Abstract
Hoxa9 and Flt3 signaling are individually important for the generation of lymphoid lineage precursors from multipotent hematopoietic progenitors (MPP) in bone marrow (BM). Mice deficient for Hoxa9, Flt3, or Flt3-ligand (FL) have reduced numbers of lymphoid-primed multipotential progenitors (LMPP), common lymphoid progenitors (CLP), and B/T cell precursors. Hoxa9 regulates lymphoid development, in part, through transcriptional regulation of Flt3. However, it was unclear if Hoxa9 has functions in lymphopoiesis independent of, or alternatively, synergistically with Flt3 signaling. Here we show that Hoxa9−/−Flt3l−/− mice have more severe deficiencies in all B lineage cells, CLP, LMPP, and total Flt3+ MPP in BM than the single knockouts. Although LMPP and Flt3+ CLP contain precursors for NK and DC lineage cells, no deficiencies in these lineages beyond that in Flt3l−/− mice was found. Thymocyte cellularity was significantly reduced in the compound knockout although peripheral T cell numbers mirrored Flt3l−/− mice. Analysis of the hematopoietic progenitor compartment revealed elevated numbers of CD150+hi CD34− CD41+ myeloid-biased stem cells in Hoxa9−/−Flt3l−/− mice. In contrast, CD150− MPP enriched for lymphoid potential were synergistically reduced, suggesting Hoxa9 and Flt3 signaling function coordinately to regulate lymphopoiesis at a very early stage. Realtime PCR analysis of CD150− Flt3+ cells from WT control, Hoxa9−/−, and Flt3l−/− single knockouts revealed decreased lymphoid transcripts, corroborating the importance of these regulators in lymphoid development. Together, these studies reveal a very early checkpoint in lymphopoiesis dependent on the combinatorial activities of Hoxa9 function and Flt3 signaling.
Keywords: Hoxa9, Flt3, cell signaling, lymphopoiesis, B cell, T cell, transcriptional regulation, development
Introduction
Steady-state production of lymphoid-lineage precursors from pluripotent hematopoietic stem cells (HSC) is a stepwise process driven by the concerted activities of transcription factors and signaling molecules (1). For example, presently, at least six developmental intermediates have been characterized between HSC and committed Pro-B cells: short-term repopulating cells, Flt3+ MPP, LMPP that are Flt3hi and evidence lymphoid priming, ALP, BLP, and Pre-Pro-B cells. The ability to resolve these developmental intermediates makes it possible to more accurately define precursor-progeny relationships and assemble unique and inter-connected gene regulatory modules that instruct the B cell fate decision. Importantly, it also allows more precise identification of developmental stage specific blocks imposed by gene-targeting strategies. The end result is a better understanding of the roles of individual molecular and cellular determinants in regulation of lymphoid development and B cell differentiation.
In BM, most HSCs are quiescent and few are actively participating in blood cell genesis at any one time (2). Pluripotent HSC are enriched in the Lineage negative (Lin−), Sca-1+, c-kit+hi (collectively referred to as LSK+) fraction of BM cells expressing the SLAM family marker CD150 (3). Within LSK+ CD150+ cells, HSC can be phenotypically distinguished from multipotential progenitors (MPP) by differential expression of a variety of cell surface markers including CD34, CD48, CD27 and Flt3 (2, 4–6). HSC are LSK+ CD150+ CD34−lo, while MPPs are CD150−lo and express CD34, CD48, CD27, and Flt3. While most HSC are not actively cycling, the majority of MPP are proliferating to maintain blood cell production.
Surface expression of Flt3 is an early event in HSC differentiation and largely coincides with downregulation of CD150 (7, 8). Acquisition of Flt3 denotes lymphoid-myeloid biased differentiation potential and reduced capacity to generate erythroid/megakaryocyte progeny (9). A subset of Flt3+ MPPs expressing high levels of Flt3 express the early program of lymphoid lineage gene expression and have been denoted early lymphoid progenitors (ELP) or lymphoid primed multipotential progenitors (LMPP) (10). Flt3 signaling plays a critical role in regulation of lymphoid priming in primitive hematopoietic progenitors (10, 16). However, a direct regulatory connection between Flt3 signaling and the expression and/or activity of any lymphoid lineage specification factor remains to be established. LMPP can be prospectively identified within the LSK+ fraction using differential expression of VCAM-1 and Flt3 (VCAM-1− Flt3+hi) or by expression of GFP using RAG-1GFP knockin reporter mice (11–13). LMPP are enriched for precursors biased for T, B, NK, and/or DC differentiation potential (14, 15). At present, it is unclear whether individual LMPP retain multilineage lymphoid differentiation potential. Regardless, data obtained from numerous gene-targeting strategies has established that failure to generate and/or maintain the LMPP pool has significant consequences on the production of B lineage lymphocytes in BM (16–21).
Downregulation of c-kit and surface expression of IL-7R denotes the transition from LMPP to CLP (15). B lineage restricted progenitors (BLP) are enriched in a subset of CLPs that express the cell surface marker Ly6D, while Ly6D− CLPs exhibit B, T, and NK potentials and are referred to as all lymphoid progenitors (ALP) (22). Resolution of ALP and BLP is an important advance as it enables more accurate determination of the roles of regulatory factors in lymphoid lineage restriction, as well as B lineage commitment from lymphoid-restricted progenitors. In that vein, this study seeks to determine individual versus combinatorial roles of two key factors, the homeodomain transcription factor Hoxa9, and the cytokine, Flt3-ligand (FL), in regulation of ALP and BLP.
Hoxa9 is a transcription factor important for the generation of normal numbers of B-lineage precursors in BM (17, 19, 23). Hoxa9 is expressed at low levels in HSCs, upregulated in MPP and CLP, then downregulated in BCP (17). Hoxa9 regulates B cell development, in part, through transcriptional regulation of Flt3 (17). It is presently unclear if the B cell deficiency in Hoxa9−/− mice is due solely to impaired Flt3 expression, or if Hoxa9 and Flt3 signaling provide non-overlapping functions that guide B cell development. We theorized that if the B lineage deficiency in Hoxa9−/− mice was due to impaired Flt3 alone, then combined loss of Hoxa9 and FL would not exacerbate the B lineage deficiency beyond that observed in the single knockouts. However, if Hoxa9 has additional nonredundant roles, or functions cooperatively with Flt3 signaling to promote lymphoid development and B cell differentiation, then combined loss could exacerbate the B lineage deficiency.
In this study, through generation and analysis of Hoxa9−/−Flt3l−/− mice, we show that Hoxa9 and Flt3 signaling function cooperatively to establish the lymphoid progenitor pool in BM. Intriguingly, LSK+ CD150hi CD34−lo CD41+ cells that are myeloid-biased HSC, are over-represented in the LSK+ compartment and increased in number in the compound knockout mice. In contrast, LSK+ CD150− Flt3+ cells that are enriched for lymphoid progenitors are significantly reduced (5, 24). ALP, BLP, and B lineage lymphocytes are ablated in BM of Hoxa9−/−Flt3l−/− mice. NK and DC lineage cells are not impacted beyond what has been documented as a consequence of FL deficiency, suggesting alternative pathways of generation (25). Thymic cellularity is significantly reduced in Hoxa9−/−Flt3l−/− mice compared to the single knockouts consistent with the ablation of LMPP in BM that seed the thymus. However, T cell development on the whole appears essentially normal and numbers of peripheral T cells mirror Flt3l−/− mice. These findings establish that there is an extremely early checkpoint in lymphoid lineage development dependent on the combinatorial activities of Hoxa9 and Flt3 signaling.
Materials and Methods
Mice
C57Bl6 (referred to herein as WT or B6) mice were purchased from The Jackson Laboratory (Bar Harbor, ME). Hoxa9−/− and Flt3l−/− mice have been described (16, 17). Hoxa9−/− and Flt3l−/− mice were bred and genotyping performed to identify compound knockouts (16, 17). All mice in this study were maintained in the Mayo Clinic animal facility and used between 8–16 weeks of age. Age-matched mice of all 4 genotypes were used in each experiment and no differences in phenotype were observed in that range or in male versus female mice. All experiments were carried out in accordance with Mayo Clinic Institutional Animal Care and Use Committee guidelines.
Antibodies and flow cytometry
Methods for flow cytometry and progenitor isolation have been described (13, 16, 17). Flow cytometric analysis was performed on the LSRII cytometer (BD Biosciences, San Jose, CA) and data analysis with FlowJo software (Tree Star, Ashland, OR). All antibodies were purchased from eBioscience, BioLegend, or Pharmingen. Antibody conjugations and combinations used to delineate progenitor subsets in BM were the following: LSK+, Lineage cocktail (FITC conjugated CD45R/B220, CD11b/Mac1, Ly6G/Gr1, TER119, CD3ε, CD8α, NK1.1), c-kit-APCefluor 780, Sca-1-PerCP-Cy5.5, CD150 Pe-Cy7, Flt3-PE, CD34-eFluor 450, and CD41-APC; CLP, Lineage cocktail (FITC conjugated CD45R/B220, CD11b/Mac1, Ly6G/Gr1, TER119, CD3ε, CD8α, CD11c, NK1.1, Ly6C), c-kit-APCefluor780, Sca1-PerCPCy5.5, biotin IL-7R (visualized with streptavidin PeCy7), Flt3-PE, and Ly6D-APC; BCP (CD19-APC, B220-PE-Cy7, IgM-FITC, CD43-PE); Myeloid cells, CD11b/Mac-1-APC and Gr-1-FITC; Erythroid cells, Ter119-PeCy7; NK subsets, CD3ε-PeCy7, CD122-FITC, and NK1.1-PerCP-Cy5.5; total DCs, CD11c-PE; thymocyte subsets, CD3ε-APC, CD4-FITC and CD8α-PE. Gates for discriminating negative and positive subsets were determined based on staining of BM mononuclear cells or previously characterized positive/negative BM subsets. Spleens and thymi were harvested and single cell suspensions made by crushing the organs through a 70 micron nylon mesh. Mononuclear cell counts were determined by counting nuclei after lysis in 3% acetic acid/water solution.
Isolation of progenitor subsets for realtime PCR analysis
BM cells were harvested and pooled from 6 B6 controls, 7 Hoxa9−/−, and 8 Flt3l−/− mice in each of 2 independent experiments. Lin− cells were enriched from the 3 mouse groups by incubating BM cell suspensions with a biotin-labeled Lin+ antibody cocktail (B220, Gr-1, Ter119, CD3e, CD8a, CD11c, NK1.1, CD19) followed by incubation with streptavidin conjugated microbeads and magnetic separation. The Lin− enriched BM fractions were incubated with c-kit-eFluor 780, Sca-1- PerCP-Cy5.5, CD150-PE, Flt3-PE, and SA-FITC (to identify residual Lin+lo cells). Two LSK+ subsets were sorted from B6, Hoxa9−/−, or Flt3l−/− Lin− cells: LSK+ CD150− Flt3lo and LSK+ CD150− Flt3+. After centrifugation, the cell pellets were resuspended in 100ul of extraction buffer then stored at −80º C or processed immediately for RNA isolation using the Arcturus PicoPure RNA isolation kit (Applied Biosystems, Foster City, CA). To remove contaminating genomic DNA, the RNA was treated with DNase I kit (Qiagen, Germantown, MD). cDNA amplification was performed using the Ovation PicoSL WTA System (NuGEN, San Carlos, CA). Amplified cDNA was purified using the MinElute Reaction Cleanup kit (Qiagen, Germantown, MD). Realtime PCR was performed using Taqman probes (Applied Biosystems) or gene specific SYBR Green assays as we previously described. Gene expression was normalized to 18S RNA for Taqman assays or gapdh for SYBR Green assays. All cDNA samples were assayed in triplicate. Relative transcript abundance was determined using the 2−ΔΔCT method with B6 transcript levels for each sorted subset assigned the comparator.
Statistical analysis
Statistical significance was determined using the Student-t test. P-values ≤0.05 were deemed significant and are indicated by asterisks.
Results
Synergistic reduction in B lymphopoiesis in Hoxa9−/−Flt3l−/− mice
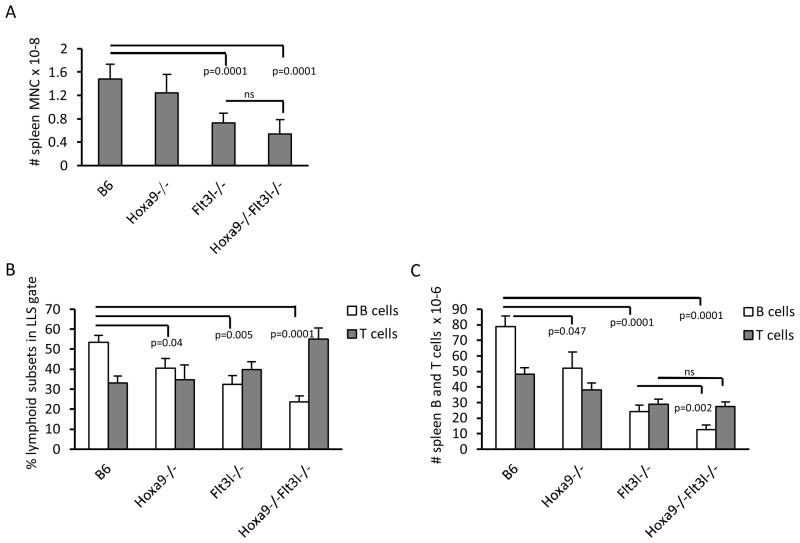
To determine if Hoxa9 provides non-redundant roles or functionally coordinates with Flt3 signaling in the generation of lymphoid progenitors and BCPs, Hoxa9−/−Flt3l−/− mice were generated. The compound knockouts were born at normal Mendelian ratios and exhibited no gross abnormalities. Peripheral blood analysis of the double knockouts mirrored Flt3l−/− mice (ie., reduced numbers and percentages of lymphocytes, data not shown). Consistent with the reduction in blood lymphocytes, spleen cellularity was reduced ~60% in Hoxa9−/−Flt3l−/− mice, compared to 39% in Flt3l−/− mice (Figure 1A). Flow cytometric analysis of T cells in the spleen showed no significant differences in percentages or absolute numbers in the compound knockouts beyond that observed in the single knockouts (Figure 1B-C). No alterations in ratios of CD4+ or CD8+ splenic T cells were observed (data not shown). In contrast, percentages of CD19+ splenic B cells were synergistically reduced in the compound knockout mice (splenic CD19+ cells, B6 = 53.4±8.4% (n=6), Hoxa9−/− = 40.6±9.4% (n=5), Flt3l−/− = 32.5±11.8% (n=6), and Hoxa9−/−Flt3l−/− = 23.8±6.6% (n=6)) (Figure 1B). The reduced percentages together with reduction in cellularity in the compound knockout culminated in a statistically significant reduction in numbers of splenic CD19+ B lymphocytes (12.5±7.4 vs. 24.1±10.2 x 106, Hoxa9−/−Flt3l−/− vs. Flt3l−/−, respectively, *p=0.047). These data suggest that combined loss of Hoxa9 and Flt3 signaling has more severe consequences on B lymphopoiesis than deficiency in Hoxa9 or FL alone. Splenic T cells are reduced, but comparable to FL deficiency alone.
Figure 1. Reduction in splenic B cells in Hoxa9−/−Flt3l−/− mice.
A) Spleen mononuclear cell counts. B) B and T cell precursor frequency in spleen. Spleen cell suspensions were incubated with anti-CD19-PE or anti-CD3ε-APC, anti-CD4-FITC, and CD8α-PE antibodies to resolve B and T lineage lymphocytes, respectively. Percentages reflect cells within the lymphoid light scatter gate. C) Absolute numbers of B or T cells in spleen. Data in A-C is representative of the mean ± standard deviation of 5–6 spleens per genotype.
Severe reduction in BM BCP in Hoxa9−/−Flt3l−/− mice
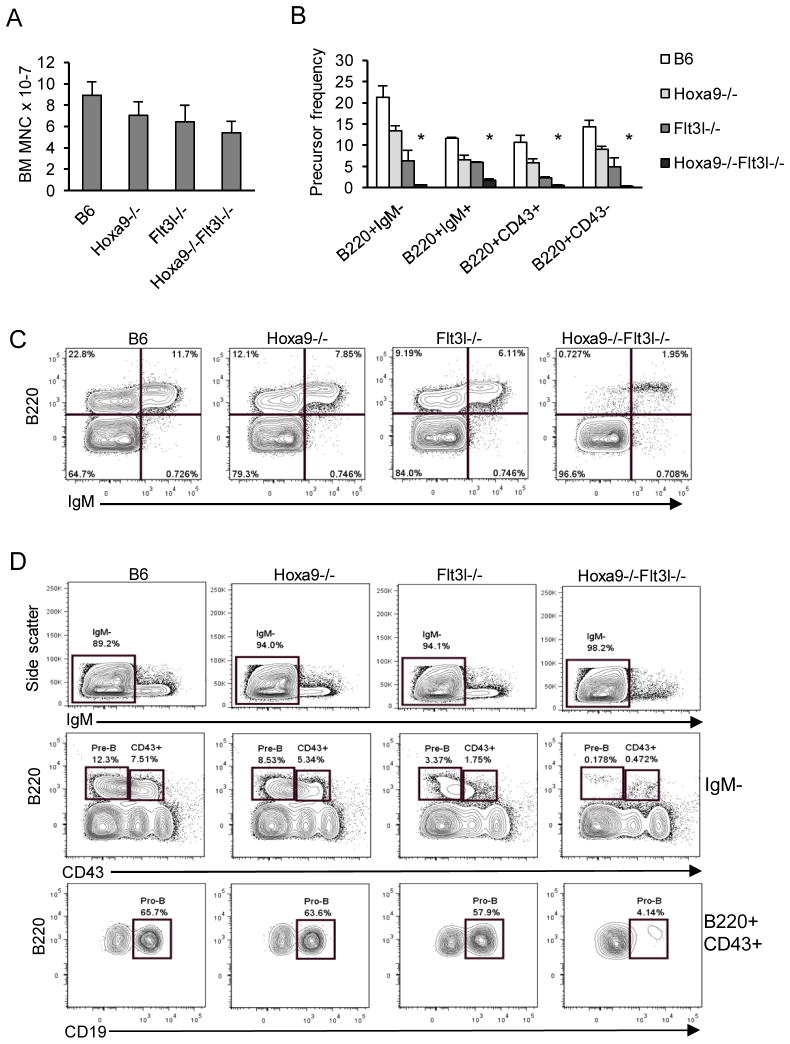
The spleen results suggested that the compound knockout mice might have selective abnormalities associated with B lymphopoiesis in BM. Hoxa9−/− and Flt3l−/− single knockout mice are known to have reduced BM cellularity, and here we determined that the same was true for Hoxa9−/−Flt3l−/− mice (Figure 2A)(23, 25). However, the reduction in BM cellularity in the compound knockouts was not statistically significant when compared to the single knockouts.
Figure 2. Synergistic defects in BM B lymphopoiesis in Hoxa9−/− Flt3l−/− mice.
A) Bone marrow mononuclear cell counts. Numbers represent total BM mononuclear cells per 4 hind limb legs bones for each mouse model analyzed. B) Summary of B lineage subset frequencies in the four mouse models. Percentages reflect cells within the lymphoid light scatter gate. The asterisks indicate statistically significant differences in percentages compared to Flt3l−/− mice. C) Representative flow cytometry profile of immature (B220+ IgM−) and mature (B220+ IgM+) BCP in the four mouse models. D) Resolution of BCP deficiencies in the four genotypes. Mature IgM− cells (gated region, top panel) were resolved into B220+ CD43− Pre-B and CD43+ (Pro-B) BCP (gated regions, middle panel). The CD43+ B220+ subset is heterogeneous and Pro-B cells within this subset express CD19. The bottom panel shows percentages of CD19+ Pro-B cells within the CD43+ B220+ subset which are severely ablated in compound knockout mice. Data represents the mean and standard deviation of 4–6 mice per genotype (n=6 Hoxa9-/Flt3l−/−).
To determine the consequence of combined loss of Hoxa9 and Flt3 signaling on B lymphopoiesis, BM cells were harvested from strain-matched C57Bl6 (B6) controls, Hoxa9−/−, Flt3l−/−, and Hoxa9−/−Flt3l−/− mice and stained with antibodies to CD45R/B220, CD19, CD43, and IgM to resolve B-lineage subsets. As summarized in Figure 2B and shown in Figure 2C-D, combined loss of Hoxa9 and FL severely ablated B lymphopoiesis. To document the stages of B lymphopoiesis affected in the compound knockout mice, mature (B220+ IgM+) and immature (B220+ IgM−) B lineage subsets were examined (Figure 2C, top panel). Indeed, the prominent subset of BCPs in marrow in the compound knockout mice were IgM+ and likely represent recirculating B lymphocytes (26). It is known that naïve B cells undergo homeostatic proliferation under conditions of B cell deficiency and the IgM+ cells in the marrow uniformly expressed high levels of B220, characteristic of recirculating B cells (26). In contrast, all IgM− BCP subsets, including B220+ CD43− Pre-B cells and B220+ CD43+ CD19+ Pro-B cells, were ablated in the compound knockout mice (Figure 2C). We conclude from these experimental findings that Hoxa9 and Flt3 function in a shared pathway to regulate B lymphopoiesis in BM.
The consequence of combined loss of Hoxa9 and Flt3 signaling was selective for B-lineage lymphocytes. We found no significant alterations in frequencies of Mac-1/Gr1+ myeloid or Ter119+ erythroid cells (data not shown). Thus, the profound deficiency in BCP in the compound knockout mice is not due to a requirement for Hoxa9 and Flt3 signaling in regulating the lymphoid versus myeloid-erythroid fate decision in primitive hematopoietic progenitors.
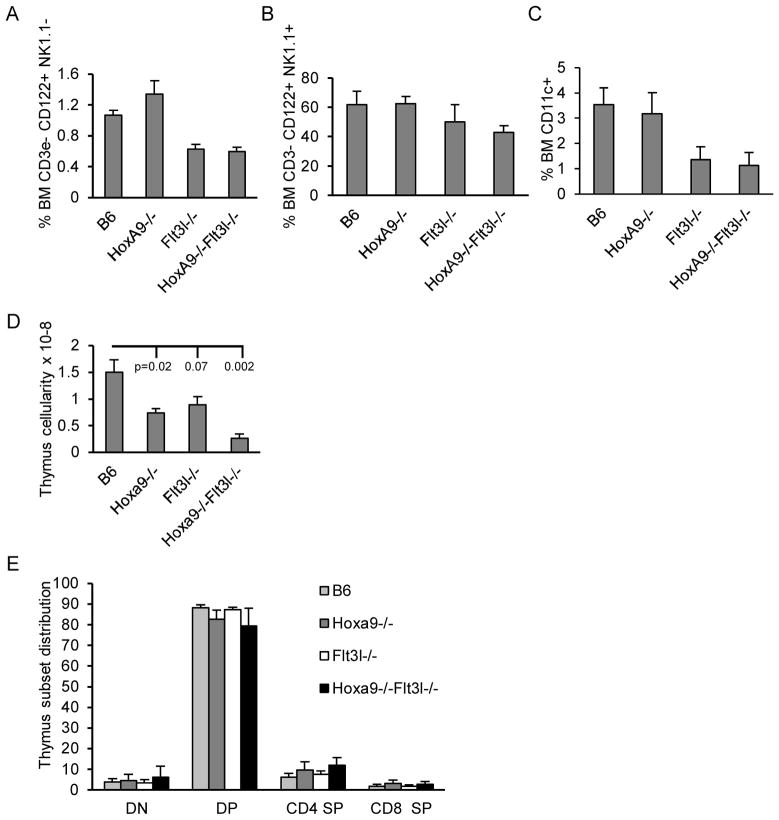
NK and DC lineage cells in BM share a common progenitor pathway with BCP (14). Flow cytometric analysis of NK precursors defined as CD3e− CD122+ NK1.1− or mature NK cells defined as CD3e− CD122+ NK1.1+ (27) revealed no significant alteration in NK development or maintenance in the compound knockouts beyond that due to Flt3l-deficiency (Figure 3A–B). The same was true for dendritic lineage cells defined broadly as CD11c+ (Figure 3C).
Figure 3. Alternate lymphoid lineage analysis of Hoxa9−/− Flt3l−/− mice.
BM cells were harvested as we previously described and incubated with combinations of antibodies to resolve NK or DC differentiation by flow cytometry. The data in A) represents precursor frequency of NK progenitors defined as CD3ε− CD122+ NK1.1−. The data in B) represents precursor frequency of mature NK cells defined as CD3ε− CD122+ NK1.1+. C) Percentages of total CD11c+ DC lineage cells in BM. The data is representative of mean frequencies ± standard deviation of 3–4 BM analyses per genotype. D) Thymi were harvested from mice of each genotype and nucleated cell counts determined. The data is representative of mean frequencies ± standard deviation of 4 animals per genotype. Statistical significance is compared to B6 controls. E) Thymic cell suspensions incubated with anti-CD4-FITC and anti-CD8-PE and flow cytometric analysis performed. Precursor frequencies of the four major T cell developmental subsets were evaluated based on differential expression of CD4 and CD8. DN=CD4− CD8−, DP=CD4+ CD8+, CD4 SP indicates CD4 single positive, and CD8 SP indicates CD8 single positive. The data is representative of mean frequencies ± standard deviation of 4 thymic analyses per genotype.
T cells and B cells arise from Flt3+ MPPs in BM (14). Numbers of total thymocytes were similarly reduced in Hoxa9−/− and Flt3l−/− mice in accord with previous reports (25, 28, 29). Compound loss of Hoxa9 and FL resulted in a more severe reduction in total thymocyte cellularity than the single knockout mice (approximately 5.8-fold reduced compared to B6 controls and 3-fold from Hoxa9−/− (p=0.0058) or Flt3l−/− mice (p=0.0078), Figure 3 D). The synergistic reduction in thymocyte cellularity is likely due to the ablation of Flt3+ MPPs in BM that seed the thymus, thus reducing the efficiency of BM progenitor seeding of the thymus (see below) (30). Although total thymocyte numbers are reduced, we found no significant alteration in major hallmarks of T cell development (Figure 3E). Percentages of CD4−CD8− (double negative, DN), CD4+CD8+ (double positive, DP), or CD4+ or CD8+ single positive (SP) thymocyte subsets were comparable in all four mouse models. Regardless, the production of T lymphocytes overall is reduced in the compound knockout compared to WT or single mutation controls.
We conclude from these analyses that compound loss of Hoxa9 and Flt3 signaling severely and synergistically reduces the generation of B and T-lineage lymphocytes.
Severe deficiency in ALP and BLP in Hoxa9−/−Flt3l−/− mice
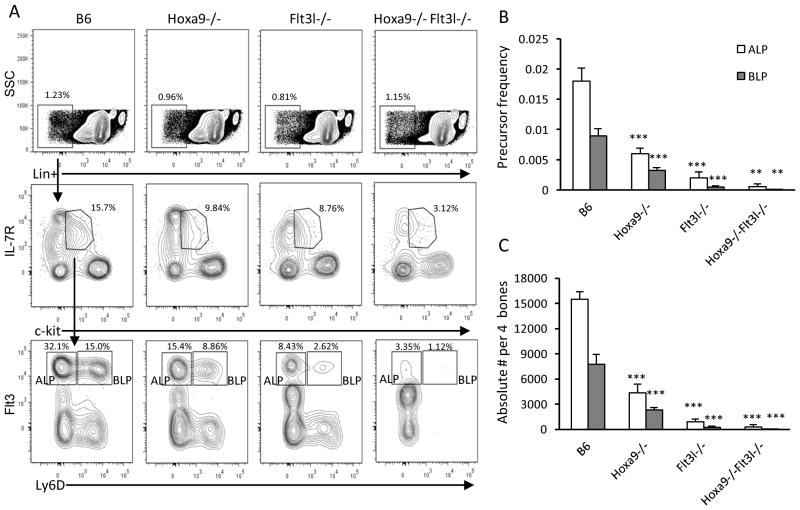
Functional BCP are enriched in Lin− ckitlo IL-7R+ Flt3+ CLPs. Hoxa9−/− and Flt3l−/− mice each have significantly reduced numbers of Flt3+ CLPs (16, 17, 19). Recently it was determined that Flt3+ CLPs could be fractionated into Ly6D− all lymphoid progenitors (ALP) and Ly6D+ B lineage restricted progenitors (BLP) (22). Both categories of Flt3+ CLPs were depressed in frequencies and numbers in the single knockouts (Figure 4A and summarized in Figure 4B-C). Combined loss of Hoxa9 and FL exacerbated the reduction of Flt3+ Ly6D− ALP (~70% reduction in Ly6D− compared to Flt3l−/− mice) and Flt3+ Ly6D+ BLP (~37-fold reduction compared to Flt3l−/− mice). We note an increase in abundance of Flt3+lo CLPs in Flt3l−/− and Hoxa9−/−Flt3l−/− mice (Figure 4A). This observation suggests that Flt3 signaling might play a role in autoregulation of Flt3 in CLPs or at an earlier stage (see below). The dramatic loss of Flt3+ CLPs in Hoxa9−/−Flt3l−/− mice suggests that Hoxa9 and Flt3 signaling are critical components of a genetic network that regulates the generation or maintenance of ALP and BLP.
Figure 4. Ablation of CLP subsets in Hoxa9−/−Flt3l−/− mice.
A) Representative flow cytometry profiles of Lineage (Lin−) IL-7R+ CLP subsets in the 4 mouse models. Lin− cells (top panels) were discriminated based on differential expression of c-kit and IL-7R to identify CLPs. Lin− c-kitlo IL-7R+ cells (middle panels) were further fractionated into ALP and BLP based on differential expression of Flt3 and Ly6D (bottom panels). The plots shown are representative of 3–4 mice per genotype. B) Precursor frequency of Flt3+ CLP subsets in the 4 mouse models. C) Absolute numbers of ALP (white box) and BLP (gray box). Data represents the mean and standard deviation of 4 mice per genotype. Statistically significance, indicated by the astericks, is determined relative to B6 WT controls.
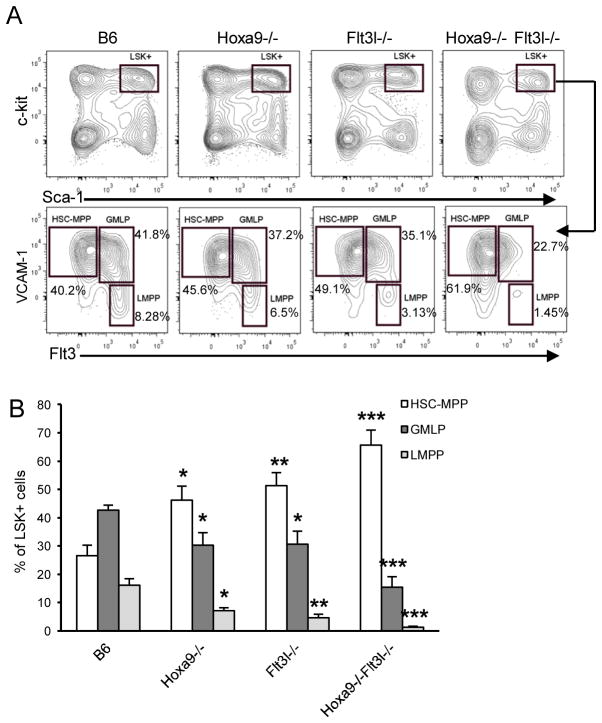
Reductions in LMPP and GMLP in Hoxa9−/−Flt3l−/− mice
We previously reported decreased Flt3+ LSK+ cells in Hoxa9−/− and Flt3l−/− mice (16, 17). The dramatic reduction in ALP in the compound knockout mice suggested critical roles for Hoxa9 and Flt3 signaling upstream of IL-7R expression. LMPP are the presumed immediate precursors of ALP as il7ra transcripts are detectable by realtime PCR at this stage (10). LMPP can be distinguished within LSK+ cells based on differential expression of VCAM-1 and Flt3 (31). As shown in Figure 5A and summarized in Figure 5B, LSK+ VCAM-1− Flt3+hi LMPP are reduced in Flt3l−/− and Hoxa9−/− mice, and ablated in Hoxa9−/−Flt3l−/− mice.
Figure 5. Reductions in GMLP and LMPP in Hoxa9−/−Flt3l−/− mice.
A) LSK+ cells were discriminated as Lin− (not shown) c-kithi Sca-1+ (boxed region). HSC/MPP, GMLP, and LMPP was resolved within the LSK+ gate based on differential expression of VCAM-1 and Flt3. B) Summary of HSC/MPP, GMLP, and LMPP subset frequencies as a percentage of LSK+ cells. Data represents the mean and standard deviation of 4–5 mice per genotype.
In addition to LMPP, Hoxa9−/− and Flt3l−/− mice have statistically significant reductions in GMLP (granulocyte-macrophage lymphoid progenitor), an LSK+ subset with combined lymphoid and myeloid differentiation potential (16, 31). Similar to LMPP, combined loss of Hoxa9 and FL results in a more severe reduction in GMLP than observed in the single knockout mice (Figure 5A and summarized in Figure 5B). Importantly, the reduction in GMLP had no significant impact on the production of myeloid cells in BM beyond that in Flt3l−/− mice (42.3±3.1% vs. 32.7±6.0% vs. 34.95±5.7% of BM mononuclear cells in B6 vs. Hoxa9−/−Flt3l−/− vs. Flt3l−/−, respectively, n=4 mice per genotype). Thus, the myeloid lineage developmental potential of VCAM-1+ Flt3+lo is not compromised.
The reductions in GMLP and LMPP coincided with a significant increase in percentages of LSK+ VCAM-1+ Flt3−/lo cells enriched for HSC and MPP (Figure 5A and summarized in Figure 5B). These observations suggest that Hoxa9 and Flt3 signaling could be important in regulation of the HSC/MPP pool.
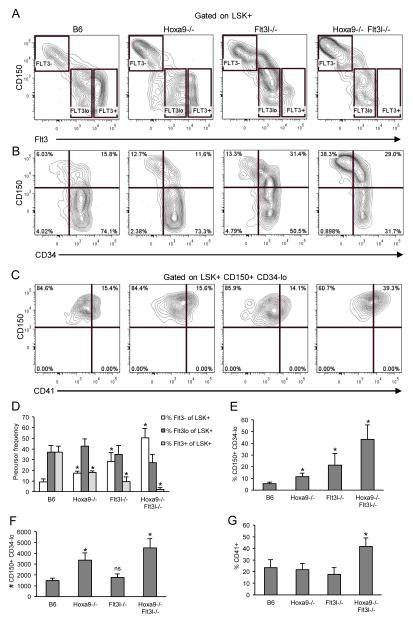
Alterations in HSC and MPP subsets in Hoxa9−/−Flt3l−/− mice
Flt3 is expressed early during hematopoietic differentiation. Two independent studies showed that all MPP progress through a Flt3+ stage, including erythroid/megakaryocyte progenitors (7, 8). However, since Flt3 expression is sensitive to loss of Hoxa9 and/or Flt3 signaling, evaluation of single or combined requirements for these regulators in subsets defined by Flt3 expression alone is problematic (16, 17). Functional HSCs are rare and express the SLAM family marker, CD150, and are CD34−lo (5, 6). MPP with lymphoid-myeloid biased differentiation potentials express Flt3 and have downregulated CD150 (3, 12). To further document the effect of combined loss of Hoxa9 and FL in the HSC/MPP compartment, we compared expression patterns of CD150, Flt3, and CD34 in the four mouse genotypes.
In accord with findings by others, upregulation of Flt3 coincided with downregulation of CD150 (Figure 6A). We observed an increase in total CD150+ LSK+ cells in the compound knockouts, compared to the single knockout mice which were also increased, but to a lesser extent (Figure 6 and Table I). The increase in frequency of CD150+ cells was accompanied by a decrease in percentages of CD150− MPP (Table I). The majority of CD150− cells in the compound knockout mice expressed Flt3, but at levels considerably reduced compared to B6 controls and the single knockouts (Figure 6A and D). Importantly, loss of Hoxa9 and FL did not abrogate expression of Flt3 on the whole as percentages of Flt3lo LSK+ cells were unchanged in the single or compound knockout mice (Figure 6A and D). With regard to Flt3, the most substantial change was in percentages of Flt3+ or Flt3− cells, which were both significantly altered. These data suggest that Hoxa9 and Flt3 signaling are coordinately required for the generation or maintenance of CD150− Flt3+ MPPs, but dispensable for CD150+ Flt3− cells enriched for HSC.
Figure 6. Alterations in HSC/MPP subsets in Hoxa9−/−Flt3l−/− mice.
A) Synergistic reduction in LSK+ CD150− Flt3+ cells in Hoxa9−/−Flt3l−/− mice. LSK+ cells were visualized based on differential expression of CD150 and Flt3 then fractionated by Flt3 density into Flt3−, Flt3lo, and Flt3+ subsets (boxed regions, left to right, respectively). The boundaries of the boxed regions were determined based on loss of CD150 expression which accompanies acquisition of Flt3. B) Increase in LSK+ CD150+ CD34−lo progenitors in Hoxa9−/−Flt3l−/− mice. C) Increased percentage of CD41+ LSK+ CD150+ CD34−lo myeloid-biased cells in Hoxa9−/−Flt3l−/− mice. D) Summary of Flt3−, Flt3lo, and Flt3+ LSK+ subsets in the four genotypes. E) Summary of LSK+ CD150+ CD34−lo progenitors in the four genotypes. F) Absolute number of LSK+ CD150+ CD34−lo cells per 4 hind limb leg bones per genotype. G) Summary of percentages of CD41+ cells within the LSK+ CD150+ CD34−lo gate. Bar graphs reflect the mean ± SD of 3–5 mice per genotype.
Table I.
Hematopoietic progenitor frequency analysis
| Mouse | % Lin− | % LSK+ | % CD150+ LSK+ | % CD150− LSK+ | % Flt3+ LSK+ | % Flt3+hi LSK+ |
|---|---|---|---|---|---|---|
| B6 | 2.8 ± 1.0 | 9.0 ± 2.5 | 9.8 ± 2.7 | 86.5 ± 1.2 | 88.8 ± 2.0 | 22.2 ± 2.9 |
| Hoxa9−/− | 3.3 ± 0.7 | 5.3 ± 1.8 | 33.2 ± 8.9** | 64.5 ± 7.5*** | 63.3 ± 11.2** | 6.6 ± 1.3*** |
| Flt3l−/− | 2.6 ± 0.9 | 4.7 ± 2.1 | 33.9 ± 9.5** | 62.7 ± 7.8*** | 76.6 ± 7.7* | 5.9 ± 2.9** |
| Hoxa9−/−Flt3l−/− | 2.1 ± 0.6 | 4.3 ± 0.6 | 55.1 ± 2.5* | 42.3 ±4.7* | 39.9± 5.4* | 1.3 ± 1.0*** |
All data represent mean ± standard deviation of 3–5 mice per genotype. LSK+ frequency reflects % LSK+ cells within the Lin− gated subset. CD150+, CD150−, Flt3+ (includes Flt3+lo cells), and Flt3+hi indicate percentages of marker positive cells within the LSK+ gated population.
Hoxa9−/− or Flt3l−/− subset statistics compared to B6.
Hoxa9−/−Flt3l−/− compared to Hoxa9−/− or Flt3l−/−
p≤0.05
p≤0.005
p≤0.0005
The LSK+ CD150+ subset is enriched for functional HSCs (5). Functional HSCs are CD34−lo (6). To determine if the CD150+ cells in the compound knockout were phenotypic HSC, we examined CD34 expression. Indeed, as shown in Figure 6B and summarized in Figures 6E–F, LSK+ CD150+ CD34−lo were significantly increased in frequency and absolute number in Hoxa9−/− and compound knockout mice. We note that although CD150+ CD34−lo represented a greater percentage of the LSK+ compartment in Flt3l−/− mice, absolute numbers of LSK+ CD150+ CD34−lo cells were not increased in these mice (Figure 6F). These data suggest that Hoxa9-deficiency, but not impaired Flt3 signaling, contributes significantly to the increased numbers of CD150+ CD34−lo HSCs in the compound knockout mice.
Finally, we consistently observed that the CD150+ cells that increased in the compound knockout mice expressed very high levels of CD150 (Figure 6). LSK+ CD150+ cells expressing high levels of CD150 have been shown to exhibit latent and myeloid-biased reconstitution potential in transplant recipients (3). Myeloid-biased HSC can be further discriminated in adult mice by expression of CD41 (32). As shown in Figure 6C, there was a significant increase in percentage of LSK+ CD150+ CD34−lo cells that expressed CD41 in the compound knockout mice (p=0.034, n=3 mice per genotype). Thus, combined loss of Hoxa9 and Flt3 signaling results in a disproportionate number of myeloid-biased HSC in BM.
Taken together, these flow cytometry analyses show that combined loss of Hoxa9 and Flt3 signaling significantly alters the HSC/MPP compartment of BM. LSK+ CD150+ CD34−lo CD41+ myeloid-biased HSC are increased while CD150− Flt3+ progenitors are severely depleted. We conclude that Hoxa9 and Flt3 signaling regulates a critical early checkpoint in hematopoietic differentiation that restricts B and T lineage developmental potential.
Differential role of Hoxa9 and/or Flt3 signaling in regulation of the early program of lymphoid gene expression
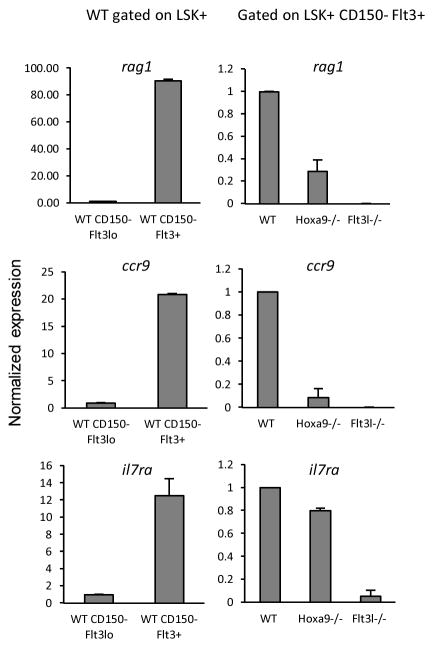
Deficiencies in Hoxa9, FL, or both, results in significant reduction in lymphoid-biased progenitors and BCP in BM. We and others have shown that the rare MPP expressing high levels of Flt3 in Flt3l−/− mice have reduced levels of lymphoid lineage transcripts, suggesting that Flt3 signaling plays a role in lymphoid priming (10, 16). Hoxa9−/− mice also have reductions in Flt3+hi MPP, although a role for Hoxa9 in lymphoid priming has not been reported. Combined roles for Hoxa9 and Flt3 signaling in regulation of lymphoid priming provide an explanation for the severe defect in lymphoid development in the compound knockout mice. To make this determination, LSK+ CD150− cells from B6 control, Flt3l−/− and Hoxa9−/− mice were sorted into Flt3lo and Flt3+ subsets. The paucity of LSK+ CD150− cells, particularly LSK+ CD150− Flt3+ cells which are severely reduced, precluded isolation of these cells from the compound knockout mice. CD150 was included as a gating parameter to exclude alterations to the results contributed by CD150+ Flt3lo cells that vary amongst the three strains. Consistent with previous findings by others, transcripts corresponding to rag1, ccr9, and il7ra are more abundant in Flt3+ than Flt3lo LSK+ cells (Figure 7) (10, 33). All three lymphoid-lineage transcripts are reduced in Flt3+ cells in Flt3l−/− mice (Figure 7) (10). Similarly, rag1 and ccr9 transcripts are reduced in Hoxa9−/− mice. However, we did not observe a significant reduction in il7ra transcripts in Flt3+ cells in Hoxa9−/− mice. These data suggest that lymphoid priming is more dependent on Flt3 signaling than Hoxa9 function.
Figure 7. Requirements for Hoxa9 and Flt3 signaling in lymphoid priming.
Realtime PCR of the indicated transcripts in CD150− LSK+ cells sorted into Flt3lo and Flt3+ subsets. The panels on the left show transcript abundance in B6 mice CD150− LSK+ Flt3lo and CD150− LSK+ Flt3+ sorted cells. The panels on the right compare the indicated transcripts in CD150− LSK+ Flt3+ cells isolated from the three indicated genotypes (B6 vs. Hoxa9−/− vs. Flt3l−/−). The data represent mean values obtained from two independent sorts. Error bars indicated SD.
Discussion
We previously showed that Hoxa9 regulates B cell development, in part, through transcriptional regulation of Flt3. Here, we sought to determine if Hoxa9 provides additional functions in BM lymphoid/B cell development, independent of, or coordinately with, Flt3 signaling. We show that Hoxa9−/−Flt3l−/− mice have a profound block in the generation of lymphoid progenitors destined to become B or T lineage lymphocytes. Importantly, the ablation of lymphoid progenitors had selective consequences on lymphoid lineage progeny as NK and DC lineage cells were not synergistically reduced. We pinpointed the block in lymphoid development to the LSK+ CD150− Flt3lo to LSK+ CD150− Flt3+ transition. Hematopoietic progenitors deficient in Hoxa9 or FL alone can mediate this developmental transition, albeit inefficiently. However, combined loss of Hoxa9 and Flt3 signaling completely abrogates the generation of LSK+ CD150− Flt3+ MPPs. The severe lymphoid deficiency was accompanied by an increase in absolute numbers of LSK+ CD150+ CD34−lo CD41+ phenotypic myeloid-biased HSC in the compound knockout mice. Taken together, these new findings uncover combinatorial and limiting requirements for Hoxa9 and Flt3 signaling in the generation of lymphoid precursors destined to become B or T lineage precursors.
Lymphoid priming is a very early event in hematopoiesis. Transcriptional priming of lymphoid genes has been demonstrated in individual HSC and one study showed that it is dependent on the Kruppel-type zinc finger transcription factor Ikaros (33, 34). Similar to Hoxa9−/− and Flt3l−/− mice, Ikaros-null mice have reductions in total Flt3+ MPP and the residual MPP in these mice are Flt3lo (35). In wildtype mice, Flt3lo MPPs show little evidence of lymphoid priming. Upregulation of Flt3 coincided with increased abundance of lymphoid lineage transcripts, including ccr9, rag1, and il7ra. The Flt3+ MPP pool, which includes LMPP, are significantly depleted by Hoxa9 or FL deficiency. Interestingly, il7ra transcripts were detectable in the residual Flt3+ MPP in Hoxa9−/− mice. Given that Lin− IL-7R+ cells are detectable by flow cytometry in the single and compound knockout mice, the reduction in il7ra transcripts in the Flt3l−/− mice likely reflects the lymphoid primed progenitor pool simply fails to be established. These results suggest that Flt3 signaling, but not Hoxa9 function, is limiting in establishment of a lymphoid primed progenitor pool. The contribution of Hoxa9 is likely indirect.
IL-7R expression in Lin− cells denotes the CLP stage and IL-7R signaling promotes lymphoid restriction (15, 36). The CLP subset on the whole is heterogeneous and at present, three distinct subsets have been resolved based on differential expression of Flt3 and Ly6D. Flt3+ Ly6D− ALP are the presumed immediate progeny of Flt3+hi LMPP, and retain full lymphoid lineage developmental potential (14, 22). Downregulation of Flt3 in CLPs accompanies NK developmental restriction (37, 38). Flt3lo− CLPs are refractory to loss of Hoxa9 function and Flt3 signaling and we recently showed that Hoxa9 is not essential for the generation or differentiation of NK lineage cells (39). At present there is no evidence for an LMPP/CLP independent pathway in NK development. IL-7R signaling is not required for NK cell genesis (40). It is possible that NKP can develop directly from LSK+ CD150− Flt3lo MPP independent of LMPP/CLP. Future studies employing adoptive transfer and in vitro assays will directly test this possibility.
Flt3+ CLPs also have DC differentiation potential (14). Like NK cells, Hoxa9 is dispensable for the generation and differentiation of DCs (39). The generation of committed DC progenitors is not dependent on Flt3 signaling although the cytokine is important for DC differentiation and homeostasis (25, 41). An IL-7R− Flt3+ common DC progenitor was recently described (42). Since DC progeny have multiple development origins and the transcription factor PU.1 is a critical regulator of Flt3 in this lineage, it is not unexpected that DCs are not perturbed in the compound mice (14, 43–45).
We previously showed that Flt3lo LSK+ cells from Flt3l−/− mice have reduced expression of pro-survival factors suggesting that Flt3 signaling plays a critical role in regulation of progenitor survival (16). Hoxa9, on the other hand, has been shown to regulate the proliferation of multipotent hematopoietic progenitors in vitro (46). Hoxa9−/− mice are deficient in the serine-threonine kinase Pim-1(47). Flt3 signaling activates Pim-1 and reductions in Pim-1 impact B lymphopoiesis (48, 49). Importantly, Pim-1 has been linked to c-Myb activity and c-Myb plays a critical role in lymphoid priming and B cell development (21, 50). Hoxa9 has been implicated in regulation of c-myb in leukemic blasts (51). These experimental findings together with the profound lymphoid/B lineage deficiency manifested by the compound knockout mice suggest that Hoxa9 and Flt3 signaling regulate lymphoid/B cell development through regulatory circuits that impact the survival and proliferation of Flt3lo MPP. Preliminary analyses of Flt3lo MPP isolated from the single knockout mice did not reveal significant alterations in expression of c-Myb or Pim-1. However, these findings do not preclude that similar analysis of Flt3lo MPP from the compound knockout mice might reveal different results. Future studies will be aimed at comparing molecular signatures of Hoxa9−/−, Flt3l−/−, and Hoxa9−/− Flt3l−/− total CD150+ and CD150− Flt3lo hematopoietic progenitors to identify genetic circuits sensitive to combinatorial inputs by Hoxa9 and Flt3 signaling.
Myeloid-biased HSC exhibit latent reconstitution capabilities in the early phase in transplant recipients and the myeloid lineage was more reconstituted than the lymphoid (3). Lymphoid reconstitution after hematopoietic stress is contingent on Hoxa9 function and Flt3 signaling (46, 52). The reduction in LSK+ Flt3+ MPP in Hoxa9−/− Flt3l−/− mice is accompanied by a marked increase in LSK+ CD150+hi CD34−lo CD41+ phenotypic myeloid-biased HSC. We note that the increase in myeloid-biased HSC did not increase myeloid-lineage output in marrow under conditions of homeostasis. However, it remains possible that the poor lymphoid reconstitution capability of this subset may contribute to the lymphoid deficiency in the compound knockout mice.
We conclude that there is an extremely early checkpoint in hematopoietic differentiation critical for B and T lymphopoiesis that can only be transversed through the cooperative action of Hoxa9 and Flt3 signaling. Our current analysis suggests that the checkpoint is localized to the LSK+ CD150− Flt3lo stage. The selective deficiency in lymphoid precursors destined to be B or T cell precursors makes the Hoxa9−/− Flt3l−/− an exemplary model to identify and characterize gene regulatory modules and their components essential for generation of cells of the adaptive immune system.
Acknowledgments
We thank Virginia Smith Shapiro, Richard Bram, and Paul W. Kincade for their helpful discussions and critical reading of the manuscript. The authors declare no competing financial interests.
Abbreviations
- LSK+
Lineage negative, Sca-1+, c-kit+
- HSC
hematopoietic stem cell
- MPP
multipotential progenitor
- LMPP
lymphoid primed multipotential progenitor
- BCP
B cell precursor
- BM
bone marrow
- ALP
all lymphoid progenitor
- BLP
B lineage restricted progenitor
- CLP
common lymphoid progenitor
- FL
Flt3-ligand
Footnotes
This work was supported by R01HL096108 from the National Heart, Lung, and Blood Institute to K.L.M.
References
- 1.Singh H, Medina KL, Pongubala JM. Contingent gene regulatory networks and B cell fate specification. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:4949–4953. doi: 10.1073/pnas.0500480102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wilson A, Laurenti E, Oser G, van der Wath RC, Blanco-Bose W, Jaworski M, Offner S, Dunant CF, Eshkind L, Bockamp E, Lio P, Macdonald HR, Trumpp A. Hematopoietic stem cells reversibly switch from dormancy to self-renewal during homeostasis and repair. Cell. 2008;135:1118–1129. doi: 10.1016/j.cell.2008.10.048. [DOI] [PubMed] [Google Scholar]
- 3.Morita Y, Ema H, Nakauchi H. Heterogeneity and hierarchy within the most primitive hematopoietic stem cell compartment. The Journal of experimental medicine. 2010;207:1173–1182. doi: 10.1084/jem.20091318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wiesmann A, Phillips RL, Mojica M, Pierce LJ, Searles AE, Spangrude GJ, Lemischka I. Expression of CD27 on murine hematopoietic stem and progenitor cells. Immunity. 2000;12:193–199. doi: 10.1016/s1074-7613(00)80172-7. [DOI] [PubMed] [Google Scholar]
- 5.Kiel MJ, Yilmaz OH, Iwashita T, Yilmaz OH, Terhorst C, Morrison SJ. SLAM family receptors distinguish hematopoietic stem and progenitor cells and reveal endothelial niches for stem cells. Cell. 2005;121:1109–1121. doi: 10.1016/j.cell.2005.05.026. [DOI] [PubMed] [Google Scholar]
- 6.Osawa M, Hanada K, Hamada H, Nakauchi H. Long-term lymphohematopoietic reconstitution by a single CD34-low/negative hematopoietic stem cell. Science (New York, NY. 1996;273:242–245. doi: 10.1126/science.273.5272.242. [DOI] [PubMed] [Google Scholar]
- 7.Boyer SW, Schroeder AV, Smith-Berdan S, Forsberg EC. All hematopoietic cells develop from hematopoietic stem cells through Flk2/Flt3-positive progenitor cells. Cell stem cell. 2011;9:64–73. doi: 10.1016/j.stem.2011.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Buza-Vidas N, Woll P, Hultquist A, Duarte S, Lutteropp M, Bouriez-Jones T, Ferry H, Luc S, Jacobsen SE. FLT3 expression initiates in fully multipotent mouse hematopoietic progenitor cells. Blood. 2011;118:1544–1548. doi: 10.1182/blood-2010-10-316232. [DOI] [PubMed] [Google Scholar]
- 9.Adolfsson J, Mansson R, Buza-Vidas N, Hultquist A, Liuba K, Jensen CT, Bryder D, Yang L, Borge OJ, Thoren LA, Anderson K, Sitnicka E, Sasaki Y, Sigvardsson M, Jacobsen SE. Identification of Flt3+ lympho-myeloid stem cells lacking erythro-megakaryocytic potential a revised road map for adult blood lineage commitment. Cell. 2005;121:295–306. doi: 10.1016/j.cell.2005.02.013. [DOI] [PubMed] [Google Scholar]
- 10.Sitnicka E, Buza-Vidas N, Ahlenius H, Cilio CM, Gekas C, Nygren JM, Mansson R, Cheng M, Jensen CT, Svensson M, Leandersson K, Agace WW, Sigvardsson M, Jacobsen SE. Critical role of FLT3 ligand in IL-7 receptor independent T lymphopoiesis and regulation of lymphoid-primed multipotent progenitors. Blood. 2007;110:2955–2964. doi: 10.1182/blood-2006-10-054726. [DOI] [PubMed] [Google Scholar]
- 11.Igarashi H, Gregory SC, Yokota T, Sakaguchi N, Kincade PW. Transcription from the RAG1 locus marks the earliest lymphocyte progenitors in bone marrow. Immunity. 2002;17:117–130. doi: 10.1016/s1074-7613(02)00366-7. [DOI] [PubMed] [Google Scholar]
- 12.Lai AY, Kondo M. Asymmetrical lymphoid and myeloid lineage commitment in multipotent hematopoietic progenitors. The Journal of experimental medicine. 2006;203:1867–1873. doi: 10.1084/jem.20060697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Medina KL, Garrett KP, Thompson LF, Rossi MI, Payne KJ, Kincade PW. Identification of very early lymphoid precursors in bone marrow and their regulation by estrogen. Nat Immunol. 2001;2:718–724. doi: 10.1038/90659. [DOI] [PubMed] [Google Scholar]
- 14.Karsunky H, Inlay MA, Serwold T, Bhattacharya D, Weissman IL. Flk2+ common lymphoid progenitors possess equivalent differentiation potential for the B and T lineages. Blood. 2008;111:5562–5570. doi: 10.1182/blood-2007-11-126219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kondo M, I, Weissman L, Akashi K. Identification of clonogenic common lymphoid progenitors in mouse bone marrow. Cell. 1997;91:661–672. doi: 10.1016/s0092-8674(00)80453-5. [DOI] [PubMed] [Google Scholar]
- 16.Dolence JJ, Gwin K, Frank E, Medina KL. Threshold levels of Flt3-ligand are required for the generation and survival of lymphoid progenitors and B-cell precursors. European journal of immunology. 2011;41:324–334. doi: 10.1002/eji.201040710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gwin K, Frank E, Bossou A, Medina KL. Hoxa9 regulates Flt3 in lymphohematopoietic progenitors. J Immunol. 2010;185:6572–6583. doi: 10.4049/jimmunol.0904203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mackarehtschian K, Hardin JD, Moore KA, Boast S, Goff SP, Lemischka IR. Targeted disruption of the flk2/flt3 gene leads to deficiencies in primitive hematopoietic progenitors. Immunity. 1995;3:147–161. doi: 10.1016/1074-7613(95)90167-1. [DOI] [PubMed] [Google Scholar]
- 19.So CW, Karsunky H, Wong P, Weissman IL, Cleary ML. Leukemic transformation of hematopoietic progenitors by MLL-GAS7 in the absence of Hoxa7 or Hoxa9. Blood. 2004;103:3192–3199. doi: 10.1182/blood-2003-10-3722. [DOI] [PubMed] [Google Scholar]
- 20.Dias S, Mansson R, Gurbuxani S, Sigvardsson M, Kee BL. E2A proteins promote development of lymphoid-primed multipotent progenitors. Immunity. 2008;29:217–227. doi: 10.1016/j.immuni.2008.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Greig KT, de Graaf CA, Murphy JM, Carpinelli MR, Pang SH, Frampton J, Kile BT, Hilton DJ, Nutt SL. Critical roles for c-Myb in lymphoid priming and early B-cell development. Blood. 2010;115:2796–2805. doi: 10.1182/blood-2009-08-239210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Inlay MA, Bhattacharya D, Sahoo D, Serwold T, Seita J, Karsunky H, Plevritis SK, Dill DL, Weissman IL. Ly6d marks the earliest stage of B-cell specification and identifies the branchpoint between B-cell and T-cell development. Genes & development. 2009;23:2376–2381. doi: 10.1101/gad.1836009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lawrence HJ, Helgason CD, Sauvageau G, Fong S, Izon DJ, Humphries RK, Largman C. Mice bearing a targeted interruption of the homeobox gene HOXA9 have defects in myeloid, erythroid, and lymphoid hematopoiesis. Blood. 1997;89:1922–1930. [PubMed] [Google Scholar]
- 24.Sambandam A, Maillard I, Zediak VP, Xu L, Gerstein RM, Aster JC, Pear WS, Bhandoola A. Notch signaling controls the generation and differentiation of early T lineage progenitors. Nat Immunol. 2005;6:663–670. doi: 10.1038/ni1216. [DOI] [PubMed] [Google Scholar]
- 25.McKenna HJ, Stocking KL, Miller RE, Brasel K, De Smedt T, Maraskovsky E, Maliszewski CR, Lynch DH, Smith J, Pulendran B, Roux ER, Teepe M, Lyman SD, Peschon JJ. Mice lacking flt3 ligand have deficient hematopoiesis affecting hematopoietic progenitor cells, dendritic cells, and natural killer cells. Blood. 2000;95:3489–3497. [PubMed] [Google Scholar]
- 26.Hardy RR, Carmack CE, Shinton SA, Kemp JD, Hayakawa K. Resolution and characterization of pro-B and pre-pro-B cell stages in normal mouse bone marrow. The Journal of experimental medicine. 1991;173:1213–1225. doi: 10.1084/jem.173.5.1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rosmaraki EE, Douagi I, Roth C, Colucci F, Cumano A, Di Santo JP. Identification of committed NK cell progenitors in adult murine bone marrow. European journal of immunology. 2001;31:1900–1909. doi: 10.1002/1521-4141(200106)31:6<1900::aid-immu1900>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 28.Izon DJ, Rozenfeld S, Fong ST, Komuves L, Largman C, Lawrence HJ. Loss of function of the homeobox gene Hoxa-9 perturbs early T-cell development and induces apoptosis in primitive thymocytes. Blood. 1998;92:383–393. [PubMed] [Google Scholar]
- 29.So CW, Karsunky H, Passegue E, Cozzio A, Weissman IL, Cleary ML. MLL-GAS7 transforms multipotent hematopoietic progenitors and induces mixed lineage leukemias in mice. Cancer Cell. 2003;3:161–171. doi: 10.1016/s1535-6108(03)00019-9. [DOI] [PubMed] [Google Scholar]
- 30.Schwarz BA, Sambandam A, Maillard I, Harman BC, Love PE, Bhandoola A. Selective thymus settling regulated by cytokine and chemokine receptors. J Immunol. 2007;178:2008–2017. doi: 10.4049/jimmunol.178.4.2008. [DOI] [PubMed] [Google Scholar]
- 31.Lai AY, Lin SM, Kondo M. Heterogeneity of Flt3-expressing multipotent progenitors in mouse bone marrow. J Immunol. 2005;175:5016–5023. doi: 10.4049/jimmunol.175.8.5016. [DOI] [PubMed] [Google Scholar]
- 32.Gekas C, Graf T. CD41 expression marks myeloid biased adult hematopoietic stem cells and increases with age. Blood. 2013 doi: 10.1182/blood-2012-09-457929. [DOI] [PubMed] [Google Scholar]
- 33.Mansson R, Hultquist A, Luc S, Yang L, Anderson K, Kharazi S, Al-Hashmi S, Liuba K, Thoren L, Adolfsson J, Buza-Vidas N, Qian H, Soneji S, Enver T, Sigvardsson M, Jacobsen SE. Molecular evidence for hierarchical transcriptional lineage priming in fetal and adult stem cells and multipotent progenitors. Immunity. 2007;26:407–419. doi: 10.1016/j.immuni.2007.02.013. [DOI] [PubMed] [Google Scholar]
- 34.Ng SY, Yoshida T, Zhang J, Georgopoulos K. Genome-wide Lineage-Specific Transcriptional Networks Underscore Ikaros-Dependent Lymphoid Priming in Hematopoietic Stem Cells. Immunity. 2009 doi: 10.1016/j.immuni.2009.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yoshida T, Ng SY, Zuniga-Pflucker JC, Georgopoulos K. Early hematopoietic lineage restrictions directed by Ikaros. Nat Immunol. 2006;7:382–391. doi: 10.1038/ni1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Purohit SJ, Stephan RP, Kim HG, Herrin BR, Gartland L, Klug CA. Determination of lymphoid cell fate is dependent on the expression status of the IL-7 receptor. The EMBO journal. 2003;22:5511–5521. doi: 10.1093/emboj/cdg522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Carotta S, Pang SH, Nutt SL, Belz GT. Identification of the earliest NK-cell precursor in the mouse BM. Blood. 2011;117:5449–5452. doi: 10.1182/blood-2010-11-318956. [DOI] [PubMed] [Google Scholar]
- 38.Fathman JW, Bhattacharya D, Inlay MA, Seita J, Karsunky H, Weissman IL. Identification of the earliest natural killer cell-committed progenitor in murine bone marrow. Blood. 2011;118:5439–5447. doi: 10.1182/blood-2011-04-348912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gwin K, Dolence JJ, Shapiro MB, Medina KL. Differential requirement for Hoxa9 in the development and differentiation of B, NK, and DC-lineage cells from Flt3+ multipotential progenitors. BMC Immunol. 2013;14:5. doi: 10.1186/1471-2172-14-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Peschon JJ, Morrissey PJ, Grabstein KH, Ramsdell FJ, Maraskovsky E, Gliniak BC, Park LS, Ziegler SF, Williams DE, Ware CB, Meyer JD, Davison BL. Early lymphocyte expansion is severely impaired in interleukin 7 receptor-deficient mice. The Journal of experimental medicine. 1994;180:1955–1960. doi: 10.1084/jem.180.5.1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Waskow C, Liu K, Darrasse-Jeze G, Guermonprez P, Ginhoux F, Merad M, Shengelia T, Yao K, Nussenzweig M. The receptor tyrosine kinase Flt3 is required for dendritic cell development in peripheral lymphoid tissues. Nat Immunol. 2008;9:676–683. doi: 10.1038/ni.1615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Onai N, Obata-Onai A, Schmid MA, Ohteki T, Jarrossay D, Manz MG. Identification of clonogenic common Flt3+M-CSFR+ plasmacytoid and conventional dendritic cell progenitors in mouse bone marrow. Nat Immunol. 2007;8:1207–1216. doi: 10.1038/ni1518. [DOI] [PubMed] [Google Scholar]
- 43.Karsunky H, Merad M, Cozzio A, Weissman IL, Manz MG. Flt3 ligand regulates dendritic cell development from Flt3+ lymphoid and myeloid-committed progenitors to Flt3+ dendritic cells in vivo. The Journal of experimental medicine. 2003;198:305–313. doi: 10.1084/jem.20030323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Manz MG, Traver D, Miyamoto T, Weissman IL, Akashi K. Dendritic cell potentials of early lymphoid and myeloid progenitors. Blood. 2001;97:3333–3341. doi: 10.1182/blood.v97.11.3333. [DOI] [PubMed] [Google Scholar]
- 45.Carotta S, Dakic A, D’Amico A, Pang SH, Greig KT, Nutt SL, Wu L. The transcription factor PU.1 controls dendritic cell development and Flt3 cytokine receptor expression in a dose-dependent manner. Immunity. 2010;32:628–641. doi: 10.1016/j.immuni.2010.05.005. [DOI] [PubMed] [Google Scholar]
- 46.Lawrence HJ, Christensen J, Fong S, Hu YL, Weissman I, Sauvageau G, Humphries RK, Largman C. Loss of expression of the Hoxa-9 homeobox gene impairs the proliferation and repopulating ability of hematopoietic stem cells. Blood. 2005;106:3988–3994. doi: 10.1182/blood-2005-05-2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hu YL, Passegue E, Fong S, Largman C, Lawrence HJ. Evidence that the Pim1 kinase gene is a direct target of HOXA9. Blood. 2007;109:4732–4738. doi: 10.1182/blood-2006-08-043356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kim KT, Baird K, Ahn JY, Meltzer P, Lilly M, Levis M, Small D. Pim-1 is up-regulated by constitutively activated FLT3 and plays a role in FLT3-mediated cell survival. Blood. 2005;105:1759–1767. doi: 10.1182/blood-2004-05-2006. [DOI] [PubMed] [Google Scholar]
- 49.Domen J, van der Lugt NM, Acton D, Laird PW, Linders K, Berns A. Pim-1 levels determine the size of early B lymphoid compartments in bone marrow. The Journal of experimental medicine. 1993;178:1665–1673. doi: 10.1084/jem.178.5.1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Leverson JD, Koskinen PJ, Orrico FC, Rainio EM, Jalkanen KJ, Dash AB, Eisenman RN, Ness SA. Pim-1 kinase and p100 cooperate to enhance c-Myb activity. Mol Cell. 1998;2:417–425. doi: 10.1016/s1097-2765(00)80141-0. [DOI] [PubMed] [Google Scholar]
- 51.Huang Y, Sitwala K, Bronstein J, Sanders D, Dandekar M, Collins C, Robertson G, MacDonald J, Cezard T, Bilenky M, Thiessen N, Zhao Y, Zeng T, Hirst M, Hero A, Jones S, Hess JL. Identification and characterization of Hoxa9 binding sites in hematopoietic cells. Blood. 2012;119:388–398. doi: 10.1182/blood-2011-03-341081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Buza-Vidas N, Cheng M, Duarte S, Nozad H, Jacobsen SE, Sitnicka E. Crucial role of FLT3 ligand in immune reconstitution after bone marrow transplantation and high-dose chemotherapy. Blood. 2007;110:424–432. doi: 10.1182/blood-2006-09-047480. [DOI] [PubMed] [Google Scholar]