Abstract
Objective
To examine multiple aspects of anger experience and expression (frequency, outward expression, suppression, control) as moderators of the association of social inequality as measured by educational status with inflammation and coagulation markers.
Methods
Following survey assessments via telephone and mail, MIDUS (Midlife in the U.S.) respondents (N = 1,054) participated in an overnight clinic visit, where they completed anger questionnaires and provided a fasting blood sample to measure IL-6, C-reactive protein (CRP), and fibrinogen.
Results
Educational status was linked to higher anger-control among men (B = .14, p = .001). Significant inverse correlations emerged between education and IL-6, CRP, and fibrinogen (r's ≥ -.09, p's < .004) and between anger-control and IL-6 and CRP (r's = -.07, p's <. 03). Controlling for demographic and health status covariates, anger-in predicted lower fibrinogen (p = .031). Interactions between education and anger measures were significant for education and trait anger as related to fibrinogen (p = .023), education and anger-out as related to IL-6 (p = 0.05) and fibrinogen (p = .05). As predicted, the inverse relationships between education and IL-6 and fibrinogen were stronger among individuals reporting high anger. Anger-control also moderated the association of education with IL-6 in women (p = .026), such that the link between education and IL-6 was attenuated among women with high anger-control.
Conclusion
Varieties of anger moderated educational gradients in inflammation: The inverse relationships between education and inflammation markers were strongest among individuals with high anger, and were attenuated among those with high anger control.
Keywords: socioeconomic status, anger, inflammation, gender differences
Anger is a primal emotion in the human repertoire. Described as varying in intensity from mild frustration to extreme fury and rage, anger has been differentiated into state (transient bouts of anger after a precipitating event) and trait components (stable affective styles characterized by frequent experiences of anger) (1). Styles of anger expression can be delineated as well, including verbal or behavioral expressions of anger (anger-out) or suppressing the expression of anger (anger-in). The management of anger (anger-control), referring to efforts to settle down and cool off, is considered a more salubrious expression style (2).
There are adverse health effects associated with high levels of anger or poorly controlled anger. This study examined if these effects were heightened in socioeconomically disadvantaged individuals. Specifically, the main objective was to test whether individual differences in multiple dimensions of anger moderated the inverse relationship between education and systemic inflammation, a physiological process involved in the etiology and pathogenesis of several major diseases, including cardiovascular disease and type II diabetes (3).
Inflammation: Links to SES and Anger
Inflammation is one biological mechanism through which psychosocial processes “get under the skin” to affect disease outcomes (4-5). It is involved in responding to the psychosocial environment as well as in the progression of many disease processes. The present study used three markers of inflammation: pro-inflammatory cytokine, interleukin-6 (IL-6), acute phase protein, C-reactive protein (CRP), and clotting factor, fibrinogen. Relevant to the present research, these biomarkers are patterned by socioeconomic status (SES) and also share meaningful variation with anger and related constructs. Lower SES individuals face higher morbidity and mortality compared to individuals in more favorable positions (6-7). These gradients are evident across numerous health outcomes, including levels of IL-6, CRP, and fibrinogen, which increase moving down the socioeconomic hierarchy (8-11). Prior work on the current sample has shown SES gradients in IL-6, CRP, and fibrinogen (12-13).
Recent work also supports a link between anger and elevated inflammation. Cross-sectional and experimental research shows positive associations between anger, hostility, and related constructs and circulating inflammatory markers and clotting factors (11,14-19) as well as with stimulated production of pro-inflammatory cytokines (20-22). However, many of these studies use smaller, homogeneous samples, limiting generalizations to the larger population (see 11,15,17 for exceptions). Still, they complement a larger literature linking anger to poor cardiovascular and metabolic health outcomes (23-27). Conversely, anger control is associated with salubrious health outcomes, including fewer cardiovascular events, faster wound healing, and higher health-related quality of life (25,28-29). Few investigations have examined its relationship with inflammation to date. In two samples, anger-control was not related to pro-inflammatory cytokine production at a wound site, despite inflammation playing a notable role in the healing process (28). The present study sought to extend the aforementioned literature by examining the relationships between several dimensions of anger and IL-6, CRP, and fibrinogen in a national sample of adults.
Need for Integration
None of the aforementioned research on inflammation and anger incorporated SES, except to use it as a control variable. Two studies, however, included SES and supported the hypothesis that the health-compromising effects of anger may be greater among low SES individuals. One showed that the link between anger and atherosclerosis was significant only in young adults from low SES backgrounds, though did not find a comparable association with adult SES (30). Another showed that trait anger was associated with ambulatory blood pressure most strongly in low SES, African-American adolescents (31). These results suggest that the risk for detrimental health effects associated with disadvantaged SES backgrounds may be exacerbated by an emotional profile marked by high anger.
Considering anger as a pertinent correlate of social inequality has distant roots. Over 2,000 years ago, Aristotle observed that frames of mind, such as being poor and having it disregarded by others, can stir men to anger (32). Contemporary research supports this view. Primary causes of anger are perceived injustices and goal blockages (33-35), which may be experienced more frequently by low SES individuals. SES is inversely related to household size and economic hardship, which both contribute to greater anger as well (35-36). We employ education as the indicator of SES because it is an individual-level variable (i.e., everyone has a level of attainment) and is a precursor to income and occupational status (37). In the Framingham Offspring Study, education inversely predicted trait anger and physical symptoms of anger (e.g., getting a headache), though it did not predict styles of anger expression (38). In another sample, cynical distrust and anger suppression were higher among the less educated; though higher education predicted greater expressions of anger (39). On the other hand, no educational differences in trait anger or anger expression were found in a sample of postmenopausal women, although mean levels of hostility decreased linearly (40). Few reports have assessed the relationship between SES and anger-control, though available evidence suggests they are positively related (36,41).
The current study brought the above literatures together by examining the interplay of anger and educational attainment on inflammatory markers, including whether such patterns might differ for men and women. Prior findings on gender differences in anger and related constructs are mixed. Some studies show no gender differences (42-43); others report that men are angrier (44-47); and still others that women report greater anger (35-36,45). Further, the extent to which trait anger and anger expression styles predict cardiovascular outcomes has differed by gender as well, with most studies reporting stronger relationships in men (23,25,48). Given these inconsistencies, we viewed gender differences as important to consider, but had no specific hypotheses about them.
Using data from MIDUS (Midlife in the U.S.), the first question was whether education was significantly related to trait anger and anger expression styles. We hypothesized that those with less education would report greater trait anger, anger-out, and anger-in and lower anger-control. Second, we hypothesized that greater trait anger, anger-out, and anger-in and lower anger-control would predict higher levels of IL-6, CRP, and fibrinogen. Regarding integrative models, which were the key focus, we posited two directional effects. The exacerbation pathway predicted that the inverse relationship between educational attainment and inflammatory markers would be strongest among individuals with high trait anger, anger-out, and/or anger-in. Thus, it is the combination of low education and high anger that is expected to predict the highest levels of inflammatory markers. The mitigation pathway, in contrast, predicted that the inverse relationship between education and inflammatory markers would be buffered among individuals who reported high anger-control.
Method
Sample
Participants were from the MIDUS survey, which began in 1995 with over 7,000 non-institutionalized adults, recruited via random digit dialing (RDD) from the 48 contiguous states, siblings of the RDD sample, and a large sample of twins (49-50). The second wave (MIDUS II) began in 2004, with 75% of surviving respondents participating. Biological data were collected from a subset of respondents (N = 1,054) who agreed to travel to one of three General Clinical Research Centers (GCRC) for an overnight visit. The response rate was 43% among those eligible (adjusted for those who could not be reached). This rate is somewhat lower than other epidemiological studies involving a clinic visit (e.g., 57% in the Cardiovascular Health Study; 51). However, the protocol is quite demanding and required extensive travel for many participants, in addition to two full days of assessment (52). This study was approved by Institutional Review boards at Georgetown University, University of California, Los Angeles, and University of Wisconsin, Madison. All participants provided informed consent. The biological sample was comparable to the MIDUS II sample on most sociodemographic and health characteristics, though was significantly better educated and less likely to smoke than nonparticipants (52). Descriptive statistics are provided in Table 1.
Table 1.
Sample Characteristics
| Mean (SD) or % | |||
|---|---|---|---|
| Total n = 1,054 | Men n = 477 | Women n = 577 | |
| Trait Anger | 23.75 (5.21) | 23.60 (5.03) | 23.88 (5.35) |
| Anger-In | 14.60 (4.07) | 14.77 (4.04) | 14.47 (4.09) |
| Anger-Out | 12.79 (3.13) | 12.79 (3.14) | 12.79 (3.13) |
| Anger-Control* | 10.09 (2.22) | 10.24 (2.18) | 9.96 (2.24) |
| IL-6 (pg/mL) | 2.66 (2.17) | 2.58 (2.03) | 2.73 (2.28) |
| CRP (μg/mL)* | 2.11 (2.08) | 1.80 (1.86) | 2.37 (2.23) |
| Fibrinogen (mg/dL)* | 340.5 (82.0) | 327.9 (79.2) | 351.1 (82.8) |
| Age | 58.0 (11.62) | 58.7 (11.87) | 57.5 (11.40) |
| Race (% non-White) | 7.2 | 6.9 | 7.5 |
| Education* | |||
| ≤ High School | 24.2 | 20.1 | 27.5 |
| Some College | 29.2 | 28.7 | 29.6 |
| ≥ College Degree | 46.6 | 51.2 | 42.9 |
| Chronic Conditions* | 2.59 (2.11) | 2.33 (1.96) | 2.80 (2.19) |
| BMI* | 29.18 (6.01) | 29.58 (5.21) | 28.85 (6.60) |
| Medication (% yes) | |||
| Anti-hypertensive | 34.8 | 34.0 | 35.5 |
| Cholesterol* | 29.4 | 37.3 | 22.9 |
| Corticosteroid* | 12.5 | 3.8 | 19.8 |
| Anti-depressant* | 15.3 | 11.5 | 18.4 |
| Smoking Status* | |||
| Never Smokers | 55.4 | 50.3 | 59.4 |
| Former Smokers | 33.2 | 37.5 | 29.6 |
| Current Smokers | 11.4 | 11.9 | 10.9 |
| Alcohol (drinks/month)* | 13.29 (23.7) | 19.33 (29.9) | 8.31 (15.3) |
| Physical Activity (minutes/week)* | 335.2 (549) | 423.69 (670) | 262.12 (412) |
Note.
Gender difference p < .05.
Measures
Education
During the telephone interviews, respondents reported the highest grade of school or year of college they had completed. Twelve response categories ranged from no schooling to completion of a professional degree.
Anger
Spielberger's State-Trait Anger Expression Inventory was completed at the GCRC (1). The Trait Anger scale contains 15 items (e.g., “I have a fiery temper”). Respondents indicated how often they generally felt the given statements on a four point scale. The anger-out (e.g., “I strike out at whatever infuriates me”) and anger-in (e.g., “I boil inside, but don't show it”) scales reflected how often respondents had such experiences when they felt angry or furious. The anger-control scale (e.g., “I control my temper”) assessed how often an individual attempts to manage the expression of their anger. The anger-out and anger-in scales contained eight items, and the anger-control scale contained four items. Internal consistency ranged from .69 - .84.
Health Covariates
Health status covariates included body mass index (BMI), medication usage, and chronic health conditions. BMI was based on measurements taken by GCRC staff. Body composition is a key predictor of inflammatory markers (53). Medication usage, including antidepressants, anti-hypertensives, cholesterol, and steroid usage was accounted for as four dummy-coded variables to indicate current use or non-use. These medications have modulatory effects on IL-6, CRP, and fibrinogen (54-55). The chronic health conditions variable was a sum score of self-reported physician diagnosed diseases in which inflammation is an important pathological mechanism (56).
Additional exploratory analyses adjusted for health behaviors, including alcohol consumption, smoking, and physical activity. Alcohol consumption was measured as the total number of drinks consumed in the past month. Smoking status was dummy coded as never-smokers (referent category), former-smokers, and current-smokers. Physical activity was quantified as the average number of minutes of moderate or vigorous physical activity per week. These health behaviors have been linked to fluctuations in inflammatory markers (57).
Inflammatory Markers
Plasma CRP levels were measured using the BNII nephelometer (Dade Behring; Deerfield, IL) utilizing a particle enhanced immunonepholometric assay. Serum IL-6 levels were measured with the Quantikine® high-sensitivity enzyme linked immunosorbent assay (ELISA) kit (R & D Systems, Minneapolis, MN). Fibrinogen antigen was measured using the BNII nephelometer (Dade Behring Inc., Deerfield, IL). All assays were completed according to manufacturer's instructions. The intra-assay and inter-assay coefficients of variance were all in an acceptable range (<12% variance).
Statistical Analyses
Biomarkers were winsorized to three standard deviations from the mean in both directions to reduce the influence of outliers without omitting data. IL-6, CRP, and BMI were log-transformed to achieve normal distributions. Individuals with CRP values over 10 μg/mL (N = 27; < 3%) were excluded as such values may indicate the presence of an acute infection (58). Generalized estimating equations (GEE) models with random intercepts for family clusters were used to address dependencies in the data from the considerable number of twins and siblings (37%) in the sample. The within-cluster covariance structure was specified as exchangeable. B values should be interpreted as unstandardized (i.e., in raw scale units).
First, gender and educational differences in anger were assessed controlling for age and race. Second, main effects of anger on the inflammatory markers were examined in models controlling for (1) sociodemographic factors and (2) health status covariates (BMI, chronic conditions, medications). An exploratory third model included health behaviors (smoking status, alcohol consumption, physical activity). We expected the relationships to be attenuated with health behaviors included because they constitute a likely mediating pathway linking combinations of education and anger to inflammation (37). To assess gender differences in the hypothesized exacerbation and mitigation pathways, three-way interactions between gender, education, and anger predicting IL-6, CRP, and fibrinogen were run in fully adjusted models. If significant, the interaction between education and anger was then analyzed in gender-stratified models. Key analyses focused on interactions between education and anger predicting inflammation. Separate models were run for each anger scale and each biomarker. The alpha level was set to .05.
Results
The first analysis examined gender and educational differences in each anger dimension, adjusting for age and race. There was a significant interaction between education and gender in predicting anger-control, Wald = 4.14, p = .042. To examine the interaction, effects of education predicting anger-control were assessed separately by gender. Education was positively related to anger-control in men (B = .14, Wald = 10.43, p = .001), but the relationship was much weaker and not significant in women (B = .02, Wald = 0.28, p = .596). No gender or educational differences emerged for trait anger, anger-in, or anger-out.
Independent relationships between education and anger with inflammation and covariates were examined next (Table 2). Relationships between education and the inflammatory markers in this sample have been previously reported (12-13). Briefly, as predicted, education inversely predicted IL-6, CRP, and fibrinogen in bivariate models. Higher IL-6, CRP, and fibrinogen were correlated with greater number of chronic conditions, higher BMI, the usage of hypertension and anti-depressant medication and less frequent physical activity. Anger-control was inversely correlated with IL-6 and CRP. Relationships between trait anger and anger-out with inflammatory markers were all non-significant.
Table 2.
Bivariate correlations among anger scales, inflammatory markers, and covariates
| Variable | 1. | 2. | 3. | 4. | 5. | 6. | 7. |
|---|---|---|---|---|---|---|---|
| 1. Trait Anger | — | — | — | — | — | — | — |
| 2. Anger-In | .49* | — | — | — | — | — | — |
| 3. Anger-Out | .53* | .20* | — | — | — | — | — |
| 4. Anger-Control | -.28* | -.16* | -.30* | — | — | — | — |
| 5. IL-6 (pg/mL) | -.00 | -.04 | .01 | -.07* | — | — | — |
| 6. CRP (μg/mL) | .02 | -.02 | .05 | -.07* | .46* | — | — |
| 7. FBGa (mg/dL) | -.04 | -.08* | -.04 | -.03 | .41* | .45* | — |
| 8. Education | -.01 | .03 | .04 | .09* | -.09* | -.11* | -.10* |
| 9. Age (years) | -.11* | -.25* | -.22* | .04 | .24* | .03 | .16* |
| 10. Gender (% Women) | .03 | -.04 | .00 | -.06* | .02 | .13* | .14* |
| 11. Race (% non-White) | -.00 | .04 | -.00 | -.07* | .00 | .03 | .07* |
| 12. Chronic Conditions | .12* | -.02 | .03 | -.10* | .28* | .18* | .19* |
| 13. BMI | .06* | .05 | .10* | -.00 | .33* | .42* | .25* |
| 14. Hypertension Medication | .04 | -.08* | -.05 | -.03 | .26* | .14* | .11* |
| 15. Cholesterol Medication | -.01 | -.05 | .00 | -.04 | .16* | -.01 | .10* |
| 16. Corticosteroid Medication | .06* | .01 | .01 | -.02 | .02 | .13* | -.03 |
| 17. Anti-depressant Medication | .09* | .03 | .06* | -.09* | .12* | .09* | .06* |
| 18. Smoking Statusb | .02 | .08* | .03 | -.03 | .04 | .06 | .04 |
| 19. Alcohol (drinks/month) | .01 | .05 | .00 | -.02 | -.04 | -.06* | -.13* |
| 20. Physical Activity (min/wk) | .01 | .01 | .01 | -.02 | -.14* | -.16* | -.12* |
Note.
p < .05.
Fibrinogen
Smoking was dichotomized as current smoker versus not.
Table 3 displays multivariate-adjusted main effect and interaction results. The relationships between education and the inflammatory markers were attenuated to non-significance after adjusting for health covariates (Model 2). The only significant relationship between anger and the inflammatory markers in fully adjusted models was between anger-in and fibrinogen, in the opposite direction of predictions: greater anger-in predicted lower fibrinogen, B = -0.63, Wald = 4.68, p = .031.
Table 3.
Unstandardized parameter estimates from GEE models of education, varieties of anger, and their interactions predicting inflammatory markers (N = 1,054)
| Model 1 | Model 2 | Model 3 | ||||
|---|---|---|---|---|---|---|
| Variable | B | p | B | p | B | p |
| Log-IL-6 | ||||||
| Education | -.008 | .042 | -.005 | .207 | -.003 | .349 |
| Trait Anger | .001 | .441 | -.001 | .505 | -.001 | .576 |
| Anger-In | .001 | .621 | .000 | .853 | -.001 | .780 |
| Anger-Out | .007 | .028 | .002 | .425 | .003 | .401 |
| Anger-Control | -.008 | .058 | -.006 | .117 | -.007 | .086 |
| Education × Trait Anger | -.001 | .057 | -.001 | .135 | -.001 | .156 |
| Education × Anger-In | -.001 | .185 | -.001 | .205 | -.001 | .345 |
| Education × Anger-Out | -.003 | .022 | -.002 | .053 | -.002 | .061 |
| Education × Anger-Control (M)a | .000 | .969 | -.002 | .336 | -.002 | .423 |
| Education × Anger-Control (W)a | .006 | .010 | .005 | .026 | .005 | .027 |
| Log-CRP | ||||||
| Education | -.015 | .012 | -.009 | .093 | -.007 | .206 |
| Trait Anger | .002 | .385 | -.002 | .417 | -.002 | .515 |
| Anger-In | -.002 | .593 | -.005 | .098 | -.005 | .087 |
| Anger-Out | .011 | .019 | .002 | .548 | .003 | .521 |
| Anger-Control | -.011 | .090 | -.009 | .118 | -.010 | .070 |
| Education × Trait Anger | -.002 | .061 | -.001 | .221 | -.001 | .268 |
| Education × Anger-In | -.002 | .195 | -.002 | .176 | -.001 | .334 |
| Education × Anger-Out | -.002 | .339 | .000 | .944 | .000 | .940 |
| Education × Anger-Control | -.001 | .792 | -.003 | .135 | -.003 | .131 |
| Fibrinogen | ||||||
| Education | -2.15 | .035 | -1.54 | .125 | -1.38 | .165 |
| Trait Anger | -.347 | .431 | -.669 | .126 | -.581 | .183 |
| Anger-In | -.875 | .144 | -1.27 | .031 | -1.19 | .040 |
| Anger-Out | -.001 | .998 | -.860 | .254 | -.767 | .318 |
| Anger-Control | -.220 | .848 | .151 | .890 | -.130 | .905 |
| Education × Trait Anger | -.421 | .016 | -.395 | .023 | -.376 | .029 |
| Education × Anger-In | -.356 | .128 | -.301 | .178 | -.252 | .250 |
| Education × Anger-Out | -.636 | .042 | -.599 | .054 | -.567 | .068 |
| Education × Anger-Control | -.081 | .851 | -.218 | .606 | -.216 | .606 |
Note.
This interaction was tested in gender-stratified models due to a significant 3-way interaction between gender, education, and anger-control predicting IL-6. Model 1 adjusted for age, race, gender, and education (education is a covariate in main effects models only). Model 2 included Model 1 and BMI, chronic health conditions, and medication usage. Model 3 included Model 2 and smoking status, alcohol consumption, and physical activity as an exploratory examination of health behavior pathways.
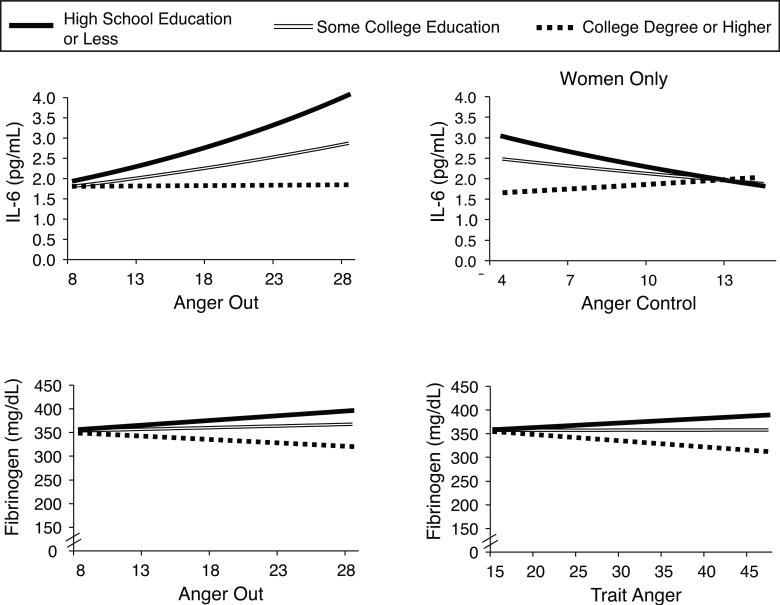
The key analyses assessed interactions between education and anger predicting the inflammatory markers. A three-way interaction between gender, education, and anger-control predicting IL-6 was significant in fully adjusted models (B = -.007, Wald = 4.52, p = .033). As such, the interaction between education and anger-control predicting IL-6 was analyzed in gender-stratified models. Men and women were combined for all other interaction models. Adjusting for demographic and health status variables (Table 3, Model 2), several significant interactions emerged, including between education and trait anger in predicting fibrinogen (Wald = 5.17, p = .023), education and anger-out in predicting both IL-6 and fibrinogen (IL-6: Wald = 3.76, p = .053; fibrinogen: Wald = 3.71, p = .054), and education and anger-control in predicting IL-6 in women only (Wald = 4.94, p = .026). These four interactions are displayed in Figure 1.
Figure 1.
Multiple aspects of anger moderated the effect of education on IL-6. Lines represent the simple effects of anger on IL-6 and fibrinogen for different levels of educational attainment. Education was modeled continuously and has been categorized for illustrative purposes only. Effects were adjusted for age, gender, medication usage, chronic health conditions, and BMI.
To assess the nature of the obtained significant interactions and whether they fit the hypothesized exacerbation and mitigation pathways, graphic displays of the results were generated. Because educational status affords meaningful subgroups (college degree earners, individuals who completed some college without earning a degree, and individuals with a high school education or less), we chose to use these groupings in displaying how education and anger interact in predicting inflammatory outcomes. We note that methodological guidelines permit graphical presentation with either the moderator or focal independent variable on the x-axis (Jaccard & Turrisi, 2003). Thus, simple slopes were obtained to reflect the relationships between anger and IL-6 and fibrinogen, respectively, for individuals grouped according to educational status. Fitting the exacerbation pathway, anger-out predicted greater IL-6 in individuals with a high school degree or less (B = .01, Wald = 3.79, p = .051). Contrary to prediction, trait anger and anger-out were related to lower fibrinogen in individuals with a college education (trait anger: B = -1.57, Wald = 8.24, p = .004; anger-out: B = -2.19, Wald = 4.78, p = .029). In line with the mitigation pathway, higher levels of anger-control were linked to lower levels of IL-6 in women with a high school education or less (B = -.02, Wald = 4.80, p = .028).
Additional analyses explored the role of health behaviors (smoking status, alcohol consumption, physical activity) in the aforementioned moderation effects. As a group, health behaviors accounted for 3% of variance in IL-6 and fibrinogen, respectively. With health behaviors added (Table 3, Model 3), two interactions became marginally significant: between education and anger-out predicting both IL-6 and fibrinogen (IL-6: Wald = 3.51, p = .061; fibrinogen: Wald = 3.33, p = .068). The decrease in coefficients for these interactions with the inclusion of health behaviors ranged from 2-6%. The interactions between education and anger-control predicting IL-6 (women only; Wald = 4.87, p = .027) and education and trait anger predicting fibrinogen (Wald = 4.75, p = .029) remained significant in fully-adjusted models, and all simple slopes were unchanged.
Discussion
The current investigation extended prior research on whether trait anger and anger expression differ as a function of education as well as whether varieties of anger relate to inflammation. As predicted, those with more education showed higher anger-control, but this outcome was driven primarily by men. These results converge with work showing that education is positively related to the effective management of anger. Data from the General Social Survey revealed that highly educated individuals who also had a high sense of control were more cognitively flexible with regard to anger-provoking situations (i.e., better able to look at the situation from a different perspective), more likely to communicate with the target of anger, and more likely to use active problem solving once angry. However, the relationship between education and anger management was much weaker among those with a lower sense of control (41). At the same time, our findings failed to replicate prior evidence of SES disparities in the experience of anger and hostility (38,59). We found no differences in trait anger, anger-in, and anger-out by education. Other sociodemographic factors, such as age or race, may be part of the relationship between SES and anger. For example, more angry profiles have been observed in younger adults and non-White minority groups (59-60). We also saw little evidence of gender differences in anger, thus adding to previous support in this regard (42-43).
Main effects of anger on inflammation were largely non-significant, with the exception of anger-in being inversely associated with fibrinogen. This implies that suppressing anger may not always have negative effects, though given the limited work on anger suppression and inflammation, further research is needed to confirm and explicate this incongruent finding. These results differ from other literature documenting positive relationships between anger and hostility and inflammation (11,14-19), though sample characteristics likely play a role in the divergent findings. Many samples finding significant associations are smaller, community samples of adults free of chronic conditions. This is one of three investigations of the relationships among anger or hostility with inflammation using a national sample (cf. 11,15).
The primary objective, however, was to examine the whether individual differences in anger moderated the link between low education and pro-inflammatory profiles, following the hypothesized exacerbation and mitigation pathways. Overall, findings were consistent with predictions. Educational gradients in IL-6 and fibrinogen were most apparent among those with high trait anger, anger-out, and low anger-control. That is, for individuals with a high school education or less, anger-out was associated with higher IL-6 and anger-control predicted lower IL-6 in women. These results support both the exacerbation and mitigation pathways, showing that the relationship between education and inflammation was more pronounced among those with more angry profiles, while the education--inflammation link was attenuated among women with high anger-control. Our results clarify who is most sensitive to psychosocial influences on IL-6 and fibrinogen, and emphasize that specific dimensions of anger may matter more for those in lower social classes.
Alternatively, and supporting the mitigation pathway, discernible educational gradients in IL-6 were absent among women with greater anger-control, suggesting that anger-control may be protective for women with low education. Women use more support seeking and anger diffusion strategies than men when angry (44) and our results suggest that low SES women who employ these techniques have a reduced inflammatory load. These findings converge with our previous work showing that educational differences in IL-6 were diminished among those with high psychological well-being (13). The combined evidence supports the view that psychological resources (i.e., high well-being, high anger-control) may confer biological benefits to individuals with less socioeconomic capital. That this effect was specific to women calls for future work on gender differences in relationships among psychosocial factors and biological mechanisms. Our findings diverge from a prior meta-analysis that documents stronger relationships between anger and cardiovascular outcomes in men than in women (23). However, few of those studies considered the control and management of anger, which may be especially beneficial for women.
For individuals with a college degree, however, higher trait anger and anger-out were associated with lower fibrinogen. These findings were unexpected and suggest that among the educationally advantaged, there may be physiological benefits to feeling and expressing anger. Such individuals may be in positions of power and have a stronger sense of control, including higher personal mastery and lower perceived constraints (61-62). These findings underscore the need to consider the context in which anger is experienced and expressed in order to understand biological concomitants. In some settings, anger may not be health-compromising. Indeed, appropriately using anger may exact desirable outcomes. Social psychological research suggests that expressing anger confers higher social status by creating impressions of competence (63). Whether these effects extend to other contexts of advantage (e.g., high income) or other health outcomes is a worthy question for future work.
No significant effects were evident in the prediction of CRP, despite prior work linking anger and cynical distrust to elevated CRP (11,15-17,19). Restriction of range may have thwarted our efforts, as those with CRP values over 10 μg/mL were excluded from analyses, per recommendations of the Centers for Disease Control and Prevention and the American Heart Association (58). Blood samples were collected only once during the GCRC visit, precluding retesting of CRP to determine if elevated levels were due to an acute infection or inflammation. Only one of the aforementioned studies (17) excluded individuals on this basis. Nonetheless, one strength of the current study was the inclusion of three related outcomes, allowing us to test the generalization of our findings across multiple outcomes.
Our moderation approach clarified who may be vulnerable or resilient to the pernicious or protective effects of various anger dimensions in the context of SES. The key argument is that those who are educationally disadvantaged are not psychologically equivalent. Rather, they bring different profiles to their experiences, and such differences appear to be meaningfully related to inflammatory outcomes. Additional work needs to address how these relationships are developed and maintained. Such work calls for mediation models, which identify pathways through which socioeconomic and psychosocial factors affect health, one being poor health behaviors (37). Several effects in these analyses were attenuated to marginal significance (p < .07) after adjusting for smoking, alcohol consumption and physical activity, indicating that lifestyle factors account for much of the variance in IL-6 and fibrinogen associated with education and anger. These health behaviors may constitute mechanisms through which the interplay of education and anger influence inflammatory outcomes, calling for further inquiry, including in intervention contexts.
Another future direction involves the interplay among related psychosocial constructs. Several studies have examined interactions between depression and hostility in predicting inflammatory outcomes. Most support joint effects, with the combination of high depression and high hostility predicting the highest inflammatory profile (64-65), but another showed that hostility predicted higher inflammation only among those with low depression (66). Further, measures of anger, anxiety, and depression can be highly correlated making it difficult to disentangle whether there are affect-specific effects that confer cardiovascular risk, or whether there is an underlying negative affectivity construct driving reported effects (27). We explored these possibilities by controlling for both depressive and anxious symptoms in fully adjusted models (data not shown). All interactions were unchanged, indicating that the obtained effects were independent and not driven primarily by a global negative affectivity factor. We also examined potential joint effects among the anger dimensions by testing interactions between trait anger and the anger expression scales, but none emerged as significant.
Several limitations warrant mention. First, study participants were better educated than the pool from which they were recruited, though more than half of the sample did not complete college (52), and only 7.2% of the sample was non-White. Limited representation of low education respondents may partially account for the lack of educational differences in reported anger. Second, the cross-sectional design makes causal claims regarding education, anger, and inflammation untenable, although use of education as an SES indicator reduces concerns of reverse causation (i.e., that poor health causes lower SES). Education is a limited measure of SES that does not address quality of education and also ignores earnings and socioeconomic capital, which may vary by age, gender, or race/ethnicity (37). Other indices of SES, especially income, would valuably extend the current inquiry, particularly given that income was previously shown to mediate the association between education and inflammation in this sample (12). Finally, numerous interactions were tested, given our interest in several dimensions of anger and multiple inflammatory markers. This allowed for testing specific hypotheses regarding the moderating role of anger in the relationship between education and inflammation. The pattern of findings underscored that not all anger dimensions are detrimental for all individuals. While future replication is needed, our confidence is strengthened by finding similar patterns of effects for several dimensions of anger and for two inflammatory outcomes.
In summary, the present work integrated anger, inequality, and inflammation in a national sample of American adults. Results underscored the psychological heterogeneity among the educationally disadvantaged, with moderation analyses exploiting such variation to identify individuals who may be particularly vulnerable, or resilient. Specifically, high anger expression exacerbated risk for elevated IL-6 among those with less education. Conversely, women with low education with high anger-control were protected from elevated IL-6. What constitutes an adaptive anger profile remains an important question; given that college educated individuals with high anger had lower fibrinogen. Such findings bring to mind ancient philosophers who mused about anger and its consequents. We sought to bring these distal observations to empirical life, by attending not only to the varieties of anger and how they are experienced by different people, but also the socioeconomic contexts in which they occur, all of which are important to understand the physiological correlates of this primal emotion.
Acknowledgments
This research was supported the National Institute on Aging (P01-AG020166) and previously by the John D. and Catherine T. MacArthur Foundation Research Network on Successful Midlife Development. J.M.B. is supported by a training grant from the National Institute of Mental Health (T32MH018931-22). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Mental Health or the National Institute of Health. We thank the staff of the Clinical Research Centers at the University of Wisconsin- Madison, UCLA, and Georgetown University for their support in conducting this study. Supported by the following grants M01-RR023942 (Georgetown), M01-RR00865 (UCLA) from the General Clinical Research Centers Program and 1UL1RR025011 (UW) from the Clinical and Translational Science Award (CTSA) program of the National Center for Research Resources, National Institutes of Health.
Acronyms
- BMI
body mass index
- CRP
C-reactive protein
- FBG
fibrinogen
- GCRC
General Clinic Research Center
- GEE
generalized estimating equations
- IL-6
Interlukin-6
- MIDUS
Midlife in the United States
- RDD
random digit dialing
- SAQ
self-administered questionnaire
- SD
standard deviation
- SES
socioeconomic status
Footnotes
Both authors declare no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Spielberger CD. State-trait anger expression inventory: professional manual. Psychological Assessment Resources; Odessa FL: 1996. [Google Scholar]
- 2.Spielberger CD, Sydeman SJ. State-trait anger inventory and state-trait anger expression inventory. In: Maruish ME, editor. The use of psychological testing for treatment planning and outcome assessment. Lawrence Erlbaum; Hillsdale NJ: 1994. pp. 292–321. [Google Scholar]
- 3.Handschin C, Spiegelman BM. The role of exercise and PGC1α in inflammation and chronic disease. Nature. 2008;454:463–69. doi: 10.1038/nature07206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Everson-Rose SA, Lewis TT. Psychosocial factors and cardiovascular diseases. Annu Rev Public Health. 2005;26:469–500. doi: 10.1146/annurev.publhealth.26.021304.144542. [DOI] [PubMed] [Google Scholar]
- 5.Miller G, Chen E, Cole SW. Health psychology: developing biologically plausible models linking the social world and physical health. Annu Rev Psychol. 2009;60:501–24. doi: 10.1146/annurev.psych.60.110707.163551. [DOI] [PubMed] [Google Scholar]
- 6.Adler NE, Rehkopf DH. US disparities in health: descriptions, causes, and mechanisms. Annu Rev Public Health. 2009;29:235–52. doi: 10.1146/annurev.publhealth.29.020907.090852. [DOI] [PubMed] [Google Scholar]
- 7.Kawachi I, Adler NE, Dow WH. Money, schooling, and health: mechanisms and causal evidence. Ann N Y Acad Sci. 2010;1186:56–68. doi: 10.1111/j.1749-6632.2009.05340.x. [DOI] [PubMed] [Google Scholar]
- 8.Koster A, Bosma H, Penninx BW, Newman AB, Harris TB, van Eijk JT, Kempen GI, Simonsick EM, Johnson KC, Rooks RN, Ayonayon HN, Rubin SM, Kritchevsky SB. Health ABC Study. Association of inflammatory markers with socioeconomic status. J Gerontol A Biol Sci Med Sci. 2006;61:284–90. doi: 10.1093/gerona/61.3.284. [DOI] [PubMed] [Google Scholar]
- 9.Petersen KL, Marsland AL, Flory J, Votruba-Drzal E, Muldoon MF, Manuck SB. Community socioeconomic status is associated with circulating interleukin-6 and C-reactive protein. Psychosom Med. 2008;70:646–52. doi: 10.1097/PSY.0b013e31817b8ee4. [DOI] [PubMed] [Google Scholar]
- 10.Pollitt RA, Kaufman JS, Rose KM, Diez-Roux AV, Zeng D, Heiss G. Cumulative life course and adult socioeconomic status and markers of inflammation in adulthood. J Epidemiol Community Health. 2008;62:484–91. doi: 10.1136/jech.2006.054106. [DOI] [PubMed] [Google Scholar]
- 11.Ranjit N, Diez-Roux AV, Shea S, Cushman M, Seeman T, Jackson SA, Ni H. Psychosocial factors and inflammation in the Multi-Ethnic Study of Atherosclerosis. Arch Intern Med. 2007;167:174–81. doi: 10.1001/archinte.167.2.174. [DOI] [PubMed] [Google Scholar]
- 12.Friedman EM, Herd P. Income, education, and inflammation: differential associations in a national sample (the MIDUS study). Psychosom Med. 2010;72:290–300. doi: 10.1097/PSY.0b013e3181cfe4c2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Morozink JA, Friedman EM, Coe CL, Ryff CD. Socioeconomic and psychosocial predictors of interleukin-6 in the MIDUS national sample. Health Psychol. 2010;29:626–35. doi: 10.1037/a0021360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Carroll JE, Low CA, Prather AA, Cohen S, Fury JM, Ross DC, Marsland AL. Negative affective responses to a speech task predict changes in interleukin (IL)-6. Brain Behav Immun. 2011;25:232–8. doi: 10.1016/j.bbi.2010.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Elovainio M, Merjonen P, Pulkki-Råback L, Kivimäki M, Jokela M, Mattson N, Koskinen T, Viikari JSA, Raitakari OT, Keltikangas-Järvinen L. Hostility, metabolic syndrome, inflammation and cardiac control in young adults: The Young Finns Study. Biol Psychol. 2011;87:234–40. doi: 10.1016/j.biopsycho.2011.03.002. [DOI] [PubMed] [Google Scholar]
- 16.Graham JE, Robles TF, Kiecolt-Glaser JK, Malarkey WB, Bissell MG, Glaser R. Hostility and pain are related to inflammation in older adults. Brain Behav Immun. 2006;20:389–400. doi: 10.1016/j.bbi.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 17.Marsland AL, Prather AA, Petersen KL, Cohen S, Manuck SB. Antagonistic characteristics are positively associated with inflammatory markers independently of trait negative emotionality. Brain Behav Immun. 2008;22:753–61. doi: 10.1016/j.bbi.2007.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shivpuri S, Gallo LC, Mills PJ, Matthews KA, Elder JP, Talavera GA. Trait anger, cynical hostility and inflammation in Latinas: variations by anger type? Brain Behav Immun. 2011;25:1256–63. doi: 10.1016/j.bbi.2011.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Suarez EC. C-reactive protein is associated with psychological risk factors of cardiovascular disease in apparently healthy adults. Psychosom Med. 2004;66:684–91. doi: 10.1097/01.psy.0000138281.73634.67. [DOI] [PubMed] [Google Scholar]
- 20.Janicki-Deverts D, Cohen S, Doyle WJ. Cynical hostility and stimulated Th1 and Th2 cytokine production. Brain Behav Immun. 2010;24:58–63. doi: 10.1016/j.bbi.2009.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Suarez EC, Lewis JG, Krishnan RR, Young KH. Enhanced expression of cytokines and chemokines by blood monocytes to in vitro lipopolysaccharide stimulation are associated with hostility and severity of depressive symptoms in healthy women. Psychoneuroendocrinology. 2004;29:1119–28. doi: 10.1016/j.psyneuen.2004.01.002. [DOI] [PubMed] [Google Scholar]
- 22.Suarez EC, Lewis JG, Kuhn C. The relation of aggression, hostility, and anger to lipopolysaccharide-stimulated tumor necrosis factor (TNF)-α by blood monocytes from normal men. Brain Behav Immun. 2002;16:675–684. doi: 10.1016/s0889-1591(02)00019-3. [DOI] [PubMed] [Google Scholar]
- 23.Chida Y, Steptoe A. The association of anger and hostility with future coronary heart disease: a meta-analytic review of prospective evidence. J Am Coll Cardiol. 2009;53:936–46. doi: 10.1016/j.jacc.2008.11.044. [DOI] [PubMed] [Google Scholar]
- 24.Everson SA, Kaplan GA, Goldberg DE, Lakka TA, Sivenius J, Salonen JT. Anger expression and incident stroke: prospective evidence from the Kuopio Ischemic Heart Disease Study. Stroke. 1999;30:523–8. doi: 10.1161/01.str.30.3.523. [DOI] [PubMed] [Google Scholar]
- 25.Haukkala A, Konttinen H, Laatikainen T, Kawachi I, Uutela A. Hostility, anger-control, and anger expression as predictors of cardiovascular disease. Psychosom Med. 2010;72:556–62. doi: 10.1097/PSY.0b013e3181dbab87. [DOI] [PubMed] [Google Scholar]
- 26.Schum JL, Jorgensen RS, Verhaeghen P, Sauro M, Thibodeau R. Trait anger, anger expression, and ambulatory blood pressure: a meta-analytic review. J Behav Med. 2003;26:395–415. doi: 10.1023/a:1025767900757. [DOI] [PubMed] [Google Scholar]
- 27.Suls J, Bunde J. Anger, anxiety, and depression as risk factors for cardiovascular disease: the problems and implications of overlapping affective dispositions. Psychol Bul. 2005;131:260–300. doi: 10.1037/0033-2909.131.2.260. [DOI] [PubMed] [Google Scholar]
- 28.Gouin JP, Kiecolt-Glaser JK, Malarkey WB, Glaser R. The influence of anger expression on wound healing. Brain Behav Immun. 2008;22:699–708. doi: 10.1016/j.bbi.2007.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Julkunen J, Ahlström R. Hostility, anger, and sense of coherence as predictors of health related quality of life. Results of an ASCOT substudy. J Psychosom Res. 2006;61:33–9. doi: 10.1016/j.jpsychores.2005.12.005. [DOI] [PubMed] [Google Scholar]
- 30.Merjonen P, Pulkki-Råback L, Puttonen S, Keskivaara P, Juonala M, Telama R, Viikari J, Raitakari OT, Keltikangas-Järvinen L. Anger is associated with subclinical atherosclerosis in low SES but not in higher SES men and women. The Cardiovascular Risk in Young Finns Study. J Behav Med. 2008;31:35–44. doi: 10.1007/s10865-007-9131-6. [DOI] [PubMed] [Google Scholar]
- 31.Beatty DL, Matthews KA. Unfair treatment and trait anger-in relation to nighttime ambulatory blood pressure in African American and white adolescents. Psychosom Med. 2009;71:813–20. doi: 10.1097/PSY.0b013e3181b3b6f8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Aristotle. Rhetoric. Roberts WR translation. Clarendon Press; Oxford: 1924. [Google Scholar]
- 33.Harmon-Jones E, Peterson CK, Harmon-Jones C. Anger, motivation, and asymmetrical frontal cortical activations. In: Potegal M, Stemmler G, Spielberger C, editors. International handbook on anger. Springer Science + Business Media, LLC; New York: 2010. pp. 61–78. [Google Scholar]
- 34.Mikula G, Scherer KR, Athenstaedt U. The role of injustice in the elicitation of differential emotional reactions. Pers Soc Psychol Bull. 1998;24:769–83. [Google Scholar]
- 35.Ross CE, Van Willigen M. Gender, parenthood, and anger. J Marriage Fam. 1996;58:572–84. [Google Scholar]
- 36.Schieman S. The sociological study of anger: basic social patterns and contexts. In: Potegal M, Stemmler G, Spielberger C, editors. International handbook on anger. Springer Science + Business Media, LLC; New York: 2010. pp. 329–48. [Google Scholar]
- 37.Matthews KA, Gallo LC. Psychological perspectives on pathways linking socioeconomic status and physical health. Annu Rev Psychol. 2011;62:501–30. doi: 10.1146/annurev.psych.031809.130711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Eaker ED, Sullivan LM, Kelly-Hayes M, D'Agostino RB, Sr, Benjamin EJ. Anger and hostility predict the development of atrial fibrillation in men in the Framingham Offspring Study. Circulation. 2004;16:1267–71. doi: 10.1161/01.CIR.0000118535.15205.8F. [DOI] [PubMed] [Google Scholar]
- 39.Haukkala A. Socio-economic differences in hostility measures—a population based study. Psychol Health. 2002;17:191–202. [Google Scholar]
- 40.Gallo LC, Matthews KA, Kuller LH, Sutton-Tyrrell K, Edmundowicz D. Educational attainment and coronary and aortic calcification in postmenopausal women. Psychosom Med. 2001;63:925–35. doi: 10.1097/00006842-200111000-00011. [DOI] [PubMed] [Google Scholar]
- 41.Schieman S. Education and the activation, course, and management of anger. J Health Soc Behav. 2000;41:20–39. [PubMed] [Google Scholar]
- 42.Costa PT, Jr, Terracciano A, McCrae RR. Gender differences in personality traits across cultures: robust and surprising findings. J Pers Soc Psychol. 2001;81:322–31. doi: 10.1037/0022-3514.81.2.322. [DOI] [PubMed] [Google Scholar]
- 43.Schieman S. Age and anger. J Health Soc Behav. 1999;40:273–89. [PubMed] [Google Scholar]
- 44.Linden W, Hogan BE, Rutledge T, Chawla A, Lenz JW, Leung D. There is more to anger coping than “in” or “out.”. Emotion. 2003;3:12–29. doi: 10.1037/1528-3542.3.1.12. [DOI] [PubMed] [Google Scholar]
- 45.Maier KJ, Goble LA, Neumann SA, Giggey PP, Suarez EC, Waldstein SR. Dimensions across measures of dispositional hostility, expressive style, and depression show some variation by race/ethnicity and gender in young adults. J Soc Clin Psychol. 2009;28:1199–1225. [Google Scholar]
- 46.Scherwitz L, Perkins L, Chesney M, Hughes G. Cook-Medley hostility scale and subsets: relationship to demographic and psychosocial characteristics in young adults in the CARDIA study. Psychosom Med. 1991;53:36–49. doi: 10.1097/00006842-199101000-00004. [DOI] [PubMed] [Google Scholar]
- 47.Yan LL, Liu K, Matthews KA, Daviglus ML, Ferguson TF, Kiefe CI. Psychosocial factors and risk of hypertension: the Coronary Artery Risk Development in Young Adults (CARDIA) Study. JAMA. 2003;290:2138–48. doi: 10.1001/jama.290.16.2138. [DOI] [PubMed] [Google Scholar]
- 48.Davidson KW, Mostofsky E. Anger expression and risk of coronary heart disease: Evidence from the Nova Scotia Health Survey. Am Heart J. 2010;159:199–206. doi: 10.1016/j.ahj.2009.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Brim OG, Ryff CD, Kessler RC. How healthy are we: a national study of well-being at midlife. University of Chicago Press; Chicago: 2004. [Google Scholar]
- 50.Radler BT, Ryff CD. Who participates? Accounting for longitudinal retention in the MIDUS National Survey of Health and Well-being. J Aging Health. 2010 Apr;22(3):307–31. doi: 10.1177/0898264309358617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fried LP. Risk factors for 5-year mortality in older adults: The Cardiovascular Health Study. JAMA. 1998;279:585–592. doi: 10.1001/jama.279.8.585. [DOI] [PubMed] [Google Scholar]
- 52.Love GD, Seeman TE, Weinstein M, Ryff CD. Bioindicators in the MIDUS National Study: protocol, measures, sample, and comparative context. J Aging Health. 2010;22:1059–80. doi: 10.1177/0898264310374355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mohamed-Ali V, Goodrick S, Rawesh A, Katz DR, Miles JM, Yudkin JS, Klein S, Coppack SW. Subcutaneous adipose tissue releases interleukin-6, but not tumor necrosis factor-α, in vivo. J Clin Endocrinol Metab. 1997;82:4196–200. doi: 10.1210/jcem.82.12.4450. [DOI] [PubMed] [Google Scholar]
- 54.Jain MK, Ridker PM. Anti-inflammatory effects of statins: clinical evidence and basic mechanisms. Nat Rev Drug Discov. 2005;4:977–87. doi: 10.1038/nrd1901. [DOI] [PubMed] [Google Scholar]
- 55.Kenis G, Maes M. Effects of antidepressants on the production of cytokines. Int J Neuropsychopharmacol. 2002;5:401–12. doi: 10.1017/S1461145702003164. [DOI] [PubMed] [Google Scholar]
- 56.Libby P. Inflammatory mechanisms: the molecular basis of inflammation and disease. Nutr Rev. 2007;65:S140–6. doi: 10.1111/j.1753-4887.2007.tb00352.x. [DOI] [PubMed] [Google Scholar]
- 57.O'Connor MF, Irwin MR. Links between behavioral factors and inflammation. Clin Pharmacol Ther. 2010;87:479–82. doi: 10.1038/clpt.2009.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pearson TA, Mensah GA, Alexander RW, Anderson JL, Cannon RO, 3rd, Criqui M, Fadl YY, Fortmann SP, Hong Y, Myers GL, Rifai N, Smith SC, Jr, Taubert K, Tracy RP, Vinicor F. Centers for Disease Control and Prevention; American Heart Association. Markers of inflammation and cardiovascular disease: application to clinical and public health practice: A statement for healthcare professionals from the Centers for Disease Control and Prevention and the American Heart Association. Circulation. 2003;107:499–511. doi: 10.1161/01.cir.0000052939.59093.45. [DOI] [PubMed] [Google Scholar]
- 59.Barefoot JC, Peterson BL, Dahlstrom WG, Siegler IC, Anderson NB, Williams RB., Jr Hostility patterns and health implications: correlates of Cook-Medley Hostility Scale scores in a national survey. Health Psychol. 1991;10:18–24. doi: 10.1037//0278-6133.10.1.18. [DOI] [PubMed] [Google Scholar]
- 60.Schieman S. Socioeconomic status and the frequency of anger across the life course. Sociol Perspect. 2003;46:207–22. 2003. [Google Scholar]
- 61.Lachman ME, Weaver SL. The sense of control as a moderator of social class differences in health and well-being. J Pers Soc Psychol. 1998;74:763–73. doi: 10.1037//0022-3514.74.3.763. [DOI] [PubMed] [Google Scholar]
- 62.Marmot MG, Fuhrer R, Ettner SL, Marks NF, Bumpass LL, Ryff CD. Contribution of psychosocial factors to socioeconomic differences in health. Milbank Q. 1998;76:403–48. doi: 10.1111/1468-0009.00097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tiedens LZ. Anger and advancement versus sadness and subjugation: the effect of negative emotional expressions on social status conferral. J Pers Soc Psychol. 2001;80:86–94. [PubMed] [Google Scholar]
- 64.Brummett BH, Boyle SH, Ortel TL, Becker RC, Siegler IC, Williams RB. Associations of depressive symptoms, trait hostility, and gender with C-reactive protein and interleukin-6 response after emotion recall. Psychosom Med. 2010;72:333–9. doi: 10.1097/PSY.0b013e3181d2f104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Stewart JC, Janicki-Deverts D, Muldoon MF, Kamarck TW. Depressive symptoms moderate the influence of hostility on serum interleukin-6 and C-reactive protein. Psychosom Med. 2008;70:197–204. doi: 10.1097/PSY.0b013e3181642a0b. [DOI] [PubMed] [Google Scholar]
- 66.Miller GE, Freedland KE, Carney RM, Stetler CA, Banks WA. Cynical hostility, depressive symptoms, and the expression of inflammatory risk markers for coronary heart disease. J Behav Med. 2003;26:501–15. doi: 10.1023/a:1026273817984. [DOI] [PubMed] [Google Scholar]