Abstract
E3 ubiquitin ligase Cbl-b is critical for establishing the threshold for T cell activation, and is essential for induction of T cell anergy. Recent studies suggest that Cbl-b is involved in the development of inducible CD4+CD25+ regulatory T cells (iTregs). In this study, we report that the optimal induction of Foxp3 by naïve CD4+CD25− T cells requires suboptimal TCR triggering. In the absence of Cbl-b, the TCR strength for optimal Foxp3 induction is down-regulated in vitro. Using TCR transgenic Rag−/− mice in combination with Cbl-b deficiency, we show that in vivo iTreg development is also controlled by Cbl-b via tuning the TCR strength. Furthermore, we show that Akt-2 but not Akt-1 regulates Foxp3 expression downstream of Cbl-b. Therefore, we demonstrate that Cbl-b regulates the fate of iTregs via controlling the threshold for T cell activation.
INTRODUCTION
CD4+CD25+ regulatory T cells (Tregs) are vital for keeping the immune system in check, which helps to avoid immune-mediated pathology and unrestricted expansion of effector T cell populations (1). It has been shown that naïve CD4+CD25− T cells, which do not express Foxp3, can be converted into CD4+CD25+Foxp3+ inducible Tregs (iTregs) in the presence of TGF-β in vitro (2). Furthermore, CD4+CD25+Foxp3+ Tregs can also be induced in TCR transgenic/Rag−/− mice, which do not have naturally-occurring Tregs (nTregs), upon chronic Ag stimulation (2-6). The conversion of CD4+CD25− T cells into CD4+CD25+ iTregs appears to be negatively regulated by PI3K/Akt/mTOR or Foxo1/3a pathway (7-10).
Casitas-B-lineage lymphoma protein-b (Cbl-b), an E3 ubiquitin ligase, is a gatekeeper involved in maintaining the balance between immunity and tolerance (11-13). Indeed, we previously demonstrated that CD28 and CTLA-4 tightly regulate Cbl-b expression which is critical for establishing the threshold for T cell activation and tolerance (14, 15). Although Cbl-b may potentiate TGF-β-induced conversion of naïve CD4+CD25− T cells into CD4+CD25+Foxp3+ iTregs (9, 16), whether T cell activation threshold controlled by Cbl-b regulates this process is unknown. Furthermore, as previous studies used the same dose of anti-CD3 to induce iTregs in vitro (9, 16), it remains to be determined whether defective iTreg generation in the absence of Cbl-b represents an artifact of the different thresholds for T cell activation. In this study, we utilized both in vitro and in vivo iTreg differentiation protocols to assess the role of Cbl-b in this process. Our data indicate that Cbl-b facilitates iTreg generation, and this process is achieved by tuning the threshold for T cell activation. We also first demonstrate that Akt-2 but not Akt-1 is involved in the regulation of Foxp3 expression downstream of Cbl-b.
Materials and Methods
Mice
BALB/c, C57BL/6 (B6), Akt-1−/−, Akt-2−/−, Scurf, DO11.10, and Rag-1−/− mice were purchased from The Jackson Laboratory (Barr Harbor, ME). Cbl-b−/− mice described previously (11) were obtained from Dr. Josef M. Penninger (University of Toronto, ON, Canada), and have been backcrossed onto BALB/c background for 14 generations. DO11.10.Rag-1−/−, DO11.10.Rag-1−/−Scurf and DO11.10.Rag-1−/−Cbl-b−/− mice were generated by extensive crossing. Cbl-b−/− mice (B6 background) were crossed onto Akt-1−/− and Akt-2−/− mice, respectively, to generate Cbl-b+/−Akt-1+/− and Cbl-b+/−Akt-2+/− mice which were then inter-crossed to generate Cbl-b−/−Akt-1−/− and Cbl-b−/−Akt-2−/− mice. Foxp3gfp reporter mice (17) were obtained from Dr. Alexander Rudensky (University of Washington; Seattle, WA), and have been crossed onto Cbl-b−/− mice to generate Cbl-b−/− Foxp3gfp mice. All experimental protocols followed NIH Guidelines and were approved by Institutional Animal Care and Use Committees of the University of Chicago and The Ohio State University. All the mice were used for experiments at ages of 6-10 wks.
Reagents
The following reagents were obtained from BD Biosciences (San Jose, CA): recombinant mouse IL-2 (rmIL-2), purified anti-CD3 (Clone 145-2C11), hamster IgG isotypic control, FITC-anti-murine CD25 (Clone 7D4), PE-anti-CD25 (Clone PC61), and APC-anti-mouse CD4 (clone RM4-5). FITC- or PE-Cy7-anti-Foxp3 (Clone FJK-16s), and the respective isotypic control mAbs were obtained from eBioscience (San Diego, CA). Recombinant human TGF-β1 was purchased from R&D Systems (Minneapolis, MN). LY294002, Akti1/2, 124029 Akt Inhibitor XII (Akt-2i), IKKγ NEMO bounding domain (NBD) inhibitory peptide (SC-514), and rapamycin were purchased from Calbiochem (San Diego, CA).
Cell Preparation
Naïve CD4+ T cells were purified from spleens using a mouse CD4+CD62L+ isolation kit II (Miltenyi Biotec.; Auburn, CA). Naïve CD4+CD25− T cells were further purified as previously described (18). The purity of naïve CD4+CD25− T cells was >95%. T cell-depleted splenocytes of BALB/c or B6 mice (irradiated, 3,000 Rad) were used as APCs. CD4+CD25−GFP− T cells from Foxp3gfp and Cbl-b−/−Foxp3gfp mice were purified by FACS sorting. Untouched naïve mature B cells were purified from mouse spleens using B cell isolation kit (Miltenyi Biotec.), and further purified by FACS sorting using a FACSAria II cell sorter (BD Biosciences).
In vitro induction of CD4+CD25+Foxp3+ iTregs from naïve CD4+CD25− T cells
Naïve CD4+CD25− T cells from WT BALB/c or B6, Cbl-b−/− (BALB/c and B6 backgrounds), Akt-1−/−, Cbl-b−/−Akt-1−/−, or Akt-2−/−, Cbl-b−/−Akt-2−/− mice, or Foxp3gfp or Cbl-b−/−Foxp3gfp mice were plated in 96-well plates coated with anti-CD3 and soluble anti-CD28 with 100 U/ml rmIL-2, 2.5 ng/ml TGF-β1 (R&D Systems). Naïve CD4+CD25− T cells from DO11.10.Rag-1−/− and DO11.10.Rag-1−/− mice were incubated with T-depleted BALB/c splenocytes as APCs pulsed with different doses of OVA323-339 peptide in the presence of TGF-β and IL-2. Cells were harvested 72 h later and stained for CD4, CD25, and Foxp3. For an in vitro suppression assay, CD4+CD25+GFP+ cells from the above culture were sorted by FACS and cultured with naïve CD4+CD25− T cells from WT B6 mice in the presence of anti-CD3 and anti-CD28 for 72 h. Alternatively, CD4+CD25+ T cells were isolated from WT and Cbl-b−/− mice, and cultured with naïve CD4+CD25− T cells from DO11.10.Rag-1−/− and DO11.10.Rag-1−/−Cbl-b−/− mice in the presence of T-depleted WT APCs and OVA323-339 peptide for 72 h. T cell proliferation was determined by [3H]thymidine incorporation.
Induction of iTregs in vivo and allergic airway inflammation
Oral tolerance to OVA was induced by offering to the animals, ad libitum, 0.1% and 1 % OVA (grade II; Sigma-Aldrich, St. Louis, MO) solution dissolved in drinking water for 5 consecutive days (4). To induce allergic airway inflammation, DO11.10.Rag-1−/− and DO11.10.Rag-1−/−Cbl-b−/− mice adoptively transferred (i.v.) with highly purified mature B cells (5 × 106/mouse) were immunized by i.p. injection of OVA323-339 peptide (667 μg/ml, peptide 2.0; Chantilly, VA) adsorbed to 2 mg of an aqueous solution of aluminum hydroxide and magnesium hydroxide (Imject®Alum; Thermo Scientific, Rockford, IL) on day 0. Challenge of OVA323-339 (10 μg in 50 μl PBS) was given on day 14 and day 21 intranasally. The mice were sacrificed and assessed for allergic inflammation of the lungs 24 h after the last aerosol exposure. Bronchoalveolar lavage (BAL) fluid was collected at the sacrifice, and the cell differentials in BAL fluid were assessed as described (19). Serum IgE level was measured by ELISA.
Western blotting analysis
Naïve CD4+CD25− T cells from WT, Akt-1−/−, and Akt-2−/−, or WT, Cbl-b−/−, Akt-1−/−, Cbl-b−/− Akt-1−/−, Akt-2−/−, and Cbl-b−/−Akt-2−/− mice were stimulated with anti-CD3 for time-points as indicated, and lysed. The cell lysates were blotted with phospho-Abs against Foxo1 and Foxo3a. The membranes were stripped and reprobed with Akt-1, Akt-2, Cbl-b, and actin. For detection of Akt activation, Naïve CD4+CD25− T cells from WT and Cbl-b−/− mice were pretreated with or without LY294002, and stimulated with anti-CD3. The cell lysates were blotted with anti-phospho-Akt (Ser473), and reprobed with anti-Akt. Quantitative reprentations of the results are shown as relative band intensities measured by The Li-Cor Odyssey® Infrared Imaging System (Li-Cor; Lincoln, NE).
Statistical analysis
A two-tailed Student’s t-test was applied for statistical comparison of two groups or, where appropriate, a Mann-Whitney test for nonparametric data (airway inflammation scoring). A P value of 0.05 or less was considered significant.
RESULTS
The threshold for T cell activation regulated by Cbl-b determines the fate of iTreg development
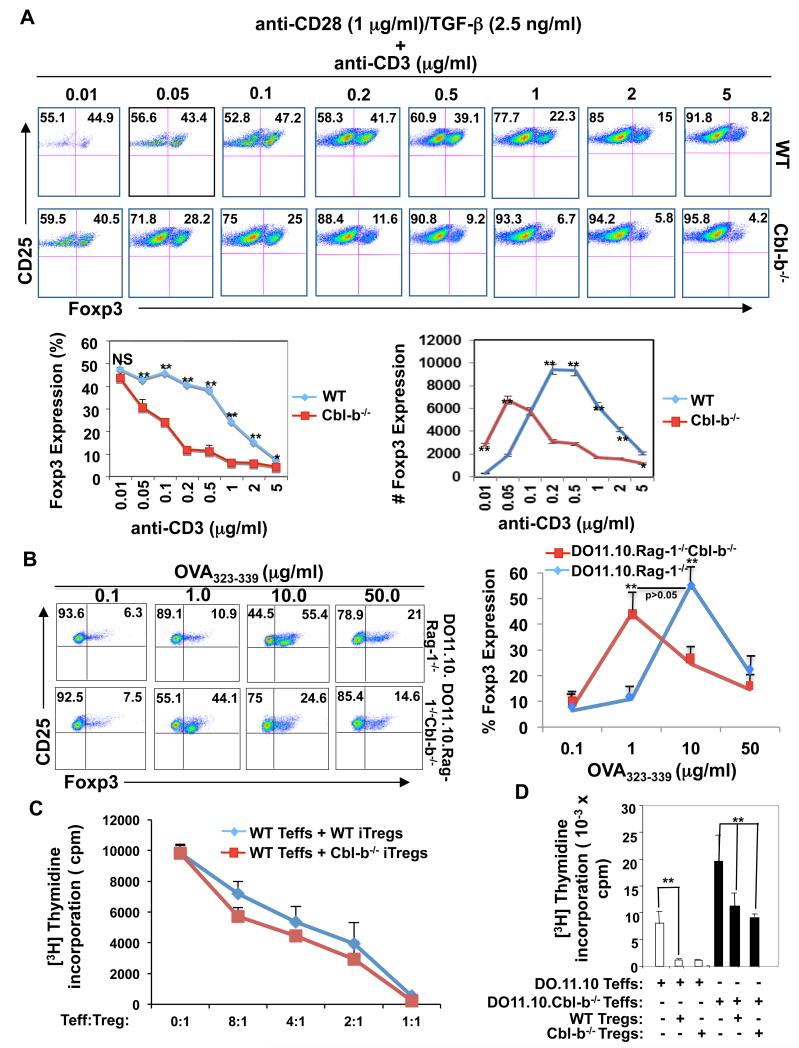
As it is well established that Cbl-b controls the threshold for T cell activation (14, 15), and regulates the affinity of antigen to their TCR (20), we decided to examine whether T cell activation threshold regulated by Cbl-b determines the fate of iTregs. To test this hypothesis, we stimulated naïve wild type (WT) and Cbl-b−/− CD4+CD25− T cells with different concentrations of anti-CD3 (0.01 to 5 μg/ml) and constant anti-CD28 (1 μg/ml) in the presence of TGF-β (2.5 ng/ml). Surprisingly, anti-CD3 at 0.01 μg/ml induced a significant iTreg population from naïve Cbl-b−/− CD4+CD25− T cells as did 0.05-0.5 μg/ml of anti-CD3 in naïve WT CD4+CD25− T cells (Fig. 1A). Increasing anti-CD3 concentration led to a dose-dependent reduction of iTregs converted from both WT and Cbl-b−/− CD4+CD25− T cells, however this reduction occurred at 0.05 μg/ml of anti-CD3 for Cbl-b−/− T cells but at 1-5 μg/ml for WT T cells (Fig. 1A). To verify this in a more physiological setting, we crossed Cbl-b−/− mice with DO11.10 transgenic mice which express CD4+ TCR specific for OVA323-339 peptide on a Rag-1−/− background. Placing a TCR transgene on the Rag-1−/− background results in all peripheral T cells expressing single Ag specificity without detectable expression of the transcription factor Foxp3 (4), which are devoid of the confounding effects of Cbl-b function in nTregs. Consistent with the data presented in Fig.1A, the absence of Cbl-b shifted the TCR strength required for iTreg conversion to much lower levels (Fig. 1B). Therefore, our data indicate that the impaired iTreg generation in vitro in the absence of Cbl-b is due to the lower threshold for T cell activation through the TCR.
FIGURE 1.
T cell activation threshold regulated by Cbl-b determines the fate of iTreg development in vitro. (A) Naïve CD4+CD25− T cells from WT and Cbl-b−/− mice were stimulated with anti-CD28 (1 μg/ml) and TGF-β (2.5 ng/ml) in the presence of increasing amounts of anti-CD3 for 72 h, and CD4+CD25+Foxp3+ T cells were determined by flow cytometry. (B) Naïve CD4+CD25− T cells from DO11.10.Rag-1−/− and DO11.10.Rag-1−/− Cbl-b−/− mice were stimulated with different doses of OVA323-339 peptide in the presence of BALB/c splenocytes depleted of T cells as APCs with or without TGF-β for 72 h. Foxp3 expression was determined by flow cytmetry. (C) Naïve CD4+CD25−GFP− T cells from Foxp3gfp or Cbl-b−/−Foxp-3gfp mice were stimulated as above. After three days, GFP+ cells were purified by FACS sorting, and co-cultured them with CD4+CD25− T cells from WT mice. T cell proliferation was determined by [3H]thymidine incorporation. (D) Naïve CD4+CD25− T cells from DO11.10.Rag-1−/− or DO11.10.Rag-1−/−Cbl-b−/− mice were stimulated by OVA323-339 (10 μg/ml) with irradiated WT T-depleted spleen cells in the presence or absence of WT or Cbl-b−/− CD4+CD25+ Tregs (the ratio of Teffs to Tregs was 2:1) for 72 h, and T cell proliferation was determined by [3H] thymidine incorporation. Student’s t test, *p<0.05 and **p<0.001, compared the same time-point of % and/or # of WT vs. Cbl-b−/− iTregs, or compared the difference in in vitro suppressive activity of WT and Cbl-b Tregs toward WT Teffs. The data are one representative of three independent experiments.
To assess the role of Cbl-b on the suppressive activity of iTregs, we isolated CD4+CD25−GFP− T cells from Foxp3gfp mice and Cbl-b−/−Foxp3gfp mice, and stimulated them with anti-CD3 and anti-CD28 in the presence of TGF-β. We found that iTregs from Cbl-b−/−Foxp3gfp mice suppressed the proliferation of WT T cells in a manner similar to iTregs from Foxp3gfp mice (Fig. 1C). Our data indicate that Cbl-b deficiency does not impair the function of iTregs. The discrepancy between our findings and those described previously may be due to the purity of iTreg isolation (9). We generated iTregs from naïve CD4+CD25−GFP− T cells of Cbl-b−/−Foxp3gfp mice, and iTregs were isolated by FACS sorting. Note that although WT and Cbl-b−/− iTregs could suppress in vitro CD4+CD25− T effector cell (Teff) proliferation, Cbl-b−/− Teffs are resistant to inhibition by both WT and Cbl-b−/− Tregs (Fig. 1D), consistent with the previous report (21).
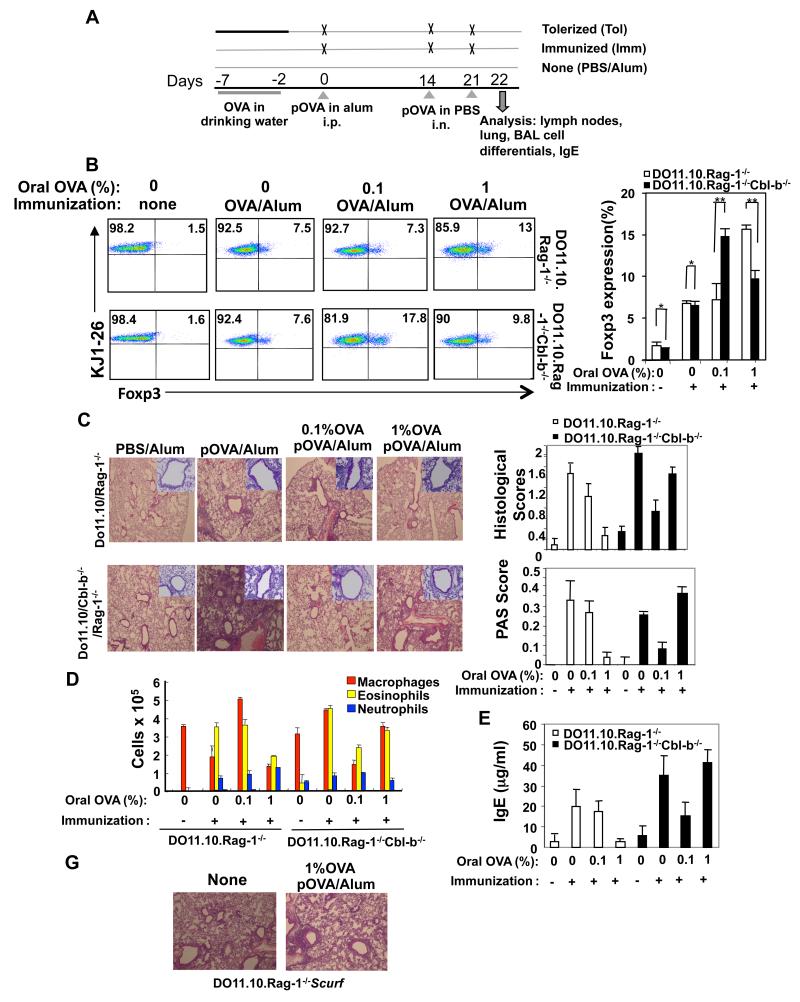
To establish that T cell activation threshold regulated by Cbl-b controls iTreg development in an in vivo setting, we generated DO11.10.Rag-1−/−Cbl-b−/− mice. As it was reported that iTregs can be induced in DO11.10.Rag-1−/− mice by oral feeding of OVA Ag (4), we fed DO11.10.Rag-1−/− and DO11.10.Rag-1−/−Cbl-b−/− mice (transferred with highly purified mature B cells from BALB/c mice) with 0.1% or 1% OVA solution dissolved in drinking water for 5 consecutive days (Fig. 2A). The transfer of mature B cells to Rag-1−/− mice allows the recipients to develop a Th2-mediated Ab response which is critical for allergic airway responses (4). As predicted, feeding DO11.10.Rag-1−/− mice with 0.1% OVA in the drinking water did not induce a significant population of KJ1-26+Foxp3+ T cells compared to DO11.10.Rag-1−/− mice without oral feeding with OVA but with OVA/alum immunization. However, the 0.1% OVA oral dose did elicit a population of KJ1-26+Foxp3+ T cells in DO11.10.Rag-1−/−Cbl-b−/− mice which was comparable with 1% OVA feeding in DO11.10.Rag-1−/− mice (Fig. 2B).
FIGURE 2.
The threshold for T cell activation controlled by Cbl-b dominates the fate of in vivo generation of iTreg which regulates allergic airway inflammation. (A) Tolerization protocol and experimental groups. DO11.10.Rag-1−/− and DO11.10.Rag-1−/−Cbl-b−/− mice (n=5) were intravenously injected with highly purified mature B cells (5 × 106/mouse), and then pretreated with different doses of OVA in drinking water (0.1% and 1 %, respectively) for 5 days. Mice were then immunized by intraperitoneal (i.p.) injection of OVA in alum (day 0). At 14 and 21 days after immunization, mice were challenged with 10 mg OVA intranasally (i.n). Analysis was performed one day after the final i.n. OVA exposure. No untreated mice represented. (B) KJ1-26+CD4+Foxp3+ T cells in lymph nodes were determined by flow cytometry. (C) Airway inflammation and mucus production in OVA-sensitized DO11.10.Rag-1−/− and DO11.10.Rag-1−/−Cbl-b−/− mice determined by H&E staining (upper panel) and PAS staining (lower panel). Original magnification, × 40 (H&E); × 100 (PAS). Semi-quantitative analysis of the severity of peribronchial inflammation and the abundance of PAS-positive mucus-containing cells was performed. (D) The inflammatory cells from BAL fluid were assessed. (E) Serum IgE was detected by ELISA. (G) DO11.10.Rag-1−/− and DO11.10. Rag-1−/−Scurf mice were orally fed with 1% OVA, followed by OVA323-339 in alum immunization, and challenged as in A. Lung inflammation was analyzed. The data shown are one representative of three independent experiments.
To monitor the function of newly-generated iTregs, we used an allergic airway inflammation model (4). We immunized the mice which received oral feeding with 0.1% or 1 % OVA with OVA323-339 in alum. To elicit an asthma-like inflammatory response, mice were challenged with OVA on day 14 and 21 after i.p. immunization (4). Analysis of serum IgE, BAL cell differentials, and lung inflammation was performed 1 day after the respiratory challenge. Oral feeding of DO11.10.Rag-1−/− mice with 1% but not 0.1% OVA inhibited airway inflammation, accumulation of eosinophils in the BAL fluid, and higher titers of serum IgE, whereas this inhibition occurred at 0.1% OVA feeding in DO11.10.Rag-1−/−Cbl-b−/− mice (Fig. 2C-E). Note that although significant inhibition of airway inflammation and serum IgE was observed in DO11.10.Rag-1−/−Cbl-b−/− mice fed 0.1% OVA in drinking water, this inhibition was not as effective when observed in DO11.10.Rag-1−/− mice fed 1% OVA in drinking water. This is possibly due to hyper-activation of effector T cells in DO11.10.Rag-1−/−Cbl-b−/− mice, which were resistant to suppression by CD4+CD25+ Tregs (Fig. 1D). Intriguingly, feeding DO11.10.Rag-1−/−Cbl-b−/− mice with 1% OVA in drinking water, impaired iTreg generation (Fig. 2B), which was associated with heightened airway inflammation, increased BAL eosinophils, and serum IgE (Fig. 2C-E). To further verify that the suppression of airway inflammation by oral feeding with OVA was mediated by iTregs, we generated DO11.10.Rag-1−/−Scurf mice. These mice carry a point mutation of Foxp3 which abrogates the expression of Foxp3 (22). Feeding these mice orally with 1% OVA did not prevent severe airway inflammation (Fig. 2G). Our data strongly indicate that Cbl-b indeed regulates the threshold for T cell activation, which determines the fate of iTregs in vivo and controls allergic airway inflammation.
Cbl-b regulates iTreg development via an Akt-but not NF-κB-dependent manner
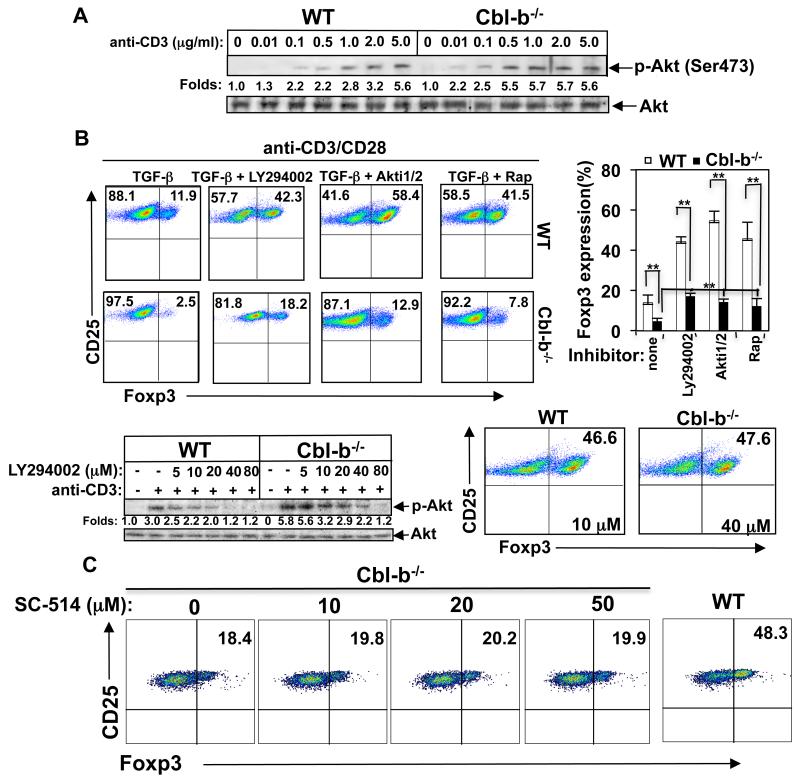
It has been documented that PI3K/Akt/mTOR or Foxo1/3a pathway may inhibit iTreg generation (7-10). As we have shown that Akt but not ERK, JNK, and p38 MAPK is hyper-activated in Cbl-b−/− T cells upon optimal TCR stimulation (23, 24), it is possible that the threshold for T cell activation regulated by Cbl-b is mediated by Akt. To test this we stimulated naïve CD4+CD25− T cells from WT and Cbl-b−/− mice with 0.01 to 5 μg/ml of anti-CD3, and cell lysates were blotted with anti-phospho-Akt. In support of our notion, Cbl-b−/− CD4+CD25− T cells required markedly less anti-CD3 to induce the activation of Akt (Fig. 3A, e.g. compare 0.01 μg/ml for Cbl-b−/− vs. 0.1 μg/ml for WT), which correlated with in vitro iTreg generation at these doses (Fig. 1A).
FIGURE 3.
Cbl-b potentiates the conversion of CD4+CD25− T cells into iTregs via an Akt-dependent manner. (A) Naïve CD4+CD25− T cells were incubated with anti-CD3 (0.01 to 5 μg/ml) on ice for 30 min, washed in pre-warmed RPMI1640 medium twice, and crosslinked with rabbit anti-mouse IgG for 5 min, and lysed. The cell lysates were blotted with anti-phospho-Akt (Ser473) and reprobed with anti-Akt. The fold changes of phospho-Akt bands in arbitrary densitometric units were determined by Li-Cor Odyssey Imaging System. (B) Naïve CD4+CD25− T cells from WT and Cbl-b−/− mice were stimulated with plate-bound anti-CD3 (0.5 μg/ml) and soluble anti-CD28 in the presence or absence of TGF-β together with or without LY294002 (10 μM), Akti1/2 (3 μM), or rapamycin (Rap; 25 nM) for 72 h. T cells were surface stained with anti-CD4 and anti-CD25, and intracellularly stained with anti-Foxp3, and analyzed by flow cytometry (upper panel). Naïve WT and Cbl-b−/− CD4+CD25− T cells were pretreated with different concentrations of LY294002 at 37°C for 30 min and stimulated with anti-CD3 for 5 min. Akt phosphorylation was determined by immunolotting with anti-phospho-Akt, and quantitated using Li-Cor Odyssey Imaging System (lower left panel). Naïve WT or Cbl-b−/− CD4+CD25− T cells were pretreated with 10 μM or 40 μM LY294002, respectively, and then cultured under iTreg conditions. iTreg expression was determined as above (lower right panel). (C) Naïve Cbl-b−/− CD4+CD25− T cells were pretreated with 10 to 50 μM IKKγ NBD inhibitory peptide (SC-514), and then cultured under iTreg conditions. iTreg expression was determined as above. The conversion of naïve WT CD4+CD25− T cells to iTregs was used as a control. The data shown are one representative of three independent experiments.
To confirm that Cbl-b regulates the development of iTregs via the PI3K/Akt/mTOR pathway, we pretreated naïve WT and Cbl-b−/− CD4+CD25− T cells with LY294002, Akti1/2 and rapamycin, and cultured them under iTreg differentiation condition. Inhibition of PI3/Akt/mTOR significantly rescued defective Foxp3 expression caused by optimal TCR stimulation in the absence of Cbl-b (Fig. 3B, upper panel). We recently showed that 40 μM LY294002 completely inhibits Akt phosphorylation induced by CD3 stimulation in WT T cells, but that this dose does not completely abolish Akt activation in Cbl-b−/− T cells (24). In support of this finding, complete inhibition of TCR-induced Akt activity in Cbl-b−/− T cells required a much higher dose of LY294002 (Fig. 3B, lower left panel). Consistent with this finding, increasing LY294002 to 40 μM to treat naïve Cbl-b−/− CD4+CD25− T cells induced a level of iTregs comparable to that seen in naïve WT CD4+CD25− T cells treated with 10 μM LY294002 (Fig. 3B, lower right panel). Taken together, our data strongly suggest that Cbl-b facilitates iTreg development by suppressing the PI3K/Akt/mTOR pathway.
We have shown that Cbl-b negatively regulates TCR-induced activation of NF-κB (23), whereas NF-κB p50 is involved in iTreg generation in vitro, possibly due to IL-2 production (25). Recent studies suggest that CARMA-1, a critical molecule involved in TCR-mediated NF-κB activation, is required for thymic but not peripheral Treg development (26-28). To test whether NF-κB signaling is involved in the regulation of iTreg generation controlled by Cbl-b, we treated naïve CD4+CD25− T cells from Cbl-b−/− mice with a NF-κB specific inhibitor. In support of this notion, we find that inhibition of NF-κB by the specific NF-κB inhibitor only minimally rescues defective TGF-β-induced Treg generation in vitro by Cbl-b−/− CD4+CD25− T cells upon optimal TCR stimulation (Fig. 3C), suggesting that Cbl-b facilitates Foxp3 expression by naïve CD4+CD25− T cells predominantly through Akt.
Akt-2 but not Akt-1 is the downstream signaling pathway of Cbl-b regulating Foxp3 expression
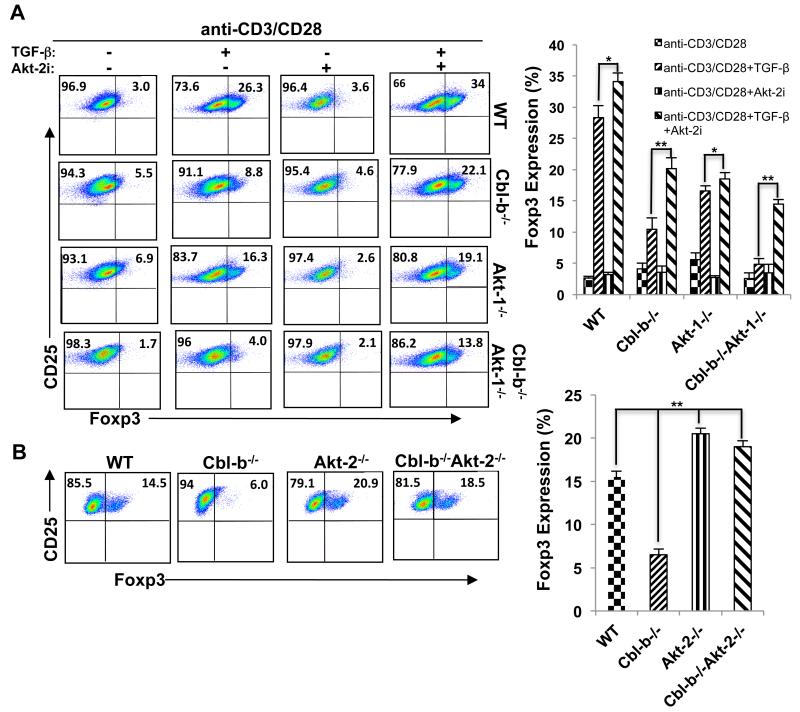
It has been reported that T cells predominantly express Akt-1, and to a lesser extent Akt-2, but do not express detectable Akt-3 (Supplemental Fig. 1)(29), and that both Akt-1 and Akt-2 are activated upon TCR stimulation (Supplemental Fig. 1). To determine which Akt isoform is the major mediator in suppressing Foxp3 expression by naïve Cbl-b−/− CD4+CD25− T cells upon optimal TCR stimulation, we introduced Akt-1 deficiency into Cbl-b−/− background. Surprisingly, Akt-1 deficiency did not overcome the defective in vitro iTreg generation caused by optimal TCR stimulation in the absence of Cbl-b (Fig. 4A), suggesting that Akt isoform(s) other than Akt-1 may be involved in iTreg generation. To assess whether Akt-2 is responsible for the inhibition of Foxp3, we pretreated naïve CD4+CD25− T cells from WT, Cbl-b−/−, Akt-1−/−, and Cbl-b−/−Akt-1−/− mice with Akt-2i, a specific Akt-2 inhibitor. This treatment significantly increased iTregs generated not only from naïve WT CD4+CD25− T cells but also from naïve CD4+CD25− T cells of Cbl-b−/− and Cbl-b−/−Akt-1−/− mice, suggesting that Akt-2 may be the isoform responsible for the inhibition of Foxp3 expression in the absence of Cbl-b (Fig. 4B). To further confirm that Akt-2 is involved in the regulation of Foxp3 expression downstream of Cbl-b, we made use of the Akt-2−/− mice and generated Cbl-b−/−Akt-2−/− mice. Elimination of Akt-2 completely rescued iTreg generation by naïve CD4+CD25− T cells from Cbl-b−/− mice induced by optimal TCR stimulation to a level comparable to that seen in naïve WT CD4+CD25− T cells (Fig. 4B).
FIGURE 4.
Akt-2 but not Akt-1 is the downstream target of Cbl-b in regulating Foxp3 expression. (A) Naïve CD4+CD25− T cells from WT, Akt-1−/−, Cbl-b−/−, and Cbl-b−/−Akt-1−/− mice in the presence or absence of Akt-2i (3 μM) were stimulated and cultured as in B, and the expression of Foxp3 in CD4+CD25+ T cells was determined. (B) Naïve CD4+CD25− T cells from WT, Cbl-b−/−, Akt-2−/−, and Cbl-b−/−Akt-2−/− mice were stimulated with anti-CD3 (0.5 μg/ml) together with anti-CD28 and TGF-β as shown in Figure 1A, and the expression of Foxp3 in CD4+CD25+ T cells was determined. The data shown are one representative of two independent experiments.
Foxo1 and Foxo3a are the downstream targets of Akt-2 but not Akt-1 in T cells
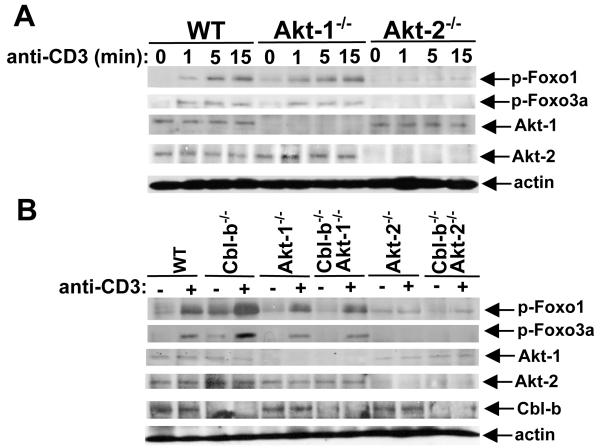
Foxo1 and Foxo3a have been shown to be crucial for iTreg development (9, 10), and are the downstream targets of Akt. Akt phosphorylates Foxo1 at Thr24, Ser256, and Ser319. In Foxo3a, the three sites phosphorylated by Akt are Thr32, Ser253, and Ser315 (30-32). The phosphorylation of Foxo1 and Foxo3a results in their export from the nucleus and prevents activation of their target genes, including Foxp3, in the nucleus (31, 32). To elucidate whether Akt-1 and Akt-2 differentially phosphorylate Foxo1 and Foxo3a, we examined the phosphorylation status of Foxo1 and Foxo3a in T cells from WT, Akt-1−/−, and Akt-2−/− mice, or WT, Cbl-b−/−, Akt-1−/−, Akt-2−/−, Cbl-b−/−Akt-1−/−, and Cbl-b−/−Akt-2−/− mice. We found that Akt-2 but not Akt-1 deficiency led to impaired phosphorylation of Foxo1 and Foxo3a (Fig. 5A). Furthermore, Akt-2 deficiency diminished hyper-phosphorylation of Foxo1 and Foxo3a in the absence of Cbl-b (Fig. 5B). Therefore, we are the first to demonstrate that Akt-2 is the isoform responsible for phosphorylating Foxo1 and Foxo3a in T cells, which regulates Foxp3 expression. We and others have shown that inhibition of mTOR facilitates Foxp3 expression (Fig. 3B, upper panel) (7, 8). As mTORC2 can phoshorylate Akt at Ser473, functioning as a PDK-2, it is speculated that mTORC2 may phosphorylate Akt-2 which phosphorylates Foxo1 and Foxo3a, thus regulating Foxp3 expression.
FIGURE 5.
Akt-2 but not Akt-1 phosphorylates Foxo1 and Foxo3a downstream of Cbl-b. Naïve CD4+CD25− T cells from WT, Akt-1−/−, and Akt-2−/− (A), or WT, Cbl-b−/−, Akt-1−/−, Cbl-b−/−Akt-1−/−, Akt-2−/−, and Cbl-b−/−Akt-2−/− mice (B) were stimulated with anti-CD3 for times indicated and lysed. The cell lysates were blotted with phospho-Abs against Foxo1 and Foxo3a, and reprobed with anti-actin. The presence or absence of Akt-1, Akt-2, and Cbl-b was determined by immunoblotting with anti-Akt-1, anti-Akt-2, and anti-Cbl-b, respectively. The data shown are one representative of three independent experiments.
DISCUSSION
Previously, we have shown that Cbl-b is crucial for CD28- and CTLA-4-mediated T cell costimulatory signaling (14, 15), suggesting that Cbl-b plays a critical role in the induction of peripheral T cell tolerance. In support of this notion, Cbl-b has been implicated in T cell anergy induction (33, 34). Recently, two reports suggest that Cbl-b facilitates the development of iTregs (9, 16). Therefore, Cbl-b may regulate peripheral T cell tolerance via multiple mechanisms. The molecular mechanism by which Cbl-b regulates Foxp3 expression by naïve CD4+CD25− T cells remains to be further defined. The study led by Dr. Clark’s group suggested that Cbl-b deficiency impairs TGF-β-mediated phosphorylation of Smad2/3 which is crucial for the induction of Foxp3 (16), whereas the latter study led by Dr. Liu’s group suggested that Cbl-b favors the Foxp3 expression by naïve CD4+CD25− T cells via inactivating Foxo1 and Foxo3a by Akt (9). As both studies used the same dose of anti-CD3 for inducing iTregs from naive WT and Cbl-b−/− CD4+CD25− T cells (9, 16), it is unknown whether the threshold for T cell activation, regulated by Cbl-b, plays a role in this process. In this study, we titrated the TCR strengths for inducing Foxp3 expression in vitro while maintaining the other conditions constant. We found that that even suboptimal doses of anti-CD3 or Ag for WT T cells are optimal for Cbl-b−/− T cells, which impair iTreg development. This observation was also true in vivo when we used DO11.10.Rag-1−/−Cbl-b−/− mice orally fed with 0.1% or 1% OVA in drinking water (Fig. 2A-F). Our data therefore indicate that lowering the TCR strengths by decreasing the doses of anti-CD3 or Ag induces normal expression of Foxp3 by naïve Cbl-b−/− CD4+CD25− T cells.
It has been documented that Foxo1 and Foxo3a can potentiate Foxp3 expression by binding to the promoter region of Foxp3 CNS 1 and 3 (9, 10). Mice lacking Foxo1 and Foxo3 develop autoimmunity due to reduced Tregs (10). Akt has been shown to phosphorylate Foxo1 and Foxo3a, thereby inactivating them (31). The hyper-activation of Akt caused by loss of Cbl-b significantly suppresses Foxo1 and Foxo3a, thus inhibiting Foxp3 expression upon suboptimal stimulation of WT T cells with anti-CD3. There are three isoforms of Akt: Akt-1, Akt-2, and Akt-3. It has been shown that Akt-1, and to a lesser extent Akt-2, are expressed in T cells, while Akt-3 is not detectable in T cells (Supplemental Fig. 1) (29). It is unknown which isoform of Akt is responsible for phosphorylating Foxo1 and Foxo3a in T cells. Our data indicate that Akt-2 but not Akt-1 phosphorylates Foxo1 and Foxo3a in response to TCR stimulation (Fig. 5A), and that inhibition of Akt-2 by a specific Akt-2 inhibitor, or loss of Akt-2, rescues defective iTreg development in the absence of Cbl-b upon an optimal dosage of stimulation with anti-CD3 (Fig. 4A and B). Consistent with this data, Akt-2 deficiency abrogates the heightened phosphorylation of Foxo1 and Foxo3a induced by TCR stimulation in the absence of Cbl-b (Fig. 5B). Taken together, our data strongly suggest that Cbl-b facilitates Foxp3 expression by suppressing Akt-2.
In summary, our data indicate that the threshold for T cell activation, regulated by Cbl-b, determines the fate of iTreg development via an Akt-2-dependent pathway, which is crucial for establishing peripheral tolerance. Our study also suggests that Cbl-b may be a potential drug target for treating autoimmune/inflammatory diseases.
Supplementary Material
Acknowledgements
We thank Dr. Josef M. Penninger (University of Toronto, Toronto, ON, Canada) for providing the Cbl-b−/− mice.
The project described was supported by grants from the National Institutes of Health (NIH) (R01 AI090901 to JZ) and from the American Heart Association (09GRNT2010084 to JZ). JZ was an American Lung Association Career Investigator.
References
- 1.Sakaguchi S. Naturally arising CD4+ regulatory T cells for immunologic self-tolerance and negative control of immune responses. Annual review of immunology. 2004;22:531–562. doi: 10.1146/annurev.immunol.21.120601.141122. [DOI] [PubMed] [Google Scholar]
- 2.Chen W, Jin W, Hardegen N, Lei KJ, Li L, Marinos N, McGrady G, Wahl SM. Conversion of peripheral CD4+CD25-naive T cells to CD4+CD25+ regulatory T cells by TGF-beta induction of transcription factor Foxp3. J. Exp. Med. 2003;198:1875–1886. doi: 10.1084/jem.20030152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Apostolou I, von Boehmer H. In vivo instruction of suppressor commitment in naive T cells. J. Exp. Med. 2004;199:1401–1408. doi: 10.1084/jem.20040249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mucida D, Kutchukhidze N, Erazo A, Russo M, Lafaille JJ, Curotto de Lafaille MA. Oral tolerance in the absence of naturally occurring Tregs. J. Clin. Invest. 2005;115:1923–1933. doi: 10.1172/JCI24487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liang S, Alard P, Zhao Y, Parnell S, Clark SL, Kosiewicz MM. Conversion of CD4+CD25− cells into CD4+CD25+ regulatory T cells in vivo requires B7 costimulation, but not the thymus. J. Exp. Med. 2005;201:127–137. doi: 10.1084/jem.20041201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cobbold SP, Castejon R, Adams E, Zelenika D, Graca L, Humm S, Waldmann H. Induction of FoxP3+ regulatory T cells in the periphery of T cell receptor transgenic mice tolerized to transplants. J. Immunol. 2004;172:6003–6010. doi: 10.4049/jimmunol.172.10.6003. [DOI] [PubMed] [Google Scholar]
- 7.Haxhinasto S, Mathis D, Benoist C. The AKT-mTOR axis regulates de novo differentiation of CD4+Foxp3+ cells. J. Exp. Med. 2008;205:565–574. doi: 10.1084/jem.20071477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sauer S, Bruno L, Hertweck A, Finlay D, Leleu M, Spivakov M, Knight ZA, Cobb BS, Cantrell D, O’Connor E, Shokat KM, Fisher AG, Merkenschlager M. T cell receptor signaling controls Foxp3 expression via PI3K, Akt, and mTOR. PNAS. 2008;105:7797–7802. doi: 10.1073/pnas.0800928105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Harada Y, Harada Y, Elly C, Ying G, Paik JH, DePinho RA, Liu YC. Transcription factors Foxo3a and Foxo1 couple the E3 ligase Cbl-b to the induction of Foxp3 expression in induced regulatory T cells. J. Exp. Med. 2010;207:1381–1391. doi: 10.1084/jem.20100004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ouyang W, Beckett O, Ma Q, Paik JH, DePinho RA, Li MO. Foxo proteins cooperatively control the differentiation of Foxp3+ regulatory T cells. Nat. Immunol. 2010;11:618–627. doi: 10.1038/ni.1884. [DOI] [PubMed] [Google Scholar]
- 11.Bachmaier K, Krawczyk C, Kozieradzki I, Kong YY, Sasaki T, Oliveira-dos-Santos A, Mariathasan S, Bouchard D, Wakeham A, Itie A, Le J, Ohashi PS, Sarosi I, Nishina H, Lipkowitz S, Penninger JM. Negative regulation of lymphocyte activation and autoimmunity by the molecular adaptor Cbl-b. Nature. 2000;403:211–216. doi: 10.1038/35003228. [DOI] [PubMed] [Google Scholar]
- 12.Chiang YJ, Kole HK, Brown K, Naramura M, Fukuhara S, Hu RJ, Jang IK, Gutkind JS, Shevach E, Gu H. Cbl-b regulates the CD28 dependence of T-cell activation. Nature. 2000;403:216–220. doi: 10.1038/35003235. [DOI] [PubMed] [Google Scholar]
- 13.Zhang J. Ubiquitin ligases in T cell activation and autoimmunity. Clin Immunol. 2004;111:234–240. doi: 10.1016/j.clim.2004.02.003. [DOI] [PubMed] [Google Scholar]
- 14.Zhang J, Bardos T, Li D, Gal I, Vermes C, Xu J, Mikecz K, Finnegan A, Lipkowitz S, Glant TT. Cutting edge: regulation of T cell activation threshold by CD28 costimulation through targeting Cbl-b for ubiquitination. J. Immunol. 2002;169:2236–2240. doi: 10.4049/jimmunol.169.5.2236. [DOI] [PubMed] [Google Scholar]
- 15.Li D, Gal I, Vermes C, Alegre ML, Chong AS, Chen L, Shao Q, Adarichev V, Xu X, Koreny T, Mikecz K, Finnegan A, Glant TT, Zhang J. Cutting edge: Cbl-b: one of the key molecules tuning CD28- and CTLA-4-mediated T cell costimulation. J. Immunol. 2004;173:7135–7139. doi: 10.4049/jimmunol.173.12.7135. [DOI] [PubMed] [Google Scholar]
- 16.Wohlfert EA, Gorelik L, Mittler R, Flavell RA, Clark RB. Cutting edge: deficiency in the E3 ubiquitin ligase Cbl-b results in a multifunctional defect in T cell TGF-beta sensitivity in vitro and in vivo. J. Immunol. 2006;176:1316–1320. doi: 10.4049/jimmunol.176.3.1316. [DOI] [PubMed] [Google Scholar]
- 17.Fontenot JD, Rasmussen JP, Gavin MA, Rudensky AY. A function for interleukin 2 in Foxp3-expressing regulatory T cells. Nat. Immunol. 2005;6:1142–1151. doi: 10.1038/ni1263. [DOI] [PubMed] [Google Scholar]
- 18.Bardos T, Czipri M, Vermes C, Finnegan A, Mikecz K, Zhang J. CD4+CD25+ immunoregulatory T cells may not be involved in controlling autoimmune arthritis. Arth. Res. & Ther. 2003;5:R106–113. doi: 10.1186/ar624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Myou S, Leff AR, Myo S, Boetticher E, Tong J, Meliton AY, Liu J, Munoz NM, Zhu X. Blockade of inflammation and airway hyperresponsiveness in immune-sensitized mice by dominant-negative phosphoinositide 3-kinase-TAT. J. Exp. Med. 2003;198:1573–1582. doi: 10.1084/jem.20030298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gronski MA, Boulter JM, Moskophidis D, Nguyen LT, Holmberg K, Elford AR, Deenick EK, Kim HO, Penninger JM, Odermatt B, Gallimore A, Gascoigne NR, Ohashi PS. TCR affinity and negative regulation limit autoimmunity. Nat. Med. 2004;10:1234–1239. doi: 10.1038/nm1114. [DOI] [PubMed] [Google Scholar]
- 21.Wohlfert EA, Callahan MK, Clark RB. Resistance to CD4+CD25+ regulatory T cells and TGF-beta in Cbl-b−/− mice. J. Immunol. 2004;173:1059–1065. doi: 10.4049/jimmunol.173.2.1059. [DOI] [PubMed] [Google Scholar]
- 22.Nakagome K, Imamura M, Kawahata K, Harada H, Okunishi K, Matsumoto T, Sasaki O, Tanaka R, Kano MR, Chang H, Hanawa H, Miyazaki J, Yamamoto K, Dohi M. High expression of IL-22 suppresses antigen-induced immune responses and eosinophilic airway inflammation via an IL-10-associated mechanism. J. Immunol. 2011;187:5077–5089. doi: 10.4049/jimmunol.1001560. [DOI] [PubMed] [Google Scholar]
- 23.Qiao G, Li Z, Molinero L, Alegre ML, Ying H, Sun Z, Penninger JM, Zhang J. T-cell receptor-induced NF-kappaB activation is negatively regulated by E3 ubiquitin ligase Cbl-b. Mol. Cell. Biol. 2008;28:2470–2480. doi: 10.1128/MCB.01505-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.uo H, Qiao G, Ying H, Li Z, Zhao Y, Liang Y, Yang L, Lipkowitz S, Penninger JM, Langdon WY, Zhang J. E3 ubiquitin ligase Cbl-b regulates Pten via Nedd4 in T cells independently of its ubiquitin ligase activity. Cell Reports. 2012;1:472–482. doi: 10.1016/j.celrep.2012.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jana S, Jailwala P, Haribhai D, Waukau J, Glisic S, Grossman W, Mishra M, Wen R, Wang D, Williams CB, Ghosh S. The role of NF-kappaB and Smad3 in TGF-beta-mediated Foxp3 expression. Eur. J. Immunol. 2009;39:2571–2583. doi: 10.1002/eji.200939201. [DOI] [PubMed] [Google Scholar]
- 26.Molinero LL, Yang J, Gajewski T, Abraham C, Farrar MA, Alegre ML. CARMA1 controls an early checkpoint in the thymic development of FoxP3+ regulatory T cells. J. Immunol. 2009;182:6736–6743. doi: 10.4049/jimmunol.0900498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Barnes MJ, Krebs P, Harris N, Eidenschenk C, Gonzalez-Quintial R, Arnold CN, Crozat K, Sovath S, Moresco EM, Theofilopoulos AN, Beutler B, Hoebe K. Commitment to the regulatory T cell lineage requires CARMA1 in the thymus but not in the periphery. PLoS Biol. 2009;7:e51. doi: 10.1371/journal.pbio.1000051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lee AJ, Wu X, Cheng H, Zhou X, Cheng X, Sun SC. CARMA1 regulation of regulatory T cell development involves modulation of interleukin-2 receptor signaling. J. Biol. Chem. 2010;285:15696–15703. doi: 10.1074/jbc.M109.095190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cheng J, Phong B, Wilson DC, Hirsch R, Kane LP. Akt finetunes NF-kappaB-dependent gene expression during T cell activation. J. Biol. Chem. 2011;286:36076–36085. doi: 10.1074/jbc.M111.259549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brunet A, Bonni A, Zigmond MJ, Lin MZ, Juo P, Hu LS, Anderson MJ, Arden KC, Blenis J, Greenberg ME. Akt promotes cell survival by phosphorylating and inhibiting a Forkhead transcription factor. Cell. 1999;96:857–868. doi: 10.1016/s0092-8674(00)80595-4. [DOI] [PubMed] [Google Scholar]
- 31.Greer EL, Brunet A. FOXO transcription factors at the interface between longevity and tumor suppression. Oncogene. 2005;24:7410–7425. doi: 10.1038/sj.onc.1209086. [DOI] [PubMed] [Google Scholar]
- 32.Tzivion G, Dobson M, Ramakrishnan G. FoxO transcription factors; Regulation by AKT and 14-3-3 proteins. Biochimica Biophysica Acta. 2011;1813:1938–1945. doi: 10.1016/j.bbamcr.2011.06.002. [DOI] [PubMed] [Google Scholar]
- 33.Jeon MS, Atfield A, Venuprasad K, Krawczyk C, Sarao R, Elly C, Yang C, Arya S, Bachmaier K, Su L, Bouchard D, Jones R, Gronski M, Ohashi P, Wada T, Bloom D, Fathman CG, Liu YC, Penninger JM. Essential role of the E3 ubiquitin ligase Cbl-b in T cell anergy induction. Immunity. 2004;21:167–177. doi: 10.1016/j.immuni.2004.07.013. [DOI] [PubMed] [Google Scholar]
- 34.Heissmeyer V, Macian F, Im SH, Varma R, Feske S, Venuprasad K, Gu H, Liu YC, Dustin ML, Rao A. Calcineurin imposes T cell unresponsiveness through targeted proteolysis of signaling proteins. Nat. Immunol. 2004;5:255–265. doi: 10.1038/ni1047. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.