Abstract
The importance for activation of innate immunity by pattern recognition receptors in forming an effective adaptive immune response is well known. Toll-like receptors (TLRs) have been demonstrated to be critical for antibody responses to a variety of immunizations. In particular, recent evidence suggests that B cell-intrinsic TLR signaling is required for optimal responses to virus-like antigens, but mechanisms by which TLR signaling impacts antibody responses during infection in vivo is unclear. In the present study, we demonstrate that deficiency of TLR7 in B cells alone is sufficient to significantly impact antibody responses in mice during chronic viral infection. This effect was independent of T follicular helper cells, and resulted in a loss of plasma cells generated later, but not early, in the response. The defect in plasma cell formation appeared to be secondary to a qualitative effect of TLR signaling on the germinal center (GC) B cell response. GC B cells in TLR7-deficient mice proliferated to a lesser extent and had a greater proportion of cells with phenotypic characteristics of light zone, relative to dark zone GC B cells. These results suggest that B cell-intrinsic TLR signaling in vivo likely affects plasma cell output by altered selection of antigen-specific B cells in the germinal center.
Introduction
In recent years, there has been much interest in harnessing the activation of innate immunity for prevention and treatment of both acute and chronic infections of major concern to global public health (1). However, much remains to be understood about how engagement of different innate immune receptors contributes to protective immune responses. How these varying recognition pathways contribute to protective adaptive immune responses is further complicated by their broad expression among many immune cell types, and further, by non-hematopoietic cells (2). This is of particular interest as vaccines, such as live-attenuated viruses, can activate different classes of innate immune pattern recognition receptors including both Toll-like receptors (TLRs) and cytoplasmic RIG-I-like receptors (RLRs) (3). Therefore, an understanding of the role innate immune receptors play in the induction of protective immune responses in the context of a live infection can lend crucial insight to both basic biology and vaccinology.
Infection of mice with lymphocytic choriomengitis virus (LCMV) has served as a useful model to interrogate immune responses during the course of both acute and chronic viral infections. Whereas infection with the Armstrong (Arm) strain of LCMV results in acute infection that is eliminated approximately 8–10 days postinfection (p.i.), infection with the genetically closely related variant clone 13 leads to persistent infection which lasts for two or more months (4). Additional manipulation of the immune response during chronic LCMV infection through either genetic means or CD4+ T cell depletion leads to sustained high levels of viremia throughout the course of the life of the animal and has demonstrated requirements for both CD4+ T cells and B cells, in addition to CD8+ T cells for long-term virus control (5–10). Although B cells may contribute to clearance in non-antibody dependent ways (7, 9), antibody-dependent requirements have also been demonstrated (6). Furthermore, chronic LCMV infection drives differentiation of CD4+ T cells into T follicular helper (Tfh) cells, and Tfh cell expression of the chemokine receptor CXCR5 was necessary for optimal antibody responses and viral clearance (11).
Recently, in an analysis of the role innate pattern recognition receptors (PRRs) play in the clearance of acute and chronic LCMV infections, we found differential roles for the cytoplasmic MAVS-dependent pathway and the nucleic acid-sensing TLR pathway (12). Whereas the MAVS pathway was important for type I interferon induction for both acute and chronic variants of LCMV, the nucleic acid-sensing TLR pathway was only necessary for successful resolution of chronic infection. Further analysis showed that when virus recognition by nucleic acid-sensing TLRs was absent, virus-specific antibody responses were greatly defective during chronic LCMV infection but mostly intact during acute infection. Although several recent studies have also highlighted the role of TLR signaling in antiviral antibody responses (13–15), the general mechanisms by which TLR signaling contributes to B cell responses in vivo, in particular during the course of a chronic infection, remain largely ill defined.
In order to more precisely address the mechanism by which TLR7, and potentially TLR3, may impact the outcome of chronic infection with LCMV and the formation of B cell responses in general, we have undertaken more detailed studies to examine at what point, and for which cell types, TLR signaling is critical. Herein, we show that B cell-intrinsic TLR7 is necessary for optimal antibody responses in chronic LCMV infection. On closer examination, chimera experiments revealed a GC B cell defect, but also a subsequent, secondary, defect in plasma cell formation. In the absence of competition, lack of TLR7 led to a delayed, but significant, decrease in plasma cell formation. Although we observed little quantitative impact of TLR7 deficiency on total GC B cell numbers, Tlr7−/− GC B cells proliferated to a lesser extent and were skewed in the distribution of cells with phenotypic characteristics of light zone (LZ) and dark zone (DZ) GC B cells. These results demonstrate that qualitative differences in the germinal center response in the absence of TLR signaling lead to defective plasma cell and antibody formation.
Materials and Methods
Mice
C57BL/6 (CD45.2+) and B6.BoyJ mice (CD45.1+) were purchased from the Jackson Laboratory or the National Cancer Institute. Unc93b1 “3d” mice on a C57BL/6 background were purchased from the Mutant Mouse Regional Resource Center (University of California, Davis, CA) (16). Tlr3−/− and Tlr7−/− mice on the C57BL/6 background were purchased from the Jackson Laboratory (17, 18). Dr. M Wabl (University of California, San Francisco, CA) generously provided JH−/− mice (19). All experiments were done in accordance with University of California, San Francisco Institutional Animal Care and Use Committee guidelines.
Virus infection and titration
Mice were infected intravenously (i.v.) with 2 × 106 PFU LCMV clone 13, or LCMV Armstrong where stated. Virus was propagated on BHK cells and titration by plaque assay was carried out on Vero cells as previously described (4).
Bone marrow chimeras
Bone marrow chimeras were generated by lethally irradiating 6–8 week old B6.BoyJ (CD45.1+) mice with γ-radiation from a Cesium source. B6.BoyJ mice were reconstituted with a 50:50 mixture of either WT (CD45.1+) and Tlr7−/− (CD45.2+) bone marrow, or WT (CD45.1+) and WT (CD45.2+) bone marrow as controls. In some experiments, irradiated mice were reconstituted with a mixture of 85% JH−/− and 15% WT, Unc93b13d/3d, or Tlr7−/− bone marrow. Mice were maintained on antibiotics for 6 wk during reconstitution then bled to determine the relative frequencies of B220+ cells from each donor population, at which time they were removed from antibiotics and maintained in the absence of antibiotics until experimentation.
Flow cytometry, antibodies, and intracellular staining
All antibodies were purchased from BioLegend except for anti-mouse CD16+CD32 (2.4G2 mAb) and straptavidin-QDot605 (University of California, San Francisco Antibody Core Facility); anti-mouse GL-7 and B220 (eBioscience), anti-CXCR4 and anti-mouse IgD (BD Biosciences) and FITC-conjugated goat anti-mouse IgG (Caltag). Control hCLIP-tetramer and MHC class II tetramer (NIH tetramer core facility, Emory University, Atlanta, GA) staining for LCMV GP66-77-specific CD4+ T cells was performed as previously described (20). Intracellular staining was performed as previously described for cytokine staining, with the following modifications: cells were blocked with anti-mouse CD16+CD32 prior to staining and FITC-conjugated anti-mouse IgG was used in place of anti-cytokine antibodies (21). Flow cytometry was performed on LSRFortessa or FACSCalibur (BD Biosciences) cytometers and analyzed with FlowJo 9.0.2 software (TreeStar).
BrdU labeling and caspase activation
5-bromodeoxyuridine (BrdU) labeling and caspase activation in cells was performed as previously described (22). For BrdU assays, mice were given a single intraperitoneal dose of 3 mg BrdU (Sigma), in sterile phosphate-buffered saline, 2 h before being euthanized. A FITC BrdU Flow Kit (BD Pharmingen) was used to detect BrdU according to the manufacturer’s instructions. To detect caspase activation, cells were incubated 30 min to 1 h at 37 °C and 5% CO2 with FITC-VAD-FMK followed by washing and surface staining with antibodies for phenotypic analysis according to manufacturer’s instructions (CaspGLOW; BioVision).
ELISA
Serum titers of LCMV-specific IgG were determined by ELISA as previously described (12).
Determination of antibody-secreting cells
LCMV-specific antibody-secreting cells (ASC) were quantified as previously described with slight modifications (23), using HA-mixed cellulose ester membrane 96-well plates (Millipore). Briefly, LCMV-infected BHK cell lysate was diluted and added to wells and then incubated overnight (O/N) at 4 °C. The next day, plates were washed once with PBS containing 0.1% Tween 20 (Bio-Rad), then blocked with 5% FBS in PBS for 30+ min. Following red blood cell lysis, three-fold dilutions of spleen and bone marrow cell preparations were added and incubated for 4 h at 37 °C and 5% CO2. Antigen-bound IgG was detected first with biotinylated donkey anti-mouse IgG (Jackson ImmunoResearch) followed by subsequent incubation with horseradish peroxidase-conjugated streptavidin (Jackson ImmunoResearch), both O/N at 4 °C. Antibody-secreting cells were visualized by using freshly prepared chromogen substrate in which 0.5 ml of 3-amino-9-ethylcarbazole (Sigma) dissolved in dimethylformamide (8 mg/ml) was added to 9.5 ml 50 mM acetate buffer (pH 5.0), passed through a 0.45 μm filter, and 5 μl of 30% H2O2 added immediately before use. The reaction was terminated using tap water, and spots were analyzed with an EliSpot Reader System and EliSpot 3.5 software (AID).
Statistical analysis
All statistical analysis was performed by using a two-tailed unpaired Student’s t-test, or one-way ANOVA where noted, with Prism software (GraphPad Software, Inc.).
Results
TLR7 but not TLR3 is important for clearance and antibody responses during chronic LCMV infection
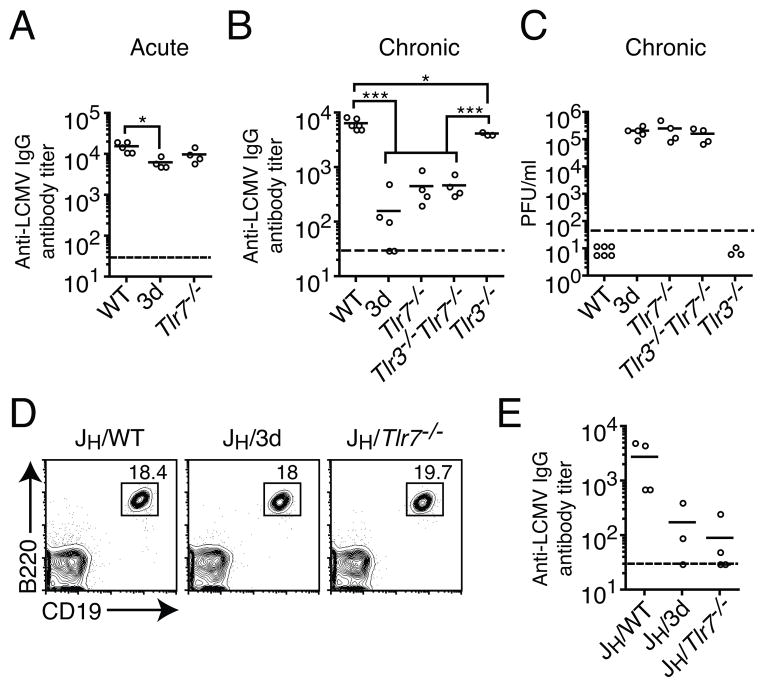
We have previously shown that mice containing a missense mutation in Unc93b1 (3d), which affects signaling through TLR3, 7, and 9, have greatly reduced levels of IgG in sera when compared to WT mice during chronic LCMV infection, and this correlates with inability to clear the virus (12). In contrast, antibody responses were much less dependent on nucleic acid-sensing TLRs during acute LCMV infection as there was only a modest decline in IgG levels in the 3d mice (12). Since LCMV is an RNA virus, the relevant nucleic acid-sensing TLRs that are defective in 3d mice are TLR3 and 7. Although TLR3 has been shown to be dispensable for control of acute LCMV infection (24), its contribution to control of chronic LCMV infection has yet to be elucidated. In light of our findings in 3d mice we sought to ascertain the contribution of TLR7 to antibody responses in acute infection and the contribution of both TLR3 and TLR7 during a chronic infection. Consistent with our previous report, when we assessed titers of LCMV-specific IgG by ELISA at day 60 following acute infection, 3d and Tlr7−/− mice had slightly lower levels of IgG when compared to wild-type (WT) mice, though in the case of TLR7, this was not statistically significant (Fig. 1A). In order to determine the contribution of the two RNA-sensing TLRs to LCMV-specific antibody responses and clearance of chronic infection, as well as to rule out the contribution of a non-TLR dependent function of Unc93b1 to virus clearance, we infected WT mice, 3d mice, or mice deficient in TLR3, TLR7, or both and assessed LCMV-specific IgG and viral loads in sera of infected mice (Fig. 1B and C). We found that at day 67 p.i. with LCMV clone 13, antibody titers were reduced ten-fold or more in 3d, Tlr7−/−, and Tlr3−/−Tlr7−/−, but not in Tlr3−/− mice (Fig. 1B). In contrast, Tlr3−/− mice had levels of LCMV-specific IgG that, though differing from those in WT mice in a statistically significant manner, this difference was generally less than two-fold in magnitude. When we assessed virus clearance at approximately two months p.i., whereas WT mice cleared virus by this time point, 3d mice and those deficient in TLR7 maintained high viral loads (Fig. 1C). In addition, mice doubly deficient in TLR3 and TLR7 had levels of virus similar to that in both 3d and Tlr7−/− mice. In contrast, Tlr3−/− mice had normal kinetics of virus clearance. This result, along with the similar virus titers between 3d, Tlr7−/−, and Tlr3−/−Tlr7−/− mice indicate that TLR3 is not necessary, and appears to play no additive or synergistic role in clearance of chronic LCMV infection. Additionally, TLR-dependent clearance of chronic LCMV infection seems to be directly correlated with the ability of mice to mount normal antibody responses, suggesting a critical role for this TLR-mediated function.
Figure 1. LCMV-specific IgG responses in chronically infected mice are dependent on B cell-intrinsic TLR7 signaling.
(A) WT, 3d, or Tlr7−/− mice were infected with LCMV Arm and anti-LCMV IgG responses were measured by ELISA on day 60 p.i. (B) The indicated mice were infected with LCMV clone 13 and LCMV-specific IgG levels were determined by ELISA at day 67 p.i. (C) Titers of LCMV in sera at day 67 of mice infected in (B) were measured by plaque assay. (D) Bone marrow chimeras reconstituted with 85% JH−/− (B cell-deficient) bone marrow and 15% WT, 3d, or Tlr7−/− bone marrow were bled at approximately six weeks and reconstitution of the B cell compartment was confirmed by flow cytometry. (E) Levels of LCMV-specific IgG in sera of chimeric mice were determined at day 30 p.i. with LCMV clone 13 by ELISA. Data in A–C are representative of two or more independent experiments. Data in D–E are representative of two independent sets of chimeric mice using either 3d or Tlr7−/− bone marrow. Dotted lines indicate levels of detection. For ELISA, if samples fell below the limit of detection they were assigned a value of 30, which was the level of least dilution. Each data point represents an individual mouse with a bar indicating the mean. Statistics were calculated using one-way ANOVA. *P < 0.05 and ***P < 0.001.
TLR7 is required in B cells for optimal LCMV-specific IgG responses
We previously hypothesized that nucleic acid-sensing TLRs may be required in a B cell-intrinsic manner to generate optimal antibody responses (12). We therefore sought to determine to what extent B cell-intrinsic TLR7 deficiency contributes to overall levels of LCMV-specific IgG, as other cell types, which may be involved in antibody responses, can also express TLR7 (2). To address the contribution of nucleic acid-sensing TLRs within B cells for LCMV-specific antibody responses, we generated bone marrow chimeras consisting of 85% JH−/− bone marrow, which cannot form B cells, and 15% WT, 3d, or Tlr7−/− bone marrow. Thus, B cells in these chimeric mice were derived entirely from the non- JH−/− bone marrow, with all other cell subsets being WT. Assessment of B cell reconstitution in these mice prior to experimentation exhibited similar proportions of CD19+B220+ cells, indicating that the B cell compartment was restored in all chimeric mice (Fig. 1D). When we assessed titers of LCMV-specific IgG at day 30 p.i. in chronically infected chimeric mice, we found that antibody levels in Tlr7−/− and 3d chimeric mice were both reduced approximately ten-fold compared to WT. Therefore, TLR7 deficiency solely within B cells is sufficient to reduce LCMV-specific IgG levels to a large extent.
T follicular helper cell differentiation is not impaired in the absence of TLR7
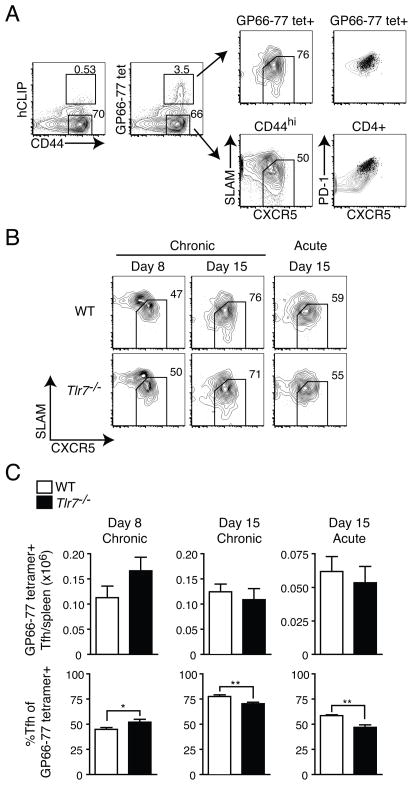
In addition to B cell-intrinsic defects in Tlr7−/− mice, the formation of Tfh cells, which help drive GC responses and the production of antibody, may also be defective. This is of particular interest in LCMV infection since chronic infection has been shown to drive the differentiation of CD4+ T cells into Tfh cells (11), and IL-6-dependent control of chronic LCMV infection was shown to be due to effects on Tfh cell differentiation (25). Furthermore, the expression of Bcl6 and differentiation of Tfh cells is driven early by dendritic cells and later by B cells, both of which may be impacted in the absence of TLR7 signaling (26–30). We therefore assessed the formation of Tfh cells in Tlr7−/− and WT mice during acute and chronic LCMV infection. We analyzed Tfh cell responses by assessing the formation of Tfh cells among the endogenous CD4+CD44hiGP66-77-tetramer+ population, using non-antigen specific hCLIP-tetramer as a negative control for our tetramer gating strategy (Fig. 2A). Tfh cells were identified as SLAMloCXCR5hiPD-1hi as previously described (27). We found that Tfh cell differentiation at days 8 and 15 during chronic infection, and day 15 during acute infection were not significantly impacted (Fig. 2B and C). The total numbers of endogenous Tfh cells per spleen were approximately equal in WT and Tlr7−/− mice regardless of infection or time point (Fig. 2C). Although the frequency of SLAMloCXCR5hi Tfh cells varied somewhat between WT and Tlr7−/− mice, it is unlikely that such small differences in frequency represent a major impediment to Tfh cell differentiation (Fig. 2B and C). Based on these results, it is unlikely that decreased antibody responses in Tlr7−/− mice are a result of insufficient numbers of Tfh cells in these mice.
Figure 2. Absence of TLR7 does not affect total Tfh cell numbers.
WT or Tlr7−/− mice were infected with LCMV Arm (acute) or clone 13 (chronic) and the numbers and frequency of endogenous GP66-77 tetramer+ SLAMloCXCR5+ Tfh cells were determined in spleens of infected mice at the indicated time points. (A) A sample gating strategy for identifying endogenous Tfh cells by flow cytometry is shown. Staining is shown for tetramer control (left); SLAMloCXCR5+ CD4+ T cells in GP66-77 tetramer+ versus CD44hi, non-tetramer+ populations; and the expression of PD-1 and CXCR5 in GP66-77 tetramer+SLAMloCXCR5+ (dot plots) overlaid on either all GP66-77 tetramer+ CD4+ T cells (right, top) or all CD4+ T cells (right, bottom) shown as contour plots. (B) Representative contour plots for Tfh cells are shown in WT and Tlr7−/− mice for the indicated time points during acute or chronic LCMV infection. Cells showing in plots were gated on CD4+CD44hiGP66-77 tetramer+ cells. (C) Numbers of CD44hiGP66-77 tetramer+ Tfh cells in spleens of WT and Tlr7−/− mice are shown (top). The frequencies of SLAMloCXCR5+ cells among the GP66-77 tetramer+ population are depicted (bottom). Data in B–C for LCMV Arm are from two experiments (n=4–6 mice per group). Data for LCMV clone 13 day 8 are from two experiments (n=5–6 mice per group) and for day 15 are from five experiments (n=11 mice per group). Statistics were calculated using two-tailed unpaired Student’s t-test. Graphs show mean ± SEM. *P < 0.05 and **P < 0.01.
B cell-intrinsic TLR7 signaling is required for efficient germinal center B cell and plasma cell formation
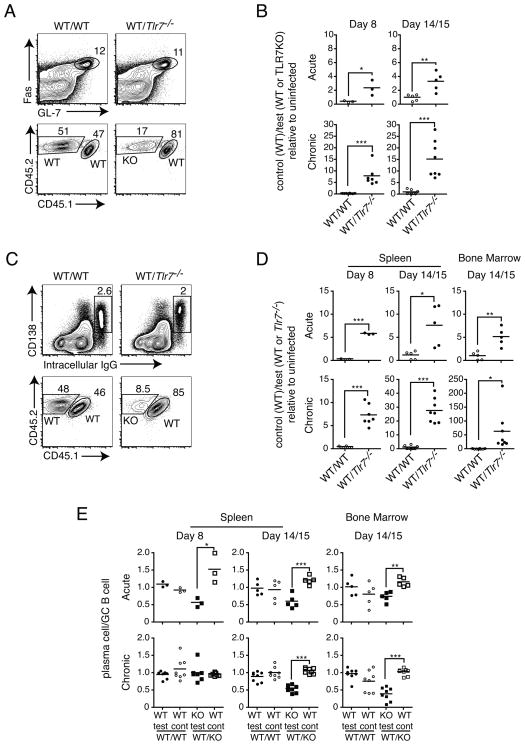
Because loss of TLR7 signaling within B cells alone was sufficient to substantially affect LCMV-specific IgG responses (Fig. 1E), we sought to address at what stage during the B cell response TLR7 was required. Briefly, following stimulation by T cells, B cells are recruited into the GC reaction during which GC B cells undergo iterative rounds of selection and proliferation leading to the production of long-lived plasma cells (31). In order to determine at what stage Tlr7−/− B cells are defective, and to isolate this defect to B cells, we generated 1:1 bone marrow chimeras in which WT mice were irradiated and reconstituted with 50% WT and 50% Tlr7−/− bone marrow marked by different CD45 congenic markers. As a control, 1:1 WT chimeras were also generated using WT bone marrow containing either CD45 congenic marker. Prior to immunization, the proportions of B220+ cells in blood derived from each donor population were determined. We first sought to assess the impact of TLR7 deficiency on the formation of GC B cells. At days 8 and 14/15 p.i. with LCMV Arm (acute) and clone 13 (chronic), we determined the frequencies of GC B cells derived from either WT or Tlr7−/− populations. We then calculated the ratios of WT (control) to WT (test) or Tlr7−/− (test) GC B cells and normalized it to the ratio within B220+ cells of the same non-immunized chimeric mice, such that the data are expressed as the fold change following immunization in the relative representation by cells from either congenic donor (Fig. 3A and B). In this case, no preference for WT (control) or Tlr7−/− would be represented by a value of 1. If WT cells constitute a larger proportion of the GC B cell population than Tlr7−/− cells, this would result in a value greater than 1. We found that at either day 8 or 14/15 p.i., there was a large preference in GC B cells derived from WT, as opposed to Tlr7−/− B cells in WT/Tlr7−/− chimeric mice (Fig. 3B). Additionally, the preference for WT-derived GC B cells increased over time in both acute and chronic infections, but to a much lesser extent during acute infection. Interestingly, the requirement for TLR7 was much greater in chronic compared to acute infection. For example at day 14 or 15 p.i., the proportion of WT cells increased three- to four-fold over that of uninfected mice following acute infection, but was increased fifteen-fold at this time point during chronic infection. The greater effect seen during chronic infection parallels the difference in LCMV-specific IgG antibody responses, which are more greatly affected during chronic infection (Fig. 1A and 1B) (12). By comparison, a relative increase in WT (CD45.1+) GC B cells was not seen in control WT/WT chimeras, indicating that this preference was not due to the difference in congenic markers used.
Figure 3. TLR7 signaling is required for effective GC B cell and plasma cell formation in mixed bone marrow chimeras.
WT/WT or WT/Tlr7−/− 1:1 bone marrow chimeras were infected with LCMV Arm (acute) or clone 13 (chronic) and the frequencies of GC B cells and plasma cells at days 8 and 15 p.i. from either donor were determined. (A) A representative example is shown for flow cytometric analysis of WT or Tlr7−/− donor-derived GC B cell populations. Cells depicted in contour plots showing GC B cells (top) were gated on CD4−B220+ cells. Later experiments gated on CD4−B220+IgD− cells, with similar results. Cells depicted in plots showing CD45.1 versus CD45.2 expression were gated on GC B cells (bottom). (B) The ratio of GC B cells derived from WT control to that derived from WT or Tlr7−/− test populations was determined and normalized to the B cell ratio in the same uninfected chimeric mouse to calculate the fold change relative to the WT control population. Results are shown for LCMV Arm (top) and clone 13 (bottom) for the indicated time points. (C) Sample contour plots to identify IgG-producing plasma cells in spleen are shown (top). Cells shown in plots were gated on IgD− B cells. CD45.1 versus CD45.2 expression identifying WT or Tlr7−/− donor-derived plasma cells is shown (bottom). (D) The fold change in WT control-derived IgG-producing plasma cells was calculated as described in (B) and determined at the indicated time points in spleen and bone marrow for Arm (top) or clone 13 (bottom) LCMV-infected chimeric mice. (E) In the same experiments as A–D, the proportions of WT or Tlr7−/− donor-derived plasma cells was normalized to the proportion of corresponding WT or Tlr7−/− donor-derived GC B cells in the same chimeric mouse for LCMV Arm (top) and clone 13 (bottom) infection. Data in A–E represent collective data from three sets of independently derived bone marrow chimeras (n=3 or more mice per group). Each data point represents an individual mouse with a bar denoting the mean. *P < 0.05, **P < 0.01 and ***P < 0.001.
We then examined the formation of plasmablasts and plasma cells in the same chimeras (Fig. 3C and D). We determined the fold change in WT (control) relative to WT (test) and Tlr7−/− populations in the same way as we did for GC B cells. Similarly to results seen for GC B cells, we found that the preference for WT over Tlr7−/− was again increased over time and to a higher degree in chronic compared to acute infection (Fig. 3D). We further noticed that the magnitude of the difference in requirement for TLR7 appeared to be greater for both the early phase plasmablasts (day 8) and the later stage plasma cells (day 14+) than for GC B cells, particularly for the formation bone marrow resident plasma cells during chronic infection. In order to address whether this was the case, we compared the frequencies of WT or Tlr7−/− plasmablasts and plasma cells to those of GC B cells in the same mouse. We hypothesized that if there were no defect in the ability of Tlr7−/− GC B cells to form plasma cells, then the WT/Tlr7−/− frequency distribution of plasmablasts or plasma cells and GC B cells in the same chimeric mice should be equivalent. Therefore, if no additional effects on plasma cell differentiation were observed, this would mean that the ratio of plasma cells to GC B cells would be the same and equal 1. However, if there were an additional defect, then the frequency of plasma cells for the same population should be lower than that of GC B cells, and therefore this ratio would be less than 1. Using this method of analysis, we determined that, as expected, there were no differences in WT/WT chimeras in the plasma cell/GC B cell ratio (Fig. 3D). However, in WT/ Tlr7−/− chimeras, Tlr7−/− GC B cells appeared to be less capable of forming plasma cells, with the exception of day 8 during chronic infection. These data indicate that TLR7 signaling is required intrinsically within B cells for the optimal formation of GC B cells, and further, for effective differentiation into plasma cells.
TLR7 signaling is crucial for the formation of late plasma cells, but not GC B cells in non-chimeric mice
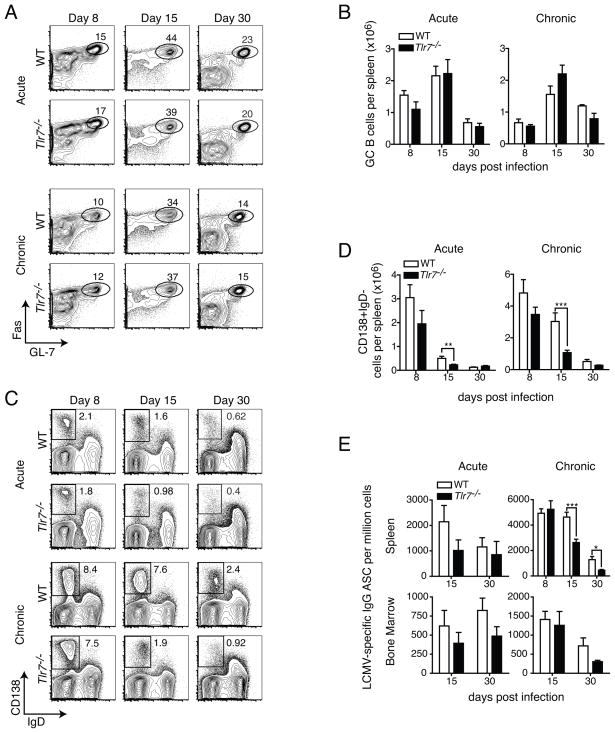
Because our experiments with chimeras indicated a significant intrinsic requirement for TLR7 in B cells for the efficient formation of GC B cells and plasma cells, we performed a more detailed analysis of the antibody response in Tlr7−/− mice in order to identify the nature of the defect. At days 8, 15 and 30 during acute or chronic LCMV infection, we analyzed the frequencies of GC B cells and plasma cells. Surprisingly, despite the severe defects in Tlr7−/− GC B cells, compared to competing WT B cells in mixed chimeric mice, we found no significant differences in the frequencies of GC B cells in spleens of WT or Tlr7−/− mice at any of the time points examined during either acute or chronic infection, although the percentage of GC B cells among IgD− cells was slightly lower at day 8 in chronic infection (Fig. 4A). We compared the total numbers of GC B cells in spleens of WT and Tlr7−/− mice during acute and chronic infection (Fig. 4B). For the most part, total numbers of GC B cells were similar between the two groups at all time points during acute and chronic infection, consistent with similar frequencies of GC B cells (Fig. 4A). Although total numbers of GC B cells at day 15 were slightly elevated in Tlr7−/− mice during chronic infection, this was not statistically significant. However, consistent with a slight reduction in frequency during chronic infection, numbers of GC B cells at day 8 p.i. in both groups of mice were reduced in chronic infection, compared to those in acute (Fig. 4B). These results indicate that although Tlr7−/− B cells are restricted in their ability to form GC B cells while in competition with WT B cells, an inability to form GC B cells in Tlr7−/− mice is unlikely the sole explanation for the severe defects in antibody production seen during chronic infection, although a slight lag in the formation of GC B cells, or qualitative differences in their functional interactions with other cells, may contribute.
Figure 4. TLR7 signaling is required for formation of plasma cells, but not GC B cells, in non-chimeric mice.
WT or Tlr7−/− mice were infected with LCMV Arm (acute) or clone 13 (chronic) and the frequencies and numbers of GC B cells and plasmablasts and plasma cells in spleens were determined. (A) Representative flow cytometry analysis of spleen GC B cells for acute or chronically infected WT and Tlr7−/− mice is shown for days 8, 15 and 30 p.i. Cells shown in plots were gated on CD4−B220+IgD− cells. (B) Numbers of GC B cells in spleens of WT and Tlr7−/− mice were calculated at the indicated time points following infection with LCMV Arm or clone 13. (C) Spleen plasma cell gating for the same mice as in (A) is shown. (D) For the same mice as in (C), total numbers of plasmablasts and plasma cells in spleen were calculated. (E) Numbers of LCMV-specific IgG secreting cells were enumerated in WT or Tlr7−/− mice during acute or chronic infection at the indicated time points. Data in A–E are compiled from two or more independent experiments (n=3–20 mice per group). Statistics were calculated using two-tailed unpaired Student’s t-test. Graphs show mean ± SEM. *P < 0.05, **P < 0.01 and ***P < 0.001.
In contrast to GC B cells, when we compared the plasmablast and plasma cell responses between WT and Tlr7−/− mice during acute and chronic infection, we found near WT levels of plasma cells at day 8 p.i. in Tlr7−/− mice, but by day 15 this decreased to about two-thirds of WT during acute infection, and about one-fourth of WT during chronic infection, and these differences were sustained at day 30 (Fig. 4C). Furthermore, the magnitude of the plasmablast and plasma cell response was larger during chronic infection, and was sustained to a greater extent than that during acute infection.
We assessed the numbers of plasma cells in WT and Tlr7−/− mice during acute and chronic infection. Consistent with the differences in frequencies, WT and Tlr7−/− mice had similar numbers of plasma cells at day 8 during the immune response, although there was small reduction in Tlr7−/− mice (Fig. 4D). However, by day 15, responses in Tlr7−/− mice were reduced approximately two-fold during acute and three-fold during chronic infection. Unlike acute infection, in which numbers were relatively similar at day 30, the reduction in splenic plasma cells in Tlr7−/− mice was also present at day 30 p.i. We also assessed numbers of LCMV-specific IgG-producing plasma cells in WT and Tlr7−/− mice by ELISpot assay (Fig. 4E). At day 8 p.i., the total numbers of IgG-producing plasma cells in spleen were relatively equivalent between WT and Tlr7−/− mice during chronic infection. However, by day 15 p.i., the presence of IgG-producing plasma cells in spleens of Tlr7−/− mice were reduced approximately two-fold, and this deficit continued to day 30 p.i. In contrast, and consistent with results from flow cytometric analysis (Fig. 4D), although numbers of LCMV-specific IgG-secreting cells in spleens were reduced about two-fold during acute infection at day 15 in Tlr7−/− mice, numbers were similar to those in WT mice by day 30 (Fig. 4E). In addition to defective plasma cell formation in the spleen at days 15 and 30 p.i., we also found lower numbers of LCMV-specific ASCs in bone marrow at these time points in Tlr7−/− mice, with the exception of day 15 during chronic infection, when little difference was observed. Therefore, although the loss of TLR7 significantly impacts the formation of plasma cells during both acute and chronic LCMV infections, this effect seems to be somewhat magnified during chronic infection in both the magnitude of the decline in numbers compared to WT mice, but also the duration for which it persists.
TLR signaling is important for GC B cell proliferation and plasma cell survival
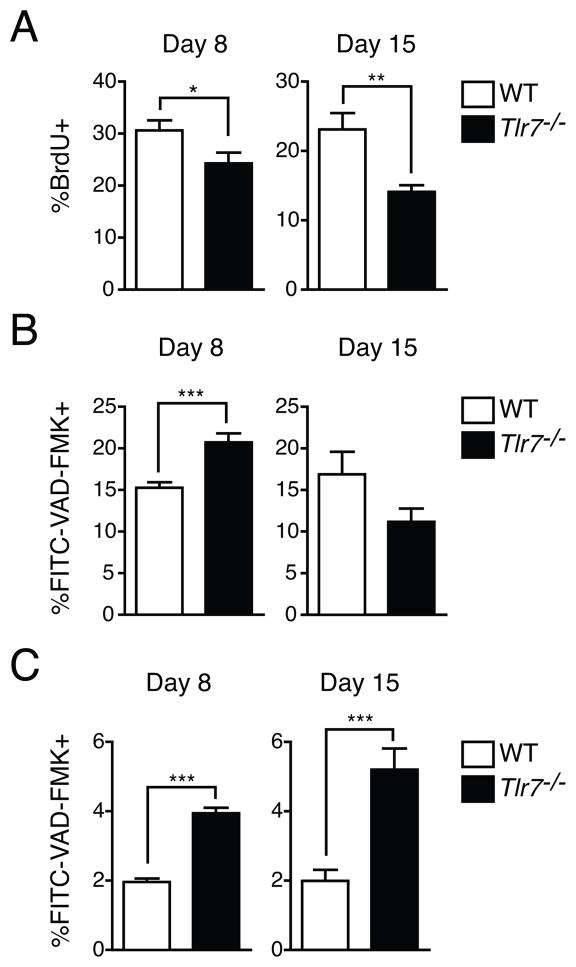
Although total numbers of spleen GC B cells were not affected in Tlr7−/−, relative to WT mice, the large reduction in plasma cell formation indicated that these cell populations might be qualitatively different with respect to survival or proliferation. We first analyzed cell proliferation in GC B cells by incorporation of BrdU following a 2 h pulse (22). While we found a small, approximately 5%, difference in BrdU incorporation between WT and Tlr7−/− GC B cells at day 8 p.i. during chronic infection, by day 15, approximately half the frequency of GC B cells incorporated BrdU in Tlr7−/−, relative to WT mice (Fig. 5A). These data indicate that chronic infection may drive differences in GC B cell proliferation between WT and Tlr7−/− GC B cells.
Figure 5. Decreased GC B cell proliferation and increased plasma cell death during chronic LCMV infection.
(A) Proliferation of GC B cells in spleens was measured by BrdU incorportation in WT or Tlr7−/− mice following infection with LCMV clone 13. The data is expressed as the frequency of GC B cells that incorporate BrdU during a 2 h pulse. (B) and (C) Caspase activation in GC B cells (B) and spleen plasmablasts and plasma cells (C) was measured by FITC-VAD-FMK, which binds to active caspases. The frequencies of GC B cells or CD138+IgD− plasmablasts and plasma cells that were marked by FITC-VAD-FMK are shown. Data in A–C are from three or more independent experiments (n=7–16 mice per group). Statistics were calculated using two-tailed unpaired Student’s t-test. Graphs show mean ± SEM. *P < 0.05, **P < 0.01 and ***P < 0.001.
We assessed cell death by using a fluorescent pan-caspase inhibitor that binds to activated caspases (22). When we analyzed the frequency of GC B cells that were labeled with this inhibitor, we observed a slight increase in Tlr7−/− GC B cells at day 8, but a small decrease at day 15 during chronic infection (Fig. 5B). Thus, as there seemed to be no consistent differences between WT and Tlr7−/− GC B cells in the frequency of cells with activated caspases, an increase in cell death by Tlr7−/− GC B cells is unlikely to lead to defective plasma cell formation. We then analyzed the frequency of CD138+IgD− plasmablasts and plasma cells that had caspase activation (Fig. 5C). Wheras only 1–2% of plasma cells at days 8 and 15 during chronic infection in WT mice were labeled positive for caspase activation, the frequency increased to approximately 4% in Tlr7−/− CD138+IgD− cells at day 8 and 5% at day 15. Therefore, the decrease in GC B cell proliferation in Tlr7−/− mice was associated temporally with defective plasma cell formation, with a larger decrease in GC B cell proliferation correlating with higher frequencies of plasmablasts and plasma cells with activated caspases.
TLR7 signaling affects the distribution of light zone and dark zone GC B cells
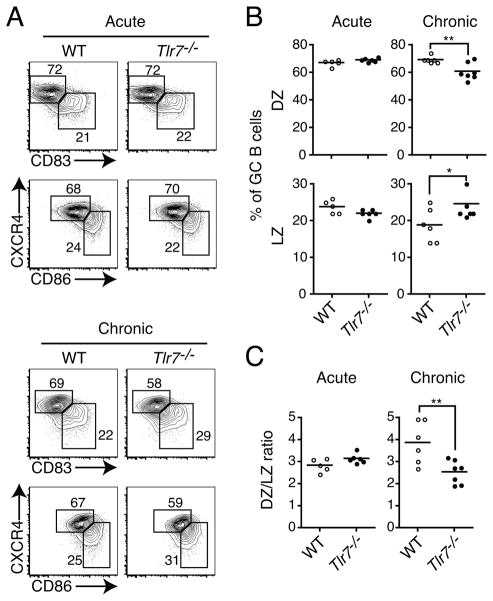
During antigen-driven selection in the GC, GC B cells are divided between a light zone (LZ), in which selection of high affinity B cells occurs, and the dark zone (DZ), during which positively selected GC B cells proliferate (31). Expression of the chemokine receptor CXCR4 is critical for localization of GC B cells to the DZ, and the expression of this receptor is associated with cell division (32, 33). Although the demarcation between LZ and DZ cells is difficult to do based solely on the expression of CXCR4 alone, additional use of the makers CD86 and CD83 has provided a useful tool for differentiating between these populations with LZ cells being CXCR4loCD86hiCD83hi and DZ cells being CXCR4hiCD86loCD83lo (34). Therefore, given the differences in proliferation between WT and Tlr7−/− GC B cells, we assessed whether the relative frequencies of GC B cells with phenotypic characteristics of DZ and LZ cells were different. At day 15 p.i. during acute LCMV infection, WT and Tlr7−/− GC B cells were similar in their frequencies of LZ and DZ GC B cells (Fig. 6A and B). In contrast, during chronic LCMV infection, the frequency distribution of LZ and DZ GC B cells in Tlr7−/− GC B cells relative to that in WT was skewed towards increased proportions of cells with phenotypic characteristics of LZ GC B cells (Fig. 6A and B). As expected from these results, the ratio of DZ to LZ GC B cells was equivalent in WT and Tlr7−/− mice at day 15 following acute infection, but was lower in Tlr7−/− GC B cells at this time during chronic infection (Fig. 6C). Consistent with the DZ being where GC B cell proliferation occurs, we found that BrdU+ GC B cells in both WT and Tlr7−/− mice expressed slightly higher levels of CXCR4 (data not shown). Therefore, consistent with our results that Tlr7−/− GC B cells proliferate to a lesser extent during the course of chronic LCMV infection (Fig. 5A), TLR7 signaling is important for driving proliferation of GC B cells in the DZ.
Figure 6. TLR7 deficiency alters GC B cell light zone/dark zone phenotype in chronic, but not acute infection.
WT and Tlr7−/− mice were infected with LCMV Arm (acute) or clone 13 (chronic) and the distribution GC B cells among light zone (LZ) and dark zone (DZ) populations at day 15 p.i. were assessed by flow cytometry. (A) Representative contour plots are shown for GC B cell LZ and DZ populations at day 15 following acute (top) or chronic (bottom) LCMV infection, using two different strategies. Cells shown in contour plots were gated on live (DAPI−), CD4−B220+IgD−Fas+GL-7+ cells. (B) Frequencies of LZ and DZ GC B cells. (C) Ratios of DZ to LZ GC B cells from mice in (A) were calculated. Data in A–C are from two to three independent experiments (n=5–7 mice per group). Statistics were calculated by using a two-tailed unpaired Student’s t-test. Data points on graphs represent individual mice with the means indicated by bars. *P < 0.05, **P < 0.01.
Discussion
In the present study, we demonstrate that B cell-intrinsic TLR7 signaling is required for optimal antibody responses during the course of both acute and chronic LCMV infection, but to a greater extent in chronic infection. In the absence of TLR7, mice infected with the chronic strain of LCMV failed to form an effective plasma cell response, though early plasmablast responses were relatively unimpaired. This defect was linked to an altered GC response in which Tlr7−/− GC B cells proliferated less and were skewed more towards cells with phenotypic characteristics of LZ rather than DZ GC B cells, with a slight increase in plasmablast and plasma cell caspase activation. These findings provide evidence that in the context of a chronic virus infection, TLR signaling in B cells augments plasma cell formation by alteration of the GC reaction.
Recently, both we and another group identified a necessity for nucleic acid-sensing TLRs in the control of infection with the chronic strain of LCMV, likely due to defective antibody responses in the absence of TLR signaling (12, 15), although we observed that this effect was not as significant during acute infection (12). Experiments using bone marrow chimeric mice revealed defective GC B cell formation during chronic infection in the absence of TLR7 signaling when in competition with WT cells (15). Examination of B cells from these chimeras also demonstrated that Tlr7−/− B cells were less capable of forming antibody-secreting cells. However, the role of TLR7 in antibody responses during acute infection, whether or not TLR7 signaling in B cells alone was sufficient for antibody responses, and whether other TLRs, such as TLR3, further contributed during chronic infection were not investigated. In this study, we demonstrate that TLR7, and not TLR3 is required for LCMV-specific antibody responses and clearance of chronic LCMV infection, and contributes to a lesser extent for antibody responses during acute infection. Despite LCMV forming dsRNA replication intermediates, it appears that signaling via TLR3 does not cooperate with TLR7, as Tlr3−/−Tlr7−/− and 3d mice were comparable to Tlr7−/− in virus load and antibody titer. Furthermore, when B cells alone were deficient in their ability to respond to TLR7 ligands, antibody responses were greatly decreased. These results were somewhat surprising given a recent study showing that immunization with multiple TLR ligands was synergistic in driving antibody responses when both were engaged on the same B cell (35), but may reflect the restriction of TLR3 expression among B cells to those of the marginal zone, and a limited role for TLR3 expression in cDCs for driving antibody responses (36). In addition, since the TLR3 and TLR7 double-deficient mice had an identical phenotype to the 3d mutant mice with regards to chronic LCMV infection, it is likely that the effect of Unc93b1 on shaping immune responses in this model is via its role in TLR signaling, as opposed to a separate role in antigen presentation (16).
We found that Tlr7−/− cells were severely impaired in their ability to form GC B cells when in competition with WT cells in both acute and chronic LCMV infection, with the differences being more exaggerated in the latter. Furthermore, our studies revealed that in the same chimeric mice, TLR7-deficient plasma cells were underrepresented relative to their proportion among GC B cells. Interestingly, the increase in preference for WT, over Tlr7−/− GC B cells and plasma cells increased over time, and was greater in chronically infected mice. Therefore, it seems as though chronic infection in particular exacerbated the need for TLR7 signaling in GC B cell and plasma cell development. Consistent with a subsequent defect in plasma cells in Tlr7−/− mice, we observed a slight increase in the frequency of plasma cells marked by a reagent that binds to activated caspases. However, as this was only 4–5% of all splenic plasma cells, it is unclear to what extent cell death alone is responsible for decreased plasma cell numbers in Tlr7−/− mice.
Although it was previously shown that Tlr7−/− mice had decreased frequencies of GC B cells relative to WT mice (15), we did not find significant differences in total numbers or GC B cells in spleens of Tlr7−/− versus WT mice, and minimal, if any, differences in frequencies. In contrast, despite robust formation of early plasmablasts at day 8 p.i., Tlr7−/− mice showed impaired formation of later stage plasma cells in both spleen and bone marrow, which was greater in both magnitude and duration in chronic, compared to acute, infection. This is similar to results seen in MyD88-deficient mice following infection with mouse polyoma virus (37). The effect of TLR7 on antibody responses and plasma cell formation was independent of an effect on endogenous Tfh cell numbers, despite recent studies demonstrating the importance of Tfh cells and IL-21, a cytokine produced by Tfh cells, for control of chronic LCMV infection (11, 25, 38–40). In light of a study demonstrating that administration of virus-like particles (VLPs) containing RNA was able to rescue antibody production by Il21r−/− B cells (41), through direct action on B cells in a MyD88-dependent manner (14), this may indicate that Tfh cells are necessary, but not sufficient, for optimal antibody responses during chronic LCMV infection when B cells lack TLR7. Alternatively, although Tfh cell numbers were unaffected, Tfh cells may be functionally impaired when B cells lack TLR7.
Upon investigation of the GC B cell response, TLR7 was shown to affect the proliferation, but not survival of GC B cells. This was due to a relative decrease in Tlr7−/− GC B cells with phenotypic characteristics of DZ relative to the LZ GC B cells. It has been previously reported that lipopolysaccharide (LPS) treatment of B cells increased proliferative responses of these cells when transferred in vivo, and that these cells preferentially localized to the DZ of GCs, compared to non-LPS-treated cells (42). However, these cells were treated in vitro prior to transfer, and LPS treatment was shown to up-regulate expression of homing receptors such as CCR7, L-selectin, CXCR4, and CXCR5, as well as expression of the GC B cell marker GL-7. Therefore, it was unclear whether or not TLR signaling is generally required for the DZ localization or phenotype, or if these results reflected an altered homing and activation status of these cells with respect to non-treated cells prior to transfer. Here, we demonstrate during the course of a chronic viral infection, a requirement for TLR7 for optimal GC B cell proliferation and normal proportion of cells with phenotypic characteristics of LZ versus DZ GC B cells.
How exactly this requirement for TLR7 signaling affects LZ versus DZ GC B cell formation is unclear, but could possibly be the result of changes in levels of genes differentially expressed between these subsets, such as those required for cell cycle entry (34). Furthermore, how this translates to the formation of plasma cells is unclear, but as proliferation and somatic hypermutation occur in the DZ (31), and long-lived plasma cells are preferentially selected from high-affinity GC B cells (43–45), decreased proliferation in Tlr7−/− GC B cells may result in a smaller pool of somatically hypermutated B cells that are less efficient than TLR7-sufficient cells at forming plasma cells. Although this may partly explain the exacerbated defect in chimeric mice, this likely does not account for defective responses in the absence of competition. Considering that Tlr7−/− plasma cell formation in chimeras was more affected than GC B cell formation, and the plasma cell and antibody defects in Tlr7−/− mice were more exacerbated than the defect in GC B cell proliferation, these results suggest that TLR7 signaling may additionally affect plasma cell output, possibly through survival or programming. Mechanistically, this may be through a qualitative difference in the ability of Tlr7−/− GC B cells to interact with and be positively selected by Tfh cells, which helps drive clonal expansion (34). Together, these results indicate that manipulation of TLR signaling in B cells may provide therapeutic benefit in generating antibody responses during immunization or as treatment for chronic viral infections.
Acknowledgments
We are grateful for the generosity of the following people: Matthias Wabl for JH−/− mice; Art Weiss and Richard Locksley for use of various equipment; Dirk Baumjohann and K. Mark Ansel for sharing of reagents; Christopher Allen for useful discussions and advice; and Anthony DeFranco and Lewis Lanier for useful discussions and critical reading of the manuscript.
J.M.C. was supported by a Graduate Research Fellowship from the National Science Foundation. M.M. was supported, in part, by the Rosalind Russell Medical Research Center for Arthritis and National Institutes of Health Grant 1RO1AI074694.
Footnotes
The authors have no conflicting financial interests.
References
- 1.Pulendran B, Ahmed R. Immunological mechanisms of vaccination. Nat Immunol. 2011;12:509–517. doi: 10.1038/ni.2039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Coffman RL, Sher A, Seder RA. Vaccine adjuvants: putting innate immunity to work. Immunity. 2010;33:492–503. doi: 10.1016/j.immuni.2010.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Querec TD, Akondy RS, Lee EK, Cao W, Nakaya HI, Teuwen D, Pirani A, Gernert K, Deng J, Marzolf B, Kennedy K, Wu H, Bennouna S, Oluoch H, Miller J, Vencio RZ, Mulligan M, Aderem A, Ahmed R, Pulendran B. Systems biology approach predicts immunogenicity of the yellow fever vaccine in humans. Nat Immunol. 2009;10:116–125. doi: 10.1038/ni.1688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ahmed R, Salmi A, Butler LD, Chiller JM, Oldstone MB. Selection of genetic variants of lymphocytic choriomeningitis virus in spleens of persistently infected mice. Role in suppression of cytotoxic T lymphocyte response and viral persistence. J Exp Med. 1984;160:521–540. doi: 10.1084/jem.160.2.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Battegay M, Moskophidis D, Rahemtulla A, Hengartner H, Mak TW, Zinkernagel RM. Enhanced establishment of a virus carrier state in adult CD4+ T-cell-deficient mice. J Virol. 1994;68:4700–4704. doi: 10.1128/jvi.68.7.4700-4704.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bergthaler A, Flatz L, Verschoor A, Hegazy AN, Holdener M, Fink K, Eschli B, Merkler D, Sommerstein R, Horvath E, Fernandez M, Fitsche A, Senn BM, Verbeek JS, Odermatt B, Siegrist CA, Pinschewer DD. Impaired antibody response causes persistence of prototypic T cell-contained virus. PLoS Biol. 2009;7:e1000080. doi: 10.1371/journal.pbio.1000080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Homann D, Tishon A, Berger DP, Weigle WO, von Herrath MG, Oldstone MB. Evidence for an underlying CD4 helper and CD8 T-cell defect in B-cell-deficient mice: failure to clear persistent virus infection after adoptive immunotherapy with virus-specific memory cells from muMT/muMT mice. J Virol. 1998;72:9208–9216. doi: 10.1128/jvi.72.11.9208-9216.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Matloubian M, Concepcion RJ, Ahmed R. CD4+ T cells are required to sustain CD8+ cytotoxic T-cell responses during chronic viral infection. J Virol. 1994;68:8056–8063. doi: 10.1128/jvi.68.12.8056-8063.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Whitmire JK, Asano MS, Kaech SM, Sarkar S, Hannum LG, Shlomchik MJ, Ahmed R. Requirement of B cells for generating CD4+ T cell memory. J Immunol. 2009;182:1868–1876. doi: 10.4049/jimmunol.0802501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zajac AJ, Blattman JN, Murali-Krishna K, Sourdive DJ, Suresh M, Altman JD, Ahmed R. Viral immune evasion due to persistence of activated T cells without effector function. J Exp Med. 1998;188:2205–2213. doi: 10.1084/jem.188.12.2205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fahey LM, Wilson EB, Elsaesser H, Fistonich CD, McGavern DB, Brooks DG. Viral persistence redirects CD4 T cell differentiation toward T follicular helper cells. J Exp Med. 2011;208:987–999. doi: 10.1084/jem.20101773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Clingan JM, Ostrow K, Hosiawa KA, Chen ZJ, Matloubian M. Differential roles for RIG-I-like receptors and nucleic acid-sensing TLR pathways in controlling a chronic viral infection. J Immunol. 2012;188:4432–4440. doi: 10.4049/jimmunol.1103656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Browne EP. Toll-like receptor 7 controls the anti-retroviral germinal center response. PLoS Pathog. 2011;7:e1002293. doi: 10.1371/journal.ppat.1002293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hou B, Saudan P, Ott G, Wheeler ML, Ji M, Kuzmich L, Lee LM, Coffman RL, Bachmann MF, DeFranco AL. Selective utilization of Toll-like receptor and MyD88 signaling in B cells for enhancement of the antiviral germinal center response. Immunity. 2011;34:375–384. doi: 10.1016/j.immuni.2011.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Walsh KB, Teijaro JR, Zuniga EI, Welch MJ, Fremgen DM, Blackburn SD, von Tiehl KF, Wherry EJ, Flavell RA, Oldstone MB. Toll-like receptor 7 is required for effective adaptive immune responses that prevent persistent virus infection. Cell Host Microbe. 2012;11:643–653. doi: 10.1016/j.chom.2012.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tabeta K, Hoebe K, Janssen EM, Du X, Georgel P, Crozat K, Mudd S, Mann N, Sovath S, Goode J, Shamel L, Herskovits AA, Portnoy DA, Cooke M, Tarantino LM, Wiltshire T, Steinberg BE, Grinstein S, Beutler B. The Unc93b1 mutation 3d disrupts exogenous antigen presentation and signaling via Toll-like receptors 3, 7 and 9. Nat Immunol. 2006;7:156–164. doi: 10.1038/ni1297. [DOI] [PubMed] [Google Scholar]
- 17.Alexopoulou L, Holt AC, Medzhitov R, Flavell RA. Recognition of double-stranded RNA and activation of NF-kappaB by Toll-like receptor 3. Nature. 2001;413:732–738. doi: 10.1038/35099560. [DOI] [PubMed] [Google Scholar]
- 18.Lund JM, Alexopoulou L, Sato A, Karow M, Adams NC, Gale NW, Iwasaki A, Flavell RA. Recognition of single-stranded RNA viruses by Toll-like receptor 7. Proc Natl Acad Sci U S A. 2004;101:5598–5603. doi: 10.1073/pnas.0400937101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen J, Trounstine M, Alt FW, Young F, Kurahara C, Loring JF, Huszar D. Immunoglobulin gene rearrangement in B cell deficient mice generated by targeted deletion of the JH locus. Int Immunol. 1993;5:647–656. doi: 10.1093/intimm/5.6.647. [DOI] [PubMed] [Google Scholar]
- 20.Johnston RJ, Poholek AC, DiToro D, Yusuf I, Eto D, Barnett B, Dent AL, Craft J, Crotty S. Bcl6 and Blimp-1 are reciprocal and antagonistic regulators of T follicular helper cell differentiation. Science. 2009;325:1006–1010. doi: 10.1126/science.1175870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wherry EJ, Blattman JN, Murali-Krishna K, van der Most R, Ahmed R. Viral persistence alters CD8 T-cell immunodominance and tissue distribution and results in distinct stages of functional impairment. J Virol. 2003;77:4911–4927. doi: 10.1128/JVI.77.8.4911-4927.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Good-Jacobson KL, Szumilas CG, Chen L, Sharpe AH, Tomayko MM, Shlomchik MJ. PD-1 regulates germinal center B cell survival and the formation and affinity of long-lived plasma cells. Nat Immunol. 2010;11:535–542. doi: 10.1038/ni.1877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Slifka MK, Matloubian M, Ahmed R. Bone marrow is a major site of long-term antibody production after acute viral infection. J Virol. 1995;69:1895–1902. doi: 10.1128/jvi.69.3.1895-1902.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Edelmann KH, Richardson-Burns S, Alexopoulou L, Tyler KL, Flavell RA, Oldstone MB. Does Toll-like receptor 3 play a biological role in virus infections? Virology. 2004;322:231–238. doi: 10.1016/j.virol.2004.01.033. [DOI] [PubMed] [Google Scholar]
- 25.Harker JA, Lewis GM, Mack L, Zuniga EI. Late interleukin-6 escalates T follicular helper cell responses and controls a chronic viral infection. Science. 2011;334:825–829. doi: 10.1126/science.1208421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Baumjohann D, Okada T, Ansel KM. Cutting Edge: Distinct waves of BCL6 expression during T follicular helper cell development. J Immunol. 2011;187:2089–2092. doi: 10.4049/jimmunol.1101393. [DOI] [PubMed] [Google Scholar]
- 27.Choi YS, Kageyama R, Eto D, Escobar TC, Johnston RJ, Monticelli L, Lao C, Crotty S. ICOS receptor instructs T follicular helper cell versus effector cell differentiation via induction of the transcriptional repressor Bcl6. Immunity. 2011;34:932–946. doi: 10.1016/j.immuni.2011.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kerfoot SM, Yaari G, Patel JR, Johnson KL, Gonzalez DG, Kleinstein SH, Haberman AM. Germinal center B cell and T follicular helper cell development initiates in the interfollicular zone. Immunity. 2011;34:947–960. doi: 10.1016/j.immuni.2011.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kitano M, Moriyama S, Ando Y, Hikida M, Mori Y, Kurosaki T, Okada T. Bcl6 protein expression shapes pre-germinal center B cell dynamics and follicular helper T cell heterogeneity. Immunity. 2011;34:961–972. doi: 10.1016/j.immuni.2011.03.025. [DOI] [PubMed] [Google Scholar]
- 30.Poholek AC, Hansen K, Hernandez SG, Eto D, Chandele A, Weinstein JS, Dong X, Odegard JM, Kaech SM, Dent AL, Crotty S, Craft J. In vivo regulation of Bcl6 and T follicular helper cell development. J Immunol. 2010;185:313–326. doi: 10.4049/jimmunol.0904023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Victora GD, Nussenzweig MC. Germinal centers. Annu Rev Immunol. 2012;30:429–457. doi: 10.1146/annurev-immunol-020711-075032. [DOI] [PubMed] [Google Scholar]
- 32.Allen CD, Ansel KM, Low C, Lesley R, Tamamura H, Fujii N, Cyster JG. Germinal center dark and light zone organization is mediated by CXCR4 and CXCR5. Nat Immunol. 2004;5:943–952. doi: 10.1038/ni1100. [DOI] [PubMed] [Google Scholar]
- 33.Allen CD, Okada T, Tang HL, Cyster JG. Imaging of germinal center selection events during affinity maturation. Science. 2007;315:528–531. doi: 10.1126/science.1136736. [DOI] [PubMed] [Google Scholar]
- 34.Victora GD, Schwickert TA, Fooksman DR, Kamphorst AO, Meyer-Hermann M, Dustin ML, Nussenzweig MC. Germinal center dynamics revealed by multiphoton microscopy with a photoactivatable fluorescent reporter. Cell. 2010;143:592–605. doi: 10.1016/j.cell.2010.10.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kasturi SP, Skountzou I, Albrecht RA, Koutsonanos D, Hua T, Nakaya HI, Ravindran R, Stewart S, Alam M, Kwissa M, Villinger F, Murthy N, Steel J, Jacob J, Hogan RJ, Garcia-Sastre A, Compans R, Pulendran B. Programming the magnitude and persistence of antibody responses with innate immunity. Nature. 2011;470:543–547. doi: 10.1038/nature09737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gururajan M, Jacob J, Pulendran B. Toll-like receptor expression and responsiveness of distinct murine splenic and mucosal B-cell subsets. PLoS One. 2007;2:e863. doi: 10.1371/journal.pone.0000863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Guay HM, Andreyeva TA, Garcea RL, Welsh RM, Szomolanyi-Tsuda E. MyD88 is required for the formation of long-term humoral immunity to virus infection. J Immunol. 2007;178:5124–5131. doi: 10.4049/jimmunol.178.8.5124. [DOI] [PubMed] [Google Scholar]
- 38.Elsaesser H, Sauer K, Brooks DG. IL-21 is required to control chronic viral infection. Science. 2009;324:1569–1572. doi: 10.1126/science.1174182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Frohlich A, Kisielow J, Schmitz I, Freigang S, Shamshiev AT, Weber J, Marsland BJ, Oxenius A, Kopf M. IL-21R on T cells is critical for sustained functionality and control of chronic viral infection. Science. 2009;324:1576–1580. doi: 10.1126/science.1172815. [DOI] [PubMed] [Google Scholar]
- 40.Yi JS, Du M, Zajac AJ. A vital role for interleukin-21 in the control of a chronic viral infection. Science. 2009;324:1572–1576. doi: 10.1126/science.1175194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bessa J, Kopf M, Bachmann MF. Cutting edge: IL-21 and TLR signaling regulate germinal center responses in a B cell-intrinsic manner. J Immunol. 2010;184:4615–4619. doi: 10.4049/jimmunol.0903949. [DOI] [PubMed] [Google Scholar]
- 42.Hwang IY, Park C, Harrison K, Kehrl JH. TLR4 signaling augments B lymphocyte migration and overcomes the restriction that limits access to germinal center dark zones. J Exp Med. 2009;206:2641–2657. doi: 10.1084/jem.20091982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Paus D, Phan TG, Chan TD, Gardam S, Basten A, Brink R. Antigen recognition strength regulates the choice between extrafollicular plasma cell and germinal center B cell differentiation. J Exp Med. 2006;203:1081–1091. doi: 10.1084/jem.20060087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Phan TG, Paus D, Chan TD, Turner ML, Nutt SL, Basten A, Brink R. High affinity germinal center B cells are actively selected into the plasma cell compartment. J Exp Med. 2006;203:2419–2424. doi: 10.1084/jem.20061254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Smith KG, Light A, Nossal GJ, Tarlinton DM. The extent of affinity maturation differs between the memory and antibody-forming cell compartments in the primary immune response. EMBO J. 1997;16:2996–3006. doi: 10.1093/emboj/16.11.2996. [DOI] [PMC free article] [PubMed] [Google Scholar]