear leukocytes. J Cell Biol. 1974 Oct;63(1):251–269. doi: 10.1083/jcb.63.1.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curnutte J. T., Kipnes R. S., Babior B. M. Defect in pyridine nucleotide dependent superoxide production by a particulate fraction from the cranulocytes of patients with chronic granulomatous disease. N Engl J Med. 1975 Sep 25;293(13):628–632. doi: 10.1056/NEJM197509252931303. [DOI] [PubMed] [Google Scholar]
- Dewald B., Baggiolini M., Curnutte J. T., Babior B. M. Subcellular localization of the superoxide-forming enzyme in human neutrophils. J Clin Invest. 1979 Jan;63(1):21–29. doi: 10.1172/JCI109273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eeckhout Y., Vaes G. Further studies on the activation of procollagenase, the latent precursor of bone collagenase. Effects of lysosomal cathepsin B, plasmin and kallikrein, and spontaneous activation. Biochem J. 1977 Jul 15;166(1):21–31. doi: 10.1042/bj1660021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granelli-Piperno A., Vassalli J. D., Reich E. Secretion of plasminogen activator by human polymorphonuclear leukocytes. Modulation by glucocorticoids and other effectors. J Exp Med. 1977 Dec 1;146(6):1693–1706. doi: 10.1084/jem.146.6.1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris E. D., Jr, Krane S. M. An endopeptidase from rheumatoid synovial tissue culture. Biochim Biophys Acta. 1972 Feb 28;258(2):566–576. doi: 10.1016/0005-2744(72)90249-5. [DOI] [PubMed] [Google Scholar]
- Kane S. P., Hoffbrand A. V., Neale G. Indices of granulocyte activity in inflammatory bowel disease. Gut. 1974 Dec;15(12):953–959. doi: 10.1136/gut.15.12.953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kane S. P., Peters T. J. Analytical subcellular fractionation of human granulocytes with reference to the localization of vitamin B12-binding proteins. Clin Sci Mol Med. 1975 Aug;49(2):171–182. doi: 10.1042/cs0490171. [DOI] [PubMed] [Google Scholar]
- Kruze D., Wojtecka E. Activation of leucocyte collagenase proenzyme by rheumatoid synovial fluid. Biochim Biophys Acta. 1972 Dec 28;285(2):436–446. doi: 10.1016/0005-2795(72)90330-3. [DOI] [PubMed] [Google Scholar]
- Lazarus G. S., Daniels J. R., Lian J., Burleigh M. C. Role of granulocyte collagenase in collagen degradation. Am J Pathol. 1972 Sep;68(3):565–578. [PMC free article] [PubMed] [Google Scholar]
- Murphy G., Bretz U., Baggiolini M., Reynolds J. J. The latent collagenase and gelatinase of human polymorphonuclear neutrophil leucocytes. Biochem J. 1980 Nov 15;192(2):517–525. doi: 10.1042/bj1920517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sellers A., Cartwright E., Murphy G., Reynolds J. J. Evidence that latent collagenases are enzyme-inhibitor complexes. Biochem J. 1977 May 1;163(2):303–307. doi: 10.1042/bj1630303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sopata I., Dancewicz A. M. Presence of a gelatin-specific proteinase and its latent form in human leucocytes. Biochim Biophys Acta. 1974 Dec 29;370(2):510–523. doi: 10.1016/0005-2744(74)90112-0. [DOI] [PubMed] [Google Scholar]
- Sopata I., Wize J. A latent gelatin specific proteinase of human leucocytes and its activation. Biochim Biophys Acta. 1979 Dec 7;571(2):305–312. doi: 10.1016/0005-2744(79)90100-1. [DOI] [PubMed] [Google Scholar]
- Werb Z., Mainardi C. L., Vater C. A., Harris E. D., Jr Endogenous activiation of latent collagenase by rheumatoid synovial cells. Evidence for a role of plasminogen activator. N Engl J Med. 1977 May 5;296(18):1017–1023. doi: 10.1056/NEJM197705052961801. [DOI] [PubMed] [Google Scholar]
- Wright D. G., Gallin J. I. Secretory responses of human neutrophils: exocytosis of specific (secondary) granules by human neutrophils during adherence in vitro and during exudation in vivo. J Immunol. 1979 Jul;123(1):285–294. [PubMed] [Google Scholar]



