Trastuzumab, a monoclonal antibody against the receptor encoded by the HER2 protooncogene, is the mainstay of treatment for patients with HER2 gene amplified breast cancer. Main mechanisms of action of the antibody are antibody-dependent, cell mediated cytotoxicity (ADCC) with reconstitution of adoptive immunity to HER2,(1, 2) downregulation of HER2 from the cell surface,(3) and disruption of ligand-independent HER2-HER3 dimers, thus partially inhibiting post-receptor PI3K signaling.(4) Despite its effectiveness in both the adjuvant and metastatic settings, therapeutic resistance to trastuzumab remains an important clinical problem. A number of preclinical studies have proposed several molecular mechanisms whereby tumors can evade the action of trastuzumab. These mechanisms include engagement of alternate signaling pathways, alterations in antibody binding to HER2, loss of the apoptotic response, or evasion of the immune modulatory effects conferred by trastuzumab; in several cases, analysis of cohorts of patients treated with trastuzumab has suggested that at least some of these mechanisms are operative in vivo.(5) Signaling to PI3K-Akt as a result of HER2-HER3 dimerization is the most important survival pathway downstream of HER2.(4, 6) Several lines of evidence suggest that inhibition of PI3K-Akt is critical for the antitumor effect of HER2-directed therapies. Indeed, several of the proposed mechanisms of resistance to trastuzumab involve persistence or reactivation of PI3K signaling via alternative amplified RTKs and/or mutations in PI3K pathway components, thus suggesting that direct and more sustained inhibition of PI3K might be a strategy to overcome or prevent resistance to trastuzumab or other HER2 inhibitors.
There are a number of therapeutic inhibitors of PI3K/AKT currently in clinical development. Several preclinical studies suggest that trastuzumab resistance is overcome by PI3K pathway inhibitors.(4, 7, 8) Inhibition of nodes in the PI3K pathway downstream of AKT may also prove to be an effective and potentially less toxic strategy. For example, there is emerging clinical evidence that blockade of mTOR downstream of HER2 with the TORC1 inhibitor everolimus in combination with trastuzumab and a taxane can induce significant clinical responses in patients with metastatic HER2+ breast cancer who had previously progressed on trastuzumab therapy.(9, 10) Thus, the recent study by Chakrabarty et al.(11) is timely and of interest because it provides further insights into why maximal inhibition of PI3K is required for optimal inhibition of HER2 positive breast cancer cells. This work also raises some important considerations for the future clinical development of PI3K inhibitors in this subtype of breast cancer that will be discussed herein.
In this study, the authors used the ATP-competitive, pan-PI3K (p110) inhibitor, XL147, to inhibit PI3K in several HER2 gene-amplified trastuzumab-resistant cell lines. These included the Herceptin-resistant HR5 and HR6 cells that escape trastuzumab action by upregulation of EGFR and HER3 ligands,(12) and two cell lines with somatic genetic alterations in the PI3K pathway: HCC1569 cells, with deletion of PTEN (phosphatase and tensin homolog), and the HCC1954 and SUM190 cells, both with `hotspot' activating mutations in PIK3CA, the gene encoding the p110α catalytic subunit of PI3K. PTEN is the lipid phosphatase that dephosphorylates PIP3, the product of PI3K activity. Both `hotspot' mutations in PIK3CA confer p110α with increased catalytic activity over that of the wild type enzyme.(13, 14) Thus, loss of PTEN and PIK3CA mutations amplify PI3K signaling beyond a level conferred by HER2 overexpression alone and, as a result, counteract the action of trastuzumab and other HER2 inhibitors. In several retrospective studies, aberrant activation of PI3K as defined by either of these alterations, i.e., PTEN loss or PIK3CA mutation, statistically correlated with decreased benefit from trastuzumab in patients with metastatic HER2+ breast cancer.(15–19) Treatment with the PI3K inhibitor XL147 prevented growth and/or induced apoptosis in all trastuzumab-resistant cells, thus confirming their dependence on PI3K. Even though trastuzumab alone had no effect, combining trastuzumab with the PI3K inhibitor resulted in additive effects compared to XL147 alone.
Induction of apoptosis of primary breast tumors after neoadjuvant trastuzumab, as measured by cleaved caspase-3 immunohistochemisty, has been reported previously.(20) The current study provides mechanistic insights into how HER2 function is connected to apoptosis by exploring differences between antibody-sensitive and resistant cells. The study first noted that survivin, a member of the inhibitor of apoptosis family of proteins (IAPs), was the only apoptosis-related protein modulated upon treatment with the combination of XL147 and trastuzumab. In antibody-sensitive cells, survivin is downregulated by trastuzumab alone whereas in resistant cells, addition of a PI3K inhibitor to trastuzumab is required to achieve such effect on survivin levels. In this case, blockade of PI3K/AKT inhibits the phosphorylation of FoxO factors which, in turn, translocate to the nucleus where they repress the transcription of survivin. Further, modulation of FoxO function using dominant-negative or constitutively active FoxO mutants uncoupled survivin from PI3K-signaling. An interesting aspect of these studies is the demonstration that downregulation of survivin was sufficient to restore sensitivity to trastuzumab in drug-resistant cells.
Another interesting finding from this work was the observation that treatment of trastuzumab-resistant lines with PI3K inhibitors reduced their cancer stem cell (CSC) fraction. These CSCs or tumor initiating cells are hypothesized to be resistant to therapy and thus able to repopulate the tumor after treatment, potentially accounting for cancer recurrences.(21) Therefore, strategies that eliminate CSCs may overcome drug resistance and prevent cancer relapses. In trastuzumab-sensitive HER2 gene-amplified tumors, the antibody has been proposed to target this CSC fraction.(22, 23) In the resistant cells used in this report, treatment with XL147 but not trastuzumab reduced CSCs as measured by mammosphere formation, ALDH activity and IL-8 expression. Again, the combination of trastuzumab with the PI3K inhibitor was more effective in some cases even though trastuzumab itself had little effect. Derepression of FoxO-mediated transcription also explained the effects of IL-8. Knockdown via siRNA of FoxO3a upregulated IL-8 mRNA levels as well as mammosphere formation. Survivin also played a role in maintenance of the cancer stem cell fraction as downregulation of survivin with siRNAs decreased mammosphere formation and ALDH activity.
Next, this study tested the combination of trastuzumab + XL147 in athymic mice with established trastuzumab-resistant human tumors. In two different resistant xenografts, treatment with trastuzumab and the PI3K inhibitor blocked PI3K signaling, reduced ALDH1 and IL-8, both markers of stem-like cells, and survivin levels. Finally, the authors explored the role of survivin in patients with HER2+ breast cancer treated with neoadjuvant trastuzumab plus chemotherapy. Survivin mRNA levels in tumors were significantly reduced after treatment in the 5 of 13 patients who exhibited a clinical response. Further, in tumors of patients who did not have a response, survivin mRNA levels were statistically higher than in patients who responded.
In summary, this work is the first to elucidate molecular mechanisms of combined inhibition of HER2+ breast cancer cells with trastuzumab and a PI3K inhibitor. This research also demonstrates a link between HER2, PI3K signaling, tumor cell apoptosis, and cancer stem-like cell maintenance through FoxO-mediated suppression of survivin and IL-8. We speculate that both of these cellular effects, induction of apoptosis and elimination of CSCs, may explain the ability of (one year) adjuvant trastuzumab to completely eradicate micrometastases and potentially induce cures. During early therapy, the major effect of trastuzumab may be to elicit an apoptotic and anti-signaling responses whereas longer therapy is required to eradicate the smaller more intrinsically refractory CSC fraction.
This preclinical study also raises some questions regarding the clinical development of combinations of PI3K and HER2 antagonists in HER2+ breast cancer. Should PI3K and HER2 inhibitors be combined up front or should the PI3K inhibitor be added at the time of progression on trastuzumab? Would a p110α-specific inhibitor be superior to a pan-PI3K inhibitor in this setting? Should all patients be treated with PI3K inhibitors in combination with trastuzumab? Or should this combinatorial approach be reserved for those patients with HER2+ tumors that also harbor mutations in the PI3K pathway? For what duration should the PI3K inhibitor be used in combination with trastuzumab? Perhaps the mechanistic insights provided by this study can assist in the answers to these questions.
First, the study noted that even in antibody-sensitive cells, addition of the PI3K inhibitor resulted in a greater magnitude of response compared to trastuzumab alone. This may not be surprising given the importance of sustained inhibition of PI3K signaling that is required for the antitumor effect of trastuzumab.(4, 6) Another rationale for the simultaneous use of both inhibitors is the ability of trastuzumab to interfere with the enhanced HER2-HER3 dimerization resulting from compensatory FoxO-dependent upregulation of HER3 mRNA and protein that has been reported following the inhibition of PI3K-Akt.(24, 25) In preclinical studies, trastuzumab is only a partial inhibitor of HER2-HER3 signaling to PI3K. Hence, not surprisingly, clinical studies have shown superior efficacy of `double HER2 blockade' with combinations of trastuzumab with lapatinib or trastuzumab with pertuzumab, compared to lapatinib or trastuzumab alone, respectively.(26, 27) Obviously, the tolerability and efficacy of a HER2-PI3K targeting strategy will require testing in the clinic. If toxicities limit such a combination, data in this report would support a sequential strategy where addition of a PI3K inhibitor at the time of progression on trastuzumab can restore sensitivity to the antibody. This would be in line with controlled clinical trials showing the merits of continuing trastuzumab at the time of progression.(27–29) An alternative approach that may also avoid the toxicity of a pan-PI3K inhibitor would be use of a p110α isoform-specific inhibitor. Recent studies in the HER2/neu transgenic mouse model of HER2+ breast cancer demonstrated the requirement for p110α for cancer growth and progression and showed that selective targeting of p110α but not p110β was effective at blocking growth of the transgenic tumors.(30)
Ideally, only patients who are likely to develop resistance to trastuzumab should be selected for treatment with the HER2-PI3K targeted combination. However, at the present time, biomarkers that predict de novo or acquired resistance are unknown. The PI3K-FOXO-survivin mechanism outlined in this study suggests that measurement of pretreatment survivin levels may aid in identifying patients who are less likely to respond to trastuzumab. Survivin expression alone has been shown to correlate with poorer outcome in breast cancer,(31) and survivin is one of 16 genes in the 21-gene Oncotype DX recurrence score used for risk stratification of patients with ER positive HER2 negative breast cancer.(32) We speculate survivin expression in addition to biomarkers of aberrant PI3K activation such as PIK3CA mutations and PTEN loss should provide a more robust predictor than any parameter alone.
Finally, the reduction in the tumor initiating cell fraction upon treatment with XL147 and trastuzumab raises the question of whether these inhibitors should be tested in the adjuvant setting as well as the metastatic setting. We speculate that by targeting the stem cell fraction by adding a PI3K antagonist to adjuvant therapy, the duration of adjuvant trastuzumab could be reduced while maintaining or even improving its efficacy. This will be a difficult question to answer directly given the required size of a clinical trial to test such a question. One alternative might be to test whether the rate of pathological complete response after neoadjuvant anti-HER2 therapy is improved by the addition of a PI3K inhibitor. The Neo-ALTTO study showed an improvement in the rate of pathological complete response using the combination of trastuzumab with lapatinib versus either drug alone,(33) so it would be feasible to compare the effect of dual blockade with or without a PI3K inhibitor in this setting. Indeed, the success of a therapeutic combination identified in the neoadjuvant setting may serve as a surrogate predictive of long term clinical benefit.(34)
In conclusion, the data presented in this paper underscore the importance of PI3K signaling in HER2-mediated tumor progression. The results extend our understanding of how the HER2-PI3K axis modulates downstream effectors of apoptosis and maintenance of tumor-initiating cells. Important questions remains as to how to best incorporate PI3K inhibitors into the treatment of HER2+ breast cancer but there is an increasing consensus they should be considered as an integral part of HER2-targeted combinations.
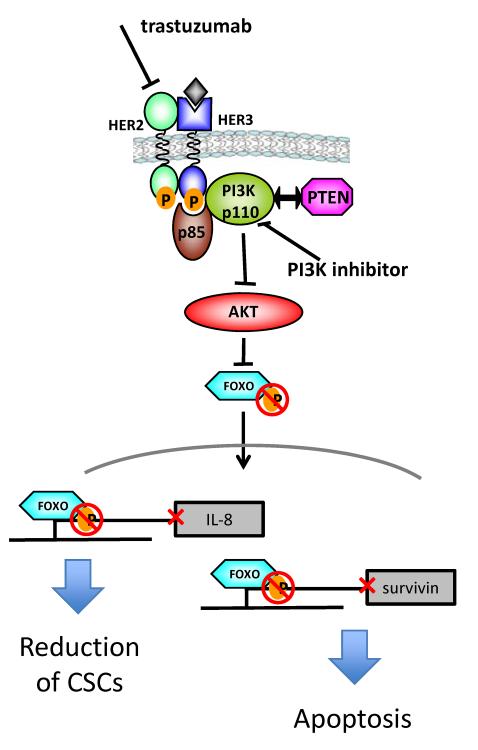
Figure. Inhibition of the HER2-PI3K-FOXO-survivin axis by trastuzumab and PI3K inhibitors.
Blockade of HER2 by trastuzumab along with PI3K inhibitors in trastuzumab-sensitive and -resistant cells results in inactivation of Akt. This results in hypophosphorylation of FOXO transcription factors which, in turn, translocate to the nucleus where they repress transcription of IL-8 and survivin. The end result is loss of maintenance of cancer stem cells and antiapoptotic proteins such as survivin.
Acknowledgments
Supported by NIH K08 CA143153, R01 grant CA80195, Vanderbilt Breast Cancer SPORE grant P50CA98131 and Vanderbilt-Ingram Cancer Center support grant P30CA68485
Footnotes
Disclaimers, conflict of interest: None
REFERENCES
- 1.Arnould L, Gelly M, Penault-Llorca F, Benoit L, Bonnetain F, Migeon C, Cabaret V, Fermeaux V, Bertheau P, Garnier J, Jeannin JF, Coudert B. Trastuzumab-based treatment of HER2-positive breast cancer: an antibody-dependent cellular cytotoxicity mechanism? Br J Cancer. 2006 Jan 30;94(2):259–67. doi: 10.1038/sj.bjc.6602930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Clynes RA, Towers TL, Presta LG, Ravetch JV. Inhibitory Fc receptors modulate in vivo cytotoxicity against tumor targets. Nat Med. 2000 Apr;6(4):443–6. doi: 10.1038/74704. [DOI] [PubMed] [Google Scholar]
- 3.Cuello M, Ettenberg SA, Clark AS, Keane MM, Posner RH, Nau MM, Dennis PA, Lipkowitz S. Downregulation of the erbB-2 receptor by trastuzumab (herceptin) enhances tumor necrosis factor-related apoptosis-inducing ligand-mediated apoptosis in breast and ovarian cancer cell lines that overexpress erbB-2. Cancer Res. 2001 Jun 15;61(12):4892–900. [PubMed] [Google Scholar]
- 4.Junttila TT, Akita RW, Parsons K, Fields C, Lewis Phillips GD, Friedman LS, Sampath D, Sliwkowski MX. Ligand-independent HER2/HER3/PI3K complex is disrupted by trastuzumab and is effectively inhibited by the PI3K inhibitor GDC-0941. Cancer Cell. 2009 May 5;15(5):429–40. doi: 10.1016/j.ccr.2009.03.020. [DOI] [PubMed] [Google Scholar]
- 5.Rexer BN, Arteaga CL. Intrinsic and acquired resistance to HER2-targeted therapies in HER2 geneamplified breast cancer: mechanisms and clinical implications. Crit Rev Oncog. 2012;17(1):1–16. doi: 10.1615/critrevoncog.v17.i1.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yakes FM, Chinratanalab W, Ritter CA, King W, Seelig S, Arteaga CL. Herceptin-induced inhibition of phosphatidylinositol-3 kinase and Akt Is required for antibody-mediated effects on p27, cyclin D1, and antitumor action. Cancer Res. 2002 Jul 15;62(14):4132–41. [PubMed] [Google Scholar]
- 7.Eichhorn PJA, Gili M, Scaltriti M, Serra V, Guzman M, Nijkamp W, Beijersbergen RL, Valero V, Seoane J, Bernards R, Baselga J. Phosphatidylinositol 3-kinase hyperactivation results in lapatinib resistance that is reversed by the mTOR/phosphatidylinositol 3-kinase inhibitor NVP-BEZ235. Cancer Res. 2008 Nov 15;68(22):9221–30. doi: 10.1158/0008-5472.CAN-08-1740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Serra V, Markman B, Scaltriti M, Eichhorn PJ, Valero V, Guzman M, Botero ML, Llonch E, Atzori F, Di Cosimo S, Maira M, Garcia-Echeverria C, Parra JL, Arribas J, Baselga J. NVP-BEZ235, a dual PI3K/mTOR inhibitor, prevents PI3K signaling and inhibits the growth of cancer cells with activating PI3K mutations. Cancer Res. 2008 Oct 1;68(19):8022–30. doi: 10.1158/0008-5472.CAN-08-1385. [DOI] [PubMed] [Google Scholar]
- 9.Andre F, Campone M, O'Regan R, Manlius C, Massacesi C, Sahmoud T, Mukhopadhyay P, Soria JC, Naughton M, Hurvitz SA. Phase I study of everolimus plus weekly paclitaxel and trastuzumab in patients with metastatic breast cancer pretreated with trastuzumab. J Clin Oncol. 2010 Dec 1;28(34):5110–5. doi: 10.1200/JCO.2009.27.8549. [DOI] [PubMed] [Google Scholar]
- 10.Morrow PK, Wulf GM, Ensor J, Booser DJ, Moore JA, Flores PR, Xiong Y, Zhang S, Krop IE, Winer EP, Kindelberger DW, Coviello J, Sahin AA, Nunez R, Hortobagyi GN, Yu D, Esteva FJ. Phase I/II study of trastuzumab in combination with everolimus (RAD001) in patients with HER2-overexpressing metastatic breast cancer who progressed on trastuzumab-based therapy. J Clin Oncol. 2011 Aug 10;29(23):3126–32. doi: 10.1200/JCO.2010.32.2321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chakrabarty A, Bhola NE, Sutton C, Ghosh R, Kuba MG, Dave B, Chang JC, Arteaga CL. Trastuzumab-Resistant Cells Rely on a HER2-PI3K-FoxO-Survivin Axis and Are Sensitive to PI3K Inhibitors. Cancer Res. 2013 Feb 1;73(3):1190–200. doi: 10.1158/0008-5472.CAN-12-2440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ritter CA, Perez-Torres M, Rinehart C, Guix M, Dugger T, Engelman JA, Arteaga CL. Human breast cancer cells selected for resistance to trastuzumab in vivo overexpress epidermal growth factor receptor and ErbB ligands and remain dependent on the ErbB receptor network. Clin Cancer Res. 2007 Aug 15;13(16):4909–19. doi: 10.1158/1078-0432.CCR-07-0701. [DOI] [PubMed] [Google Scholar]
- 13.Burke JE, Perisic O, Masson GR, Vadas O, Williams RL. Oncogenic mutations mimic and enhance dynamic events in the natural activation of phosphoinositide 3-kinase p110alpha (PIK3CA) Proc Natl Acad Sci U S A. 2012 Sep 18;109(38):15259–64. doi: 10.1073/pnas.1205508109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhao L, Vogt PK. Helical domain and kinase domain mutations in p110alpha of phosphatidylinositol 3-kinase induce gain of function by different mechanisms. Proc Natl Acad Sci U S A. 2008 Feb 19;105(7):2652–7. doi: 10.1073/pnas.0712169105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Berns K, Horlings HM, Hennessy BT, Madiredjo M, Hijmans EM, Beelen K, Linn SC, Gonzalez-Angulo AM, Stemke-Hale K, Hauptmann M, Beijersbergen RL, Mills GB, van de Vijver MJ, Bernards R. A functional genetic approach identifies the PI3K pathway as a major determinant of trastuzumab resistance in breast cancer. Cancer Cell. 2007 Oct 1;12(4):395–402. doi: 10.1016/j.ccr.2007.08.030. [DOI] [PubMed] [Google Scholar]
- 16.Chandarlapaty S, Sakr RA, Giri D, Patil S, Heguy A, Morrow M, Modi S, Norton L, Rosen N, Hudis C, King TA. Frequent Mutational Activation of the PI3K-AKT Pathway in Trastuzumab-Resistant Breast Cancer. Clin Cancer Res. 2012 Dec 15;18(24):6784–91. doi: 10.1158/1078-0432.CCR-12-1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Esteva FJ, Guo H, Zhang S, Santa-Maria C, Stone S, Lanchbury JS, Sahin AA, Hortobagyi GN, Yu D. PTEN, PIK3CA, p-AKT, and p-p70S6K status: association with trastuzumab response and survival in patients with HER2-positive metastatic breast cancer. Am J Pathol. 2010 Oct;177(4):1647–56. doi: 10.2353/ajpath.2010.090885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Razis E, Bobos M, Kotoula V, Eleftheraki AG, Kalofonos HP, Pavlakis K, Papakostas P, Aravantinos G, Rigakos G, Efstratiou I, Petraki K, Bafaloukos D, Kostopoulos I, Pectasides D, Kalogeras KT, Skarlos D, Fountzilas G. Evaluation of the association of PIK3CA mutations and PTEN loss with efficacy of trastuzumab therapy in metastatic breast cancer. Breast Cancer Res Treat. 2011 Jul;128(2):447–56. doi: 10.1007/s10549-011-1572-5. [DOI] [PubMed] [Google Scholar]
- 19.Wang L, Zhang Q, Zhang J, Sun S, Guo H, Jia Z, Wang B, Shao Z, Wang Z, Hu X. PI3K pathway activation results in low efficacy of both trastuzumab and lapatinib. BMC Cancer. 2011;11:248. doi: 10.1186/1471-2407-11-248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mohsin SK, Weiss HL, Gutierrez MC, Chamness GC, Schiff R, Digiovanna MP, Wang CX, Hilsenbeck SG, Osborne CK, Allred DC, Elledge R, Chang JC. Neoadjuvant trastuzumab induces apoptosis in primary breast cancers. J Clin Oncol. 2005 Apr 10;23(11):2460–8. doi: 10.1200/JCO.2005.00.661. [DOI] [PubMed] [Google Scholar]
- 21.Liu S, Wicha MS. Targeting breast cancer stem cells. J Clin Oncol. 2010 Sep 1;28(25):4006–12. doi: 10.1200/JCO.2009.27.5388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Korkaya H, Paulson A, Iovino F, Wicha MS. HER2 regulates the mammary stem/progenitor cell population driving tumorigenesis and invasion. Oncogene. 2008 Oct 16;27(47):6120–30. doi: 10.1038/onc.2008.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Magnifico A, Albano L, Campaner S, Delia D, Castiglioni F, Gasparini P, Sozzi G, Fontanella E, Menard S, Tagliabue E. Tumor-initiating cells of HER2-positive carcinoma cell lines express the highest oncoprotein levels and are sensitive to trastuzumab. Clin Cancer Res. 2009 Mar 15;15(6):2010–21. doi: 10.1158/1078-0432.CCR-08-1327. [DOI] [PubMed] [Google Scholar]
- 24.Chakrabarty A, Sanchez V, Kuba MG, Rinehart C, Arteaga CL. Feedback upregulation of HER3 (ErbB3) expression and activity attenuates antitumor effect of PI3K inhibitors. Proc Natl Acad Sci U S A. 2012 Feb 21;109(8):2718–23. doi: 10.1073/pnas.1018001108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chandarlapaty S, Sawai A, Scaltriti M, Rodrik-Outmezguine V, Grbovic-Huezo O, Serra V, Majumder PK, Baselga J, Rosen N. AKT inhibition relieves feedback suppression of receptor tyrosine kinase expression and activity. Cancer Cell. 2011 Jan 18;19(1):58–71. doi: 10.1016/j.ccr.2010.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Baselga J, Cortes J, Kim SB, Im SA, Hegg R, Im YH, Roman L, Pedrini JL, Pienkowski T, Knott A, Clark E, Benyunes MC, Ross G, Swain SM. Pertuzumab plus trastuzumab plus docetaxel for metastatic breast cancer. N Engl J Med. 2012 Jan 12;366(2):109–19. doi: 10.1056/NEJMoa1113216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Blackwell KL, Burstein HJ, Storniolo AM, Rugo HS, Sledge G, Aktan G, Ellis C, Florance A, Vukelja S, Bischoff J, Baselga J, O'Shaughnessy J. Overall survival benefit with lapatinib in combination with trastuzumab for patients with human epidermal growth factor receptor 2-positive metastatic breast cancer: final results from the EGF104900 Study. J Clin Oncol. 2012 Jul 20;30(21):2585–92. doi: 10.1200/JCO.2011.35.6725. [DOI] [PubMed] [Google Scholar]
- 28.von Minckwitz G, Schwedler K, Schmidt M, Barinoff J, Mundhenke C, Cufer T, Maartense E, de Jongh FE, Baumann KH, Bischoff J, Harbeck N, Luck HJ, Maass N, Zielinski C, Andersson M, Stein RC, Nekljudova V, Loibl S. Trastuzumab beyond progression: overall survival analysis of the GBG 26/BIG 3-05 phase III study in HER2-positive breast cancer. Eur J Cancer. 2011 Oct;47(15):2273–81. doi: 10.1016/j.ejca.2011.06.021. [DOI] [PubMed] [Google Scholar]
- 29.Cortes J, Fumoleau P, Bianchi GV, Petrella TM, Gelmon K, Pivot X, Verma S, Albanell J, Conte P, Lluch A, Salvagni S, Servent V, Gianni L, Scaltriti M, Ross GA, Dixon J, Szado T, Baselga J. Pertuzumab monotherapy after trastuzumab-based treatment and subsequent reintroduction of trastuzumab: activity and tolerability in patients with advanced human epidermal growth factor receptor 2-positive breast cancer. J Clin Oncol. 2012 May 10;30(14):1594–600. doi: 10.1200/JCO.2011.37.4207. [DOI] [PubMed] [Google Scholar]
- 30.Utermark T, Rao T, Cheng H, Wang Q, Lee SH, Wang ZC, Iglehart JD, Roberts TM, Muller WJ, Zhao JJ. The p110alpha and p110beta isoforms of PI3K play divergent roles in mammary gland development and tumorigenesis. Genes Dev. 2012 Jul 15;26(14):1573–86. doi: 10.1101/gad.191973.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ryan BM, Konecny GE, Kahlert S, Wang HJ, Untch M, Meng G, Pegram MD, Podratz KC, Crown J, Slamon DJ, Duffy MJ. Survivin expression in breast cancer predicts clinical outcome and is associated with HER2, VEGF, urokinase plasminogen activator and PAI-1. Ann Oncol. 2006 Apr;17(4):597–604. doi: 10.1093/annonc/mdj121. [DOI] [PubMed] [Google Scholar]
- 32.Paik S, Tang G, Shak S, Kim C, Baker J, Kim W, Cronin M, Baehner FL, Watson D, Bryant J, Costantino JP, Geyer CE, Jr., Wickerham DL, Wolmark N. Gene expression and benefit of chemotherapy in women with node-negative, estrogen receptor-positive breast cancer. J Clin Oncol. 2006 Aug 10;24(23):3726–34. doi: 10.1200/JCO.2005.04.7985. [DOI] [PubMed] [Google Scholar]
- 33.Baselga J, Bradbury I, Eidtmann H, Di Cosimo S, de Azambuja E, Aura C, Gomez H, Dinh P, Fauria K, Van Dooren V, Aktan G, Goldhirsch A, Chang TW, Horvath Z, Coccia-Portugal M, Domont J, Tseng LM, Kunz G, Sohn JH, Semiglazov V, Lerzo G, Palacova M, Probachai V, Pusztai L, Untch M, Gelber RD, Piccart-Gebhart M. Lapatinib with trastuzumab for HER2-positive early breast cancer (NeoALTTO): a randomised, open-label, multicentre, phase 3 trial. Lancet. 2012 Feb 18;379(9816):633–40. doi: 10.1016/S0140-6736(11)61847-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Prowell TM, Pazdur R. Pathological complete response and accelerated drug approval in early breast cancer. N Engl J Med. 2012 Jun 28;366(26):2438–41. doi: 10.1056/NEJMp1205737. [DOI] [PubMed] [Google Scholar]