Abstract
Purpose
To compare the long-term outcomes after intraarterial cytoreductive chemotherapy (IACC) to conventional treatment for lacrimal gland adenoid cystic carcinoma (ACC).
Design
Retrospective case series.
Participants
Nineteen consecutive patients treated with IACC, followed by orbital exenteration, chemoradiotherapy, and intravenous chemotherapy.
Interventions
Analyses of the histologic characteristics of biopsy specimens, extent of disease at the time of diagnosis, diagnostic surgical procedures, incidence of locoregional recurrences or distant metastases, disease-free survival time, response to IACC, tumor margins at definitive surgery, and toxicity and complications.
Main Outcome Measures
Disease relapse, disease-free survival, and chemotherapeutic complications.
Results
Eight patients with an intact lacrimal artery had significantly better outcomes for survival (100% vs. 28.6% at 10 years), cause-specific mortality, and recurrences (all p=0.002, log-rank test) than conventionally treated patients from this institution. These eight patients (Group 1) had cumulative 10 year disease-free survival of 100% compared to 50% for 11 patients (Group 2) who had an absence of the lacrimal artery and/or deviated from the treatment protocol (p=0.035) and 14.3% for conventionally treated patients (p<0.001). Likewise, Group 2 was associated with lower cause-specific mortality than the institutional comparator group (p=0.038). Prior tumor resection with lateral wall osteotomy, delay in IACC implementation or exenteration, and failure to adhere to protocol are risk factors for suboptimal outcomes.
Conclusions
Neoadjuvant IACC appears to improve overall survival and decrease disease recurrence. An intact lacrimal artery, no disruption of bone barrier or tumor manipulation other than incisional biopsy, and protocol compliance are factors responsible for favorable outcomes. The chemotoxicity complication rate is limited and manageable.
The grave prognosis for patients with adenoid cystic carcinoma (ACC) of the lacrimal gland is well recognized.1–8 Font and Gamel4 reported an actuarial survival rate of less than 50% at 5 years and 20% at 10 years regardless of the local treatment regimens. The difficulty in achieving a long-term disease-free survival in this disease is attributed to the complex regional orbital anatomy and the aggressive biological behavior of the tumor. A lacrimal gland ACC has a proclivity for soft tissue and bone infiltration, retrograde perineural extension, and hematogenous and lymphatic spread. Because of these characteristics, permutations of the use of radical surgery and/or radiation therapy have not produced stepwise incremental improvements in treatment outcome.1,9–11 The principal shortcoming of locoregional treatments is related to the inherent limitations of the different therapies to address occult metastases even after surgery and radiation therapy have achieved local disease control.
In 1998, Meldrum, Tse and Benedetto12 introduced the neoadjuvant intraarterial cytoreductive chemotherapy (IACC) protocol as a multimodal approach to augment conventional treatments that failed to improve disease-free survival. Neoadjuvant chemotherapy implies chemotherapy administered before any definitive surgical procedure in patients without evidence of metastatic disease but at high risk for such. The rationale of the neoadjuvant regional treatment was to administer a high concentration of a chemotherapeutic agent to the lacrimal gland tumor through the vascular system, prior to surgical excision of the tumor, to enhance tumor cell death.12 Because the drug is delivered through the intraarterial (IA) route, its concentration is considerably higher than that with intravenous (IV) delivery. The higher drug concentration increases its cytotoxic effect while preserving the therapeutic levels for chemotherapy to the systemic circulation.13,14 IACC for lacrimal gland ACC is given through the external carotid artery which has anastomotic branches to the lacrimal artery that lead to direct perfusion of the lacrimal gland. This route of administration avoids direct brain perfusion that would result from internal carotid artery infusion.
The neoadjuvant phase of the protocol12 consisted of two cycles of drug infusion at three week intervals. Each cycle included IA delivery of a high dose of cisplatin over a one hour period on day 1 followed by daily IV infusions of doxorubicin (Adriamycin) for 3 days. After two cycles of chemotherapy, orbital imaging was used to assess the response of the tumor to this therapeutic regimen. A third cycle was given if the surgeon felt that further tumor shrinkage would enhance tumor margin clearance at the time of exenteration. This final decision is hinged on the location of the posterior margin of the tumor relative to the superior orbital fissure on imaging study.
Following hematologic recovery, orbital exenteration was performed generally within 3–4 weeks. Two to four weeks later or enough time for surgical healing, radiation therapy (55–60 Gy) was given in a standard daily fractional protocol. A weekly IV infusion of cisplatin was given as a radiosensitizer. Upon completion of the chemoradiation therapy, four adjuvant cycles of IV cisplatin and doxorubicin were administered for a total of 6 cycles of systemic therapy. Adjuvant chemotherapy refers to chemotherapy administered to augment the preceding surgical or radiation therapy to prevent disease relapse in patients considered to be at high risk for primary treatment failure.
In 2006, Tse and co-workers15 reported the salutary effect of this treatment protocol on nine patients compared to a historical cohort of seven patients treated by conventional local therapies (surgical resection and post-operative irradiation) at the same institution. The authors demonstrated that IACC was effective in achieving preoperative cytoreduction by down-staging the disease and enhancing the ability to resect the entire lesion for local disease control. The cumulative 5-year cancer cause-specific death rate in the IACC treated group was 16.7% compared to 57.1% in the conventional treatment group.
However, controversy still remained regarding the optimal local therapy for lacrimal gland IACC, ranging from eye sparing surgery followed by external beam radiation therapy,16 brachytherapy,17,18 fast neutron radiotherapy,9,19 and radical multidisciplinary surgical interventions.20–23 The reluctance of many orbital surgeons and oncologists to integrate IACC into the primary treatment is due to the lack of long-term survival data, chemotoxicity, and the desire to preserve the globe.
The purpose of this paper is to report the outcomes of IACC using a non-randomized protocol in a cohort at a single institution. The goal of this report is to provide information on the safety and efficacy of this treatment protocol, and to identify factors contributing to treatment failures that will assist in the future management of lacrimal gland ACC.
METHODS AND MATERIALS
The study protocol was approved by the University of Miami Miller School of Medicine Institutional Review Board. The study was conducted in accordance with the provisions of the Declaration of Helsinki and was performed in compliance with the Health Insurance Portability and Accountability Act.
Participants
The clinical records of 19 consecutive patients (Cases 1–19) with lacrimal gland ACC treated with IACC (IACC treatment group) were retrieved from the Medical Records Department of the Bascom Palmer Eye Institute (BPEI) and University of Miami Hospital and Clinics (UMHC). The study period extended from 1988 to 2012. An archival search of the Ophthalmic Pathology Laboratory records identified sixteen patients with a diagnosis of lacrimal gland ACC who were treated by conventional therapies between 1967 and 1984; 9 patients were treated outside the medical center and were pathology consults only. The remaining 7 patients were treated at BPEI in a consecutive fashion for which follow-up information and archival pathology specimens were available. These patients were considered as the conventional treatment group.
The information analyzed included histological characteristics of the carcinoma, extent of disease at the time of diagnosis, diagnostic surgical procedures, response to IACC which was defined as any clinical or radiographic evidence of tumor size reduction, tumor margins at the time of the definitive surgery, incidence of locoregional recurrences or distant metastases, disease-free survival time, treatment toxicity, and complications. Disease-related mortality was defined as those patients who died as a direct result of the ACC including preoperative death. Disease-free survival time was measured from the beginning of the treatment protocol to the date of a recurrence or the most recent follow-up examination.
The cohort receiving the IACC treatments were divided into two groups. Group 1 comprised of 8 patients with an intact lacrimal artery who underwent the IACC treatment protocol in sequential order and within a timeframe of implementation. The protocol consisted of 2–3 cycles of IA cisplatin and IV doxorubicin, orbital exenteration, chemoradiation, and 3–4 cycles of IV cisplatin and doxorubicin. A total of six cycles of chemotherapy was delivered unless dose limiting toxicity prevented continuation of chemotherapy. Group 2 consisted of 11 patients who had tumor resection prior to the IACC and were thus without an intact lacrimal artery (10) and/or patients whose protocol was altered, e.g., refused or delayed the time of exenteration until a recurrence developed, or refused to complete 6 cycles of chemotherapy (1).
Two patients with an alternate histology, adenocarcinoma of the lacrimal gland, were treated in a parallel fashion, and their data were included in the chemotherapy toxicity analyses but not in the survival analyses.
Statistical Analyses
Analysis of variance and chi-square tests were used to determine the significance of differences in the outcomes between the treatment groups. The survival time was calculated as the number of days from protocol initiation to the date of death or last follow-up examination or contact. The survival times were analyzed for the IACC groups and conventional treatment group with the Kaplan-Meier method and compared between treatments with the log-rank test. Similar calculations and analyses were performed for time to a recurrence.
Data from two published case series (PCS), PCSW11 and PCSE25 that were treated by conventional methods (surgery and radiation) at other institutions and described in sufficient detail to allow computation of the Kaplan-Meier estimates and log-rank test are also included in the survival analyses as additional comparator groups. It was not possible to ascertain disease specific mortality from the PCSW study.11
RESULTS
The demographics and treatment characteristics of the patients are summarized in Table 1. The patient number is the chronological order of treatment and is displayed by the treatment group. The diagnosis of ACC was made from a histopathological examination of an incisional biopsy of the lacrimal gland in 8 patients (Group 1) for whom the lacrimal artery was preserved. None had a disruption of the bone barrier. These 8 patients completed all parts of the protocol in sequential order and without delay. Examination of exenteration specimens showed tumor cells that extended to the tissue margins in two patients.
TABLE 1.
Patient Characteristics of IA Cytoreductive Chemotherapy and Conventional Treatment for Adenoid Cystic Carcinoma of the Lacrimal Gland
| Patient No. | Age/Gender/Affected Side | ACC Subtype/Perineural/Bone Infiltration | IACC Cycles/Total Cycles | Prior Tumor Resection | Radiographic Tumor Response/Tissue Margins | Treatment | Time (mo) of and Status at Last Follow-Up | Time (mo) of Local Recurrence/Met | Remarks |
|---|---|---|---|---|---|---|---|---|---|
| Group 1 | |||||||||
| 1 | 29/M/OD | Basa/P/B | 3/4 | NO | Yes/− | Exent + RT | 288; Alive, no D | No | Intracranial extension |
| 3 | 73/F/OS | Basa/P | 2/6 | NO | Yes/− | Exent + RT | 193; Alive, no D | No | Kidney transplant |
| 4 | 58/M/OS | Crib/P | 2/6 | NO | Yes/− | Exent + RT + GK | 156; Alive, no D | No | Intracranial, ethmoid sinus ACC |
| 5 | 35/M/OS | Crib/P | 3/6 | NO | Yes/+ | Exent + RT | 149; Alive, no D | No | Non-healing apex; free flap |
| 7 | 30/M/OS | Basa/P | 2/4 | NO | Yes/− | Exent + Bone + RT | 116; Alive, no D | No | |
| 10 | 29/F/OD | Basa | 2/6 | NO | Yes/− | Exent + RT | 96; Alive, no D | No | |
| 17 | 53/F/OD | Basa | 2/6 | NO | Yes/− | Exent + RT | 29; Alive, no D | No | Neck dissection negative |
| 18 | 44/M/OS | Basa/Crib/P/B | 3/6 | NO | Yes/+ | Exent + RT | 20; Alive, no D | No | Lung Bx negative |
| Group 2 | |||||||||
| 2 | 32/M/OD | Crib/P/B | 3/6 | YES | Yes/− | Exent + Bone + RT | 167; Died, no D | No | HIV. Died of oral SCC |
| 6 | 42/F/OD | Crib/P/B | 3/6 | YES | Yes/− | Exent + Bone + RT | 127; Alive, with D | 50; Lung | Bone necrosis, hearing deficit; 7th N palsy |
| 8 | 36/F/OS | Basa/P/B | 2/2 | NO | Yes/+ | Exent + RT | 28; Died of D | 18; LR/Liver | Delayed exent, refused final 4 cycles |
| 9 | 64/F/OD | Crib/B | 2/2 | YES | Yes/+ | Exent orbit/sinuses + Bone + RT | 112; Alive, no D | 13; LR/Sinus | Delayed exent, refused final 4 cycles, ACC in sinus mucosa resected |
| 11 | 54/M/OS | Crib/Basa | 2/6 | YES | Yes/+ | Exent + Bone + TM +RT | 87; Died of D | 20; LR/Lung/Brain | Extensive disease at presentation |
| 12 | 32/F/OD | Crib | 2/5 | YES | Yes/+ | Exent + Bone + RT + GK | 85; Alive, no D | No | |
| 13 | 34/M/OD | Crib | 2/6 | YES | Yes/− | Exent + Bone + RT | 89; Alive, no D | No | Ophthal artery infusion, vessel thrombosis |
| 14 | 49/M/OS | Crib/Basa | 2/5 | YES | Yes/− | Exent + Bone + RT | 82; Alive, no D | 60; Lung | NED after lung nodule resection |
| 15 | 20/M/OD | Crib/Basa/P/B | 3/5 | YES | Yes/− | Exent + Bone + RT | 73; Alive; no D | No | |
| 16 | 38/F/OS | Basa | 2/6 | YES | Yes/+ | Exent + RT | 52; Alive; no D | No | |
| 19 | 56M/OS | Basa/P | 5/5 | YES | Yes/+ | Exent orbit/sinuses + RT | 20; Alive; no D | No | Intracranial and ethmoid sinus ACC, free flap |
| Alternate Histology | |||||||||
| 20 | 43/F/OS | Adenocar/P/B | 2/6 | YES | Yes/− | Exent + RT | 41; Died; no D | No | No ACC detected in exenteration specimen; AML 3 years post IACC; 11q23 translocation + margin at superior orbital fissure |
| 21 | 45/F/OD | Adenocar/P/B | 2/2 | NO | Yes/+ | Exent + RT | 30; Died of D | 26; Brain | |
| Conventional Treatment Group | |||||||||
| 1 | 37/M/OS | Basa | N/A | N/A | Exent + RT | 22; Died of D | 3; LR/Sinus | ||
| 2 | 57/F/OD | Crib/P/B | N/A | N/A | Resect + RT | 94; Died of D | 36; LR/Lung | ||
| 3 | 48/M/OD | Basa | N/A | N/A | Resect + Exent | 157; Died of D | No | ||
| 4 | 42/F/OD | Basa | N/A | N/A | Resect | 18; Died of D | 7; Brain | ||
| 5 | 69/M/OS | Basa | N/A | N/A | Exent + RT | 198; Died of D | 84; Brain | ||
| 6 | 53/F/OS | Crib | N/A | N/A | Exent + RT | 8; Died of D | 5; Brain | ||
| 7 | 37/M/OD | Crib/P/B | N/A | N/A | Exent + Bone + RT | 44; Died of D | 16; Lung | ||
ACC = adenoid cystic carcinoma; Basa = basaloid subtype; Crib = cribriform subtype; B = bone infiltration; P = perineural infiltration; Radiographic Tumor Response/Tissue Margins = radiographic evidence of tumor shrinkage after IA chemotherapy cycles/presence of tumor cells at soft tissue surgical margins; No D = No ACC; LR = local recurrence; Met = distant metastases; D = disease (local or metastatic ACC); Exent + bone + RT = exenteration with removal of lateral orbital wall fragment plus postoperative radiation therapy; TM = temporalis muscle resection; Exent + RT = exenteration plus postoperative radiation therapy; GK = gamma knife therapy; NED = no evidence of disease; N/A = not applicable; Resect = primary resection of mass; SCC = squamous cell carcinoma; 7th N = facial nerve palsy; Adenocar = adenocarcinoma; AML = acute myelocytic leukemia.
Eleven patients (Group 2) deviated from the protocol design. Ten patients had excisional biopsy by a lateral orbitotomy approach with the diagnosis of ACC made after the resection of the tumor. Thus, intraarterial chemotherapy was delivered in the absence of an intact lacrimal artery in these ten patients. A preoperative diagnosis of the lacrimal gland mass was pleomorphic adenoma in five cases (Cases 2, 6, 9, 12, 14), and an in toto dacryoadenectomy was performed through a lateral orbitotomy with bone take-down. The other five patients (Cases 11, 13, 15, 16, 19) underwent unplanned resection without an incisional biopsy.
Gross residual orbital disease or positive tumor margins of the resected mass was present in all ten cases at the time of referral for IACC. Case 19 developed a large recurrent mass with disease extension to the ipsilateral ethmoid and nasal sinuses following globe-sparing surgery without postoperative irradiation 10 years earlier. One patient (Case 8) was diagnosed with ACC from an incisional biopsy and received 2 cycles of IACC with a noticeable response,15 but the exenteration was delayed for more than 5 months and the patient refused the remaining 4 cycles of chemotherapy.
Five patients (Cases 1, 7, 12, 14, 15) were unable to complete all six cycles of chemotherapy because of severe cytopenia, renal dysfunction, or ototoxicity. In addition, one case (Case 19) received all chemotherapy cycles in the preoperative setting because of socioeconomic issues, and two cases (Case 8, 9) refused adjuvant chemotherapy. In patients who had a prior resection procedure, the titanium fixation plate/screws or sutures securing the bone fragment were removed at the time of the definitive surgery.
Of the 19 patients, six cases had evidence of bone erosion detected either by preoperative imaging or by intraoperative observations (Cases 1, 2, 6, 8, 9, 11), three had intracranial extension (Cases 1, 4, 19), and three had infiltration of the tumor into the temporalis muscle (Cases 8, 9, 11).
Eighteen of the 19 lacrimal gland ACC and the 2 adenocarcinoma patients received the intraarterial chemotherapy through the internal maxillary artery of the external carotid artery (ECA), and one patient in Group 2 (Case 13) through the ophthalmic artery of the internal carotid artery (ICA). Eight of the 19 ACC patients returned to the referring orbital surgeons for exenteration and completion of the treatment protocol. Eleven patients underwent exenteration at the Bascom Palmer Eye Institute (BPEI). Seven of the 11 returned to the referring physicians to complete the adjuvant chemotherapy protocol.
All 8 patients in Group 1 are alive and disease-free with a median survival of 10 years (range 2–24 years; Table 1). Four have survived for more than 10 years and one (Case 7) is 4 months short of 10 years. The duration of the longest survivor is 24 years (Case 1).
Of the 11 patients in Group 2, eight are disease-free with a median survival of 7 years (range 2–13 years). Two had resection of solitary sites of recurrent tumor; Case 9 underwent resection of tumor extension into the ethmoid sinus mucosa, and Case 14 had a resection of a lung metastasis. Two patients died as a direct result of ACC; Case 8 from metastatic liver disease and Case 11 from relentless locoregional recurrences and intracranial extension. Case 2 succumbed to oral squamous cell carcinoma likely related to human immunodeficiency virus (HIV), and without evidence of ACC relapse. One patient (Case 6) is alive with no evidence of locoregional disease, but has unresectable lung metastases.
We previously reported on a subset of ACC patients in 199812 and in 2006.15 The following two unreported cases highlight factors contributing to the treatment failure and intraarterial catheter complications.
Case 11: Pitfalls of extraorbital disease. 26
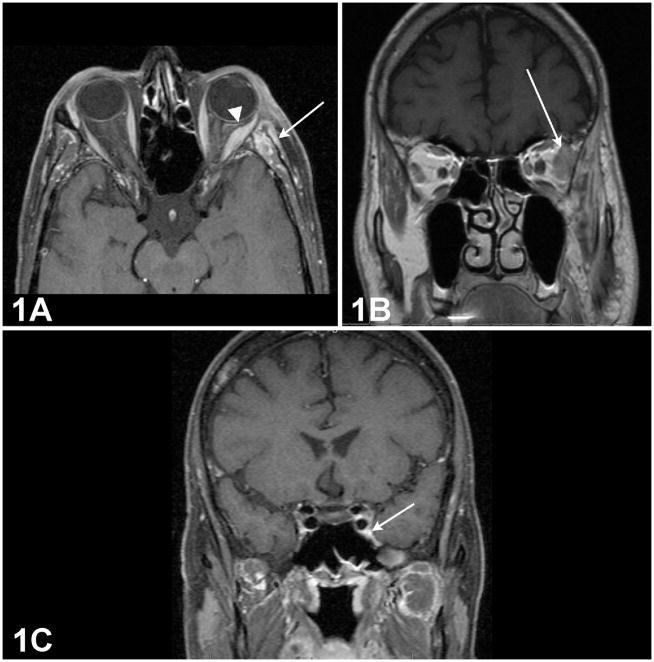
A 54-year-old man underwent a lateral orbitotomy procedure for subtotal resection of a left lateral extraconal lesion that had eroded the bone and infiltrated into the temporal fossa. Histopathological examination revealed a lacrimal gland ACC in a cribriform pattern. Post-resection orbital magnetic resonance imaging (MRI) showed enhancement of the left lateral rectus muscle, lacrimal gland remnant, and temporal fossa (Figure 1A and 1B). An imaging study obtained following the second cycle of IACC showed a persistent enlargement of the V2 segment within the inferior orbital fissure extending to the foramen rotundum and an asymmetrical enlargement of the left cavernous sinus (Figure 1C).
Figure 1. Orbital magnetic resonance imaging (MRI) of a patient with lacrimal gland adenoid cystic carcinoma.
1A: Post-resection orbital MRI showing thickening of the left lateral rectus muscle (Arrowhead) and increased soft tissue enhancement of the temporal fossa. (Arrow)
1B: Coronal view showing tumor remnant superior to lateral rectus at orbital apex. (Arrow)
1C: Enlargement of the V2 segment within the inferior orbital fissure extending to the foramen rotundum, and asymmetrical enlargement of the left cavernous sinus. (Arrow)
After IACC, he underwent extended radical orbital exenteration with removal of the temporalis muscle, resection of the pterygopalatine fossa and the left cavernous sinus. Histopathological examination of the major specimen demonstrated extensive tumor in the left orbit as well as disease in the left pterygopalatine fossa and perineural invasion of V2. All of the surgical margins, including that of the cavernous sinus specimens and the lateral orbital wall were negative.
Twenty months following completion of the full chemotherapy protocol, the patient developed a local recurrence in the temporal fossa requiring eight more maxillofacial and neck procedures, multiples lines of chemotherapy (nab-paclitaxel, mitoxantrone, sunitinib, docetaxel/cisplatin/5-FU), one round of radiation, and two Cyberknife treatments. He also participated in many clinical trials. Two years later, he succumbed to the disease due to extensive locoregional recurrence with intracranial extension.
Case 13: Catheter placement complication
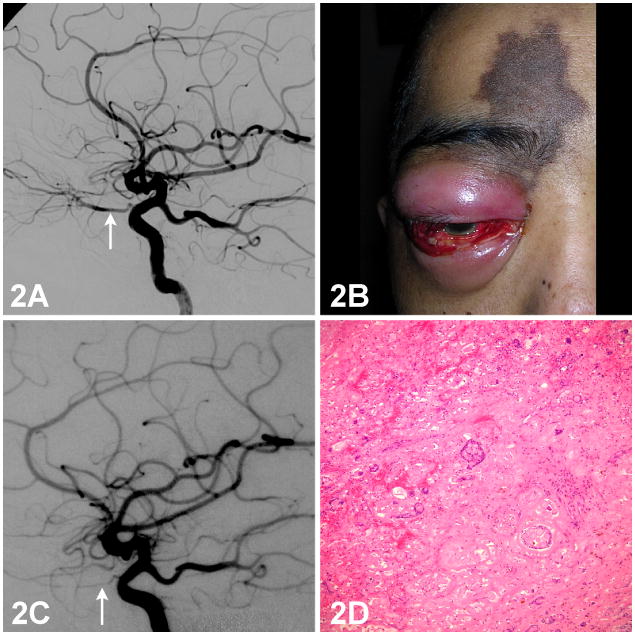
A 34-year-old man underwent subtotal resection through a lateral orbitotomy of a large lacrimal gland mass infiltrating into the orbital apex. Histopathologic examination revealed an ACC with tumor cells present at multiple surgical margins. Diagnostic external carotid artery angiography did not show vascular staining of the tumor, but internal carotid artery angiography showed tumor remnants supplied by several ophthalmic artery branches (Figure 2A). The catheter was positioned at the origin of the ophthalmic artery, and cisplatin (100 mg/m2) was infused continuously per protocol. Post procedure intracranial ICA angiography showed no evidence of vascular rupture, thrombosis, or spasm.
Figure 2. Internal carotid artery angiogram, external photograph of the eye, and photomicrograph of a histopathological section of the lacrimal gland from a patient with lacrimal gland adenoid cystic carcinoma.
2A: Diagnostic internal carotid artery angiogram showing tumor remnants supplied by the ophthalmic artery. (Arrow)
2B: Skin hyperpigmentation over the medial right brow and forehead. Anterior segment examination showing conjunctival injection, chemosis, hemorrhage, corneal edema, shallow anterior chamber, and hypotony.
2C: Repeat angiogram shows a hypoplastic right ophthalmic artery. (Arrow) Collateral flow from the middle meningeal artery and the distal maxillary artery of the external carotid artery (ECA) is present.
2D: Histopathologic examination of an exenterated specimen shows foci of tumor necrosis and blood vessel thrombosis. H/E 100X.
Two days after the ophthalmic artery delivery of cisplatin, the patient noted a complete loss of vision in the right eye. Over the ensuing 5 days, he complained of progressive orbital swelling, proptosis, forehead numbness, and darkening of the forehead skin. Ophthalmic examination showed no light perception (NLP) vision oculus dexter (OD) and 20/20 oculus sinister (OS). Ophthalmoplegia of the right eye, reduced corneal sensation, and a well-demarcated patch of hyperpigmented skin over the medial right brow and forehead were noted (Figure 2B). Slit-lamp examination showed signs of anterior segment ischemia. Over the next two weeks, the orbital inflammation and skin hyperpigmentation resolved without soft tissue necrosis or eschar. A second angiogram performed 3 weeks later disclosed a hypoplastic right ophthalmic artery (Figure 2C). Collateral flow toward the orbit from the middle meningeal artery and the distal maxillary artery of the external carotid artery was present. The microcatheter was now positioned in the internal maxillary artery of the external carotid artery to deliver the second cycle of cisplatin. Histopathologic examination of the exenterated specimen revealed marked tumor necrosis and blood vessel thrombosis (Figure 2D).
Efficacy
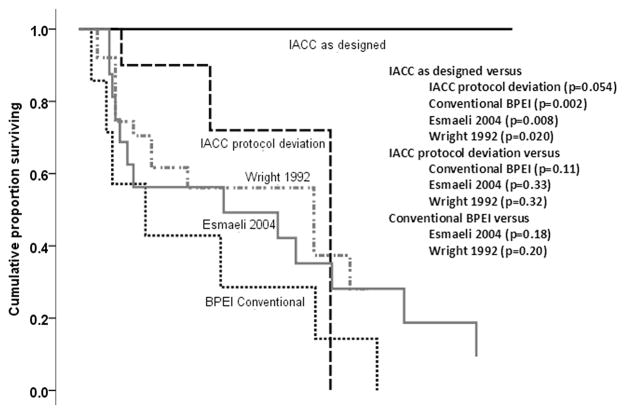
Analyses of the overall survival of Group 1 patients demonstrated that the IACC treatment was significantly better than the BPEI conventional treatment (P=0.002, log-rank test), and it was also better than the two published conventionally treated case series11,25 (PPCSW=0.020, PPCSE=0.008, log-rank test). The IACC treatment had a borderline better survival for Group 1 compared to that of Group 2 patients (P=0.054, log-rank test). However, the survival in Group 2 was not significantly different from that after conventional treatment (PBPEI=0.11, PPCSW=0.32, PPCSE=0.33, log-rank test). These data are displayed in Figure 3 and Table 2 (available at http://aaojournal.org).
Figure 3.
Kaplan-Meier survival curves irrespective of the cause of death in patients treated for lacrimal gland adenoid cystic carcinoma (ACC). Comparison of survival proportions of patients treated with intraarterial cytoreductive chemotherapy (IACC) as designed, IACC with protocol deviation, historical control that were conventionally treated patients at Bascom Palmer Eye Institute (BPEI), and two case series from the literature.
The median survival of patients in Group 1 (IACC with intact lacrimal artery) could not be conclusively determined because all of the patients remain alive without recurrences at a median follow-up of >10 years (100%). The cumulative survival at 10 years in Group 2 was 72% with 2 patients alive at >10 years. However, one of these 2 patients died of a non-ACC related malignant disease at 14 years, and the second is alive with unresectable metastatic disease at 11 years. The conventional therapy group had a 28.6% cumulative survival at 10 years.
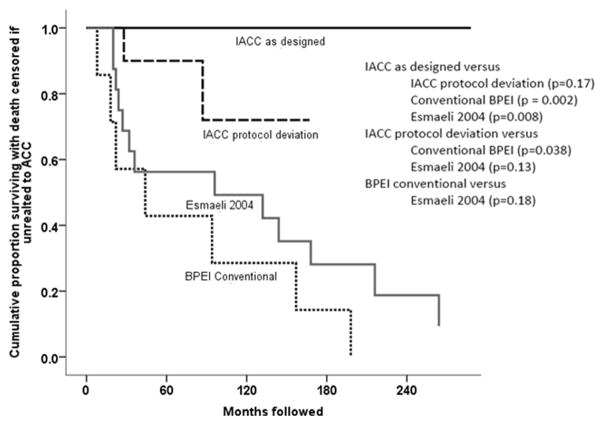
In the ACC disease-specific mortality analyses (Figure 4 and Table 3 [available at http://aaojournal.org]), Group 1 patients had significantly lower disease specific mortality than the conventional treatment group (PBPEI=0.002) and the Esmaeli report25 (PPCSE=0.008, log-rank test). However, the disease specific mortality was not significantly different from that of Group 2 patients (P=0.17). Group 2 patients did have significantly lower cause-specific mortality than that of the BPEI conventional treatment group (P=0.038), but not the published cases series of Esmaeli (PSCE; P=0.13, log-rank test).
Figure 4.
Kaplan-Meier survival curves of disease specific mortality in patients treated for lacrimal gland adenoid cystic carcinoma (ACC). A comparison of the cumulative Kaplan-Meier survival proportions, when mortality was due to ACC, of patients treated with intraarterial cytoreductive chemotherapy (IACC) as designed, IACC with protocol deviation, historical control conventionally treated patients at Bascom Palmer Eye Institute (BPEI), and one case series from the literature. Deaths unrelated to disease were censored in this analysis.
The recurrence rate for Group 1 patients was significantly lower than that for the conventional treatment group (P<0.001, log-rank test) and that for the Group 2 patients (P=0.035, log-rank test). Group 2 had significantly lower recurrence rates than that of conventionally treated patients (P=0.044). These data are displayed in Figures 5 and Table 4 (available at http://aaojournal.org).
Figure 5.
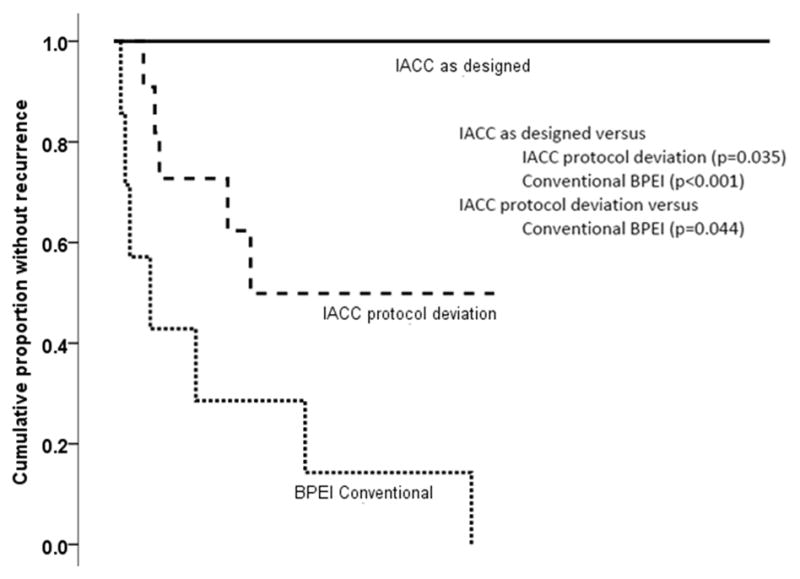
Kaplan-Meier analysis of recurrences in patients treated for lacrimal gland adenoid cystic carcinoma (ACC). Compares the cumulative Kaplan-Meier proportions of patients without recurrence treated with intraarterial cytoreductive chemotherapy (IACC) as designed, IACC with protocol deviation, and historical control conventionally treated patients at Bascom Palmer Eye Institute (BPEI).
Safety
Intraarterial Chemotherapy Complications Among all Patients
Fifty-one intraarterial chemotherapy procedures were performed in 21 patients. The total number of chemotherapy cycles was 106 including all IA and IV cycles. The complications were divided into the following groups: chemotherapy-related, local, catheter-related, neurological, and cardiovascular (Table 5, available at http://aaojournal.org).
IACC was safe and well tolerated by most patients. There were five episodes of febrile neutropenia (5/51 or 9.8%) related to the intraarterial procedures. There were no catheter-related and/or angiographic complications including thrombotic stroke, hemorrhage, or ophthalmologic toxicity when chemotherapy was delivered through the external carotid artery. A vessel thrombosis occurred in one case when cisplatin was infused through the ophthalmic artery of ICA resulting in NLP vision, anterior segment ischemia, and forehead skin depigmentation (Case 13).
DISCUSSION
Our results show that the integration of neoadjuvant and adjuvant chemotherapy to the conventional approach of orbital exenteration and radiation for the treatment of lacrimal gland ACC was effective in improving the overall survival and in reducing disease recurrences. Implementing the IACC protocol for patients whose tumor had an intact lacrimal artery resulted in significantly better outcomes by all three measures of success: all-cause mortality, cause-specific mortality, and recurrence, than the BPEI conventional therapy group. Comparisons of the survival data to those in two previously published studies11,25 revealed a significantly better outcome for our Group 1 patients compared to those reported in these papers from outside institutions. The outcomes in Groups 2 and BPEI conventional treatment group did not differ significantly from these published data. These comparisons validate the BPEI conventional therapy group, a historical cohort, as an appropriate comparator group for the protocol treated population.
The patients in Group 1 had excellent therapeutic outcomes with no relapses after a median follow-up of 10 years although the sample size was small and the subjects were non-randomized. The outcomes were significantly better than that in the ACC patients who were treated with surgery and/or radiotherapy at the same institution. The long-term survival findings affirm that the integrated neoadjuvant and adjuvant chemotherapy approach is an appropriate chemotherapeutic technique for the treatment of lacrimal gland ACCs. Our results are consistent with the proven clinical utility of similar preoperative cytoreductive chemotherapy strategies for many types of tumors including osteosarcoma, breast cancer, and gastric cancer.27–30
The benefit of IACC for lacrimal gland ACC is related to the delivery of high concentrations of cisplatin in its active form to the target for greater biologic effects, including overwhelming the tumor repair mechanisms. Cisplatin functions in vivo in a way similar to alkylating compounds by binding to the nitrogen in purines of the DNA to produce adducts, preventing replication. However, tumor cells with competent nucleotide excision repair pathway (NER) mechanisms can repair these DNA adducts and may survive a platinum-based chemotherapeutic challenge.31,32 Theoretically, the substantial pharmacologic advantage of intraarterial delivery of cisplatin can overwhelm the molecular resistance, thus enhancing therapeutic response as compared to intravenous administration.33
In addition to the reduction of the tumor size to optimize resection with negative margins, IACC also decreases the tumor burden to minimize the likelihood of viable tumor cell dissemination through the vascular spaces during surgical manipulation and introduces systemic therapy early in the course of treatment to address micrometastatic disease. In 3 patients (Cases 1, 4, 19) with intracranial extension and 3 patients (Cases 8, 9, 11) with tumor infiltration into the temporal fossa, the desired effect of down-staging the disease to an intra-orbital process for exenteration was realized. Cases 1, 4 and 9 have achieved disease-free survival of 24, 13, and 9 years respectively, which supports the rationale of neoadjuvant therapy in down-staging extraorbital tumor to result in excellent locoregional control and theoretically in treating unrecognized micrometastatic disease early in the course of illness.
In our patients, several factors which led to suboptimal treatment responses to the IACC protocol were identified:
1, extent of disease or breach of orbital boundaries;
2, failure to obtain a preoperative incisional biopsy prior to surgical planning;
3, surgical disruption of the lateral orbital wall, tumor manipulation, and incomplete resection;
4, absence of a lacrimal artery to maximize drug delivery; and
5, failure to implement all parts of the IACC protocol.
Five patients in Group 2 (Cases 6, 8, 9, 11, 14) had unfavorable outcomes (5 recurrences, 2 died of disease), and each had one or more of these risk factors. Case 2 died of a disease unrelated to ACC.
Six patients (Groups 1 and 2) had tumor extension beyond the orbit at presentation; 3 had intracranial extension (Cases 1, 4, 19), 3 had temporalis muscle infiltration through the lateral orbital wall (Cases 8, 9, 11), and one had both of these factors (Case 11). Case 11 had orbital bone erosion, temporal fossa infiltration, and extension along the lacrimal nerve to the trigeminal ganglion and skull base. Confounding the cytoreductive intent of IACC was an unplanned tumor resection with lateral wall disruption and severing of the lacrimal artery thus minimizing the full benefits of intraarterial drug delivery. With the “horse out-of-the barn”,26 IACC was not helpful in improving the outcome even with extensive local and regional extirpations. In contrast, both Cases 1 and 4 in Group 1 were judged to be unresectable because of intracranial infiltration. While preserving the lacrimal artery and avoiding tumor manipulation to minimize seeding, IACC reduced the mass and brought the intracranial tumor margins within the orbit, rendering surgical resection of the entire tumor mass possible by exenteration and without disrupting the bony barrier. The duration of the disease-free survival of these two patients was 24 years and 13 years which is unprecedented for unresectable lesions with evidence of perineural infiltration and basaloid histologic subtype features known to have a poor prognosis.15
Ten of the 11 patients in Group 2 underwent primary tumor resection through a standard lateral orbitotomy with bone take down because the diagnosis of ACC was not suspected; five had planned dacryoadenectomy for presumed pleomorphic adenoma and 5 had limited resection without prior tissue diagnosis. Such unplanned surgical manipulation with disruption of the bony barrier could contribute to an inadvertent tumor implantation and vascular dissemination. More critically, all had either gross residual orbital disease or positive tumor margins on inspection of the excised specimens, and an unintentional removal of the lacrimal artery which undermined the effectiveness of IACC.
Five patients (Cases 2, 6, 9, 12, 14) who underwent dacryoadenectomy for presumed pleomorphic adenoma had procedures performed by three different experienced orbital surgeons. However, a preoperative incisional biopsy was not performed. Each specimen showed an ACC with focal perineural invasion and positive margins which prompted referral for IACC treatment. Had the tissue diagnosis of ACC been available preoperatively, the surgeon may not have used the lateral orbitotomy procedure which could result in tumor seeding. Anatomically, a lacrimal gland ACC cannot be dissected in toto to achieve tumor margin clearance without sacrificing the lateral horn of the levator aponeurosis and conjunctiva. These procedures would not be included in the resection of a pleomorphic adenoma.34 Among these five patients, IACC conferred benefit for two cases (Cases 2, 12) with disease-free survival of 14 and 7 years. The other three cases (Cases 6, 9, 14) developed metastatic disease despite completion of the IACC protocol and local disease control.
To determine a treatment protocol for any lacrimal gland lesion suspicious of malignancy, we recommend an initial transcutaneous incisional biopsy to make a tissue diagnosis. The concern of cutting into a lesion exhibiting clinical and radiographic features of a pleomorphic adenoma is controversial but justified. To address this dilemma, Tse suggested the use of cyanoacrylate glue to cover the lacrimal gland incisional biopsy surface immediately to minimize tumor cell spillage while waiting for an intraoperative tissue diagnosis.35 If the transcutaneous biopsy confirms a pleomorphic adenoma, then a planned lateral orbitotomy for en bloc resection is implemented. If the biopsy reveals an ACC, the incision is closed and a “no further touch” technique is adopted. Further treatment should be defined by a multidisciplinary team approach with IACC as the core element of a coordinated plan. At the time of exenteration, Tse also has outlined a protocol for intraoperative frozen section assessment with an orbital form of Mohs microscopically-controlled tumor margin surveillance.34 In patients whose tumor has breached the lateral wall with extensions into the temporalis muscle, a head/neck surgeon should be consulted for possible regional neck dissection. This initial biopsy specimen can also serve as a “genomic biopsy” to provide tumor genomic information to guide a future personalized targeted cancer therapy.
The findings in Cases 6, 9, 11 and 19 illustrate the importance of minimizing the tumor manipulation and achieving tumor margin clearance at the initial surgery. Cases 6 and 9 had lateral wall take-down and incomplete primary tumor resection. Despite undergoing the IACC protocol, Case 6 developed metastatic lung disease and Case 9 had tumor extension into the ipsilateral ethmoid sinus. Case 19 had prior globe-sparing surgery but developed a recurrence of a large mass and extension of the disease to the ipsilateral sinuses. The recurrence of the orbital disease was most likely due to an incomplete tumor clearance at the time of the globe-sparing surgery. The residual disease served as a nidus to spawn tumor cells to the sinus mucosa probably by retrograde tracking along the lacrimal nerve toward the trunk of the ophthalmic nerve (V1) in the trigeminal ganglion. The tumor cells then crossed to the nasociliary nerve, a branch of V1 in the cavernous sinus and tracked in an antegrade manner toward the anterior and posterior ethmoidal branches to involve the sinus mucosa.36
Completing all elements of the IACC protocol in a timely manner was important to the success of this treatment. Case 8 had an infiltrative lesion extending outside the orbital boundaries. She had an incisional biopsy establishing the tumor diagnosis, and an intact artery suitable for IACC. After an initial dramatic local response to IACC, the patient refused surgical intervention and delayed the remaining 4 cycles of chemotherapy until an orbital recurrence developed 1 year later at which time she also had metastatic disease to the liver, from which she ultimately succumbed.15 We believe the lack of maximum disease control after demonstrating initial tumor chemosensitivity supports the need for surgical intervention and systemic therapy to augment the success of the neoadjuvant approach. The failure of this case also illustrates the concept of time-limited effect of neoadjuvant chemotherapy37 with most favorable outcomes dependent on the timely application of therapy to avoid tumor regrowth between cycles or phases of the treatment design even in a highly sensitive tumor. We recommend that exenteration should be performed within 6 weeks or less following neoadjuvant chemotherapy to allow for adequate bone marrow and patient recovery. Patient 19 received multiple pre-operative IACC cycles (>3) due to socioeconomic issues resulting in treatment interruptions and tumor regrowth between cycles despite significant initial chemosensitivity. Both cases demonstrate that optimal management requires adherence to the protocol specifications including rapid cycling of therapy, treatment intensity, and timely surgery to avoid interval tumor regrowth.
While the overall survival and relapse frequency of Group 2 patients were not significantly better than the conventionally treated group for cause-specific mortality and recurrences, the outcomes favored the intraarterial chemotherapy-treated cohort. The lack of statistical significance may in part be related to the small sample size.
The toxicity of the chemotherapy was manageable by trained medical oncologists. The intensity of treatment and the agents had a common spectrum of side effects including alopecia, which was universal, and nausea and vomiting which was frequent. Over the extended time course of the treated cohort, cytopenias requiring transfusion, episodes of febrile neutropenia dependent in part on the introduction of growth factor support, renal dysfunction without significant long-term sequelae, oropharyngeal mucositis, and ototoxicity were noted. Specific to the intraarterial approach, 9 patients developed limited degree of trismus, 2 patients with transient facial nerve palsy on the side ipsilateral to the catheter, and local tissue inflammation (facial swelling). We emphasize that the catheter placement and arterial infusion of chemotherapy were not associated with stroke, seizure, hemorrhage, or catheter complications. Although no catheter-related complications occurred in this series of patients, it is prudent for practitioners to be aware that such possibilities do exist. An ophthalmological adverse event related to the superselective infusion of drug through the internal and not the external carotid circulation was noted in Case 13, but this complication did not compromise the overall success of the therapy. Instead, this tumor demonstrated an enhanced response to treatment.
The toxicity from chemotherapy was expected and manageable, and the long-term complication rate was low (Table 5, available at http://aaojournal.org). However, one unfortunate but serious complication was a possible secondary malignancy with fatal outcome. Case 20, which was included for toxicity assessment, did not have ACC but was treated with IACC and developed a possible treatment-related acute myeloid leukemia (AML). Chromosomal analysis identified an 11q23 mutation.38–40 Although not an infrequent occurrence in de novo AML, it is a recognized complication of exposure to cytotoxic chemotherapy most notably alkylating agents and intercalating topoisomerase II inhibitors.39 The relationship of cisplatin and acute myeloid leukemia development has not been established. While the incidence is low, the risk of secondary malignancy should not deter the consideration of IACC therapy for lacrimal gland ACC; the decision needs to be balanced by the noted improvement in survival outcome as identified in the treatment cohort when therapy is administered with curative intent.
One limitation of this study is the small sample size, a consequence of the rarity of ACC of the lacrimal gland. Other limitations include the lack of randomization, concurrent controls, and independent validation by other investigators with comparable numbers of patients and length of follow-up. We acknowledge that our BPEI comparator group is not ideal as follow-up information and archival pathology specimens were available for only 7 patients. While secular trends such as changes in imaging modalities and oncologic care of IACC patients between the different timeframes may confound the differences in survival, it should be noted that no patient in our experience over 20+ years has metastatic disease at presentation. Thus it is unlikely that imaging improvement played any role in patient selection or that we excluded patients with worse prognosis by not accounting for the nine patients. Likewise surgical techniques have not changed considerably to account for better outcome. Designing a prospective, randomized control study would be difficult because of the rarity of this tumor and at the very least would require a national collaborative effort and long-term longitudinal follow-up. The focus of the paper, however, is to present new information which we believe should change clinical care for this condition. While the power of these observations is limited by size, the results remain positive in striking contrast to alternative conventional therapies reported over past decades.
The significance of our findings is the incremental knowledge gained that can add up to treatment advances in the management of lacrimal gland ACC. Primary tumor resection and/or disruption of the natural bony barriers with lateral orbitotomy without an incisional biopsy should be avoided before cytoreduction chemotherapy. We recognize that further investigation of this treatment protocol is warranted, however we believe the results of our retrospective analysis should define a new treatment paradigm and form the foundation for a future globe-sparing strategy by incorporating IACC as the central element in managing this devastating disease.
Strategies to increase neoadjuvant cytoreductive response by superselective lacrimal artery infusion to enhance complete tumor resection with the aim of preserving the globe should be considered. Elucidation of the genomic profile of lacrimal gland ACC could help diagnose, stage and identify personalized targeted adjunctive therapies to augment the beneficial effects of IACC established in this study.
Supplementary Material
Acknowledgments
Financial Support: This work was supported in part by: NIH Center Core Grant P30EY014801; Research to Prevent Blindness Unrestricted Grant, Inc, New York, New York; Department of Defense (DOD - Grant W81XWH-09-1-0675); Plum Foundation, Los Angeles, California, and the Dr. Nasser Ibrahim Al-Rashid Orbital Vision Research Fund. The sponsor or funding organization had no role in the design or conduct of this research.
Footnotes
Meeting Presentation: American Academy of Ophthalmology Annual Meeting, November, 2012
Conflict of Interest: No conflicting relationship exists for any author.
This article contains online-only material. The following should appear online-only: Table 2, Table 3, Table 4, Table 5.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Lee DA, Campbell RJ, Waller RR, Ilstrup DM. A clinicopathologic study of primary adenoid cystic carcinoma of the lacrimal gland. Ophthalmology. 1985;92:128–34. doi: 10.1016/s0161-6420(85)34081-2. [DOI] [PubMed] [Google Scholar]
- 2.Font RL, Gamel JW. Epithelial tumors of the lacrimal gland: an analysis of 265 cases. In: Jakobiec FA, editor. Ocular and Adnexal Tumors. Birmingham, AL: Aesculapius Pub. Co; 1978. pp. 787–805. [Google Scholar]
- 3.Font RL, Smith SL, Bryan RG. Malignant epithelial tumors of the lacrimal gland: a clinicopathologic study of 21 cases. Arch Ophthalmol. 1998;116:613–6. doi: 10.1001/archopht.116.5.613. [DOI] [PubMed] [Google Scholar]
- 4.Font RL, Gamel JW. Adenoid cystic carcinoma of the lacrimal gland: a clinicopathologic study of 79 cases. In: Nicholson DH, editor. Ocular Pathology Update. New York: Masson; 1980. pp. 277–83. [Google Scholar]
- 5.Byers RM, Berkeley RG, Luna M, Jesse RH. Combined therapeutic approach to malignant lacrimal gland tumors. Am J Ophthalmol. 1975;79:53–5. doi: 10.1016/0002-9394(75)90455-9. [DOI] [PubMed] [Google Scholar]
- 6.Wright JE, Stewart WB, Krohel GB. Clinical presentation and management of lacrimal gland tumours. Br J Ophthalmol. 1979;63:600–6. doi: 10.1136/bjo.63.9.600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gamel JW, Font RL. Adenoid cystic carcinoma of the lacrimal gland: the clinical significance of a basaloid histologic pattern. Hum Pathol. 1982;13:219–25. doi: 10.1016/s0046-8177(82)80180-9. [DOI] [PubMed] [Google Scholar]
- 8.Wright JE. Factors affecting the survival of patients with lacrimal gland tumours. Can J Ophthalmol. 1982;17:3–9. [PubMed] [Google Scholar]
- 9.Douglas JG, Laramore GE, Austin-Seymour M, et al. Treatment of locally advanced adenoid cystic carcinoma of the head and neck with neutron radiotherapy. Int J Radiat Oncol Biol Phys. 2000;46:551–7. doi: 10.1016/s0360-3016(99)00445-9. [DOI] [PubMed] [Google Scholar]
- 10.Polito E, Leccisotti A. Epithelial malignancies of the lacrimal gland: survival rates after extensive and conservative therapy. Ann Ophthalmol. 1993;25:422–6. [PubMed] [Google Scholar]
- 11.Wright JE, Rose GE, Garner A. Primary malignant neoplasms of the lacrimal gland. Br J Ophthalmol. 1992;76:401–7. doi: 10.1136/bjo.76.7.401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Meldrum ML, Tse DT, Benedetto P. Neoadjuvant intracarotid chemotherapy for treatment of advanced adenocystic carcinoma of the lacrimal gland. Arch Ophthalmol. 1998;116:315–21. doi: 10.1001/archopht.116.3.315. [DOI] [PubMed] [Google Scholar]
- 13.Stewart DJ, Wallace S, Feun L, et al. A phase I study of intracarotid artery infusion of cis-diamminedichloroplatinum(II) in patients with recurrent malignant intracerebral tumors. Cancer Res. 1982;42:2059–62. [PubMed] [Google Scholar]
- 14.Stephens FO, Harker GJ, Crea P. The intraarterial infusion of chemotherapeutic agents as “basal” treatment of cancer: evidence of increased drug activity in regionally infused tissues. Aust N Z J Surg. 1980;50:597–602. doi: 10.1111/j.1445-2197.1980.tb04205.x. [DOI] [PubMed] [Google Scholar]
- 15.Tse DT, Benedetto P, Dubovy S, et al. Clinical analysis of the effect of intraarterial cytoreductive chemotherapy in the treatment of lacrimal gland adenoid cystic carcinoma. Am J Ophthalmol. 2006;141:44–53. doi: 10.1016/j.ajo.2005.08.068. [DOI] [PubMed] [Google Scholar]
- 16.Natanegara IA, Koornneef L, Veenhof K, et al. An alternative approach for the management of adenocystic carcinoma of the lacrimal gland. Orbit. 1990;9:101–5. [Google Scholar]
- 17.Shields JA, Shields CL, Eagle RC, Jr, et al. Adenoid cystic carcinoma of the lacrimal gland simulating a dermoid cyst in a 9-year-old boy. Arch Ophthalmol. 1998;116:1673–6. doi: 10.1001/archopht.116.12.1673. [DOI] [PubMed] [Google Scholar]
- 18.Tyl JW, Blank LE, Koornneef L. Brachytherapy in orbital tumors. Ophthalmology. 1997;104:1475–9. doi: 10.1016/s0161-6420(97)30113-4. [DOI] [PubMed] [Google Scholar]
- 19.Buchholz TA, Shimotakahara SG, Weymuller EA, Jr, et al. Neutron radiotherapy for adenoid cystic carcinoma of the head and neck. Arch Otolaryngol Head Neck Surg. 1993;119:747–52. doi: 10.1001/archotol.1993.01880190043009. [DOI] [PubMed] [Google Scholar]
- 20.Henderson JW, Neault RW. En bloc removal of intrinsic neoplasms of the lacrimal gland. Am J Ophthalmol. 1976;82:905–9. doi: 10.1016/0002-9394(76)90068-4. [DOI] [PubMed] [Google Scholar]
- 21.Rootman J, Lapointe JS. Tumors of the lacrimal gland. In: Rootman J, editor. Diseases of the Orbit: A Multidisciplinary Approach. Philadelphia, PA: Lippincott; 1988. pp. 384–95. [Google Scholar]
- 22.Marsh JL, Wise DM, Smith M, Schwartz H. Lacrimal gland adenoid cystic carcinoma: intracranial and extracranial en bloc resection. Plast Reconstr Surg. 1981;68:577–85. doi: 10.1097/00006534-198110000-00017. [DOI] [PubMed] [Google Scholar]
- 23.Janecka I, Housepian E, Trokel S, et al. Surgical management of malignant tumors of the lacrimal gland. Am J Surg. 1984;148:539–41. doi: 10.1016/0002-9610(84)90384-2. [DOI] [PubMed] [Google Scholar]
- 24.Esmaeli B, Golio D, Kies M, DeMonte F. Surgical management of locally advanced adenoid cystic carcinoma of the lacrimal gland. Ophthal Plast Reconstr Surg. 2006;22:366–70. doi: 10.1097/01.iop.0000232164.00208.b4. [DOI] [PubMed] [Google Scholar]
- 25.Esmaeli B, Ahmadi MA, Youssef A, et al. Outcomes in patients with adenoid cystic carcinoma of the lacrimal gland. Ophthalmic Plast Reconstr Surg. 2004;20:22–6. doi: 10.1097/01.IOP.0000105518.72611.4F. [DOI] [PubMed] [Google Scholar]
- 26.Bartley GB, Harris GJ. Adenoid cystic carcinoma of the lacrimal gland: is there a cure...yet? Ophthalmic Plast Reconstr Surg. 2002;18:315–8. doi: 10.1097/00002341-200209000-00001. [DOI] [PubMed] [Google Scholar]
- 27.Hugate RR, Wilkins RM, Kelly CM, et al. Intraarterial chemotherapy for extremity osteosarcoma and MFH in adults. Clin Orthop Relat Res. 2008;466:1292–301. doi: 10.1007/s11999-008-0252-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hohn DC, Stagg RJ, Friedman MA, et al. A randomized trial of continuous intravenous versus hepatic intraarterial floxuridine in patients with colorectal cancer metastatic to the liver: the Northern California Oncology Group trial. J Clin Oncol. 1989;7:1646–54. doi: 10.1200/JCO.1989.7.11.1646. [DOI] [PubMed] [Google Scholar]
- 29.Kajanti M, Rissanen P, Virkkunen P, et al. Regional intra-arterial infusion of cisplatin in primary hepatocellular carcinoma. A phase II study. Cancer. 1986;58:2386–8. doi: 10.1002/1097-0142(19861201)58:11<2386::aid-cncr2820581105>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 30.Koyama H, Nishizawa Y, Wada T, et al. Intra-arterial infusion chemotherapy as an induction therapy in multidisciplinary treatment for locally advanced breast cancer. A long-term follow-up study. Cancer. 1985;56:725–9. doi: 10.1002/1097-0142(19850815)56:4<725::aid-cncr2820560404>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 31.Metzger R, Leichman CG, Danenberg KD, et al. ERCC1 mRNA levels complement thymidylate synthase mRNA levels in predicting response and survival for gastric cancer patients receiving combination Cisplatin and fluorouracil chemotherapy. J Clin Oncol. 1998;16:309–16. doi: 10.1200/JCO.1998.16.1.309. [DOI] [PubMed] [Google Scholar]
- 32.Britten RA, Liu D, Tessier A, et al. ERCC1 expression as a molecular marker of cisplatin resistance in human cervical tumor cells. Int J Cancer. 2000;89:453–7. [PubMed] [Google Scholar]
- 33.Cloughesy TF, Gobin YP, Black KL, et al. Intra-arterial carboplatin chemotherapy for brain tumors: a dose escalation study based on cerebral blood flow. J Neurooncol. 1997;35:121–31. doi: 10.1023/a:1005856002264. [DOI] [PubMed] [Google Scholar]
- 34.Tse DT. Clinical and microdissection genotyping analyses of the effect of intra-arterial cytoreductive chemotherapy in the treatment of lacrimal gland adenoid cystic carcinoma. Trans Am Ophthalmol Soc. 2005;103:337–67. [PMC free article] [PubMed] [Google Scholar]
- 35.Tse DT, Folberg R. Technique for incisional biopsy of a lacrimal gland mass when the diagnosis of benign mixed tumor cannot be excluded clinically. Ophthal Surg. 1988;19:321–4. [PubMed] [Google Scholar]
- 36.Tse DT, Benedetto P, Morcos JJ, et al. An atypical presentation of adenoid cystic carcinoma of the lacrimal gland. Am J Ophthalmol. 2006;141:187–9. doi: 10.1016/j.ajo.2005.08.070. [DOI] [PubMed] [Google Scholar]
- 37.Simek J, Ehrmann J, Pazdera J. Inter-arterial chemotherapy and its significance in the treatment of oropharyngeal carcinoma. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 2007;151:219–24. doi: 10.5507/bp.2007.037. [DOI] [PubMed] [Google Scholar]
- 38.Pedersen-Bjergaard J, Sigsgaard TC, Nielsen D, et al. Acute monocytic or myelomonocytic leukemia with balanced chromosome translocations to band 11q23 after therapy with 4-epi-doxorubicin and cisplatin or cyclophosphamide for breast cancer. J Clin Oncol. 1992;10:1444–51. doi: 10.1200/JCO.1992.10.9.1444. [DOI] [PubMed] [Google Scholar]
- 39.Sandoval C, Pui CH, Bowman LC, et al. Secondary acute myeloid leukemia in children previously treated with alkylating agents, intercalating topoisomerase II inhibitors, and irradiation. J Clin Oncol. 1993;11:1039–45. doi: 10.1200/JCO.1993.11.6.1039. [DOI] [PubMed] [Google Scholar]
- 40.Chaplain G, Milan C, Sgro C, et al. Increased risk of acute leukemia after adjuvant chemotherapy for breast cancer: a population-based study. J Clin Oncol. 2000;18:2836–42. doi: 10.1200/JCO.2000.18.15.2836. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.