Abstract
Dendritic cells (DCs) play a crucial role in launching protective adaptive immunity against pathogens while maintaining immune tolerance to self-antigens. However, how intracellular signaling pathways program DCs to mediate tolerogenic responses remains largely unexplored. Here we describe that p38α signaling in CD103+ mesenteric lymph node (MLN) DCs reciprocally regulates the differentiation of anti-inflammatory induced regulatory T (iTreg) cells and proinflammatory Th1 cells from naïve precursors, and promotes mucosal tolerance. Deficiency of p38α in CD103+ DCs inhibited the generation of iTreg cells while promoting Th1 cell development in a TGF-β2-dependent manner. Consequently, loss of p38α in DCs prevented induction of oral tolerance in vivo. Moreover, p38α in CD103+ DCs was required for optimal expression of retinaldehyde dehydrogenase (Aldh1a2), a key enzyme for retinoic acid synthesis, which in turn imprinted gut homing receptors on responding T cells. Consistent with a crucial role of p38α to program the tolerogenic activity of CD103+ DCs, such DC subset contained constitutive activity of p38α and abundant expression of TGF-β2 and Aldh1a2. Our studies identify a key mechanism of DC-mediated coupling of T cell differentiation and trafficking that orchestrates mucosal immune tolerance.
Keywords: iTreg cell, Dendritic cell, TGF-β, Retinoic acid, Oral tolerance
Introduction
The primary function of the immune system, comprised of the interconnected innate and adaptive arms, is to launch a robust immune response towards pathogens while maintaining tolerance to self-antigens. Although much progress has been made in understanding the central role of innate immunity in engaging adaptive immune reactions for defense against pathogens, we are just beginning to appreciate how innate signals contribute to immune tolerance. The most important cell type that bridges innate and adaptive immunity is dendritic cells (DCs). DCs serve as potent antigen-presenting cells to mediate the activation and differentiation of naïve T cells (1, 2). Recent studies have also established a crucial role of DCs in maintaining immune tolerance (3, 4), and depletion of DCs disrupts self-tolerance and results in myeloid inflammation and autoimmunity (5, 6). However, the underlying mechanisms by which DCs dictate protective immunity versus immune tolerance remain incompletely understood.
The gut-associated lymphoid tissue (GALT) is the largest immune organ in the body. Because the intestine is constantly exposed to copious amounts of food antigens, foreign pathogens and commensal microbes, intestinal homeostasis arises from a dynamic balance between protective immunity and regulatory mechanisms (7). One of the major regulatory mechanisms in the GALT is de novo induction of Foxp3 expression from conventional CD4 T cells in response to intestinal antigens (8, 9). These induced regulatory T cells (iTreg) play an important role in intestinal immune homeostasis under steady state (10, 11), and contribute to tolerance induced by ingested antigens, namely oral tolerance (12). Induction of oral tolerance also relies on mesenteric lymph nodes (MLNs) and antigen carriage by DCs (13). A major DC subset in the intestinal lamina propria (LP) is CD103+ DCs, which constitutively traffic to MLNs where they promote tolerogenic responses (14, 15). Specifically, these DCs produce high levels of retinoic acid (RA), TGF-β, and other immunoregulatory molecules to induce iTreg cell generation and imprint gut homing receptors, thereby facilitating intestinal immune tolerance (16-18). Despite these exciting advances on the role of DCs in intestinal tolerance, the intracellular signaling networks that program DCs to become tolerogenic are largely unexplored.
Mitogen-activated protein kinases (MAPKs), including ERK, JNK and p38, constitute one of the central pathways activated by innate immune signals (19, 20). Excessive activation of MAPKs is associated with many autoimmune and inflammatory diseases. Negative regulation of MAPK activities is effected mainly through a group of phosphatases known as MAPK phosphatases (MKPs). Our recent studies have established that an intracellular signaling axis comprised of p38α and MKP-1 acts in DCs to dictate T cell fates especially Th17 differentiation, and thus program effector T cell-mediated inflammatory and autoimmune diseases (21, 22). In contrast, the roles of this signaling pathway in DC-mediated tolerogenic responses are poorly defined.
To investigate the function of p38 signaling in DC-mediated intestinal immune tolerance, we used a genetic model with DC-specific ablation of p38α (p38αΔDC). Loss of p38α signaling in DCs impaired induction of oral tolerance and generation of antigen-specific iTreg cells in vivo. Mechanistically, p38 signaling is constitutively activated in CD103+ MLN DCs to mediate the generation of iTreg cells while inhibiting Th1 cell development in a TGF-β2-dependent manner. Moreover, p38α signaling in CD103+ DCs was important for the induction of gut homing molecules on responding T cells, partly by regulating the expression of a rate-limiting enzyme in RA synthesis. Therefore, DC-derived p38α signaling coordinates T cell differentiation and trafficking by affecting the expression of distinct downstream effector molecules. Altogether, our studies identify a key mechanism of DC-mediated control of T cell fates and immune tolerance in intestinal immune homeostasis.
Methods
Mice
C57BL/6, Thy1.1+, OT-II, Rag1–/–, Il6–/–, Il12a–/–, and CD4-dnTGFβRII mice were from the Jackson Laboratory. CD11c-Cre, CD4-Cre and p38αfl/fl mice have been described (22). Foxp3YFP-Cre mice were generously provided by Dr. Alexander Rudensky (23). All mice had been backcrossed to the C57BL/6 background for at least eight generations and were used at an age of 6–12 weeks. Wild-type (WT) control mice were of the same genetic background and, where relevant, included Cre+ mice to account for the effects of Cre. All mice were kept in specific pathogen-free conditions in Animal Resource Center at St. Jude Children's Research Hospital. Animal protocols were approved by Institutional Animal Care and Use Committee of St. Jude Children's Research Hospital.
Oral tolerance
OT-II or p38αΔDC OT-II mice were administered intragastrically with ovalbumin (OVA) protein (2 mg/mouse) daily for 5 days. Control mice were given PBS alone. All mice were then immunized subcutaneously with 100 μg of OVA protein emulsified in CFA. Seven days after immunization, splenocytes were isolated and stimulated with OVA for 3 days; proliferation was assayed after pulsing with [3H]-thymidine for the final 8 h, and IFN-γ secretion was analyzed by Bio-plex (Millipore).
Adoptive transfer and in vivo challenges
Naïve T cells (CD4+CD62LhiCD44loCD25–) were sorted from mice and transferred into recipient mice (donor and recipient cells were distinguished by the congenic markers Thy1.1 and Thy1.2). For oral antigen challenge, after 24 h, recipients were fed with OVA (20 mg/ml Grade VI OVA; Sigma-Aldrich) in the drinking water for 5 days, followed by analysis of MLN cells by FACS. For Rag1–/– recipients, at 7 days after transfer, MLN cells were analyzed by FACS.
Cell purification and culture
Mouse spleen and MLNs were digested with collagenase D, and DCs (CD11c+MHC II+TCR–CD19–DX5– for spleen DCs; CD11c+MHC II+TCR–CD19–DX5–CD103+ or CD103– for MLN DCs, and where indicated, CD103+ DCs were further divided into CD11b+ and CD11b– subsets) were sorted on a Reflection (i-Cyt). Lymphocytes were sorted for naïve T cells, and were labeled with CFSE (Invitrogen) where indicated. For DC–T cell co-cultures, 2.5 × 104 DCs and 2.5 × 105 T cells were mixed in the presence of the cognate peptide (0.05 or 50 μg/ml OVA) or 0.1 or 10 μg/ml αCD3 (2C11; Bio X Cell). After 5 days of culture, live T cells were collected for Foxp3 staining (FJK-16S; eBioscience) or RNA analysis directly; or were stimulated with PMA (phorbol 12-myristate 13-acetate) and ionomycin (Sigma) plus monensin (BD Biosciences) for intracellular cytokine staining, or with plate-bound αCD3 (5 h) for RNA analysis. For antibody or cytokine treatment, cultures were supplemented with TGF-β2 (2 ng/ml; R&D Systems), αIL-27 (10 μg/ml; AF1834; R&D Systems), αTGF-β (10 μg/ml; 1D11, Bio X Cell), IL-27 (100 ng/ml; R&D Systems), or RA (10 nM; Sigma). For cytokine-mediated T cell differentiation, naïve T cells were activated for 5 days with αCD3, αCD28 (37.51; Bio X Cell) and IL-2 (100 U/ml), in the presence of TGF-β1 (2 ng/ml; R&D Systems) for iTreg differentiation, or in the presence of IL-12 (0.5 ng/ml) and αIL-4 (10 μg/ml; 11B11; Bio X Cell) for Th1 differentiation.
Isolation of LP DCs
The isolation of LP DCs was as described (24) with slight modifications. Briefly, after excising Peyer's patches, the small and large intestine was opened longitudinally and washed twice in PBS. Epithelial cells were separated from the underlying LP by incubation in HBSS containing 5 mM EDTA for 15 min at 37°C with vigorous shaking. LP tissue was pulse-vortexed and washed two times in PBS. The remaining tissue was finely chopped with a razor blade and digested in a solution of 1 mg/ml Collagenase type IV (Worthington) and 5% FBS in HBSS for 20 min at 37°C. Tissue digestion was repeated two times. Leukocytes were isolated from the supernatant with a Percoll (GE Healthcare) gradient separation method in which the cells were resuspended in 40% Percoll and underlayered with 80% Percoll followed by centrifugation at 2,500 rpm for 20 min. The interface was collected for FACS analysis and sorting.
Flow cytometry
For analysis of surface markers, cells were stained in PBS containing 2% (wt/vol) BSA and surface antibodies, as described (22). For intracellular phosphorylation assays, cells were stained with antibodies for phosphorylated p38 (4552; Cell Signaling Technology) and ERK (612593; BD) according to the manufacturer's instructions (BD Biosciences). Flow cytometry data were acquired on an upgraded five-color FACScan or LSRII (BD) and were analyzed with FlowJo software (Treestar).
RNA and protein analysis
Primer and probe sets from Applied Biosystems were used for real-time PCR; results were normalized to those of the endogenous control gene encoding HPRT as described (25). For Bio-plex assays, cytokines in supernatants were measured with MILLIPLEX kits for mouse cytokines according to the manufacturer's instructions (Millipore). Immunoblot analyses were performed as described (26), with antibodies for p-Smad3 at Ser423 (ab51451; Abcam) and β-actin (AC-15; Sigma).
Statistical analysis
p values were calculated using Student's t-test. p values of less than 0.05 were considered significant. All error bars in graphs represent SEM calculated from at least 3 replicates.
Results
p38α activity in DCs is required for immune tolerance and iTreg generation in vivo
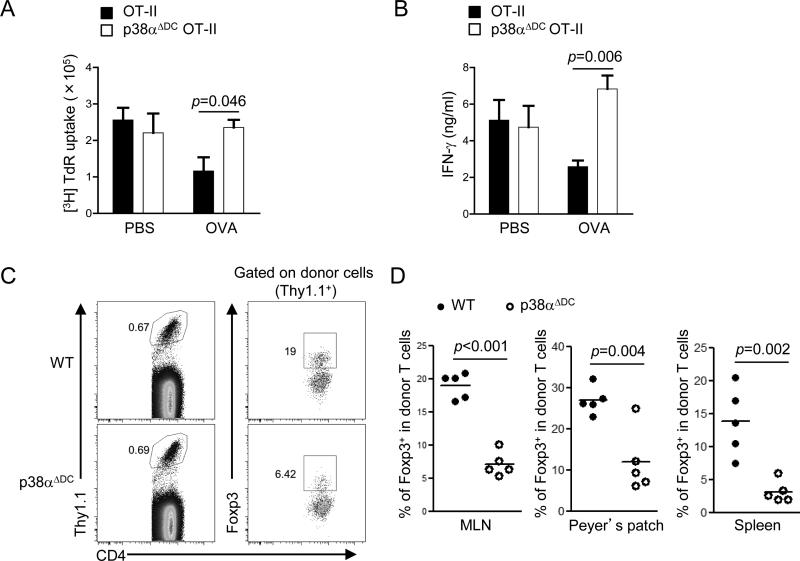
To determine the role of p38α signaling in DC-mediated immune tolerance, we crossed p38αΔDC mice with OT-II TCR transgenic mice (a TCR-transgenic model with T cells recognizing ovalbumin (OVA) amino acids 323–339). We administered OVA protein (or PBS control) intragastrically for five consecutive days to induce oral tolerance, followed by subcutaneous immunization with OVA emulsified in CFA. After seven days, proliferation and cytokine production of splenic T cells were examined in an ex vivo recall response. As expected, tolerance induction by oral OVA treatment actively suppressed both proliferation and IFN-γ production in T cells from wild-type (WT) mice (Figure 1A-B). Although mice with DC-specific deletion of p38α did not show defects under the PBS treatment conditions, they failed to respond to oral induction by suppressing T cell proliferation or IFN-γ production (Figure 1A-B). These data indicate that p38α activity in DCs is important for oral tolerance induction in vivo.
Figure 1. Loss of p38α in DCs prevents induction of oral tolerance and generation of iTreg cells in vivo.
(A–B) OT-II and p38αΔDC OT-II mice were administered intragastrically with OVA protein or PBS as controls, and then immunized with OVA emulsified in CFA; splenocytes were isolated 7 days later for ex vivo stimulation with OVA to measure T cell proliferation (A) and IFN-γ production (B). (C) Naïve OT-II T cells (Thy1.1+) were transferred into wild-type (WT) or p38αΔDC mice that were subsequently fed with OVA in the drinking water for 5 days, followed by analysis of Foxp3 expression in the donor population in MLNs (C). (D) The proportions of Foxp3+ population among donor CD4+ T cells in MLNs, Peyer's patches and spleen from (C). Data are representative of 2 (A, B, n=5 mice per group) and 4 (C, D, n≥5 mice per group) independent experiments. Error bars indicate SEM.
Since Treg cells are important regulators of oral tolerance (12), we next analyzed expression of Foxp3 in p38αΔDC mice. Importantly, development and homeostasis of thymus-derived natural Treg cells were undisturbed in these mice (supplemental Figure 1A). We therefore focused on the role of DC-mediated p38α signaling for the induction of antigen-specific iTreg cells, in a model of oral antigen-induced generation of iTreg cells (27, 28). To this end, we transferred congenically marked naïve CD4+ T cells (CD62LhiCD44loCD25–) from OT-II mice into WT or p38αΔDC mice that were subsequently fed with OVA protein in the drinking water for a total of 5 days. As compared with donor-derived T cells from MLNs of WT mice, those in p38αΔDC mice developed into Foxp3+ iTreg cells at one third of the efficiency (Figure 1C-D). Similar defects were observed in Peyer's patches and spleen (Figure 1D). These data collectively establish an important role of p38α activity in DCs to mediate oral tolerance and iTreg generation in vivo.
CD103+ DCs employ p38α signaling to drive iTreg generation
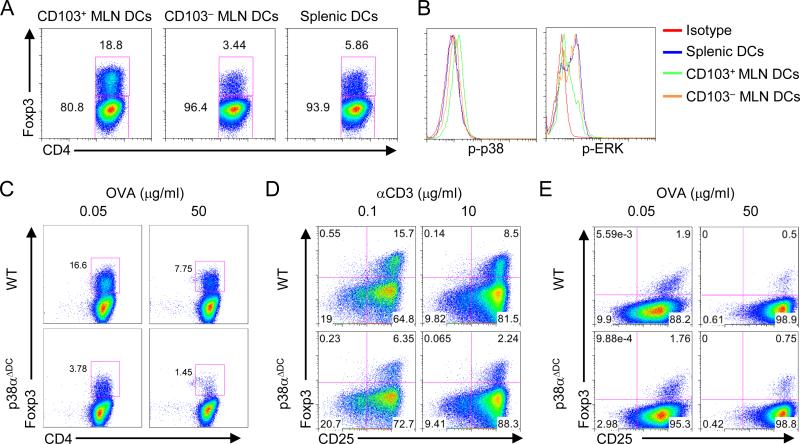
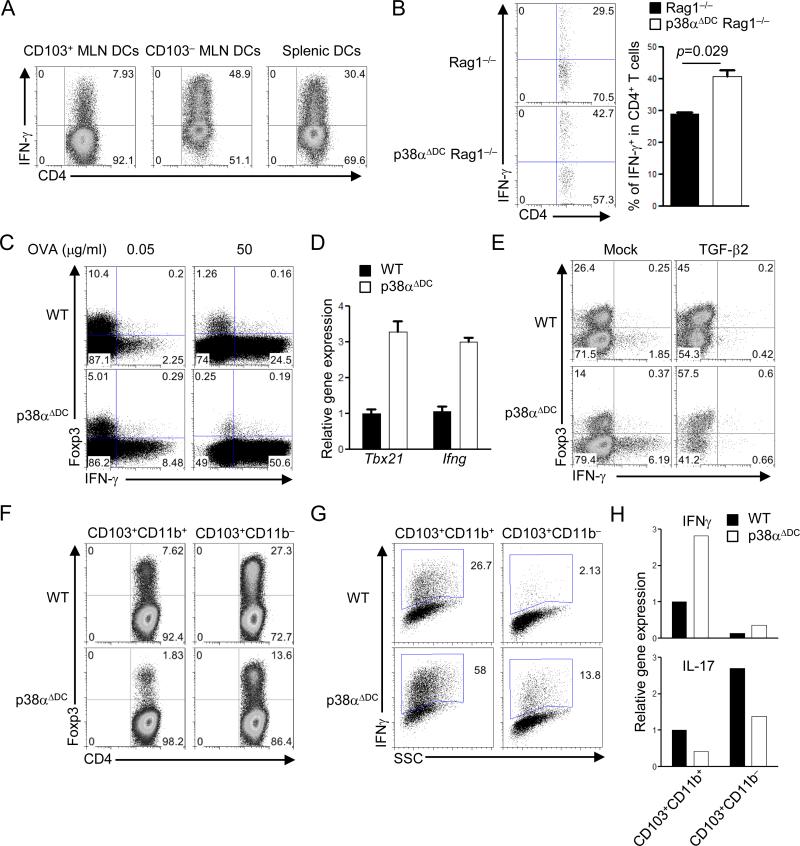
To explore the specific DC subset that depends upon p38α-dependent mechanisms for iTreg induction, we first examined whether DCs from various immune compartments had different abilities to drive iTreg generation. Specifically, we purified CD103+ and CD103– MLN DCs using the gating strategy described in supplemental Figure 1B, as well as splenic DCs, and co-cultured them with naïve OT-II CD4+ T cells, in the presence of antigen but no exogenous cytokines to mimic the physiological interaction between DCs and T cells. After 5 days of culture, T cells activated with CD103+ MLN DCs developed into a Foxp3+ population at a 3-5 fold higher frequency than those activated with CD103– or splenic DCs (Figure 2A). These results indicate a strong tolerogenic property of CD103+ MLN DCs for iTreg induction, in agreement with previous observations (27-29). We next examined signaling pathways in DC subsets. Importantly, CD103+ MLN DCs contained a higher p38 activity than other DC populations under steady-state conditions (Figure 2B). Further, p38α was expressed at much higher levels than other family members including p38β, p38α and p38α in CD103+ DCs (supplemental Figure 1C). In contrast, activity of the related ERK pathway was reduced in these DCs (Figure 2B). These results highlight a likely involvement of p38α in mediating the tolerogenic activity of CD103+ DCs.
Figure 2. p38α is selectively activated in CD103+ MLN DCs and is required for these DCs to mediate iTreg generation in vitro.
(A) Expression of Foxp3 in OT-II T cells activated with CD103+ and CD103– MLN DCs and splenic DCs, in the presence of OVA (0.05 μg/ml), for 5 days. (B) p38 and ERK activity in CD103+ and CD103– MLN DCs and splenic DCs. Pooled samples of all 3 DC populations were used for isotype staining. (C) Expression of Foxp3 in OT-II T cells activated with WT or p38α-deficient CD103+ MLN DCs, in the presence of the indicated doses of OVA, for 5 days. (D) Expression of Foxp3 in polyclonal T cells activated with WT or p38α-deficient CD103+ MLN DCs, in the presence of the indicated doses of αCD3, for 5 days. (E) Expression of Foxp3 in OT-II T cells activated with WT or p38α-deficient CD103– MLN DCs, in the presence of the indicated doses of OVA, for 5 days. Data are representative of 2 (A, D, E, n=4 mice per group), 3 (B, n=4 mice per group) and 4 (C, n≥5 mice per group) independent experiments.
To directly test this hypothesis, we analyzed MLN DCs from p38αΔDC mice. Staining with surface markers CD103, CD11b and CD8 did not reveal obvious defects in the development or homeostasis of DC subsets (supplemental Figure 1D-E). We next determined whether p38α mediated the tolerogenic activity of CD103+ DCs by co-culturing naïve OT-II T cells with CD103+ MLN DCs from WT or p38αΔDC mice. Under these conditions, proliferation and apoptosis of T cells were comparable when stimulated with WT or p38α-deficient DCs, as assessed by CFSE dilution and 7-AAD staining, respectively (supplemental Figure 2A-B). However, T cells activated by p38α-deficient DCs contained a markedly lower frequency of Foxp3+ cells than those activated by WT DCs (Figure 2C). This defect was observed in the presence of varying doses of antigen, although a lower dose of antigen favored iTreg generation regardless of p38α activity (Figure 2C). To ensure that the effect on iTreg induction is not restricted to a specific type of antigen-specific T cells, we used naïve T cells from a polyclonal background and again found impaired iTreg generation mediated by p38α-deficient DCs (Figure 2D). Mechanistically, the effect of DC-derived p38α signaling could be explained, at least partially, by its role in the maintenance of Foxp3 expression in mature iTreg cells, considering the emerging evidence that iTreg cells are more plastic than previously appreciated and may not stably maintain Foxp3 expression (30). To test this possibility, we first activated naïve T cells isolated from Foxp3YFP-Cre mice (23) with WT CD103+ DCs for 4 days, and then sorted YFP+ cells and stimulated them with WT or p38α-deficient CD103+ DCs in the second-round culture. We found that ~50% of iTreg cells lost Foxp3 expression independent of p38α activity, indicating that p38α in CD103+ DCs promotes the induction but not maintenance of iTreg cells (supplemental Figure 2C).
In contrast to the role of p38α in CD103+ MLN DCs, T cells activated with CD103– DCs from WT or p38αΔDC mice expressed low but comparable levels of Foxp3 (Figure 2E). Thus, consistent with a lower p38 activity in CD103– DCs (Figure 2B), p38α in these cells is dispensable for iTreg generation. We therefore conclude that p38α is selectively activated in CD103+ MLN DCs as an important mechanism to mediate iTreg induction in vitro.
p38α-dependent TGF-β2 expression in CD103+ DCs promotes iTreg generation
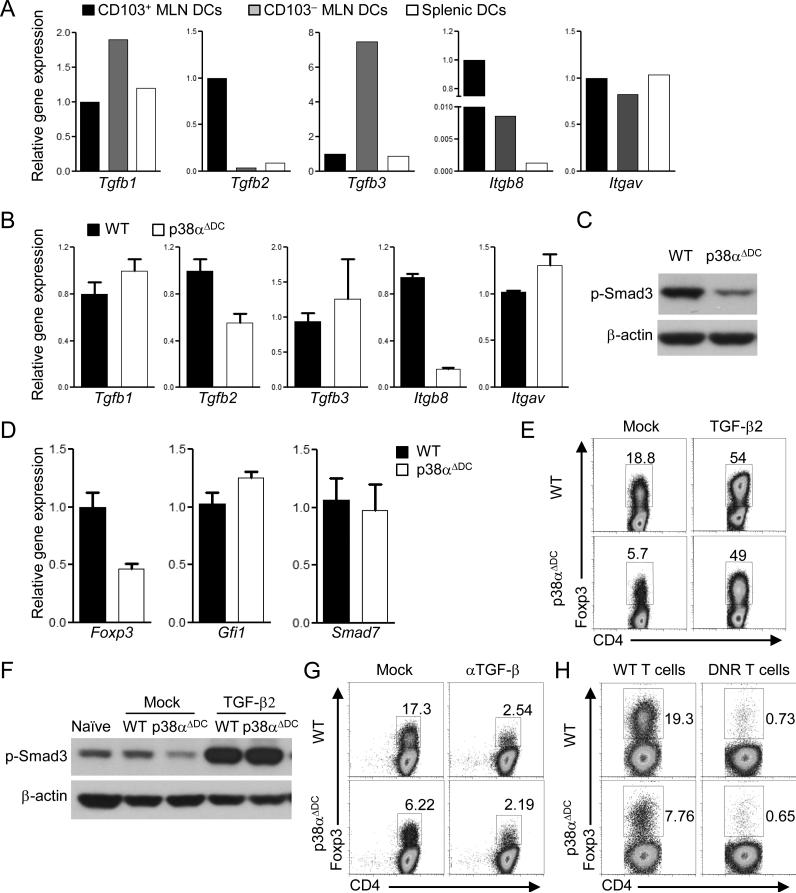
We explored the molecular mechanisms whereby p38α acted in CD103+ DCs to regulate iTreg differentiation. Arguably the most potent cytokine for iTreg differentiation is TGF-β (31). Among the three TGF-β isoforms, Tgfb2 was expressed at a much higher level in CD103+ MLN DCs as compared with other DC subsets, as previously reported (27), while Tgfb1 or Tgfb3 was not selectively expressed by CD103+ DCs (Figure 3A). Notably, TGF-β proteins are synthesized as latent precursors that require integrin-dependent activation, in a process dependent upon the integrin alpha(v)beta8 (αvβ8) expressed by DCs (32, 33). We found that integrin β8 (encoded by Itgb8), but not αv (Itgav), was selectively expressed by CD103+ MLN DCs (Figure 3A). Importantly, deletion of p38α in CD103+ DCs considerably diminished the expression of Tgfb2 and Itgb8, but not Tgfb1, Tgfb3 or Itgav (Figure 3B), further supporting a model that p38α endows such DCs with a selective tolerogenic activity. Consistent with the requirement of p38α in TGF-β2 mRNA expression and functional activation, CD103+ DCs lacking p38α were impaired to induce Smad3 activation in co-cultured T cells (Figure 3C). Interestingly, while Foxp3 mRNA induction was defective in T cells activated with p38α-deficient CD103+ DCs, expression of other TGF-β target genes including Gfi1 and Smad7 was unaltered (Figure 3D). These results are in agreement with a crucial and selective role of Smad3 in transducing TGF-β signals for iTreg generation (34). Therefore, p38α activates the TGF-β2–Smad3 axis at the DC–T cell interface.
Figure 3. DC-derived p38α signaling promotes iTreg generation by regulating TGF-β2 expression in CD103+ DCs.
(A) RNA analysis of CD103+ and CD103– MLN DCs and splenic DCs. (B) RNA analysis of WT or p38α-deficient CD103+ DCs. (C) Phosphorylation of Smad3 in OT-II T cells stimulated with WT or p38α-deficient CD103+ DCs, in the presence of OVA (0.05 μg/ml), for 2 days. (D) RNA analysis of OT-II T cells activated with WT or p38αΔDC DCs, in the presence of OVA (0.05 μg/ml), for 5 days. (E) Expression of Foxp3 in OT-II T cells activated with WT or p38αΔDC DCs, in the presence of OVA (0.05 μg/ml), with or without TGF-β2 for 5 days. (F) Phosphorylation of Smad3 in OT-II T cells stimulated with WT or p38αΔDC DCs, in the presence of OVA (0.05 μg/ml), with or without TGF-β2 for 2 days. (G) Expression of Foxp3 in OT-II T cells activated with WT or p38αΔDC DCs, in the presence of OVA (0.05 μg/ml), with or without αTGF-β for 5 days. (H) Expression of Foxp3 in WT and CD4-dnTGFβRII (DNR) polyclonal T cells activated with WT or p38αΔDC DCs, in the presence of αCD3 (0.1 μg/ml), for 5 days. Data are representative of 2 (n=4 mice per group) independent experiments. Error bars indicate SEM.
We next assessed the functional importance of p38α-dependent TGF-β2 expression. Addition of TGF-β2 to the DC–T cell co-cultures restored the defective Foxp3 induction, as well as the decreased phosphorylation of Smad3, in T cells activated by p38α-deficient CD103+ DCs (Figure 3E-F). Conversely, neutralizing TGF-β activity in the co-cultures abrogated iTreg development mediated by CD103+ DCs from either WT or p38αΔDC mice (Figure 3G). Similar findings were obtained when we used T cells expressing the dominant negative TGF-βR2 molecule to block TGF-β signaling (Figure 3H) (35). Therefore, p38α-dependent TGF-β2 expression in CD103+ DCs plays a crucial role in mediating iTreg generation.
p38α in CD103+ DCs mediates iTreg induction independently of proinflammatory cytokines
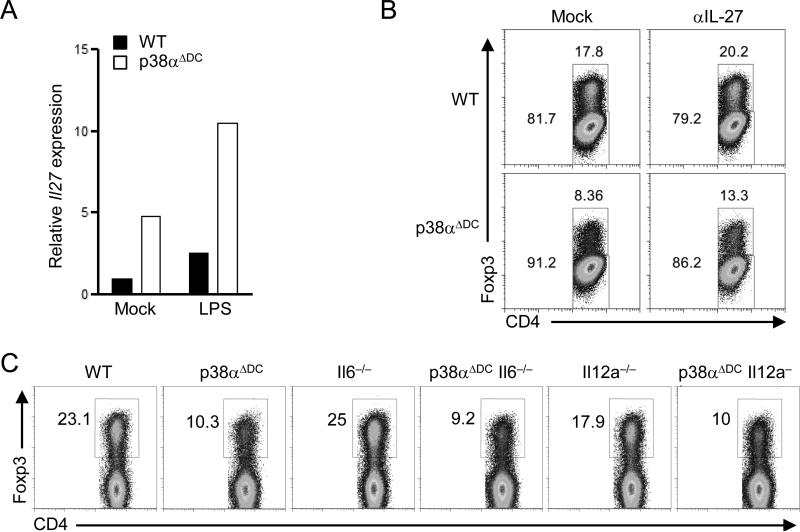
The differentiation of iTreg cells is not only driven by tolerogenic cytokines such as TGF-β, but is also shaped by proinflammatory cytokines. IL-27 is a potent cytokine limiting iTreg differentiation (36). IL-27 expression was increased in p38α-deficient CD103+ DCs (Figure 4A), similar as our recent observations in splenic DCs.(22) However, blockade of IL-27 had little effect on restoring the defective iTreg differentiation mediated by the mutant DCs (Figure 4B). IL-6 is another proinflammatory cytokine for inhibiting iTreg differentiation (30), and its secretion by splenic DCs is affected by p38α deficiency (22). To test the role of IL-6 in p38α-dependent DC–T cell crosstalk, we crossed p38αΔDC mice with mice deficient in IL-6. Compared with CD103+ DCs deficient in IL-6, those lacking both IL-6 and p38α showed a decreased ability to drive iTreg differentiation, similar as the effects of p38α deficiency on an IL-6-sufficient background (Figure 4C). Similarly, we did not find a role of IL-12 produced from p38α-deficient DCs in this process (Figure 4C). Altogether, p38α signaling in DCs regulates iTreg generation largely independently of the proinflammatory cytokines IL-27, IL-6 and IL-12, further highlighting a key role for p38α in specifically programming the tolerogenic activity of CD103+ DCs.
Figure 4. p38α signaling in CD103+ MLN DCs drives iTreg differentiation independently of IL-27, IL-6 and IL-12.
(A) Expression of IL-27 in CD103+ MLN DCs isolated from WT and p38αΔDC mice. (B) Expression of Foxp3 in OT-II T cells activated with WT or p38α-deficient CD103+ DCs, in the presence of OVA (0.05 μg/ml), with or without αIL-27for 5 days. (C) Foxp3 expression in OT-II T cells activated with CD103+ MLN DCs isolated from WT, p38αΔDC, Il6–/–, p38αΔDC Il6–/–, Il12a–/–, or p38αΔDC Il12a–/– mice, in the presence of OVA (0.05 μg/ml), for 5 days. Data are representative of 2 independent experiments (n=3 mice per group).
DC-derived p38α signaling reciprocally regulates iTreg and Th1 differentiation
We recently described that naïve T cells can engage reciprocal developmental pathways to generate iTreg and Th1 cells (25, 37). Indeed, associated with enhanced ability to drive iTreg generation (Figure 2A), CD103+ MLN DCs were less potent at mediating Th1 differentiation as compared with CD103– MLN or splenic DCs (Figure 5A). We next determined whether CD103+ DCs employed p38α signaling to actively suppress the differentiation of Th1 cells. First, we adoptively transferred naïve T cells from C57BL/6 mice into Rag1–/– or p38αΔDC Rag1–/– recipients. Seven days later, donor cells developed into a greater IFN-γ+ Th1 population in MLNs from p38αΔDC Rag1–/– recipients as compared with Rag1–/– mice (Figure 5B), indicating that p38α activity in DCs suppresses IFN-γ production in T cells in vivo. Second, in the CD103+ DC–T cell co-cultures, T cells activated with p38α-deficient DCs showed a greater degree of Th1 differentiation (Figure 5C), associated with higher Tbx21 (encoding T-bet) and Ifng expression (Figure 5D). Therefore, p38α activity in CD103+ DCs exerts reciprocal effects on Th1 and iTreg differentiation.
Figure 5. DC-derived p38α signaling regulates reciprocal iTreg and Th1 differentiation in a TGF-β2-dependent manner.
(A) Expression of IFN-γ in OT-II T cells activated with CD103+ and CD103– MLN DCs and splenic DCs, in the presence of OVA (0.05 μg/ml), for 5 days, followed by intracellular cytokine staining. (B) T cells were transferred into Rag1–/– and p38αΔDC Rag1–/– mice, and expression of IFN-γ from donor cells in MLNs was analyzed at day 7. Right, the proportions of IFN-γ+ population among donor CD4+ T cells. (C) Expression of Foxp3 and IFN-γ in OT-II T cells stimulated with WT or p38α-deficient CD103+ DCs, in the presence of the indicated doses of OVA, for 5 days. (D) Gene expression in T cells from (C). (E) Expression of Foxp3 and IFN-γ in OT-II T cells stimulated with WT or p38α-deficient CD103+ DCs, in the presence of OVA (0.05 μg/ml), with or without TGF-β2 for 5 days. (F–G) Expression of Foxp3 (F) and IFN-γ (G) in OT-II T cells stimulated with WT or p38α-deficient CD103+CD11b+ or CD103+CD11b– MLN DCs, in the presence of OVA (0.05 μg/ml), for 5 days. (H) RNA analysis of IFNγ and IL-17 expression from (F–G). Data are representative of 2 (n=5 mice per group) independent experiments. Error bars indicate SEM.
We next determined the mechanistic basis for this altered differentiation. Because p38α is required for TGF-β2 expression in CD103+ DCs (Figure 3B), we asked whether this pathway coordinated the development of iTreg and Th1 cells. To this end, we primed naïve T cells with CD103+ DCs in the presence or absence of TGF-β2. TGF-β2 inhibited IFN-γ production while promoting Foxp3 expression, and largely corrected the altered iTreg and Th1 differentiation mediated by p38α-deficient DCs (Figure 5E). These results collectively indicate that p38α signaling in CD103+ DCs regulates reciprocal iTreg and Th1 differentiation in a TGF-β2-dependent manner.
Role of p38α in distinct subsets and origins of intestinal CD103+ DCs
The intestinal CD103+ DCs can be further divided into two subsets on the basis of CD11b expression (38, 39). To determine the roles of p38α signaling in these two DC subsets in mediating T cell differentiation, we purified CD103+CD11b+ and CD103+CD11b– MLN DCs and co-cultured them with naïve OT-II CD4+ T cells. Compared with T cells activated by CD103+CD11b+ DCs, those activated by CD103+CD11b– DCs developed into more Foxp3+ but less IFN-γ+ cells, indicating a stronger tolerogenic activity of CD103+CD11b– DCs (Figure 5F-G). Importantly, loss of p38α in both DC subsets diminished iTreg but enhanced Th1 differentiation, thereby reinforcing the key role of p38α in mediating DC-dependent reciprocal differentiation of these T cell lineages (Figure 5F-G). Under these conditions, IL-17+ cells were barely detectable by intracellular staining (data not shown). However, RNA analysis indicated that p38α signaling in both DC subsets was required for Th17 differentiation (Figure 5H), in agreement with our recent finding for the requirement of p38α signaling in splenic DCs for driving Th17 differentiation (22).
Recent studies have identified discrete immune functions of DC subsets in LP. For example, the CD103+CD11b+ DC subset in LP plays an important role in mediating Th17 responses (24, 40). However, how intracellular signaling programs the tolerogenic activity of LP DC subsets remains poorly understood. We therefore purified CD103+CD11b+ and CD103+CD11b– LP DCs from WT and p38αΔDC mice, and examined their ability to drive iTreg differentiation. Similar as our observations in MLN DCs, CD103+CD11b– LP DCs had a stronger ability to induce T cell Foxp3 induction than their CD103+CD11b+ counterpart, but both DCs subsets depended upon p38α activity for iTreg differentiation (supplemental Figure 3). We conclude that p38α signaling represents a crucial pathway to program the tolerogenic activity of mucosal CD103+ DCs of distinct subsets and origins.
T cell-intrinsic p38α signaling is dispensable for iTreg or Th1 differentiation
Published studies using p38 inhibitors have revealed an important role for T cell-intrinsic p38 activity in the generation of iTreg cells (41) and Th1 cells (19), but these effects are yet to be genetically defined. To this end, we crossed p38αfl/fl mice with CD4-Cre mice to achieve T cell-specific deletion of p38α (called p38αΔT mice) (22). Naïve T cells from WT or p38αΔT mice had a similar ability to develop into iTreg cells when stimulated with CD103+ or CD103– MLN DCs (Figure 6A). Also, p38α-deficient T cells showed normal iTreg differentiation in response to in vitro polarizing conditions mediated by TGF-β (Figure 6B). Furthermore, p38α-deficient T cells had no defects in Th1 differentiation in vitro (Figure 6C). Collectively, our results exclude a T cell-intrinsic function of p38α in iTreg and Th1 generation, and further highlight a selective requirement of p38α activity for DC-mediated T cell lineage differentiation.
Figure 6. T cell-intrinsic p38α activity is dispensable for Th1 or iTreg differentiation.
(A) Expression of Foxp3+ in WT and p38αΔT naïve T cells activated with CD103+ and CD103– MLN DCs, in the presence of αCD3(0.5 μg/ml), for 5 days. (B) Expression of Foxp3 in WT and p38αΔT naïve T cells differentiated under iTreg conditions for 5 days. (C) Expression of IFN-γ in WT and p38αΔT naïve T cells differentiated under Th1 conditions for 5 days, followed by PMA and ionomycin stimulation and intracellular staining. Data are representative of 2 (n=4 mice per group) independent experiments.
p38α in CD103+ DCs imprints induction of gut homing receptors on responding T cells
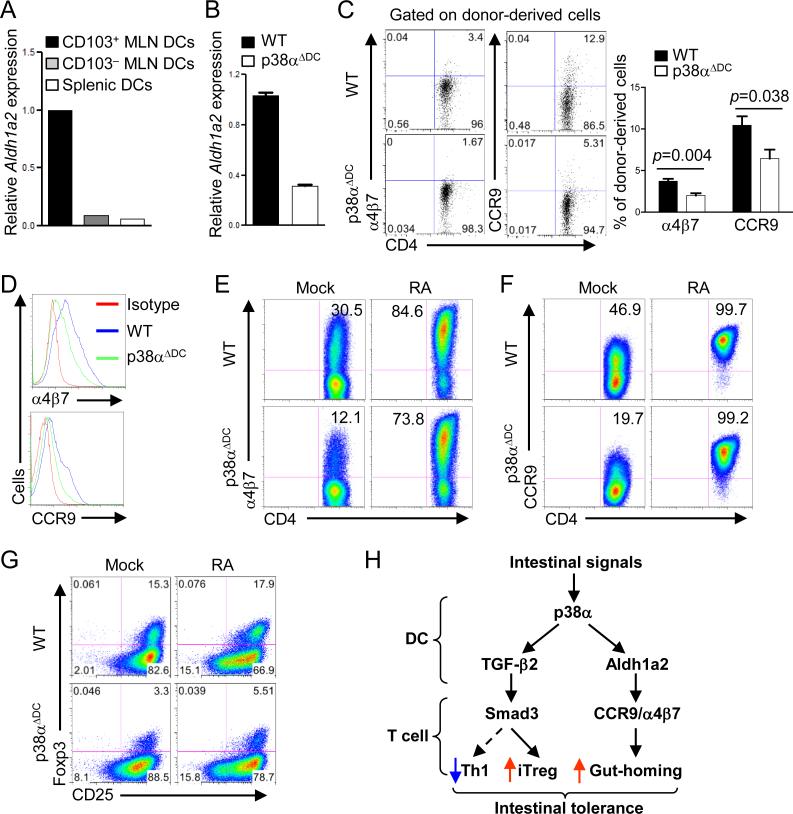
To perform immune functions, activated T cells in the GALT migrate preferentially to LP of the intestine, in a process dependent upon induction of gut homing receptors including the integrin α4β7 and chemokine receptor CCR9. MLN DCs are essential for imprinting gut homing specificity on T cells by producing RA to promote T cell expression of these trafficking molecules (16-18). Synthesis of RA from its precursor, vitamin A, depends upon the enzyme retinaldehyde dehydrogenases, especially the major isoform Aldh1a2 in intestinal DCs (16-18). Indeed, Aldh1a2 was highly expressed in CD103+ MLN DCs compared with CD103– or splenic DCs (Figure 7A), but its level was considerably decreased following p38α deletion (Figure 7B). To examine whether this defect alters trafficking molecules on responding T cells, we transferred naïve OT-II T cells into WT or p38αΔDC mice, followed by feeding with OVA protein in the drinking water. Expression of α4β7 and CCR9 on donor T cells from p38αΔDC recipients was substantially reduced as compared with those from WT mice (Figure 7C). Consistent with the in vivo findings, T cells stimulated with p38α-deficient CD103+ DCs expressed lower levels of α4β7 and CCR9 than those stimulated with WT DCs (Figure 7D). These results indicate that p38α is important for the expression of Aldh1a2 in CD103+ DCs and for DC-dependent induction of T cell trafficking molecules.
Figure 7. p38α is required for Aldh1a2 expression in CD103+ DCs and DC-mediated imprinting of gut homing receptors on responding T cells.
(A) RNA analysis of Aldh1a2 from CD103+ and CD103– MLN DCs and splenic DCs. (B) Expression of Aldh1a2 in CD103+ DCs from WT or p38αΔDC mice. (C) Naïve OT-II T cells (Thy1.1+) were transferred into WT or p38αΔDC mice that were subsequently fed with OVA in the drinking water for 5 days, followed by analysis of α4β7 and CCR9 expression on the donor population in MLNs. Right, the proportions of α4β7+ and CCR9+ populations among donor CD4+ T cells. (D) Expression of α4β7 and CCR9 on OT-II T cells stimulated with WT or p38α-deficient CD103+ DCs, in the presence of OVA (0.05 μg/ml), for 5 days. (E, F) Expression of α4β7 (E) and CCR9 (F) on OT-II T cells stimulated with WT or p38α-deficient CD103+ DCs, in the presence of OVA (0.05 μg/ml), with or without RA for 5 days. (G) Expression of Foxp3 in OT-II T cells activated with WT or p38α-deficient CD103+ DCs, in the presence of OVA (0.05 μg/ml), with or without RA for 5 days. (H) Proposed model of p38α-dependent programming of CD103+ mucosal DCs to mediate reciprocal differentiation of Th1 and iTreg cells, and the induction of gut-homing receptors on T cells. Data are representative of 2 (A, C, E, F, n≥5 mice per group; G, n=3 mice per group) and 3 (B, D n=4 mice per group) independent experiments. Error bars indicate SEM.
To identify the causative relationship between these two effects, we added exogenous RA into the co-cultures. RA treatment restored the defective α4β7 and CCR9 expressions on T cells activated by p38αΔDC DCs (Figure 7E-F). In agreement with previous observations that RA acts as a co-factor for TGF-β-dependent Foxp3 induction (16-18), RA alone had minimal effects at promoting iTreg generation and was unable to rectify the diminished iTreg generation mediated by p38α-deficient CD103+ DCs (Figure 7G). Therefore, p38α-dependent Aldh1a2 expression in CD103+ DCs mainly contributes to the induction of gut homing receptors but not Foxp3 expression from responding T cells (Figure 7H).
Discussion
A fundamental question in immunology is how the decision between protective immunity and immune tolerance is made. DCs, the most potent cells for bridging innate and adaptive immunity, have emerged as a central regulator of this process. Much emphasis has been placed on DC-mediated cellular and molecular mechanisms that program T cell activation and effector differentiation (1, 2). Our recent identification of the p38α/MKP-1 axis for DC-dependent Th17 differentiation contributes to our understanding of the signaling mechanisms orchestrating this process (20-22). In contrast, we have little information on how intracellular signaling networks program DCs to become tolerogenic, a crucial function of DCs under steady state (3, 4, 42). Here we describe that p38α is constitutively activated in CD103+ MLN DCs to program the tolerogenic activity of these DCs in the intestinal immune system. Loss of p38α activity in DCs prevents the induction of oral tolerance, highlighting the physiological significance of this novel signaling mechanism in vivo.
Recent studies have revealed specialized functions of CD103+ MLN DCs, relative to the CD103– counterpart and other DC subsets, in imprinting T cell gut homing receptors and differentiative capacity toward iTreg cells (27, 29, 43, 44). Such tolerogenic properties of CD103+ DCs are mediated by the secretory factors especially RA and TGF-β, which can be further attributed to the exposure to the local conditioning factors in LP prior to their migration to MLN (14). Despite the advances on the biology and origin of CD103+ DCs, what remains undefined is the molecular mechanism orchestrating such unique profiles. We found that p38α is constitutively activated in CD103+ MLN DCs relative to other DC populations. Moreover, p38α activity in CD103+ DCs is required for the expression of Tgfb2 and Aldh1a2, two molecules crucial for mucosal DC functions (18, 27, 28). This synergistic effect shapes T cell differentiation and further couples it to the induction of gut homing receptors on T cells. To our knowledge, p38α is the first signaling pathway identified that programs the tolerogenic properties of CD103+ MLN DCs.
Emerging evidence suggests a greater complexity in T cell lineage commitment and plasticity than previously appreciated (30). The most notable example is the shared circuitry between iTreg and Th17 cells, as the differentiation of both lineages relies on TGF-β and the balance between them is dictated by the inflammatory environment (45). Despite extensive progress in this area, it remains poorly understood how the differentiation of naïve precursors into opposing lineages is regulated. Also, as many of the studies rely on the use of cytokine supplement or ablation, how T cell lineage commitment and plasticity are coordinated by physiological stimuli remains to be established. Using DC–T cell co-cultures and in vivo DC-specific deletion systems to recapitulate the physiological interactions between these cells, we have identified that DC-derived p38α signaling directs the reciprocal differentiation of iTreg and Th1 cells by modulating the TGF-β2/Smad3 axis at the DC–T cell interface. This function is operative in mucosal CD103+ DCs of distinct subsets and origins, but not in CD103– MLN DCs, highlighting the important role of p38α in mediating the tolerogenic activity of CD103+ DCs. This extrinsic control mechanism is reminiscent of our recent studies implicating TGF-β/Smad3 signaling in T cell-intrinsic regulation of these two related lineages (25, 37), as well as the dichotomy of iTreg and Th17 lineage decisions (45). Notably, because of the tolerogenic properties of CD103+ MLN DCs, these mucosal DCs do not polarize Th17 differentiation regardless of p38α activity, and this is distinct from the roles of p38α activity in programming DCs in the peripheral immune compartment to drive Th17 differentiation under inflammatory conditions (22). Furthermore, p38α is not required in CD103– MLN DCs for iTreg generation; nor is it important for T cell-intrinsic regulation of iTreg and Th1 differentiation. Taken together, p38α activity in DCs integrates both inflammatory and anti-inflammatory signals in a context-dependent manner, with a dominant effect at mediating intestinal tolerogenic responses under steady state.
Aside from lineage specification, proper trafficking of antigen-specific T cells is also crucial for the outcome of immune responses and homeostasis. In the intestinal immune system, naïve T cells traffic to MLNs where they are activated by DCs to differentiate into effector or regulatory T cells, which then home to the LP to mediate immune reactions and maintain intestinal homeostasis. CD103+ MLN DCs metabolize vitamin A to produce RA, which facilitates both iTreg generation and gut homing properties of T cells (18, 27, 28). RA has also been shown to suppress Th17 differentiation (46), although a recent study has described an obligatory role of RA in T cell activation and proinflammatory Th17 and Th1 responses (47). Consistent with a complex role of RA in T cell lineage decisions, supplement with RA did not affect iTreg differentiation mediated by p38α-deficient CD103+ DCs, but it completely restored the defective induction of gut-homing receptors on responding T cells. In contrast, exogenous TGF-β2 rectified the altered abilities of these DCs to mediate the differentiation of iTreg and Th1 cells. Therefore, p38α signaling in DCs coordinately regulates T cell differentiation and trafficking by orchestrating the expression of two distinct effector molecules, Tgfb2 and Aldh1a2, respectively (Figure 5H).
Aside from DC-derived signals, T cell-intrinsic signaling pathways also contribute to cell fate decisions. In particular, the p38 pathway has been shown to contribute to the generation of iTreg (41) and Th1 cells (19). However, T cell-specific deletion of p38α did not impact the differentiation of these cells, at least until the in vitro polarizing conditions. We speculate that the apparent functions of p38 previously observed in T cells were likely due to nonspecific actions of pharmacological approaches, or the inability to distinguish the effects in T cells from those in APCs. Our results support a selective role of p38α in DC-mediated, but not T cell-intrinsic, iTreg and Th1 differentiation.
Collectively, our results identify p38α as a central pathway for programming the tolerogenic activity of mucosal CD103+ DCs. Although T cell responses can be influenced by the specific type of DC subsets, our results suggest that at the fundamental level, it is the intracellular signaling pathways, especially those mediated by p38α, that endow DC subsets with specific abilities to control T cell fates. Given the key roles of CD103+ DCs in intestinal tolerance (14), the identification of this novel control mechanism has important implications for therapeutic intervention of intestinal inflammation and other diseases. From this perspective, p38α is by far the most extensively investigated protein kinase target for the development of anti-inflammatory drugs in the pharmaceutical industry, but severe side effects have prevented clinical advancement of p38α inhibitors (48). Identification of the tolerogenic activity and downstream effectors mediated by p38α will be instrumental to the design of more selective strategies for therapeutic intervention.
Supplementary Material
Acknowledgements
The authors acknowledge C. Cloer and N. Brydon for help with animal colony management, B. Reizis for CD11c-Cre mice, K. Otsu for p38αfl/fl mice, A. Rudensky for Foxp3YFP-Cre mice, and St. Jude Immunology FACS core facility for cell sorting.
Footnotes
This work was supported by US National Institutes of Health (R01 NS064599 and R21 AI094089), National Multiple Sclerosis Society RG 4691-B-2, and Cancer Research Institute (to H.C.).
Conflict of interest statement
The authors declare no conflict of interest.
References
- 1.Iwasaki A, Medzhitov R. Regulation of adaptive immunity by the innate immune system. Science. 2010;327:291–295. doi: 10.1126/science.1183021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Joffre O, Nolte MA, Sporri R, Reis e Sousa C. Inflammatory signals in dendritic cell activation and the induction of adaptive immunity. Immunol Rev. 2009;227:234–247. doi: 10.1111/j.1600-065X.2008.00718.x. [DOI] [PubMed] [Google Scholar]
- 3.Steinman RM, Hawiger D, Nussenzweig MC. Tolerogenic dendritic cells. Annu Rev Immunol. 2003;21:685–711. doi: 10.1146/annurev.immunol.21.120601.141040. [DOI] [PubMed] [Google Scholar]
- 4.Manicassamy S, Pulendran B. Dendritic cell control of tolerogenic responses. Immunol Rev. 2011;241:206–227. doi: 10.1111/j.1600-065X.2011.01015.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Birnberg T, Bar-On L, Sapoznikov A, Caton ML, Cervantes-Barragan L, Makia D, Krauthgamer R, Brenner O, Ludewig B, Brockschnieder D, Riethmacher D, Reizis B, Jung S. Lack of conventional dendritic cells is compatible with normal development and T cell homeostasis, but causes myeloid proliferative syndrome. Immunity. 2008;29:986–997. doi: 10.1016/j.immuni.2008.10.012. [DOI] [PubMed] [Google Scholar]
- 6.Ohnmacht C, Pullner A, King SB, Drexler I, Meier S, Brocker T, Voehringer D. Constitutive ablation of dendritic cells breaks self-tolerance of CD4 T cells and results in spontaneous fatal autoimmunity. J Exp Med. 2009;206:549–559. doi: 10.1084/jem.20082394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Maloy KJ, Powrie F. Intestinal homeostasis and its breakdown in inflammatory bowel disease. Nature. 2011;474:298–306. doi: 10.1038/nature10208. [DOI] [PubMed] [Google Scholar]
- 8.Bilate AM, Lafaille JJ. Induced CD4+Foxp3+ regulatory T cells in immune tolerance. Annu Rev Immunol. 2012;30:733–758. doi: 10.1146/annurev-immunol-020711-075043. [DOI] [PubMed] [Google Scholar]
- 9.Josefowicz SZ, Lu LF, Rudensky AY. Regulatory T cells: mechanisms of differentiation and function. Annu Rev Immunol. 2012;30:531–564. doi: 10.1146/annurev.immunol.25.022106.141623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Haribhai D, Williams JB, Jia S, Nickerson D, Schmitt EG, Edwards B, Ziegelbauer J, Yassai M, Li SH, Relland LM, Wise PM, Chen A, Zheng YQ, Simpson PM, Gorski J, Salzman NH, Hessner MJ, Chatila TA, Williams CB. A requisite role for induced regulatory T cells in tolerance based on expanding antigen receptor diversity. Immunity. 2011;35:109–122. doi: 10.1016/j.immuni.2011.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Josefowicz SZ, Niec RE, Kim HY, Treuting P, Chinen T, Zheng Y, Umetsu DT, Rudensky AY. Extrathymically generated regulatory T cells control mucosal TH2 inflammation. Nature. 2012;482:395–399. doi: 10.1038/nature10772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Weiner HL, da Cunha AP, Quintana F, Wu H. Oral tolerance. Immunol Rev. 2011;241:241–259. doi: 10.1111/j.1600-065X.2011.01017.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Worbs T, Bode U, Yan S, Hoffmann MW, Hintzen G, Bernhardt G, Forster R, Pabst O. Oral tolerance originates in the intestinal immune system and relies on antigen carriage by dendritic cells. J Exp Med. 2006;203:519–527. doi: 10.1084/jem.20052016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Scott CL, Aumeunier AM, Mowat AM. Intestinal CD103+ dendritic cells: master regulators of tolerance? Trends Immunol. 2011;32:412–419. doi: 10.1016/j.it.2011.06.003. [DOI] [PubMed] [Google Scholar]
- 15.Milling S, Yrlid U, Cerovic V, MacPherson G. Subsets of migrating intestinal dendritic cells. Immunol Rev. 2010;234:259–267. doi: 10.1111/j.0105-2896.2009.00866.x. [DOI] [PubMed] [Google Scholar]
- 16.Agace WW, Persson EK. How vitamin A metabolizing dendritic cells are generated in the gut mucosa. Trends Immunol. 2012;33:42–48. doi: 10.1016/j.it.2011.10.001. [DOI] [PubMed] [Google Scholar]
- 17.Iwata M. Retinoic acid production by intestinal dendritic cells and its role in T-cell trafficking. Semin Immunol. 2009;21:8–13. doi: 10.1016/j.smim.2008.09.002. [DOI] [PubMed] [Google Scholar]
- 18.Hall JA, Grainger JR, Spencer SP, Belkaid Y. The role of retinoic acid in tolerance and immunity. Immunity. 2011;35:13–22. doi: 10.1016/j.immuni.2011.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huang G, Shi LZ, Chi H. Regulation of JNK and p38 MAPK in the immune system: signal integration, propagation and termination. Cytokine. 2009;48:161–169. doi: 10.1016/j.cyto.2009.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Huang G, Wang Y, Chi H. Regulation of T(H)17 cell differentiation by innate immune signals. Cell Mol Immunol. 2012;9:287–295. doi: 10.1038/cmi.2012.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huang G, Wang Y, Shi LZ, Kanneganti TD, Chi H. Signaling by the phosphatase MKP-1 in dendritic cells imprints distinct effector and regulatory T cell fates. Immunity. 2011;35:45–58. doi: 10.1016/j.immuni.2011.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Huang G, Wang Y, Vogel P, Kanneganti TD, Otsu K, Chi H. Signaling via the kinase p38alpha programs dendritic cells to drive T(H)17 differentiation and autoimmune inflammation. Nat Immunol. 2012;13:152–161. doi: 10.1038/ni.2207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rubtsov YP, Rasmussen JP, Chi EY, Fontenot J, Castelli L, Ye X, Treuting P, Siewe L, Roers A, Henderson WR, Jr., Muller W, Rudensky AY. Regulatory T cell-derived interleukin-10 limits inflammation at environmental interfaces. Immunity. 2008;28:546–558. doi: 10.1016/j.immuni.2008.02.017. [DOI] [PubMed] [Google Scholar]
- 24.Kinnebrew MA, Buffie CG, Diehl GE, Zenewicz LA, Leiner I, Hohl TM, Flavell RA, Littman DR, Pamer EG. Interleukin 23 production by intestinal CD103(+)CD11b(+) dendritic cells in response to bacterial flagellin enhances mucosal innate immune defense. Immunity. 2012;36:276–287. doi: 10.1016/j.immuni.2011.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu G, Burns S, Huang G, Boyd K, Proia RL, Flavell RA, Chi H. The receptor S1P1 overrides regulatory T cell-mediated immune suppression through Akt-mTOR. Nat Immunol. 2009;10:769–777. doi: 10.1038/ni.1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yang K, Neale G, Green DR, He W, Chi H. The tumor suppressor Tsc1 enforces quiescence of naive T cells to promote immune homeostasis and function. Nat Immunol. 2011;12:888–897. doi: 10.1038/ni.2068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Coombes JL, Siddiqui KR, Arancibia-Carcamo CV, Hall J, Sun CM, Belkaid Y, Powrie F. A functionally specialized population of mucosal CD103+ DCs induces Foxp3+ regulatory T cells via a TGF-beta and retinoic acid-dependent mechanism. J Exp Med. 2007;204:1757–1764. doi: 10.1084/jem.20070590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sun CM, Hall JA, Blank RB, Bouladoux N, Oukka M, Mora JR, Belkaid Y. Small intestine lamina propria dendritic cells promote de novo generation of Foxp3 T reg cells via retinoic acid. J Exp Med. 2007;204:1775–1785. doi: 10.1084/jem.20070602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jaensson E, Uronen-Hansson H, Pabst O, Eksteen B, Tian J, Coombes JL, Berg PL, Davidsson T, Powrie F, Johansson-Lindbom B, Agace WW. Small intestinal CD103+ dendritic cells display unique functional properties that are conserved between mice and humans. J Exp Med. 2008;205:2139–2149. doi: 10.1084/jem.20080414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhou L, Chong MM, Littman DR. Plasticity of CD4+ T cell lineage differentiation. Immunity. 2009;30:646–655. doi: 10.1016/j.immuni.2009.05.001. [DOI] [PubMed] [Google Scholar]
- 31.Chen W, Jin W, Hardegen N, Lei KJ, Li L, Marinos N, McGrady G, Wahl SM. Conversion of peripheral CD4+CD25- naive T cells to CD4+CD25+ regulatory T cells by TGF-beta induction of transcription factor Foxp3. J Exp Med. 2003;198:1875–1886. doi: 10.1084/jem.20030152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Travis MA, Reizis B, Melton AC, Masteller E, Tang Q, Proctor JM, Wang Y, Bernstein X, Huang X, Reichardt LF, Bluestone JA, Sheppard D. Loss of integrin alpha(v)beta8 on dendritic cells causes autoimmunity and colitis in mice. Nature. 2007;449:361–365. doi: 10.1038/nature06110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lacy-Hulbert A, Smith AM, Tissire H, Barry M, Crowley D, Bronson RT, Roes JT, Savill JS, Hynes RO. Ulcerative colitis and autoimmunity induced by loss of myeloid alphav integrins. Proc Natl Acad Sci U S A. 2007;104:15823–15828. doi: 10.1073/pnas.0707421104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tone Y, Furuuchi K, Kojima Y, Tykocinski ML, Greene MI, Tone M. Smad3 and NFAT cooperate to induce Foxp3 expression through its enhancer. Nat Immunol. 2008;9:194–202. doi: 10.1038/ni1549. [DOI] [PubMed] [Google Scholar]
- 35.Gorelik L, Flavell RA. Abrogation of TGFbeta signaling in T cells leads to spontaneous T cell differentiation and autoimmune disease. Immunity. 2000;12:171–181. doi: 10.1016/s1074-7613(00)80170-3. [DOI] [PubMed] [Google Scholar]
- 36.Cox JH, Kljavin NM, Ramamoorthi N, Diehl L, Batten M, Ghilardi N. IL-27 promotes T cell-dependent colitis through multiple mechanisms. J Exp Med. 2011;208:115–123. doi: 10.1084/jem.20100410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liu G, Yang K, Burns S, Shrestha S, Chi H. The S1P(1)-mTOR axis directs the reciprocal differentiation of T(H)1 and T(reg) cells. Nat Immunol. 2010;11:1047–1056. doi: 10.1038/ni.1939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Helft J, Ginhoux F, Bogunovic M, Merad M. Origin and functional heterogeneity of non-lymphoid tissue dendritic cells in mice. Immunol Rev. 2010;234:55–75. doi: 10.1111/j.0105-2896.2009.00885.x. [DOI] [PubMed] [Google Scholar]
- 39.Merad M, Sathe P, Helft J, Miller J, Mortha A. The dendritic cell lineage: ontogeny and function of dendritic cells and their subsets in the steady state and the inflamed setting. Annu Rev Immunol. 2013;31:563–604. doi: 10.1146/annurev-immunol-020711-074950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lewis KL, Caton ML, Bogunovic M, Greter M, Grajkowska LT, Ng D, Klinakis A, Charo IF, Jung S, Gommerman JL, Ivanov II, Liu K, Merad M, Reizis B. Notch2 receptor signaling controls functional differentiation of dendritic cells in the spleen and intestine. Immunity. 2011;35:780–791. doi: 10.1016/j.immuni.2011.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Huber S, Schrader J, Fritz G, Presser K, Schmitt S, Waisman A, Luth S, Blessing M, Herkel J, Schramm C. P38 MAP kinase signaling is required for the conversion of CD4+CD25- T cells into iTreg. PLoS ONE. 2008;3:e3302. doi: 10.1371/journal.pone.0003302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Manicassamy S, Reizis B, Ravindran R, Nakaya H, Salazar-Gonzalez RM, Wang YC, Pulendran B. Activation of beta-catenin in dendritic cells regulates immunity versus tolerance in the intestine. Science. 2010;329:849–853. doi: 10.1126/science.1188510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Johansson-Lindbom B, Svensson M, Pabst O, Palmqvist C, Marquez G, Forster R, Agace WW. Functional specialization of gut CD103+ dendritic cells in the regulation of tissue-selective T cell homing. J Exp Med. 2005;202:1063–1073. doi: 10.1084/jem.20051100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Annacker O, Coombes JL, Malmstrom V, Uhlig HH, Bourne T, Johansson-Lindbom B, Agace WW, Parker CM, Powrie F. Essential role for CD103 in the T cell-mediated regulation of experimental colitis. J Exp Med. 2005;202:1051–1061. doi: 10.1084/jem.20040662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bettelli E, Carrier Y, Gao W, Korn T, Strom TB, Oukka M, Weiner HL, Kuchroo VK. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006;441:235–238. doi: 10.1038/nature04753. [DOI] [PubMed] [Google Scholar]
- 46.Mucida D, Park Y, Kim G, Turovskaya O, Scott I, Kronenberg M, Cheroutre H. Reciprocal TH17 and regulatory T cell differentiation mediated by retinoic acid. Science. 2007;317:256–260. doi: 10.1126/science.1145697. [DOI] [PubMed] [Google Scholar]
- 47.Hall JA, Cannons JL, Grainger JR, Dos Santos LM, Hand TW, Naik S, Wohlfert EA, Chou DB, Oldenhove G, Robinson M, Grigg ME, Kastenmayer R, Schwartzberg PL, Belkaid Y. Essential Role for Retinoic Acid in the Promotion of CD4(+) T Cell Effector Responses via Retinoic Acid Receptor Alpha. Immunity. 2011;34:435–447. doi: 10.1016/j.immuni.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cohen P. Targeting protein kinases for the development of anti-inflammatory drugs. Curr Opin Cell Biol. 2009;21:317–324. doi: 10.1016/j.ceb.2009.01.015. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.