Abstract
Knowledge of how neutrophils respond to chemotactic signals in a complex inflammatory environment is not completely understood. Moreover, even less is known about factors in physiological fluids that regulate the activity of chemoattractants. The vitamin D binding protein (DBP) has been shown to significantly enhance chemotaxis to complement activation peptide C5a using purified proteins in vitro, and by ex vivo depletion of DBP in physiological fluids, but this function has not been determined in vivo. DBP null (-/-) mice were used to investigate how a systemic absence of this plasma protein affects leukocyte recruitment in alveolitis models of lung inflammation. DBP-/- mice had significantly reduced (~50%) neutrophil recruitment to the lungs compared to their wild-type DBP+/+ counterparts in three different alveolitis models, two acute and one chronic. The histology of DBP-/- mouse lungs also showed significantly less injury than wild-type animals. The chemotactic cofactor function of DBP appears to be selective for neutrophil recruitment, but in contrast to previous in vitro results, in vivo DBP can enhance the activity of other chemoattractants including CXCL1. The reduced neutrophil response in DBP-/- mice could be rescued to wild-type levels by administering exogenous DBP. Finally, in inflammatory fluids DBP binds to G-actin released from damaged cells and this complex may be the active chemotactic cofactor. Results show for the first time that DBP is a significant chemotactic cofactor in vivo and not specific for C5a, suggesting that this ubiquitous plasma protein may have a more significant role in neutrophil recruitment than previously recognized.
INTRODUCTION
Migration of neutrophils from the bloodstream into tissues is a key stage during inflammation and is essential for host survival. However, inappropriate and/or excessive recruitment of these cells is known to be a critical step in the pathogenesis of both acute and chronic inflammatory disorders (1-3). Products of complement activation, most notably the pro-inflammatory peptide C5a, also have been strongly correlated with the pathogenesis of many conditions (4). C5a (as well as its stable degradation product C5a des Arg) is a very robust chemotactic factor for many cell types including most leukocytes, and is a particularly potent neutrophil chemoattractant (5). But the regulation of C5a activity in a complex physiological environment is poorly understood. There is abundant in vitro evidence that the vitamin D binding protein (DBP) functions as a chemotactic cofactor for C5a and C5a des Arg, significantly augmenting cell migration to suboptimal concentrations of these C5-derived peptides (hereafter referred to as C5a) (6-13). More recently, ex vivo analysis of human blood and bronchoalveolar lavage (BAL) fluid showed that DBP, and to a lesser extent platelet-derived thrombospondin-1, are required for the maximal chemotactic activity of C5a in biological fluids (14). However, the role of DBP as a chemotactic cofactor has not been investigated in vivo.
DBP, also known as Gc-globulin, is an abundant multifunctional 56-58 kDa plasma protein that is part of the albumin gene family (15). The protein has at least four distinct functions: binding and transport of vitamin D metabolites, binding and clearance of monomeric G-actin released from dead cells, a deglycosylated form functions as a macrophage activating factor, and DBP acts as a chemotactic cofactor for C5a (15). Although the protein by itself lacks chemotactic activity, it associates with the plasma membrane of many cell types and appears to bind with low avidity to multiple cell surface ligands such as chondroitin sulfate proteoglycans (16), CD44 (17), megalin (18), and cubulin (19). Furthermore, the interaction of DBP with the neutrophil cell surface is essential for C5a chemotaxis enhancement (16, 17, 20-23). Formation of this DBP binding site complex in neutrophils is a dynamic, multi-step and transient process requiring cell activation (23) and perhaps several distinct macromolecules. The complex appears to function independent of C5a binding to the C5a receptor (C5aR1/CD88) since DBP does not alter C5a receptor-ligand interactions (24), and DBP does not bind to C5a or the C5a receptor (20, 25). The sequences in DBP that mediate both the cell binding (25) and chemotactic cofactor functions (26) have been identified, however, the precise mechanism by which this protein enhances chemotaxis is not known.
There are no known natural deficiencies of DBP in any vertebrate species but a DBP null (-/-) mouse, fully backcrossed on a C57BL/6 background, has been generated. These mice are healthy and develop and reproduce similar to their wild-type counterparts when fed a vitamin D sufficient mouse chow diet (27, 28). Studies using DBP-/- mice have shown that the primary role of DBP is to maintain circulating vitamin D levels within a physiological range to protect against transient vitamin deficiencies (29). In contrast to the vitamin D carrier role, other functions of DBP have not been investigated in the DBP-/- mouse and the objective of this study was to characterize the chemotactic cofactor function in these animals. Utilizing an alveolitis (inflammation of small airways and airspaces) model of lung inflammation, results demonstrate for the first time that DBP is a significant chemotactic cofactor for neutrophils in vivo, and this function is not specific for C5a. Furthermore, the reduced neutrophil response in DBP-/- mice can be rescued to wild-type levels by administering exogenous DBP. Finally, evidence is presented to suggest that the chemotactic cofactor function of DBP is mediated via DBP-actin complex. These results indicate that DBP may play a more significant role in recruiting neutrophils to inflamed tissue than previously realized.
MATERIALS AND METHODS
Reagents
Purified recombinant mouse C5a was purchased from R&D Systems (Minneapolis, MN), purified natural human C5a was purchased from CompTech (Tyler, TX), purified murine KC (CXCL1) was purchased from Peprotech, Inc. (Rocky Hill, NJ), purified human DBP was obtained from Athens Research & Technology (Athens, GA). Chicken anti-human DBP antibody was purchased from Gallus Immunotech (Cary, NC). Highly purified skeletal muscle actin was obtained from Cytoskeleton, Inc. (Denver, CO) and type I collagen from Elastin Products, Inc. (Owensville, MO). Goat anti-actin (I-19) was purchased from Santa Cruz Biotechnology (Santa Cruz, CA) and pan anti-actin mAb ACTN05 was purchased from Thermo Scientific/Lab Vision (Kalamazoo, MI). Multi-Analyte ELISArray kits for analysis of inflammatory cytokines and chemokines were obtained from Qiagen/SA BioSciences (Germantown, MD). Rabbit anti-chicken ovalbumin was purchased from Biodesign (Saco, ME); ovalbumin was purchased from Worthington Biochemicals (Lakewood, NJ). Rat anti-mouse monoclonal antibodies for flow cytometry, and their corresponding labeled isotype controls, were purchased from Biolegend (San Diego, CA): PE-labeled anti-Gr-1 (RB6-8C5), FITC-labeled anti-F4/80 (BM8), APC-labeled anti-CD11b (M1/70), Pacific Blue-labeled anti-CD44 (IM7), PE/Cy7-labeled anti-CD88 (20/70), and Alexafluor 647-labeled anti-CXCR2 (TG11). HBSS, DPBS and RPMI 1640 were purchased from Mediatech, Inc. (Manassas, VA). EDTA solution was purchased from Life Technologies-Gibco (Grand Island, NY). Fetal bovine serum (FBS) and bovine serum albumin (BSA) were purchased from Gemini BioProducts (West Sacramento, CA). Collagenase A and DNase I were purchased from Roche (Indianapolis, IN).
Mice
The DBP-/- mouse line has been fully backcrossed on a C57BL/6 background for 10 generations (27, 28). Frozen sperm from DBP-/- mice was used to revive the line at the University of Pennsylvania's Transgenic and Chimeric Mouse Facility. DBP+/- heterozygotes were generated by in vitro fertilization of eggs from wild-type C57BL/6J mice (Jackson Labs, Bar Harbor, ME) with DBP-/- sperm. The DBP+/- hemizygotes were bred to produce the DBP-/- (null) and DBP+/+ (wild-type) homozygous mouse colonies at the Stony Brook University animal facility. Mice were genotyped using tail tissue as previously described (27). The DBP null phenotype of -/- mice was also confirmed by western blot analysis of sera using chicken anti-human DBP that cross-reacts strongly with mouse DBP. In some experiments, wild-type C57BL6/J mice were purchased from Jackson Labs. All mice were housed in a maximum isolation facility at Stony Brook and all experiments utilized 8 to 10 week old male mice. Animal experiments were performed using protocols approved by the Institutional Animal Care and Use Committee at Stony Brook University.
Immune Complex Alveolitis
A well-characterized model for complement dependent inflammation is the reverse passive Arthus reaction which is initiated by intravenous injection of antigen followed by local injection (intratracheal) of the specific IgG antibody (30). A prominent C5a-dependent neutrophil infiltrate typically is observed 4 hours after antibody injection (31). DBP-/-, +/- and +/+ 8 to 10 week old male mice were anesthetized by an i.p. injection (0.3 ml) of ketamine (90 mg/kg) and xylazine (10 mg/kg) solution. Anesthetized mice were secured to a small animal surgery board (Kent Scientific, Torrington, CT) for the maintenance of a 60° angle in a head up position to prevent instillate from flowing out of the trachea. Ophthalmic ointment was applied to the eyes to prevent drying. A small midline incision was made in the suprasternal region and the trachea exposed by blunt dissection. A sterile BD angiocath (18 gauge needle stylet in a 1.3 × 44 mm long I.V. catheter) was inserted into the anterior portion of the exposed trachea between cartilage rings. Once the catheter was properly placed with the tip just above the carina (bifurcation point of the trachea), the stylet needle was removed and a Tridek stepper pipettor (Fisher Scientific) fitted with a 1 ml syringe was attached to the catheter. Exactly 50 μl of rabbit anti-chicken egg albumin (150 μg) in PBS was instilled into the lungs, the catheter was removed once the animal gasps (within 5-10 seconds) indicating that the fluid had been delivered to the airspaces. The incision was then closed with 9 mm wound clips. Next, the animal immediately was injected i.v. in the tail vein with 20 μg ovalbumin/g body weight in 0.2 ml of PBS. Mice were allowed to recover in a heated recovery cage and were observed frequently until they were alert enough to be moved back to their home cages. Controls were an Ab control (PBS i.v., Ab in the lungs) and Ag control (Ag i.v., PBS in the lungs). At 4 hours after induction of injury, anesthetized animals were killed by cervical dislocation. An 18-gauge BD angiocath was inserted between the tracheal rings and the lungs were lavaged four times, each with 1.0 ml aliquots of HBSS-EDTA. The aliquots were pooled and the resulting BAL fluid stored on ice until analyzed for total and differential counts. BAL fluid was centrifuged at 200 × g for 7 min at 4°C to pellet the cells. BAL cell pellets were resuspended in 1 ml of PBS-1% BSA and duplicate counts of total cell number were made using a BioRad TC10 automated cell counter. Differential analysis of the white blood cells were performed using duplicate cytocentrifuge preparations stained with Wright-Geimsa (Diff-Quick), a total of 300 cells per duplicate slide were counted to determine the percent differential. Differential counts were also performed by flow cytometry (see that section for details).
C5a-Induced Alveolitis
Purified recombinant mouse C5a (and in separate experiments purified mouse KC) was instilled into the lungs of 8 to 10 week old anesthetized DBP+/+ or DBP-/- mice at a in a total volume of 50 μl at a concentration of 1 μg/24g mouse or 0.25 μg/24g mouse via oropharyngeal aspiration technique (32). As a control, the same volume (50 μl) of PBS was instilled into the lungs. Mice were allowed to recover and sacrificed at 2.5, 4, 6, 24 or 48 hours, their lungs lavaged four times using 1 ml of sterile HBSS (no ions) + 5 mM EDTA. The BAL fluid was then centrifuged to pellet the cells. The supernatant was aspirated and the cells were counted and then resuspended in 110 μl PBS+1% BSA for flow cytometric analysis.
Bleomycin-induced chronic lung injury model
The bleomycin model of murine lung injury has been described in detail previously (33, 34). Bleomycin (sterile clinical-grade Blenoxane, Bristol-Meyers Squibb) was dissolved in PBS and instilled into the lungs of anesthetized DBP-/- and DBP+/+ mice. Animals received 0.05 U of bleomycin in a total volume of 50 μl via oropharyngeal aspiration method described above. Controls received 50 μl of PBS. Mice were sacrificed and their tissues analyzed 7 days after treatment. Lungs were lavaged and the BAL cells and supernatant processed as described above for the alveolitis model. The percent macrophages, neutrophils and lymphocytes in the BAL fluid were calculated by counting 500 cells in duplicate cytospin preparations, and then verified by flow cytometry staining.
Preparing single cell suspension from the lungs
Four hours after instillation of C5a, lungs were isolated and placed into a well in a 24 well plate containing 1 ml of complete culture medium (RPMI 1640 + 10% FBS) and thoroughly minced using a small surgical scissor. Minced tissue was transferred into a 15 ml conical tube containing 5 ml of fresh collagenase solution (1 mg/ml type IV collagenase, 25 U/ml DNase I, 5% FBS and the volume was brought up to 5 ml with RPMI). The samples were then incubated at 37°C for 45 minutes with shaking every 10 minutes. After the incubation, the tissue was broken up into cellular suspension by passing it through an 18-gauge needle 20 times. Then the samples were spun down at 500 × g for 5 minutes. The supernatant was aspirated and the pellet was resuspended in RBC lysis buffer and immediately spun at 50 × g for 5 minutes. The supernatant was saved and the pellet, containing large tissue aggregates, was discarded. The supernatant was then again centrifuged at 500 × g for 5 minutes and the cells were resuspended in 500 μl of flow cytometry staining buffer. The samples were then counted using an automatic cell counter then stained and analyzed by flow cytometry.
Histological analysis of the lung
Two to three animals from each group had their lungs prepared for histology 4 hours after instillation of C5a. Mice were euthanized and the trachea exposed as above but lungs were inflated with 10% PBS-buffered formalin for 15 min at 25 cm water pressure to ensure the proper degree of lung inflation. The lungs then were removed from the thoracic cavity en bloc and submerged in 10% PBS-buffered formalin for 24 hours at 4°C. The fixed lungs were transferred to the Stony Brook University Hospital Histology Laboratory for preparation of thin-sectioned slides stained with hematoxylin and eosin (H&E). Digital photos were taken using a Nikon Eclipse Ti Inverted microscope with an Insight Spot2 digital camera using 20x and 100x objectives.
Flow cytometry staining
The cells were prepared as previously described in the alveolitis methods section. Cells were counted using a TC10 automated cell counter and resuspended in 110 μl of flow staining buffer (PBS + 1% BSA). The cells were then blocked using TruStain FcX (Biolegend) for 15 minutes on ice and then stained with the appropriate antibody for 15 minutes on ice. The cells were then washed twice with flow cytometry staining buffer and then fixed in 2% paraformaldehyde. Flow cytometric data was then collected (a minimum of 10,000 events were analyzed) using a BD FACS Calibur II flow cytometer and data was analyzed using FlowJo software (Tree Star Inc., Ashland, OR). Neutrophils were defined as Gr-1 high staining cells and macrophages were defined as F4/80 positive staining cells. The total number of neutrophils and macrophages was determined by multiplying the total number of leukocytes in the BAL by the percentage of Gr-1 high cells and F4/80 positive cells respectively.
Preparation of complement-activated serum
Untreated DBP+/+ and DBP-/- mice were sacrificed, and blood was collected by cardiac puncture. Serum was prepared by transferring the collected blood into coagulation activating tubes (Terumo Medical Corp., Somerset, NJ) and incubating them at room temperature for 15-30 minutes or until the clot had begun to retract. Then the tubes were centrifuged at 6500 × g for 10 minutes at 4°C. 300 μl of DBP-/- or +/+ serum was transferred into a new tube containing MgCl2 (2 mM final concentration) and 10.5 μl of 1 mg/ml cobra venom factor (CVF, Complement Technology Inc., Tyler, TX). The serum was incubated at 37°C for 60 minutes with gentle mixing every 15 minutes to activate complement. After activation the amount of C5a/C5a des Arg generated was quantified using a sandwich ELISA (see below) and the samples were aliquoted and stored at -80°C until further use. In control experiments, serum was first heat-inactivated at 56°C for 45 minutes before CVF was added.
Mouse C5a sandwich ELISA
Measurement of C5a/C5a des Arg levels was determined by sandwich ELISA as previously described (35) with a slight modification. Maxi-sorb 96 well plates were coated with rat anti-mouse C5a monoclonal capture antibody (clone I52-1486, BD-Pharmingen, San Diego, CA), diluted to 5 μg/ml in pH 9.6 coating buffer overnight at 4 °C. Each well was then blocked with 300 μl of blocking buffer (3% non fat dry milk in PBS + 0.05% Tween-20) for 1 hour at room temperature. Next, each well was washed 3x with wash buffer (PBS-Tween). Standards (0.1 to 50 ng/ml of purified mouse C5a) and serum samples (diluted 1:500-1:1000) were added to the appropriate wells and incubated at room temperature with shaking for 90 minutes then the wells were washed 4 times with PBS-Tween. The captured C5a was detected by adding 100 μl of 0.5 μg/ml of biotin conjugated rat anti-mouse C5a detection antibody (clone I52-278, BD-Pharmingen) to each well for 1 hour at room temperature with shaking followed 4 washes with PBS-Tween. Next, 100 μl of 160 ng/ml of HRP conjugated streptavidin (KPL, Gaithersburg, MD) was added to each well for 30 minutes at room temperature with shaking. After washing 5 times with wash buffer, 100 μl of substrate solution (KPL) was added to each well, and the plate was incubated for 10 minutes or until strong color development in the wells. Stop solution (KPL) was added to each well and the plate was read using a Spectramax M2 plate reader (Molecular Devices, Sunnyvale, CA) at 450 nm. The data were analyzed using Softmax Pro version 5.0 (Molecular Devices). The specificity of the assay was verified by using serum from C5-deficient mice (B10.D2/oSnJ) obtained from Jackson Laboratories. This serum had no detectable C5a following in vitro complement activation.
Chemotaxis Assays
Cell movement was measured with a 48 well microchemotaxis chamber (Neuroprobe, Cabin John, MD) using a 5.0 μm pore size cellulose nitrate filters (purchased from Neuroprobe) as previously described (21). Human neutrophils were isolated from venous blood of healthy, medication-free donors who gave informed consent (21). Mouse DBP +/+ and -/- neutrophils were isolated from the bone marrow of femur and tibia bones as previously described (36). In each assay, the migration of 200,000 neutrophils (50 μl of 4×106/ml) was evaluated. Cell movement was quantitated microscopically by measuring the distance in microns (μm) that the leading front of cells had migrated into the filter according to the method described by Zigmond and Hirsch (37). In each experiment, five fields per duplicate filter were measured at 400x magnification. The values of the background controls for random cell movement (cells responding to buffer) were subtracted in all cases so that the data are presented as net movement in μm.
The under agarose migration assay was performed as previously described (38) with some modifications. All plates were prepared in a tissue culture hood to maintain a sterile environment. Briefly, glass cover slips (22 × 22 mm) were coated with type I collagen (0.005% collagen solution in 1% acetic acid) and incubated at 4°C overnight, after which the collagen solution was removed and each plate was washed 3x with DPBS. The agarose solution was prepared by mixing a preheated (70°C) 20 ml solution of 1% BSA in RPMI and 10 ml of 2x HBSS with 10 ml of 5% UltraPure Agarose (Invitrogen, Carlsbad, CA) dissolved in dH2O. Immediately after mixing, 3 ml was added to each plate and allowed to solidify for 1 hour at room temperature. Next, 3 holes (in a line 2.3 mm apart) were punched in duplicate using a custom-fabricated hole punching apparatus, the agarose plugs were removed using an aspirator and the plates were incubated at 37°C and 5% CO2 for 1 hour to equilibrate the pH. Mouse bone marrow neutrophils were isolated from the femurs and tibias of 3 DBP +/+ and DBP -/- mice as previously described (36). Center wells were loaded with 20 μl of mouse bone marrow cells (20 × 106/ml) while the left and right sample wells were loaded with 20 μl of chemoattractant or assay buffer (negative control). Plates were incubated at 37°C for 4.5 hours after which non-adherent cells were removed by aspiration and each plate was fixed using 1.3 ml of 2% paraformaldehyde. Images of migrating cells were obtained using a Nikon Eclipse Ti microscope at 100x magnification and then analyzed with Macnification software (Interface Design Software) and ImageJ (NIH). The net distance migrated was determined by subtracting the distance migrated toward the buffer well from the distance cells migrated to the chemoattractant well. For each sample, 12 measurements of net migration distance in μm were obtained, and each sample was analyzed in duplicate.
Data Analysis and Statistics
Each experiment had a minimum of 5 mice per treatment group. Results of several experiments were analyzed for significant differences among group means using either an unpaired T-test (comparing two groups) or analysis of variance (ANOVA) followed by a multiple comparisons post-test (comparing three or more groups). Statistical testing was performed using the software program InStat (GraphPad Software, San Diego, CA).
RESULTS
DBP Functions as a Significant Chemotactic Cofactor for C5a In Vivo
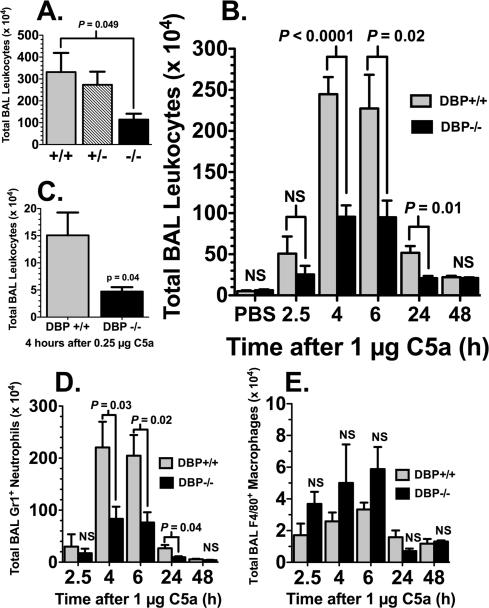
The in vivo role of DBP in augmenting neutrophil recruitment was assessed first using the immune complex lung injury model. This well-established murine model of inflammation is dependent on immune complex activation of complement and production of C5a to generate a short-term alveolitis (31). The total number of leukocytes recovered by BAL 4 hours after intravenous (i.v.) injection of ovalbumin and intratracheal (i.t.) instillation of rabbit anti-ovalbumin showed a robust inflammatory injury that was significantly diminished in null (-/-) mice compared to the hemizygous (+/-) or wild-type (+/+) animals (Fig. 1A). The total BAL leukocytes recovered from the antigen controls (ovalbumin i.v., PBS i.t.) 5.3 ± 0.87 × 104 (n=6), and the antibody controls (PBS i.v., anti-ovalbumin i.t.) 18.1 ± 2.88 × 104 (n=5), were significantly less (P < 0.01) than animals receiving both antigen and antibody (Fig. 1A). These results demonstrate that DBP-/- animals have diminished recruitment of leukocytes to the lungs following an inflammatory injury. The immune complex-induced alveolitis model relies on precise timing of the i.v. injections and i.t. instillations so that proper concentrations of antigen and antibody interact at the correct place and time to activate complement, consequently this model can produce variable results. In addition, the mechanisms of cell recruitment and tissue injury in immune complex-induced models of inflammation are multifaceted and involve inhibitory and activating Fc gamma receptors, chemokines as well as different complement activation products, so the response is not exclusive to C5a. Therefore, to target a C5a response specifically, we developed a simpler lung injury model by instilling purified mouse C5a directly into the airspaces; this approach produced a very reproducible transient alveolitis with 100% survival. Figure 1B shows the time course of leukocyte influx into the lung in response to instilled C5a (1 μg), DBP-/- mice had less than 50% the number of leukocytes in the BAL than DBP+/+ animals, a difference that was statistically significant at the 4, 6 and 24 hour time points. A lower concentration of C5a (0.25 μg) produced a less robust alveolitis but the DBP-/- phenotype of a significantly diminished leukocyte influx was still observed (Fig. 1C). Analysis of the BAL cells in figure 1B by flow cytometry revealed that the overwhelming majority of cells recruited into the airspaces in response to C5a were Gr-1+ neutrophils, and these cells accounted for the significant differences between DBP+/+ and -/- mice at 4, 6 and 24 hours (Fig. 1D). Interestingly, DBP-/- mice showed a slight but not significant increase in F4/80+ macrophages in the BAL at the 2.5, 4 and 6-hour timepoints (Fig. 1E).
Figure 1.
Cellular response of DBP-/- mice to complement-dependent alveolitis. (A) Total leukocytes in the BAL 4 hours after immune complex alveolitis in wild-type (+/+), hemizygotes (+/-) and DBP null (-/-) mice. (B) Time course of total leukocytes in the BAL of DBP+/+ and DBP-/- after instillation of 1 μg purified mouse C5a in a total volume of 50 μl. (C) Total leukocytes in the BAL of DBP+/+ and DBP-/- mice 4 hours after instillation of 0.25 μg purified mouse C5a in a total volume of 50 μl. (D) Time course of Gr-1+ neutrophils in the BAL after 1 μg C5a. (E) Time course of F4/80+ macrophages in the BAL after 1 μg C5a. Numbers represent mean ± SEM, n = 5-12 mice per treatment group. Statistical significance is indicated, NS = not significant.
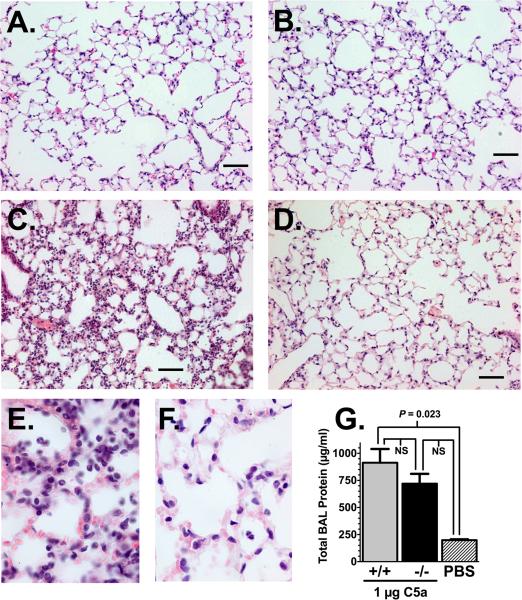
Examination of lung histology from DBP+/+ and -/- mice at the peak of C5a-induced alveolitis (4 hours after C5a) supported the BAL cell results in figure 1. Figure 2 shows H&E stained sections of lung parenchyma from DBP+/+ (panels A, C, E) and DBP-/- (panels B, D, F) mice. PBS controls show normal lung architecture in the DBP +/+ (Fig. 2A) and -/- (Fig. 2B) animals. In contrast, 4 hours after instillation of 1 μg C5a, the DBP+/+ mice (Fig. 2C) had a marked increase in the number of inflammatory cells, interstitial thickening and hemorrhage that was not observed in DBP-/- mice (Fig. 2D). High power (1000x) images (DBP+/+, panel E; DBP-/-, panel F) clearly demonstrate the increased cellularity in DBP+/+ animals. To further examine C5a-induced alveolitis, the protein concentration in the cell-free BAL was measured as an indicator of vascular leakage into the airspaces. As shown in figure 2G there was a significant increase in the total cell-free BAL protein in DBP+/+ C5a challenged mice versus the PBS controls (protein values of +/+ and -/- mice were identical and were combined). DBP-/- mice treated with C5a had lower total BAL protein than corresponding DBP+/+ mice but the value did not reach statistical significance. These results demonstrate for the first time that DBP functions as a significant C5a chemotactic cofactor in vivo.
Figure 2.
Histology of alveolitis in DBP+/+ and DBP-/- mice 4 hours after instillation of 1 μg purified mouse C5a or PBS in a total volume of 50 μl. Panels A to D, images at 200x magnification, bar = 100 μm. (A) DBP+/+ mouse treated with PBS. (B) DBP-/- mouse treated with PBS. (C) DBP+/+ mouse treated with C5a. (D) DBP-/- mouse treated with C5a. (E) DBP+/+ mouse treated with C5a at 1000x magnification. (F) DBP-/- mouse treated with C5a at 1000x magnification. (G) Total protein content of cell-free BAL fluid from C5a and PBS-treated mice. Numbers represent mean ± SEM, n= 5-7. Statistical significance is indicated, NS = not significant.
C5a is known to induce alveolar macrophages to synthesize and release numerous chemoattractants (31). Thus, it is possible that purified C5a instilled into the lung could have triggered macrophage release of secondary chemotactic factors, and these would function as the main chemotactic stimulus. To test for this potential indirect effect of C5a, cell-free BAL fluids collected from DBP+/+ and -/- mice 4 hours after instillation of 1 μg C5a were analyzed using multi-analyte ELISA array kits to detect 13 different inflammatory cytokines and chemokines: IL-1β, IL-6, IL-10, IL-12, IL-17A, G-CSF, TNFα, KC (CXCL1), MCP-1 (CCL2), MIP-1α (CCL3), MIP-1β (CCL4), RANTES (CCL5), and SDF-1 (CXCL12). Supplemental Table I presents the data from six individual mice and shows that all BAL fluids were negative for these cytokines/chemokines with the exception of one wild-type mouse that had elevated TNFα and KC (CXCL1) levels. However, the elevated cytokine levels in this mouse did not correspond with an increased number of BAL neutrophils. These results confirm that C5a is the primary chemotactic signal in the lungs 4 hours after it is instilled.
Chemotactic Cofactor Function of DBP is Not Specific for C5a In Vivo
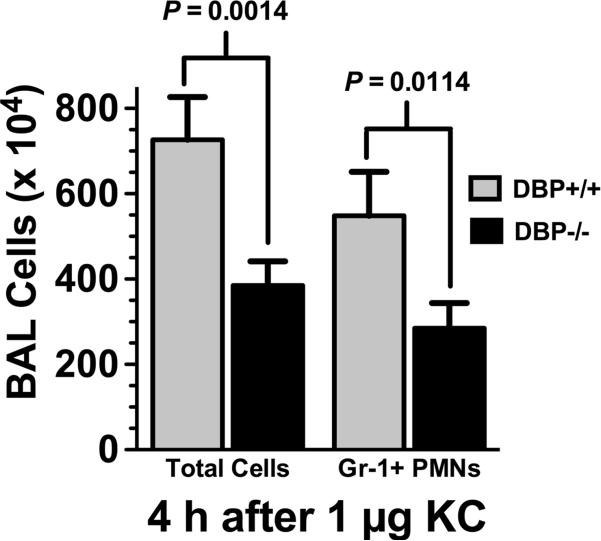
Numerous in vitro studies have shown unequivocally that DBP enhances leukocyte chemotaxis specifically to C5a (6-13). However, the possibility remains that the cofactor function of DBP for other chemoattractants may not have been uncovered due to the limitations of the in vitro assay (i.e., fixed migration depth of filters). In vivo assays are not constrained by these limitations and should reveal if DBP enhances cell recruitment in response to other chemoattractants besides C5a. Using the alveolitis model we next tested several purified neutrophil chemoattractants (KC, formylated peptides, platelet-activating factor) in wild type C57BL/6 mice to find a stimulus that could mimic the alveolitis at 4 hours induced by 1 μg C5a. The mouse chemokine KC (CXCL1) was the only chemoattractant that produced a similar alveolitis as C5a after 4 hours. Figure 3 shows that 1 μg purified mouse KC instilled into the lungs induced robust leukocyte recruitment at 4 hours, and the majority of these BAL cells were Gr-1+ neutrophils. Interestingly, a significant decrease in the number of BAL neutrophils was observed in DBP-/- mice compared to DBP+/+ animals (Fig. 3). The results with KC (~50% decrease in neutrophil recruitment) are similar to what was observed with purified C5a in DBP-/- mice (Fig. 1), and clearly indicate that diminished neutrophil recruitment into the lungs of DBP-/- mice is not specific for C5a. These results suggest that in vivo DBP could have a significant role in recruiting neutrophils to sites of inflammation by functioning as a cofactor for multiple chemoattractants.
Figure 3.
Analysis of BAL cells in KC-induced alveoltis. Total BAL leukocytes and Gr-1+ neutrophils (PMNs) were measured in DBP+/+ (n = 16) and DBP-/- (n = 15) mice 4 hours after instillation of 1 μg purified mouse KC (CXCL1) in a total volume of 50 μl. Numbers represent mean ± SEM, statistical significance is indicated.
DBP Deficiency Results in a Neutrophil Recruitment Defect
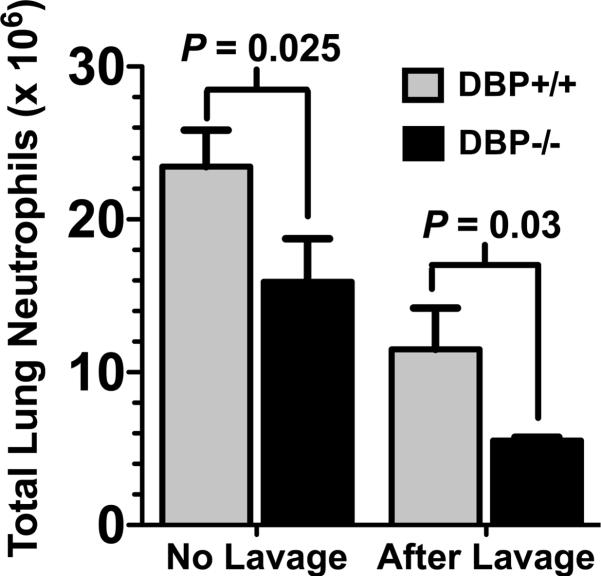
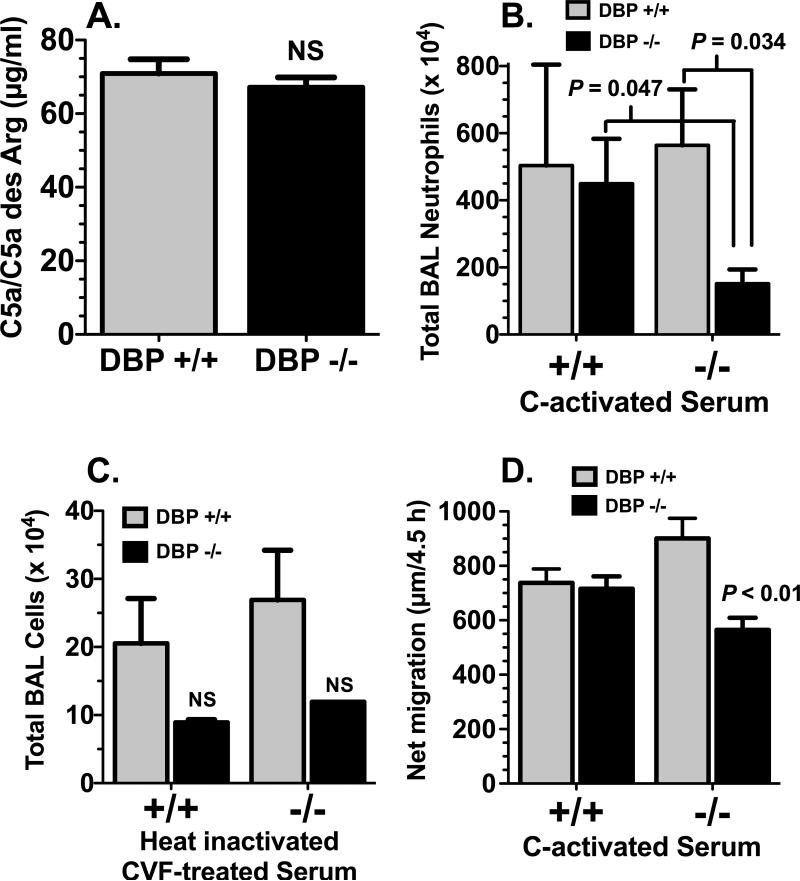
The reduced number of BAL neutrophils in DBP-/- mice could be due to a diminished recruitment to the lung or a defect in migration across the pulmonary microvasculature into the airspaces. This question was investigated by analyzing the total number of neutrophils in whole lung homogenates 4 hours after instillation of 1 μg C5a (Fig. 4). There was a significant diminution both in total Gr-1+ lung neutrophils (no lavage) and residual neutrophils in the lung after lavage in DBP-/- mice (Fig. 4), indicating that significantly fewer neutrophils were recruited to the lungs of DBP-/- mice in response to C5a. There are several possible mechanisms that could explain why these mice have a significantly diminished neutrophil recruitment to the lungs. For example, DBP-/- mice could be neutropenic as compared to wild type mice, or DBP-/- neutrophils may have reduced expression of chemoattractant receptors and/or adhesion molecules, or cells could have an inherent chemotactic defect. Analysis of the Gr-1 high, side-scatter (SSc) high population in the blood (Supplemental Figure, panel A) of DBP+/+ and -/- mice showed the same number of circulating neutrophils (Supplemental Figure, panel B) and identical expression of CD88, the C5a receptor (Supplemental Figure, panel C), CD44, an adhesion receptor and a DBP binding site (Supplemental Figure, panel D), and the major neutrophil adhesion integrin CD11b (Supplemental Figure, panel E). Furthermore, both DBP+/+ and -/- neutrophils migrated essentially the same distance in 30 minutes toward purified C5a or complement-activated (C-activated) serum (Supplemental Figure, panel F) when evaluated in vitro using a Boyden-type chemotaxis chamber and 120 μm thick tortuous pore cellulose nitrate filters. These results support the premise that a systemic absence of DBP results in defective neutrophil recruitment to the lungs.
Figure 4.
Analysis of total neutrophils in lung homogenates after C5a-induced alveolitis. Gr-1+ neutrophils were measured in homogenized lungs from DBP+/+ (n = 5) and DBP-/- (n = 5) mice 4 hours after instillation of 1 μg purified mouse C5a in a total volume of 50 μl. Total neutrophils recruited were measured in lungs that were not lavaged, residual neutrophils remaining in the lung after lavage was measured in separate group of animals. Numbers represent mean ± SEM, statistical significance is indicated.
Exogenous DBP Can Correct the Chemotactic Cofactor Deficiency in DBP-/- Mice
Next, the question of whether the diminished neutrophil recruitment in DBP-/- mice could be corrected with exogenous DBP was investigated. Figure 5 shows alveolitis in DBP+/+ and -/- mice induced by either +/+ or -/- C-activated mouse serum. Mouse serum was collected from several untreated DBP+/+ and -/- mice and complement was activated with cobra venom factor (CVF) for 60 min at 37°C. Figure 5A indicates that CVF-induced activation of complement generated the same level of C5a in both DBP+/+ and -/- serum. Diluted (50% with PBS) complement-activated serum was instilled into the lungs and total recruited BAL Gr-1+ neutrophils were measured after 4 hours. Figure 5B shows that there was no difference in the number of cells in the BAL of wild type DBP+/+ mice exposed to either +/+ or -/- complement-activated serum. In contrast, DBP-/- animals exposed to -/- complement-activated serum had significantly fewer cells in the BAL than all other groups, whereas reconstitution of DBP-/- mice with +/+ complement-activated serum completely reversed this reduced number of neutrophils (Fig. 5B). To control for serum components and CVF, serum was first heat-inactivated (56°C for 45 min) to prevent complement activation and then subsequently treated with CVF (Fig. 5C). Mice treated with these serum samples had less than 5% the number of total BAL leukocytes (and less than 0.2% of neutrophils) as compared to animals treated with complement-activated serum, indicating that C5a is the primary chemotactic factor in these samples. Finally, an in vitro correlate of this in vivo experiment was performed using the under agarose chemotaxis assay (Fig. 5D). This assay permits cells to migrate longer (4.5 h) and over much greater distances as opposed to the filter-based Boyden chamber-style chemotaxis method (Supplemental Figure, panel F), and this increased assay time may be required to reveal migratory differences. Indeed, results from the under-agarose assay confirm the in vivo findings that DBP-/- neutrophils show significantly reduced chemotaxis compared to all other groups when migrating toward -/- complement-activated serum, but this migratory defect can be reversed with DBP+/+ complement-activated serum (Fig. 5D). These results indicate that diminished recruitment of DBP-/- neutrophils can be corrected by exogenous DBP, further highlighting the importance of DBP as a chemotactic cofactor.
Figure 5.
Alveolitis induced by instillation of complement-activated serum. (A) C5a/C5a des Arg levels in DBP+/+ and DBP-/- mouse serum activated with CVF as measured by ELISA (n = 3). (B) Total Gr-1+ neutrophils in the BAL of DBP+/+ and DBP-/- mice 4 hours after instillation of 50 μl of 50% dilution (in PBS) of +/+ or -/- C-activated serum (n = 5). (C) Total leukocytes in the BAL from DBP+/+ and DBP-/- mice measured 4 hours after instillation of 50 μl of 50% dilution (in PBS) of +/+ or -/- heat-inactivated (56°C for 45 min) CVF-treated serum (n = 4). Numbers represent mean ± SEM, statistical significance is indicated, NS = not significant. (D) In vitro under-agarose chemotaxis assay using bone-marrow derived neutrophils from DBP+/+ and -/- mice. Numbers represent the net (buffer control subtracted) migration distance of cells toward either DBP+/+ or -/- complement-activated serum (n = 12). DBP-/- cells migrated significantly less (P < 0.01) toward DBP-/- complement-activated serum than all other groups.
DBP Functions as a Chemotactic Cofactor for Neutrophils in a Model of Chronic Inflammatory Injury
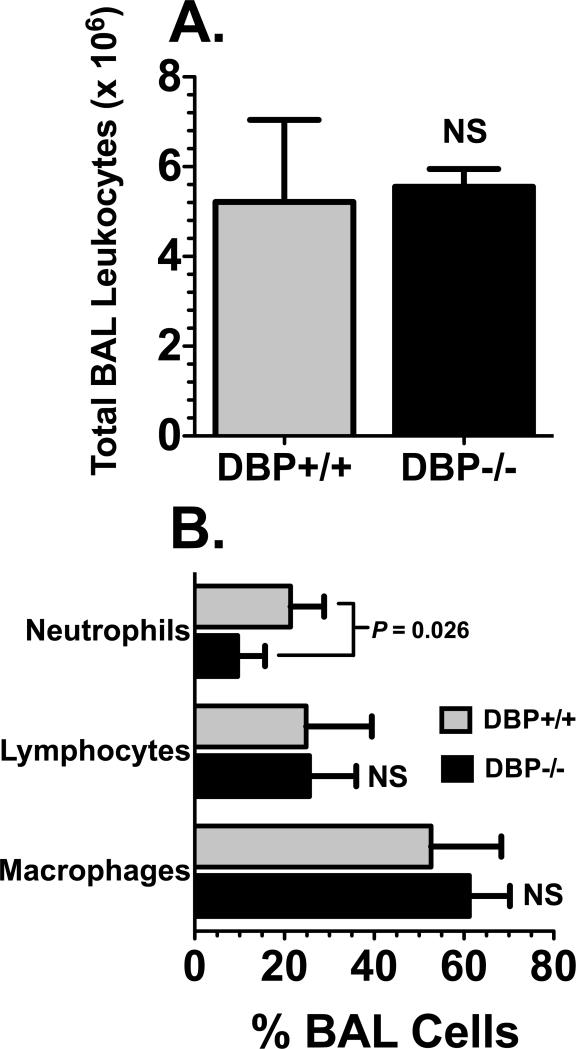
The question of whether DBP can function to augment tissue recruitment of leukocytes in a chronic inflammatory lesion was investigated using the bleomycin-induced model of lung injury, a well-characterized murine model of pulmonary inflammation and fibrosis (34, 39). In this complex multi-phasic injury model numerous chemoattractants, cytokines and growth factors have been shown to mediate different aspects of the inflammatory and fibrotic phases (34, 39). Figure 6 shows leukocyte recruitment into the airspaces at the peak of the chronic inflammatory phase (7 days after bleomycin instillation). There was no difference in the total numbers of BAL cells between DBP+/+ and DBP-/- mice (Fig. 6A). However, consistent with the findings of the acute alveolitis models, the number of neutrophils in DBP-/- BAL was significantly reduced (again by about 50%) compared to their DBP+/+ counterparts (Fig. 6B). There was no difference in the number of BAL macrophages or lymphocytes between the two strains, suggesting that the DBP chemotactic cofactor effect may be selective for augmenting neutrophil recruitment. Moreover, there is a diverse range of molecules that mediate chemotaxis in this chronic inflammatory model, further supporting the concept that DBP functions broadly as a neutrophil chemotactic cofactor and is not specific for C5a.
Figure 6.
Analysis of BAL cells in a bleomycin-induced alveolitis model. Lungs from DBP+/+ (n = 5) and DBP-/- (n = 5) mice were lavaged 7 days after 0.05 U of bleomycin was instilled into the lungs. (A) Total BAL leukocytes. (B) Percent neutrophils, lymphocytes or macrophages in the total BAL cell population. The percent BAL neutrophils in DBP-/- mice was significantly less (P = 0.026) compared to DBP+/+ animals. NS = not significant.
DBP-Actin Complexes may be the Functional Chemotactic Cofactor
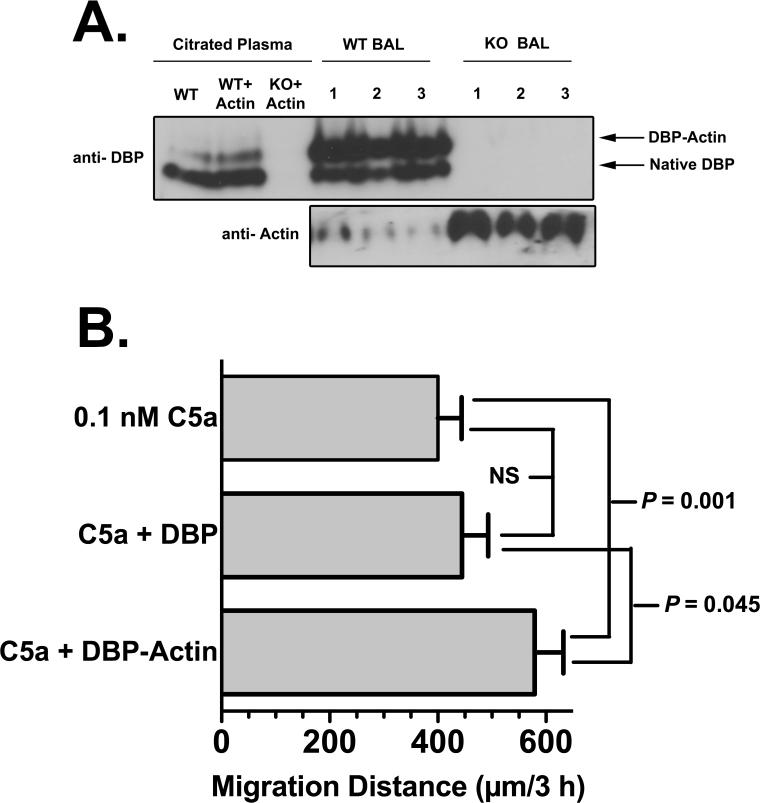
Mechanistically, the enhancement of neutrophil tissue recruitment by DBP may be associated with one of the two primary binding functions of DBP: sequestration of monomeric G-actin and transport of vitamin D. The actin binding property of DBP is the more plausible function to investigate since the protein binds with high affinity (Kd of 10-9 M) to G-actin released from cells at sites tissue injury (40, 41). Moreover, circulating DBP-actin complexes have been observed in several forms of severe trauma (42-44). A large percentage of the circulating DBP pool (>50%) can be complexed with actin during tissue injury, whereas no more than 1-2% of total DBP is ever bound with vitamin D (15). BAL fluids obtained from DBP+/+ and -/- mice four hours after instillation of 1 μg C5a were analyzed by native (non-denaturing) polyacrylamide gel electrophoresis and immunoblotting to determine if DBP is complexed with actin. Figure 7A upper panel (DBP blot) shows that in the BAL of all wild-type mice the majority of DBP is complexed with actin. As a reference, the position of DBP-actin complexes formed in plasma by the addition of purified actin is shown (compare WT plasma+actin versus WT BALs). DBP-/- mice as expected have no DBP in plasma or BAL fluid. BAL fluid from PBS–treated control DBP+/+ mice showed very little or no DBP (data not shown), indicating that most of the DBP in BAL fluid originates from exudated plasma. An immunoblot of the same BAL samples using anti-actin (Fig. 7A, lower panel) demonstrates that DBP-/- mice had uncomplexed actin in the BAL fluid as evidenced by the significant immunoreactive bands. (Note that anti-actin Ab does not detect actin when it is bound to DBP.) The DBP+/+ mice had strikingly less of these free actin bands (lower panel) since most actin was complexed with DBP (upper panel). Finally, the question of whether DBP-actin complexes function as an active chemotactic cofactor was investigated. To test this premise, an in vitro neutrophil chemotaxis assay using the under agarose method and purified C5a with DBP or DBP-actin was performed (Fig. 7B). Results show that DBP-actin can enhance C5a-induced chemotaxis to a significantly greater degree than DBP alone. These results suggest that DBP may need to bind G-actin to manifest the chemotactic cofactor function.
Figure 7.
DBP complexes with actin in BAL fluid. (A) Cell-free BAL fluid from DBP+/+ wild-type (WT) and DBP-/- knock-out (KO) mice obtained 4 hours after instillation of 1 μg purified mouse C5a was analyzed by native (non-denaturing) polyacrylamide (8%) gel electrophoresis. Complex formation in pooled citrated plasma from WT and KO mice spiked with 1 μM purified actin (this concentration will bind 15-20% of the total plasma pool of DBP) was included as a reference marker. Upper panel is an immunoblot with chicken anti-human DBP, lower panel is an immunoblot of the same BAL samples using pan anti-actin mAb (clone ACTN05). Note that when actin is bound to DBP it is not detected by the anti-actin mAb, only unbound actin is detected. Numbers indicate BAL samples from individual WT or KO mice. (B) Chemotaxis of normal human neutrophils to purified C5a (0.1 nM), C5a + 1 μM purified DBP, or C5a + 1 μM purified DBP-actin complex (n = 6). Chemotaxis was performed using the under agarose method for 3 h at 37°C. Statistical significance is indicated.
DISCUSSION
Complement proteins and neutrophils are the primary humoral and cellular components of innate immunity that respond very rapidly to inflammatory stimuli. Activation of complement and subsequent recruitment of neutrophils not only serve to neutralize pathogens but also direct and shape the ensuing immune and wound healing responses (2, 4). Therefore, factors that regulate complement-mediated tissue recruitment of neutrophils would have clear physiological relevance. Prior to this report, the chemotactic cofactor function of DBP was strictly an in vitro phenomenon and consequently was not widely appreciated. This paper presents the first in vivo evidence that a systemic deficiency of DBP in mice results in diminished recruitment of neutrophils to the lung in both acute and chronic models of inflammation, and the chemotactic cofactor function of DBP is not chemoattractant (C5a) specific as was previously believed. Furthermore, results show that DBP complexed with G-actin may function as the active chemotactic cofactor. These findings should have broad implications since DBP is an abundant plasma protein that is ubiquitous in all fluid compartments (15), and accordingly, it would always be present to enhance neutrophil chemotaxis during inflammation. Data reported herein also confirms the previous ex vivo analysis of human blood and BAL where immunodepletion of DBP reduced C5a dependent chemotactic activity by more than 60% (14). One of the most interesting aspects of this work is that DBP-/- mice show a significant and selective reduction in neutrophil recruitment to the lung, both acutely in response to C5a and the murine chemokine KC (CXCL1), and chronically in a bleomycin-induced injury model. This data verifies what had been suspected for several years but were not able to demonstrate due to the limitations of the filter-based in vitro chemotactic assay, i.e., that the chemotactic cofactor function of DBP is not specific to C5a. Previously, mechanistic models to explain the specificity for C5a have been difficult to construct since DBP does not alter C5a receptor-ligand interactions (24) and DBP does not bind to C5a or the C5a receptor (20, 25). The broader role of DBP as a chemotactic cofactor for other neutrophil chemoattractants is a fundamental piece of information that will enhance the understanding of this process and clarify mechanistic models. Another important observation is that addition of exogenous DBP to the lungs of DBP-/- mice completely rescued their neutrophil recruitment defect. These results indicate that DBP-/- mice have impaired neutrophil recruitment due to lack of DBP and not a cellular defect since the total number, receptor expression and chemotaxis of circulating DBP-/- neutrophils are essentially identical to cells from their wild-type DBP+/+ counterparts.
Murine models of alveolitis were employed to determine the role of DBP in neutrophil recruitment for two reasons. First, the lung is a major target of inflammatory injury in response to both air-borne and blood-borne pathogens and these models have well-defined disease relevance. Second, the lung has several unique features that make it an ideal organ to study inflammation (reviewed in detail (31), and rodent models of alveolitis are well established and have been extensively utilized to demonstrate the importance of complement and neutrophils as mediators of lung injury (31). This study showed that neutrophils are the primary cell type that display enhanced recruitment in the presence of DBP. The F4/80+ macrophage population showed only a slight increase that was not sustained at 24 or 48 hours after instillation of C5a. This macrophage data confirms a previous study using a thioglycolate-induced model of peritonitis that showed there was no difference between DBP+/+ and -/- mice in recruitment of macrophages to the peritoneal cavity 4 days post treatment (28). However, further studies will be needed to confirm the role of DBP as a specific neutrophil chemotactic cofactor in other organs and types of injury.
Although this report provides strong evidence that DBP functions as a chemotactic cofactor in vivo, the precise mechanism of chemotaxis enhancement is not known. The chemotactic cofactor function of DBP requires a chemotactic signal, neutrophil activation, and binding of DBP to the cell surface, all of which generally does not occur in the circulating blood. Thus, in this regard, DBP functions in exudated plasma at sites of inflammation. Both previous in vitro data and the current in vivo observations are consistent with DBP functioning as a direct positive regulator of neutrophil chemotaxis. Alternatively, the data also would be consistent with DBP functioning to neutralize an endogenous inhibitor of chemotaxis. The later concept is supported by growing evidence that tissue recruitment of cells is a balance between attractive and repulsive factors. Malawista et. al. showed that plasma oleic acid functions as a tonic inhibitor of neutrophil chemotaxis (45). DBP is known to bind fatty acids including oleic acid (46, 47) and could function to scavenge inhibitory fatty acids in physiological fluids. Other chemotaxis inhibitors are expressed on neutrophils such as the repulsive guidance molecule-A (RGM-A) which functions as a chemorepulsive factor by binding to its receptor neogenin (48), and DBP could possibly disrupt that interaction. Indeed, previous in vitro studies have shown that DBP needs to interact with the surface of neutrophils in order to function as a chemotactic cofactor for C5a (16, 17, 20-23). But the possibility that DBP acts as “an inhibitor of an inhibitor” remains to be investigated.
Previously, we determined the effect of ligating DBP with its primary physiological ligands (vitamin D and G-actin) on C5a chemotactic cofactor activity in vitro (49). It was demonstrated that only the active form of vitamin D (1,25 dihydroxy-vitamin D3) bound to DBP, but not other forms of vitamin D or G-actin, completely abolishes the chemotactic cofactor function for human neutrophils in vitro (49). Unfortunately, these experiments were designed only to examine inhibition (not enhancement) of chemotactic cofactor activity. Results reported herein (Fig. 7B) show that DBP-actin complexes are significantly better at enhancing neutrophil chemotaxis than DBP alone, suggesting that DBP-actin may function as the active chemotactic cofactor. Furthermore, we have very recently observed that DBP binds to actin on the plasma membrane of activated and/or migrating neutrophils and these complexes are shed from the cell surface (D.M. Habiel et. al., manuscript in preparation), which may explain how DBP alone enhances chemotaxis in vitro (Fig. 7B). Extracellular DBP-actin complexes are found in the blood and fluids (BAL, wound fluids) of humans and animals after traumatic injury (42-44) and could function as a type of danger associated molecular pattern (DAMP) to signal immune cells of ongoing tissue injury and augment recruitment of neutrophils. Indeed, it has recently been reported that F-actin released from necrotic cells can function as a DAMP by binding to Clec9A on dendritic cells (50, 51). However, the role of DBP-actin in tissue injury will require further investigation.
In summary, the information presented herein indicates that a systemic deficiency of DBP in mice results in significantly reduced (~50%) neutrophil recruitment to the lungs in both acute and chronic alveolitis models. In addition, in vivo DBP appears to function more broadly as a chemotactic cofactor since it is not restricted to enhancing the activity of C5a. These results suggest that the regulation of chemotactic activity in physiological fluids is more complex than previously thought and may involve an intricate balance between yet unrecognized inhibitory and enhancing cofactor signals. This work also validates the concept that DBP could be a novel therapeutic target to reduce tissue injury in inflammatory disorders by limiting excessive neutrophil recruitment.
Supplementary Material
Acknowledgments
This work was supported by grants from the U.S. National Institutes of Health (NIH) to R.R.K (GM063769), N.E.C. (GM032035) and the University of Pennsylvania's Transgenic Mouse Facility P30 CA016520, P30 DK050306, P30 DK019525. D.M.H. was supported by NIH training grant T32-GM008468.
Abbreviations used in this article
- BAL
bronchoalveolar lavage
- CVF
cobra venom factor
- DAMP
danger-associated molecular pattern
- DBP
vitamin D binding protein
- i.t.
intratracheal
REFERENCES
- 1.Amulic B, Cazalet C, Hayes GL, Metzler KD, Zychlinsky A. Neutrophil function: from mechanisms to disease. Annu Rev Immunol. 2012;30:459–489. doi: 10.1146/annurev-immunol-020711-074942. [DOI] [PubMed] [Google Scholar]
- 2.Nathan C. Neutrophils and immunity: challenges and opportunities. Nature reviews. Immunology. 2006;6:173–182. doi: 10.1038/nri1785. [DOI] [PubMed] [Google Scholar]
- 3.Kolaczkowska E, Kubes P. Neutrophil recruitment and function in health and inflammation. Nature reviews. Immunology. 2013;13:159–175. doi: 10.1038/nri3399. [DOI] [PubMed] [Google Scholar]
- 4.Ricklin D, Hajishengallis G, Yang K, Lambris JD. Complement: a key system for immune surveillance and homeostasis. Nature immunology. 2010;11:785–797. doi: 10.1038/ni.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Woodruff TM, Nandakumar KS, Tedesco F. Inhibiting the C5-C5a receptor axis. Mol Immunol. 2011;48:1631–1642. doi: 10.1016/j.molimm.2011.04.014. [DOI] [PubMed] [Google Scholar]
- 6.Kew RR, Webster RO. Gc-globulin (vitamin D-binding protein) enhances the neutrophil chemotactic activity of C5a and C5a des Arg. J Clin Invest. 1988;82:364–369. doi: 10.1172/JCI113596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Perez HD, Kelly E, Chenoweth D, Elfman F. Identification of the C5a des Arg cochemotaxin. Homology with vitamin D-binding protein (group-specific component globulin). J.Clin.Invest. 1988;82:360–363. doi: 10.1172/JCI113595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Petrini M, Azzara A, Carulli G, Ambrogi F, Galbraith RM. 1,25-dihydroxycholecalciferol inhibits the cochemotactic activity of Gc (vitamin D binding protein). J.Endocrinol.Invest. 1991;14:405–408. doi: 10.1007/BF03349090. [DOI] [PubMed] [Google Scholar]
- 9.Metcalf JP, Thompson AB, Gossman GL, Nelson KJ, Koyama S, Rennard SI, Robbins RA. Gc-globulin functions as a cochemotaxin in the lower respiratory tract. A potential mechanism for lung neutrophil recruitment in cigarette smokers. Am.Rev.Respir.Dis. 1991;143:844–849. doi: 10.1164/ajrccm/143.4_Pt_1.844. [DOI] [PubMed] [Google Scholar]
- 10.Binder R, Kress A, Kan G, Herrmann K, Kirschfink M. Neutrophil priming by cytokines and vitamin D binding protein (Gc-globulin): impact on C5a-mediated chemotaxis, degranulation and respiratory burst. Mol Immunol. 1999;36:885–892. doi: 10.1016/s0161-5890(99)00110-8. [DOI] [PubMed] [Google Scholar]
- 11.Zwahlen RD, Roth DR. Chemotactic competence of neutrophils from neonatal calves. Functional comparison with neutrophils from adult cattle. Inflammation. 1990;14:109–123. doi: 10.1007/BF00914034. [DOI] [PubMed] [Google Scholar]
- 12.Senior RM, Griffin GL, Perez HD, Webster RO. Human C5a and C5a des Arg exhibit chemotactic activity for fibroblasts. Journal of Immunology. 1988;141:3570–3574. [PubMed] [Google Scholar]
- 13.Piquette CA, Robinson-Hill R, Webster RO. Human monocyte chemotaxis to complement-derived chemotaxins is enhanced by Gc-globulin. Journal of Leukocyte Biology. 1994;55:349–354. doi: 10.1002/jlb.55.3.349. [DOI] [PubMed] [Google Scholar]
- 14.Trujillo G, Zhang J, Habiel DM, Ge L, Ramadass M, Ghebrehiwet B, Kew RR. Cofactor regulation of C5a chemotactic activity in physiological fluids. Requirement for the vitamin D binding protein, thrombospondin-1 and its receptors. Mol Immunol. 2011;49:495–503. doi: 10.1016/j.molimm.2011.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chun RF. New perspectives on the vitamin D binding protein. Cell biochemistry and function. 2012;30:445–456. doi: 10.1002/cbf.2835. [DOI] [PubMed] [Google Scholar]
- 16.DiMartino SJ, Kew RR. Initial characterization of the vitamin D binding protein (Gc-globulin) binding site on the neutrophil plasma membrane: evidence for a chondroitin sulfate proteoglycan. J Immunol. 1999;163:2135–2142. [PubMed] [Google Scholar]
- 17.McVoy LA, Kew RR. CD44 and annexin A2 mediate the C5a chemotactic cofactor function of the vitamin D binding protein. J Immunol. 2005;175:4754–4760. doi: 10.4049/jimmunol.175.7.4754. [DOI] [PubMed] [Google Scholar]
- 18.Nykjaer A, Dragun D, Walther D, Vorum H, Jacobsen C, Herz J, Melsen F, Christensen EI, Willnow TE. An endocytic pathway essential for renal uptake and activation of the steroid 25-(OH) vitamin D3. Cell. 1999;96:507–515. doi: 10.1016/s0092-8674(00)80655-8. [DOI] [PubMed] [Google Scholar]
- 19.Nykjaer A, Fyfe JC, Kozyraki R, Leheste JR, Jacobsen C, Nielsen MS, Verroust PJ, Aminoff M, de la Chapelle A, Moestrup SK, Ray R, Gliemann J, Willnow TE, Christensen EI. Cubilin dysfunction causes abnormal metabolism of the steroid hormone 25(OH) vitamin D(3). Proc Natl Acad Sci U S A. 2001;98:13895–13900. doi: 10.1073/pnas.241516998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.DiMartino SJ, Shah AB, Trujillo G, Kew RR. Elastase controls the binding of the vitamin D-binding protein (Gc-globulin) to neutrophils: a potential role in the regulation of C5a co-chemotactic activity. J Immunol. 2001;166:2688–2694. doi: 10.4049/jimmunol.166.4.2688. [DOI] [PubMed] [Google Scholar]
- 21.Kew RR, Fisher JA, Webster RO. Co-chemotactic effect of Gcglobulin (vitamin D binding protein) for C5a. Transient conversion into an active co-chemotaxin by neutrophils. J Immunol. 1995;155:5369–5374. [PubMed] [Google Scholar]
- 22.Trujillo G, Kew RR. Platelet-derived thrombospondin-1 is necessary for the vitamin D-binding protein (Gc-globulin) to function as a chemotactic cofactor for C5a. J Immunol. 2004;173:4130–4136. doi: 10.4049/jimmunol.173.6.4130. [DOI] [PubMed] [Google Scholar]
- 23.DiMartino SJ, Trujillo G, McVoy LA, Zhang J, Kew RR. Upregulation of vitamin D binding protein (Gc-globulin) binding sites during neutrophil activation from a latent reservoir in azurophil granules. Mol Immunol. 2007;44:2370–2377. doi: 10.1016/j.molimm.2006.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Perez HD. Gc globulin (vitamin D-binding protein) increases binding of low concentrations of C5a des Arg to human polymorphonuclear leukocytes: An explanation for its cochemotaxin activity. Inflammation. 1994;18:215–220. doi: 10.1007/BF01534562. [DOI] [PubMed] [Google Scholar]
- 25.Zhang J, Habiel DM, Ramadass M, Kew RR. Identification of two distinct cell binding sequences in the vitamin D binding protein. Biochim Biophys Acta. 2010;1803:623–629. doi: 10.1016/j.bbamcr.2010.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang J, Kew RR. Identification of a region in the vitamin D-binding protein that mediates its C5a chemotactic cofactor function. J Biol Chem. 2004;279:53282–53287. doi: 10.1074/jbc.M411462200. [DOI] [PubMed] [Google Scholar]
- 27.Safadi FF, Thornton P, Magiera H, Hollis BW, Gentile M, Haddad JG, Liebhaber SA, Cooke NE. Osteopathy and resistance to vitamin D toxicity in mice null for vitamin D binding protein. Journal of Clinical Investigation. 1999;103:239–251. doi: 10.1172/JCI5244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.White P, Liebhaber SA, Cooke NE. 129X1/SvJ mouse strain has a novel defect in inflammatory cell recruitment. J Immunol. 2002;168:869–874. doi: 10.4049/jimmunol.168.2.869. [DOI] [PubMed] [Google Scholar]
- 29.Zella LA, Shevde NK, Hollis BW, Cooke NE, Pike JW. Vitamin D-binding protein influences total circulating levels of 1,25-dihydroxyvitamin D3 but does not directly modulate the bioactive levels of the hormone in vivo. Endocrinology. 2008;149:3656–3667. doi: 10.1210/en.2008-0042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shushakova N, Eden G, Dangers M, Menne J, Gueler F, Luft FC, Haller H, Dumler I. The urokinase/urokinase receptor system mediates the IgG immune complex-induced inflammation in lung. J Immunol. 2005;175:4060–4068. doi: 10.4049/jimmunol.175.6.4060. [DOI] [PubMed] [Google Scholar]
- 31.Gao H, Neff T, Ward PA. Regulation of lung inflammation in the model of IgG immune-complex injury. Annual review of pathology. 2006;1:215–242. doi: 10.1146/annurev.pathol.1.110304.100155. [DOI] [PubMed] [Google Scholar]
- 32.De Vooght V, Vanoirbeek JA, Haenen S, Verbeken E, Nemery B, Hoet PH. Oropharyngeal aspiration: an alternative route for challenging in a mouse model of chemical-induced asthma. Toxicology. 2009;259:84–89. doi: 10.1016/j.tox.2009.02.007. [DOI] [PubMed] [Google Scholar]
- 33.Trujillo G, O'Connor EC, Kunkel SL, Hogaboam CM. A novel mechanism for CCR4 in the regulation of macrophage activation in bleomycin-induced pulmonary fibrosis. Am J Pathol. 2008;172:1209–1221. doi: 10.2353/ajpath.2008.070832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Moore BB, Hogaboam CM. Murine models of pulmonary fibrosis. American journal of physiology. Lung cellular and molecular physiology. 2008;294:L152–160. doi: 10.1152/ajplung.00313.2007. [DOI] [PubMed] [Google Scholar]
- 35.Huber-Lang M, Sarma JV, Zetoune FS, Rittirsch D, Neff TA, McGuire SR, Lambris JD, Warner RL, Flierl MA, Hoesel LM, Gebhard F, Younger JG, Drouin SM, Wetsel RA, Ward PA. Generation of C5a in the absence of C3: a new complement activation pathway. Nat Med. 2006;12:682–687. doi: 10.1038/nm1419. [DOI] [PubMed] [Google Scholar]
- 36.Boxio R, Bossenmeyer-Pourie C, Steinckwich N, Dournon C, Nusse O. Mouse bone marrow contains large numbers of functionally competent neutrophils. J Leukoc Biol. 2004;75:604–611. doi: 10.1189/jlb.0703340. [DOI] [PubMed] [Google Scholar]
- 37.Zigmond S, Hirsch J. Leukocyte locomotion and chemotaxis. New methods for evaluation and demonstration of a cell-derived chemotactic factor. Journal of Experimental Medicine. 1973;137:387–410. doi: 10.1084/jem.137.2.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Heit B, Kubes P. Measuring chemotaxis and chemokinesis: the under-agarose cell migration assay. Science's STKE : signal transduction knowledge environment. 20032003:PL5. doi: 10.1126/stke.2003.170.pl5. [DOI] [PubMed] [Google Scholar]
- 39.Matute-Bello G, Frevert CW, Martin TR. Animal models of acute lung injury. American journal of physiology. Lung cellular and molecular physiology. 2008;295:L379–399. doi: 10.1152/ajplung.00010.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Swamy N, Head JF, Weitz D, Ray R. Biochemical and preliminary crystallographic characterization of the vitamin D sterol- and actin-binding by human vitamin D-binding protein. Arch Biochem Biophys. 2002;402:14–23. doi: 10.1016/S0003-9861(02)00033-4. [DOI] [PubMed] [Google Scholar]
- 41.Verboven C, Bogaerts I, Waelkens E, Rabijns A, Van Baelen H, Bouillon R, De Ranter C. Actin-DBP: the perfect structural fit? Acta crystallographica. Section D, Biological crystallography. 2003;59:263–273. doi: 10.1107/s0907444902021455. [DOI] [PubMed] [Google Scholar]
- 42.Haddad JG, Harper KD, Guoth M, Pietra GG, Sanger JW. Angiopathic consequences of saturating the plasma scavenger system for actin. Proceeding of the National Academy of Sciences. 1990;87:1381–1385. doi: 10.1073/pnas.87.4.1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dahl B, Schiodt FV, Kiaer T, Ott P, Bondesen S, Tygstrup N. Serum Gc-globulin in the early course of multiple trauma. Critical Care Medicine. 1998;26:285–289. doi: 10.1097/00003246-199802000-00027. [DOI] [PubMed] [Google Scholar]
- 44.Dahl B, Schiodt FV, Nielsen M, Kiaer T, Williams JG, Ott P. Admission level of Gc-globulin predicts outcome after multiple trauma. Injury. 1999;30:275–281. doi: 10.1016/s0020-1383(99)00080-7. [DOI] [PubMed] [Google Scholar]
- 45.Malawista SE, de Boisfleury Chevance A, van Damme J, Serhan CN. Tonic inhibition of chemotaxis in human plasma. Proc Natl Acad Sci U S A. 2008;105:17949–17954. doi: 10.1073/pnas.0802572105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Williams MH, Van Alstyne EL, Galbraith RM. Evidence of a novel association of unsaturated fatty acids with Gc (vitamin D-binding protein). Biochem Biophys Res Commun. 1988;153:1019–1024. doi: 10.1016/s0006-291x(88)81330-5. [DOI] [PubMed] [Google Scholar]
- 47.Ena JM, Esteban C, Perez MD, Uriel J, Calvo M. Fatty acids bound to vitamin D-binding protein (DBP) from human and bovine sera. Biochemistry international. 1989;19:1–7. [PubMed] [Google Scholar]
- 48.Mirakaj V, Brown S, Laucher S, Steinl C, Klein G, Kohler D, Skutella T, Meisel C, Brommer B, Rosenberger P, Schwab JM. Repulsive guidance molecule-A (RGM-A) inhibits leukocyte migration and mitigates inflammation. Proc Natl Acad Sci U S A. 2011;108:6555–6560. doi: 10.1073/pnas.1015605108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shah AB, DiMartino SJ, Trujillo G, Kew RR. Selective inhibition of the C5a chemotactic cofactor function of the vitamin D binding protein by 1,25(OH)2 vitamin D3. Mol Immunol. 2006;43:1109–1115. doi: 10.1016/j.molimm.2005.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ahrens S, Zelenay S, Sancho D, Hanc P, Kjaer S, Feest C, Fletcher G, Durkin C, Postigo A, Skehel M, Batista F, Thompson B, Way M, Reis e Sousa C, Schulz O. F-actin is an evolutionarily conserved damage-associated molecular pattern recognized by DNGR-1, a receptor for dead cells. Immunity. 2012;36:635–645. doi: 10.1016/j.immuni.2012.03.008. [DOI] [PubMed] [Google Scholar]
- 51.Zhang JG, Czabotar PE, Policheni AN, Caminschi I, Wan SS, Kitsoulis S, Tullett KM, Robin AY, Brammananth R, van Delft MF, Lu J, O'Reilly LA, Josefsson EC, Kile BT, Chin WJ, Mintern JD, Olshina MA, Wong W, Baum J, Wright MD, Huang DC, Mohandas N, Coppel RL, Colman PM, Nicola NA, Shortman K, Lahoud MH. The dendritic cell receptor Clec9A binds damaged cells via exposed actin filaments. Immunity. 2012;36:646–657. doi: 10.1016/j.immuni.2012.03.009. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.