Abstract
Objective
Xeroderma pigmentosum (XP) is a rare autosomal recessive disease caused by mutations in DNA repair genes. Clinical manifestations of XP include mild to extreme sensitivity to ultraviolet radiation resulting in inflammation and neoplasia in sun-exposed areas of the skin, mucous membranes, and ocular surfaces. This report describes the ocular manifestations of XP in patients systematically evaluated in the Clinical Center at the National Institutes of Health.
Design
Retrospective Observational Case Series
Participants
Eighty-seven participants, aged 1.3 to 63.4 years, referred to the National Eye Institute for examination from 1964 to 2011. Eighty-three had XP, 3 had XP/Cockayne Syndrome complex, and 1 had XP/trichothiodystrophy complex.
Methods
Complete, age- and developmental stage-appropriate ophthalmic examination.
Main Outcome Measures
Visual acuity; eyelid, ocular surface and lens pathology; tear film and tear production measures; and cytological analysis of conjunctival surface swabs.
Results
Of the 87 patients, 91% had at least one ocular abnormality. The most common abnormalities were conjunctivitis (51%), corneal neovascularization (44%), dry eye (38%), corneal scarring (26%), ectropion (25%), blepharitis (23%), conjunctival melanosis (20%), and cataracts (14%). Thirteen percent of patients had some degree of visual axis impingement and 5% had no light perception in one or both eyes. Ocular surface cancer or a history of ocular surface cancer was present in 10% of patients. Patients with an acute sunburning skin phenotype were less likely to develop conjunctival melanosis and ectropion but more likely to develop neoplastic ocular surface lesions than non-burning patients. Some patients also showed signs of limbal stem cell deficiency.
Conclusions
Our longitudinal study reports the ocular status of the largest group of XP patients systematically examined at one facility over an extended period of time. Structural eyelid abnormalities, neoplasms of the ocular surface and eyelids, tear film and tear production abnormalities, ocular surface disease and inflammation, as well as corneal abnormalities were present in this population. Burning and non-burning XP patients exhibit different rates of important ophthalmologic findings, including neoplasia. Additionally, ophthalmic characteristics can help refine diagnoses in the case of XP complex phenotypes. DNA repair plays major role in protection of the eye from sunlight induced damage.
Xeroderma pigmentosum (XP) is an autosomal recessive disease caused by mutations in DNA repair genes.1 XP is found in approximately 1 per million people in the United States and Europe and 1:20,000 people in Japan.2–4 Clinical manifestations of XP include extreme sun sensitivity (blistering burns with just a few minutes exposure at most severe) and freckle-like pigmentation, ocular abnormalities, and a greater than 10,000 fold increased risk of developing neoplasms in sun-exposed areas of the skin, mucous membranes, and eyes.2, 5 In addition, approximately 20 to 30 percent of patients 1–2, 6 develop progressive neurologic disease, clinically manifesting as progressive ataxia and spasticity, cognitive deterioration, and abnormal hearing, reflexes and speech.2, 6–7
Patients with XP can be classified into complementation groups depending on which gene is disrupted. In 7 complementation groups, referred to as XP-A through XP-G (Online Mendelian Inheritance in Man-OMIM # 278700, 610651, 278720, 278730, 278740, 278760, 278780), a component of the nucleotide excision repair (NER) pathway is disrupted.8 One additional complementation group, XP-variant group (XPV, OMIM #278750) is characterized by mutations in polymerase eta.8 The clinical manifestations of XP depend both on molecular abnormality and environmental damage sustained and therefore, can be quite variable, not only between complementation groups, but also within a complementation group or even within an individual family.9 In North America, XP-C group patients are the most common and tend to have severe skin abnormalities, but rarely have neurological symptoms.2, 8 XP-A and XP-D patients are the most prone to neurological involvement.2, 8–9 Ocular symptoms appear to occur at the same rate in patients with or without neurological involvement.2
A few individuals have been identified with complex syndromes that combine the phenotypes of XP and the related disorders Cockayne syndrome (CS, characterized by post-natal growth failure and developmental delay as well as neurological dysfunction/deterioration, and retinal degeneration but with no sun sensitivity1; XP/CS10–16 OMIM #278780 or #610651), or trichothiodystrophy (TTD, characterized by sulphur-deficient brittle hair, intellectual impairment, short stature, recurrent infections, , sun sensitivity and cataracts and 1; TTD OMIM#601675 [photosensitive] and #234050 [non-photosensitive]; XP/TTD OMIM #27873017–19,20). XP/TTD and one form of XP/CS are due to pathogenic variations within the XPD gene; XP/CS can alternately involve pathogenic mutations in the XPG (OMIM 278780) or XPB genes (OMIM 610651). Patients with overlap syndromes may manifest some, but not all, of the classic features of each component syndrome.
Ocular manifestations are a major component of XP9 and early descriptions of the disease by Kaposi in the late 19th century included xerosis and clouding of the cornea.21 The majority of reported ocular findings are from case reports or small case series. Ocular manifestations of XP mainly involve the eyelids and ocular surface, which are exposed to ultraviolet (UV) radiation. Optical radiation is absorbed by or transmitted through the components of the eye depending on wavelength, and as it is absorbed can cause photochemical reactions that produce free radicals.22 Short wavelengths in the 200 to 315 nm range are absorbed by the cornea and sclera, while the 295–400 nm range is absorbed by the lens, and light from 400–1400 nm range penetrate to the retina.22 Ocular findings in XP include photophobia, conjunctivitis, ectropion, exposure keratitis leading to corneal opacification or vascularisation, pterygium, and neoplasia.2, 23–26
In this paper we report the ocular characteristics of all XP patients examined at the National Eye Institute (NEI) from 1964 to 2011(Table 1) including visual acuity (VA), eyelid, ocular surface and lens pathology, tear film and tear production measures, and cytological analysis of conjunctival surface swabs.
Table 1.
Xeroderma pigmentosum patients examined in the National Eye Institute Clinic from 1964 to 2011
| total | Gender | Race/Ethnicitya | ||||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| female | male | NHW | African/AA | Asian | HW | NA/1st Nation | ||
| Total | 87 | 45 | 42 | 65 | 12 | 2 | 6 | 2 |
| Age at most recent observation, years | ||||||||
| median | 17 | 20 | 15 | 20 | 15 | 15 | 10 | 22 |
| youngest | 1 | 1 | 3 | 1 | 5 | 8 | 5 | 14 |
| oldest | 63 | 58 | 63 | 63 | 58 | 22 | 39 | 31 |
| # patients, multiple ophthalmic exams | 36 | 16 | 20 | 27 | 5 | 0 | 3 | 1 |
| duration of follow-up, months | ||||||||
| median | 44 | 53 | 38 | 54 | 16 | 4 | 165 | |
| shortest | 0.3 | 4 | 0.3 | 4 | 0.3 | 0.4 | ||
| longest | 363 | 363 | 224 | 363 | 86 | 17 | ||
| Ocular abnormalities | ||||||||
| yes | 79 | 39 | 40 | 58 | 11 | 2 | 6 | 2 |
| no | 8 | 6 | 2 | 7 | 1 | 0 | 0 | 0 |
| History of ocular surface cancer? | ||||||||
| yes | 5 | 2 | 3 | 2 | 3 | 0 | 0 | 0 |
| no | 82 | 43 | 39 | 63 | 9 | 2 | 6 | 2 |
| # of deceased patientsb | 21 | 11 | 10 | 16 | 3 | 0 | 1 | 1 |
| age at death, years | ||||||||
| median | 31 | 34 | 31 | 31 | 27 | 37 | 31 | |
| youngest | 6 | 24 | 6 | 6 | 24 | |||
| oldest | 50 | 50 | 37 | 50 | 33 | |||
| Burning phenotype | ||||||||
| non-burning | 32 | 13 | 19 | 22 | 4 | 1 | 3 | 2 |
| burning | 50 | 29 | 21 | 41 | 6 | 1 | 2 | 0 |
| unknown | 5 | 3 | 2 | 2 | 2 | 0 | 1 | 0 |
| History of skin cancer?c | ||||||||
| yes | 65 | 35 | 30 | 51 | 6 | 2 | 4 | 2 |
| no | 22 | 10 | 12 | 14 | 6 | 0 | 2 | 0 |
| Neurological Phenotype | ||||||||
| Degeneration present | 19 | 12 | 7 | 17 | 2 | 0 | 0 | 0 |
| No degeneration | 59 | 30 | 29 | 41 | 8 | 2 | 6 | 2 |
| Non-XP neuro abnormality | 5 | 2 | 3 | 3 | 2 | 0 | 0 | 0 |
| Unknown | 2 | 1 | 1 | 2 | 0 | 0 | 0 | 0 |
| Complementation group (phenotype) | ||||||||
| A (XP) | 8 | 5 | 3 | 5 | 2 | 1 | 0 | 0 |
| B (XP/CS) | 1 | 1 | 0 | 1 | 0 | 0 | 0 | 0 |
| C (XP) | 40 | 18 | 22 | 26 | 6 | 1 | 5 | 2 |
| D (XP) | 18 | 12 | 6 | 15 | 2 | 0 | 1 | 0 |
| D (XP/TTD) | 1 | 0 | 1 | 1 | 0 | 0 | 0 | 0 |
| E (XP) | 3 | 2 | 1 | 3 | 0 | 0 | 0 | 0 |
| G (XP) | 1 | 1 | 0 | 1 | 0 | 0 | 0 | 0 |
| G (XP/CS) | 2 | 0 | 2 | 1 | 1 | 0 | 0 | 0 |
| Variant (XP) | 7 | 3 | 4 | 7 | 0 | 0 | 0 | 0 |
| Unknown (XP) | 6 | 3 | 3 | 5 | 1 | 0 | 0 | 0 |
Non-Hispanic White (NHW), African American (AA), Hispanic White (HW), Native American (NA).
Causes of death: neurologic degeneration (7), metastatic melanoma (4), invasive squamous cell carcinoma (3), unknown (2), glioblastoma (1), uterine cancer (1), atheriosclerosis (1), drug overdose (1), struck by lightening (1). Causes of death have been previously reported (Bradford et al, 2011).
including cancers of the lids. AA = African American, CS = Cockayne syndrome, HW = Hispanic White, NA = Native American, NHW = non-Hispanic White, TTD = trichothiodystrophy, and XP = Xeroderma pigmentosum.
Methods
The patients were evaluated under protocols approved by the National Cancer Institute (NCI) Institutional Review Board and the research adhered to the tenets of the Declaration of Helsinki. The work is Health Insurance Portability and Accountability Act (HIPAA)-compliant, and informed consent from patients (including consent for use of patient photographs) was obtained. The most current version of the clinical trials these patients have been studied under is registered under trial identifier NCT00004044 in the public database http:www.clinicaltrials.gov (last accessed 2/25/2012). This natural history diagnostic protocol studies clinical and genetic features of patients with XP, TTD or CS.
Since 1971 all patients were evaluated by NCI dermatologists (JJD and/or KHK) and found to have diagnostic features of XP. The patients had a formal diagnosis of XP and attempted determination of XP complementation group. Complementation group status was assigned as previously described using peripheral blood lymphocytes or cultured fibroblasts or lymphoblastoid cells (DiGiovanna J. Trichothiodystrophy with and without Xeroderma pigmentosum. Paper presented at: Society for Investigative Dermatology Annual Meeting, May 15,2002; Los Angeles, California).18, 27–28. Patient identification numbers used in this study were preceded by a code for the diagnosis XP, and followed by a location code “BE” indicating Bethesda, MD or “DC” indicating Washington, DC as used in previous manuscripts (DiGiovanna J. Trichothiodystrophy with and without Xeroderma pigmentosum. Paper presented at: Society for Investigative Dermatology Annual Meeting, May 15,2002; Los Angeles, California).27–28 XP-type neurologic degeneration observed in these patients includes deterioration of neurologic status, impaired hearing, abnormal speech, ataxia, peripheral neuropathy, and loss of the ability to walk and talk.28 Non-XP related neurologic abnormalities observed included hypoglycinemia, traumatic hearing loss, intellectual impairment without loss of coordination, and systemic lupus erythematosus.28
All patients with XP who had an ophthalmic examination between 6/1/1964 and 12/15/2011 at the National Institutes of Health Clinical Center were included in this report. Patients seen from 2001 to present were examined by one or more of the authors of this paper (BPB, ET, JC, RB, or WZ).
The complete ophthalmic examinations included (when possible) best-corrected Snellen visual acuity (VA) measurement, slit-lamp biomicroscopy, corneal endothelial cell density by specular microscopy (Konan NONCON ROBO Pachy Specular Microscope with the KC-3009 Konan Fully Automatic Cell Analysis System), and dilated fundus examination with an indirect ophthalmoscope. Corneal fluorescein staining,29 Schirmer test II (Schirmer test with topical anesthesia),30 tear film breakup time (TBUT), corneal topography and thickness (Orbscan, Bausch & Lomb, Model DP-3002), and axial length measurements were performed as the patients were able, according to their age and developmental stage. For the TBUT, 5 microliters of fluorescein was instilled using a micro-pipette and the average of three measurements per eye is reported. Whenever possible a central area of the cornea without structural abnormality was chosen for assessment. A TBUT ≤5 seconds (s) is considered abnormally rapid.31 Schirmer test II values > 10 millimeters (mm)/5 minutes (min) indicate normal baseline tear production, values from 6 mm/5 min to 10 mm/5 min is considered borderline deficiency, and ≤5 mm/5 min confirms an aqueous-deficient dry eye categorization.32 Ocular surface staining was graded according to the Oxford scale.29 Charts, consult letters, and data in the patients’ medical records were reviewed and the data abstracted for this report.
Conjunctival cytology specimens were obtained by gently rubbing a cotton-tip applicator across the locally-anesthetized conjunctival surface. Cells were transferred by soaking the applicator tip in phosphate buffered saline, pH 7.4. Slides of patient cells were stained and examined by an ophthalmic pathologist (CCC).
IBM® SPSS® Statistics software, Version 19 was used for the statistical analysis. Fisher’s exact test was used for comparing rates of ectropion, lid pigmentation, conjunctival melanosis, and ocular surface cancers between patient groups (either burning versus non-burning patient groups, or non Hispanic white patients versus patients of all other race/ethnicities. The odds ratio between lid margin keratinization and conjunctival scarring was calculated by use of logistic regression.
Results
A total of 87 patients with XP, XP/CS or XP/TTD underwent examination by an NEI ophthalmologist during this study (Table 1). The patients ranged in age from 1.3 to 63.4 years at the time of their most recent visit, with a median age of 17 years. Fifty patients had one visit to the NEI, and 36 patients (43%) were examined at the NEI at least twice. Multiple visit patients had a median duration of follow-up of 44 months (range 1 to 363 months, Table 1); a collective total of 331 visits. Twenty-one patients are deceased; causes of death are listed in the footnotes of Table 1. There were five pairs of siblings and one set of three brothers among the patients. Fifty-seven percent of the XP patients we studied had a skin phenotype of acute burning on minimal sun exposure,28 referred to as a “burning phenotype” (Table 1). Because of the greatly increased risk of skin neoplasms with XP, all patients were counselled to avoid or mitigate sun exposure and use extensive protection of the skin and eyes against ultraviolet radiation daily, regardless of burning phenotype.
Case Studies
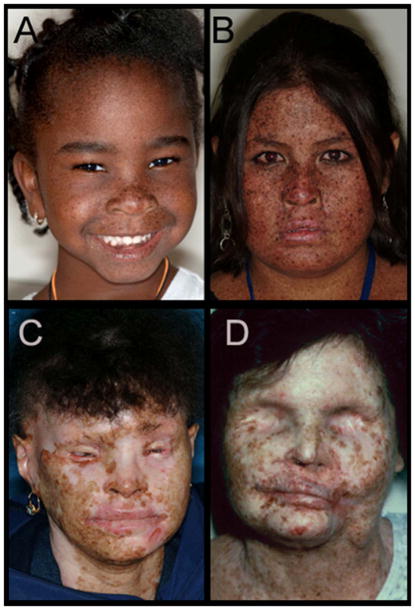
The range of clinical l phenotypes is illustrated in Figure 1 in order of increasing severity. Case 1 (Figure 1A) is a 5-year old African American (AA) girl. Despite her race and youth she shows lentigines on her face and presented with blepharitis and conjunctival injection. Case 2 (Figure 1B) is 13-year old First Nations patient. Her face and neck are extensively covered with lentigines, and she had multiple non-melanoma skin cancers removed since age 5. She was sunburned at the time the photo was taken. Case 3 (Figure 1C) is a 39 year old Hispanic patient. She had multiple non-melanoma skin cancers removed, the first at age 7. She has severe conjunctivitis, bilateral ectropion and entropion, with a history of ectropion surgery on the left lower lid. Her left eye has severe pannus, and the cornea is completely opacified. Her right eye was enucleated at age 20 secondary to sun-induced damage, and she has a prosthesis. Case 4 (Figure 1D) is a 46-year old non-Hispanic White patient who died at age 49 of uterine cancer.33 She had a history of multiple melanoma and non-melanoma skin cancers, including over two hundred biopsy-proven basal cell and squamous cell carcinomas.33–35 Due to recurring ocular surface squamous cell carcinomas and basal cell carcinomas of the lids, the patient underwent complete orbital exenterations at age 32 for the right eye (OD), and age 36 for the left eye(OS).36
Figure 1. Facial lentigines and ocular abnormalities in Xeroderma pigmentosum (XP) patients of different ethnicities.
A) Case 1 is 5-year old African American XP-C patient (XP444BE) with lentigines on her skin. B) Case 2 is 13-year old First Nations XP-C patient (XP83BE) with many lentigines, ocular surface injection, and sunburn. C) Case 3 is 39-year old Hispanic XP-C patient (XP131BE). She is originally from the Dominican Republic. The patient has a history of skin cancer, but no history of eyelid or ocular surface cancer. Many prominent lentigines are seen on her face and neck. The patient was fairly darkly pigmented when younger, however now pigmentation has been lost, resulting in hypopigmented areas. D) Case 4 is 46-year old Caucasian XP-C patient (XP1BE).33–35 She underwent bilateral orbital exenteration at ages 32 and 36 due to recurring invasive squamous cell carcinoma of the ocular surfaces and basal cell carcinoma of the lids, including one squamous cell carcinoma that occurred on the palpebral conjunctiva of the upper eyelid—a non-sun-exposed area.36 This patient, originally light-skinned, has many darkly pigmented lentiginous areas on her face. She died at age 49 from uterine cancer.28,33
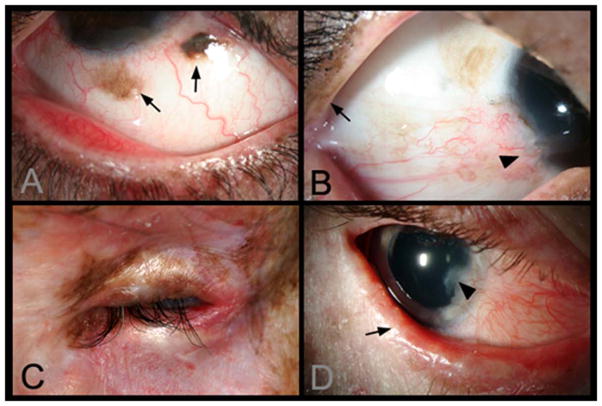
Typical ocular manifestations of XP are shown in Figure 2. Conjunctival melanosis with feeder vessels is shown in Panel A in an 8-year old Asian Indian patient. Panels B and C are closer views of one eye of each patient shown in Figure 1B and C. Panel B shows early pterygium and increased lid pigmentation. Panel C shows severe ectropion, entropian and inflammation. Panel D shows the eye of a 14 year old non-Hispanic White patient with lentigines on his eyelids and also bilateral pterygium and ectropion. The lid margin is keratinized and there is loss of lashes. Localized corneal clouding at the leading edge of the pterygium was suspicious for early malignancy, and biopsy was recommended. An expanded case history for this patient is available in Appendix 1, available at http://aaojournal.org.
Figure 2. Lid and ocular surface manifestations in Xeroderma pigmentosum (XP) patients.
A) Conjunctival melanosis (arrows) in Case 5, 8-year old Asian Indian XP-C patient (XP417BE). Note the feeder vessels to lesions (arrows). B) Early pterygium (arrowhead) and lid pigmentation (arrow) in Case 2. C) Severe ectropion, entropion, and ocular inflammation in Case 3. D) Lid margin keratinisation (arrow), loss of lashes in Case 6, a 14 year old patient (XP243BE). The patient has a history of skin cancer but no history of ocular surface cancer. Lentigines are present on eyelids and patient has bilateral pterygium, and ectropion. Patient has decreased best corrected visual acuity, possibly due to amblyopia. Localized corneal clouding at leading edge of pterygium was suspicious for early malignancy, biopsy recommended.
Ocular involvement in African patients
Figure 3 (available at http://aaojournal.org) shows Case 6, a particularly severe presentation of UV-induced ocular manifestations of XP as seen in one of two Northern African brothers in the study (Patient XP394BE; XP393BE is his sibling). 37 Case 6 did not have a burning phenotype and by history did not utilize sun protection measures. His parents were second cousins and from the same tribe in the Sudan.37 He developed lentigines on his face by age 8, followed by bilateral progressive loss of vision. The patient had been unilaterally blind since age 14, and was first seen at the NEI clinic at age 16. He had extensive neovascularization and a large elevated corneal plaque with a white, papillomatous growth nasally, which enlarged rapidly. The patient is shown in Figure 3A and B at age 17(available at http://aaojournal.org). At this point, due to extreme photophobia, the patient was nearly unable to fully open his eyes and his vision was count fingers at 2′. After the patient was encouraged to use sunglasses, there was partial improvement of the photophobia.
Although the appearance of this lesion was alarming and globe removal was initially considered, biopsy showed the lesion was not cancerous, and therefore could be resected. Lesion pathology and immunohistochemistry are shown in panels E-H. After resection the patient began daily aggressive UV protection. This entailed sun avoidance (staying indoors when the sun was above the horizon) but also application of sunblock over the entire body in the morning, then every 3 hours for uncovered skin; wearing tightly woven, long sleeved shirts and long pants, and closed shoes with socks; a broad brimmed hat, UV blocking sun glasses with side shield or a UV blocking hood with face shield and gloves when going outside. VA was improved to 20/250 seven months after resection of lesion. Panels C and D show the patient 7 months post surgery. The patient at that time was able to open his right lid nearly fully, and his VA OD had improved to 20/250.
An AA XP-G patient, case 7, was seen at ages 10, 13 and 17 (Figure 4A, B and C, available at http://aaojournal.org). Conjunctival melanosis OS was pronounced at age 10 (D) and showed substantial progression in severity by age 17 (E). Until his visit at age 17, this patient had been classified as an XP patient; however fundus abnormalities --macular granularity with a few yellowish dots and mild arteriolar attenuation-- observed during that examination led to further testing. On electroretinogram (ERG), photopic flash responses were abnormal at 52–63 μV (normal >101 μV), consistent with early cone-rod degeneration. ERG also showed dark adapted (scotopic) responses (amplitude and implicit time) at the low end of normal. Maximum combined responses were borderline with an a-wave amplitude of 125–146μV (normal >188 μV) and a b-wave amplitude of 236–279μV (normal > 373 μV). The presence of annular macular autofluorescence was also suggestive of early cone-rod degeneration (Figure 4F and G, available at http://aaojournal.org). CAT scan of the patient’s brain in 2011 showed basal ganglion calcification consistent with the diagnosis of CS: earlier scans were normal. The patient has been re-classified as combined XP/CS due to this cone-rod degeneration and basal ganglion calcification.
The one XP/TTD patient in this report (XP/TTD465BE) also had been originally classified as an XP patient. TTD is a multisystem disorder which can be caused by mutations in the same genes that cause XP.1 XP and TTD share the feature of photosensitivity. However, TTD patients typically have short, brittle hair, short stature, developmental delay, recurrent infections, a variety of ophthalmologic findings19 and other abnormalities (Faghri S. Trichothiodystrophy includes a broad spectrum of multisystem abnormalities and may have a high mortality at a young age. Paper presented at: Society for Investigative Dermatology Annual Meeting, May 9, 2007; Los Angeles, California).38 These features may be attenuated in the XP/TTD overlap syndrome 18–19, 28 making the diagnosis challenging on the basis of general physical examination. However, the ophthalmologic features of XP and TTD are sufficiently different that they may be important in distinguishing between the XP and TTD phenotypes. The patient had an early history of burning and a history of multiple skin cancers including basal cell carcinomas, squamous cell carcinomas and primary and metastatic melanomas beginning at age 13 years, typical of XP. Lens examination at the patient’s first ophthalmic visit at the NIH at age 55 revealed bilateral non-visually significant punctate cataracts typically seen in TTD patients. These are not typical features of XP, and subsequent examination of the patients’ hair under polarizing microscopy found tiger tail banding characteristic of TTD, thus his diagnosis was changed to XP/TTD.
Ocular Characteristics of the XP Patients
Seventy nine (91%) of the 87 patients (age 1–63, mean age 24) were found to have abnormalities on one or more eye exams (Tables 1 and 2). Abnormalities can be broadly divided into four categories: 1) structural eyelid abnormalities; 2) neoplasms of the ocular surface and eyelids; 3) ocular surface disease and inflammation and 4) corneal abnormalities.
Table 2.
High frequencies of periocular and ocular pathologies in Xeroderma pigmentosum patients.
| Patientsa # (%) | Kraemer lit review, 1987 # (%) | Goyal retrospective study, 1994 # (%) | Anttinen prospective study, 1998 # (%) | Alfawaz, case series, 2011 # (%) | ||
|---|---|---|---|---|---|---|
| Ocular Abnormalities | Present | 79 (91%) | 328 (40%) | 10 (100%) | 11(85) | |
| Absent | 8 (9%) | 9 (1%) | 0 | 2(15%) | ||
| Not stated | - | 493 (59%) | - | - | 27 j | |
|
| ||||||
| Lids and Lashes | Ectropion b,c | 22 (25%) | 3 (70%) | 8 (30%) n | ||
| Entropion | 2 (2%) | |||||
| Lagophthalmos | 9 (10%) | |||||
| Eyelid abnormalities | - | 135 (16%) | 6 (46%) | 23 (85%)k | ||
| Blepharitis | 20 (23%) | |||||
| Lid Pigmentationd,e,e | 9 (10%) | 10 (100%) | 12 (44%) | |||
| Lid Keratinizationf | 11 (13%) | |||||
|
| ||||||
| Ocular Surface | Conjunctivitis | 44 (51%) | 149 (18%) | 4 (40%)h | 7(54%) | 8 (30%)l |
| Conjunctival Melanosis | 17 (20%) | |||||
| Pannus | 38 (44%) | |||||
| Pterygium | 27 (31%) | 4 (40%) | 9 (69%) | 8 (30%) | ||
| Corneal scarringf | 23 (26%) | 4 (40%)i | ||||
| Corneal abnormalities | - | 141 (17%) | 5(38%) | 18 (66%)m | ||
| Reports Tearing | 10 (11%) | |||||
| Ocular Surface Cancer | 9 (10%) | 73 (9%) | 2 (20%) | 7 (26%) | ||
|
| ||||||
| Other | Cataracts | 12 (14%) | ||||
| Reports Photophobia | 31 (36%) | 178 (21%) | 5 (50%) | 18 (67%) | ||
| Dry Eyeg | 33 (38%) | |||||
A patient may have more than one type of abnormality.
Non-burning patients (15 pts, 47%) experienced a significantly higher rate of ectropion than burning phenotype patients (7 pts, 14%; p < 0.05).
African, African American, Native American, First Nations, Indian and/or Hispanic patients (6 pts, 27%) experience ectropion at a greater rate that Non-Hispanic White patients (3 pts, 5%; p < 0.05).
Patients of the non-burning phenotype (12 pts, 38%) experienced melanosis at a greater rate than burning phenotype patients (5 pts, 10%; p < 0.05).
African, African American, Native American, First Nations, Indian and/or Hispanic patients also experience melanosis at greater rate than Non-Hispanic White (9 pts, 41%; p < 0.05).
Patients with corneal scarring were more likely to also have lid keratinization (odds ratio = 9.18).
Dry eye was indicated if the patient reported symptoms of dry eye and was currently using artificial tears and/or had Schirmer’s baseline aqueous tear production with anesthesia test values that were borderline (≥6 mm to ≤10 mm/5 min) or abnormal (≤5 mm/5 min).
reported as conjunctival congestion or pigmentation.
reported as corneal opacity with vascularisation.
it was not indicated if there were any patients free of ocular abnormalities.
atrophic skin changes.
number of patients with conjunctival congestion or pigmentation.
opacity 18 patients, 9 superficial punctate keratitis, 4 ulcer, or 2 perforation.
number of ectropian or entropian combined.
Eyelid abnormalities were a relatively common finding in the XP patients, with 22 (25%) presenting with ectropion, 2 (2%) with entropion, 9 (10%) with lagophthalmos, and 20 (23%) with blepharitis (Table 2). Non-burning patients (pts) (15 pts, 47%) experienced a significantly higher rate of ectropion than burning phenotype patients (7 pts, 14%; p=0.022, Fisher’s exact test). An approximately equal number of burning and non-burning phenotype patients reported experiencing photophobia. Hispanic, African/African American, Native American/First Nations, and Asian patients as a group experience lid pigmentation abnormalities at greater rate (6/22 pts, 27%) than non-Hispanic White patients (3/65 pts, 5%; p = 0.031, Fisher’s exact) (Figure 2B). Keratinized lids were present in 11 (13%) patients. Eyelashes were missing or abnormal in 21 (24%) patients.
The ocular surface is vulnerable to UV-induced damage, and so the ocular surface was evaluated in all patients. Conjunctivitis, ranging from mild to severe, was the symptom experienced by the largest number of patients, 44 (51%).
XP patients can experience the growth of melanin-containing conjunctival lesions on their ocular surface, particularly seen in (but not limited to) UV-exposed areas. These lesions can extend beyond the conjunctiva to the cornea as well, and patients in this study were evaluated for the presence and extent of this type of melanotic lesions. Conjunctival melanosis as seen in Figure 2A was present in 17 patients (20%). Patients of the non-burning phenotype (12/32 pts, 38%) experienced this melanosis at a greater rate than burning phenotype patients (5/50 pts, 10%; p=0.047, Fisher’s exact), and the group of African/African American/Native American/Indian/Hispanic patients experienced melanosis at greater rate than non-Hispanic White patients(9/22 pts, 41% vs. 8/65 pts, 12% p = 0.048, Fisher’s exact).
Corneal neovascularization was also a common finding. Thirty-eight patients (44%) presented with pannus, and in 27 patients this neovascularization extended into pterygium – a growth onto the cornea of fibrovascular tissue contiguous with the conjunctiva.
Corneal scarring and stromal infiltrates were observed in 23 patients. Patients with scarring were more likely to also have keratinized lids (Odds ratio = 9.18, p=0.003, Fisher’s exact). Eleven patients (13%) had their visual axis affected by either corneal scarring or neovascularization. While much of the melanosis and bulbar conjunctival inflammation or scarring was found on UV-exposed regions of the ocular surface, it was not limited to these areas and occurred on non-UV-exposed regions as well.
Ocular neoplasms
Five patients had a history of ocular surface cancers - squamous (4) or basal cell (1) carcinomas – that had been excised prior to their admission in the study. The age that the lesions were identified was not available from their records, but these patients entered the study at ages ranging from 14 to 28. An additional 4 patients were diagnosed with ocular surface cancer as the study progressed; 2 patients with squamous cell carcinoma, one melanoma, and one with a neoplasm of unknown type. Their ages at identification of the ocular surface lesion ranged from 5 to 28 years, median 16. Seven of these 9 patients also had a history of skin cancers, 7 are deceased, and 5 had a burning phenotype, with one unknown burning phenotype. An additional 6 patients developed ocular surface lesions suspicious of malignancy and underwent biopsy during the study and the lesions were benign. All 6 patients with benign lesions had a history of skin cancer, and two had a burning phenotype.
Twelve patients had 18 lid lesions needing biopsy during the study period. Biopsies performed either at the NEI or at the patients’ own doctor showed 1 squamous cell carcinoma (1 pt, buning phenotype), 7 basal cell carcinomas (4 pts, 2 burning phenotype), 2 unknown type neoplasms (2 pts, 1 burning, 1 unknown burning), 7 benign lesions (4 pts, 1 burning), and one lesion with an unknown outcome (non-burning patient). Because lid lesions are likely to have been addressed by the patients’ family physicians, we expect them to be under-reported in this study.
Tear film abnormalities
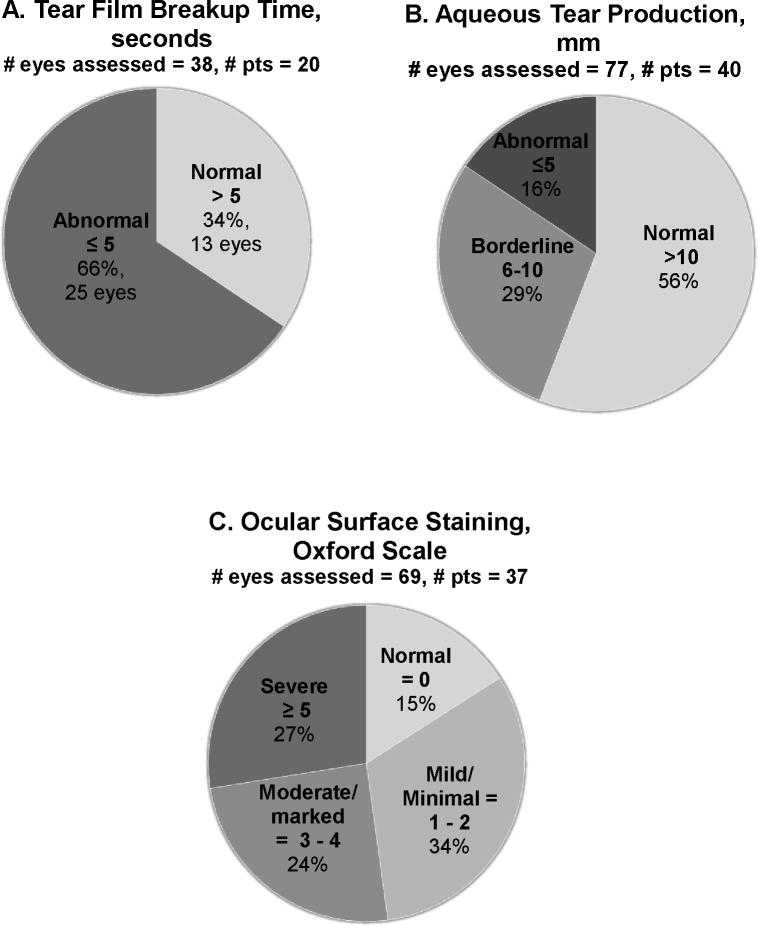
Tear film abnormalities were common in our cohort of XP patients; despite the fact that the mean age of the patients was only 23 (range 1.3 – 63.3, Table 1). To better characterize these symptoms, tear film stability and aqueous tear production were assessed via tear film breakup times and Schirmer test II with topical anesthesia, respectively. Results are reported in Figure 5 with the number of eyes assessed. Note that only a subset of patients was able to tolerate these tests.
Figure 5. High frequency of abnormalities of the tear film, tear production, and ocular surface damage in Xeroderma pigmentosum (XP)-affected patient eyes.
A) Tear film breakup times (TBUTs) ≤5 seconds are considered abnormal. No patients had TBUT measured over multiple visits. B) Baseline aqueous tear production measured by Schirmer test II. Schirmer test II values > 10 mm/5 minutes (min) indicate normal baseline tear production, values from 6 mm/5 min to 10 mm/5 min is considered borderline aqueous tear deficiency, and ≤ 5 mm/5 min confirms an aqueous-deficient dry eye categorization.32 C) Quantification of ocular surface damage in XP-affected patients via epithelial surface staining with fluorescein. The state of the ocular surface was assessed in each patient able to undergo the procedure and graded according to the Oxford Scale. Because not all patients were able to cooperate with this testing, data are available on only a subset of patients.
Tear film breakup time (TBUT) was assessable in 20 patients (Figure 5A). 85% of the patients had at least one eye had a TBUT below the normal threshold of >5 s 31, 39 (mean = 4.5s). Low TBUTs were bilateral in 9 patients and unilateral in 8 patients. Three patients were bilaterally normal with respect to TBUTs. Patients of all ages had these abnormally rapid TBUTs indicating tear film instability which contributes to rapid tear evaporation and dry eye.
When baseline aqueous tear production was assessed, 16% percent of eyes were dry (Schirmer test II <5 mm at 5 min), 29% were borderline (between 5mm and 10mm, Figure 5B). 17 of patients assessed had normal values for both eyes (43%). Four patients were bilaterally deficient and four more had one eye with deficient tear production, a total of 20%with at least one eye with aqueous tear deficiency. The remaining 15 patients had borderline values in one (11 pts, for 3 patients no data was available for second eye) or both (4 pts) eyes (30%). One adult patient presented with a severe aqueous tear deficiency (TBUT <5 s, Schirmer test II ≤1 mm) and a 22 month-old female patient presented with hypolacrimation -- a complete absence of reflex tears even while appearing to cry.
Ocular Surface Status
In order to further assess the state of the corneal and conjunctival surface, epithelial surface staining with fluorescein and lissamine green was performed on 37 of the 87 patients (Figure 5C). Results are reported by number of eyes. Eleven eyes had no staining, 24 had mild or minimal staining. The median age of patients with no staining or mild/moderate staining was 14 (range 2 to 48). The median age of patients with moderate/marked or severe staining was 35 years (range 7 to 63). Five patients had no staining in either eye. Only one patient had one normal eye and one with mild staining. All the remaining patients with staining were mild in both eyes (5 pts), moderate in both (5 pts), severe in both (6 pts), or some combination of mild, moderate and severe (16 pts).
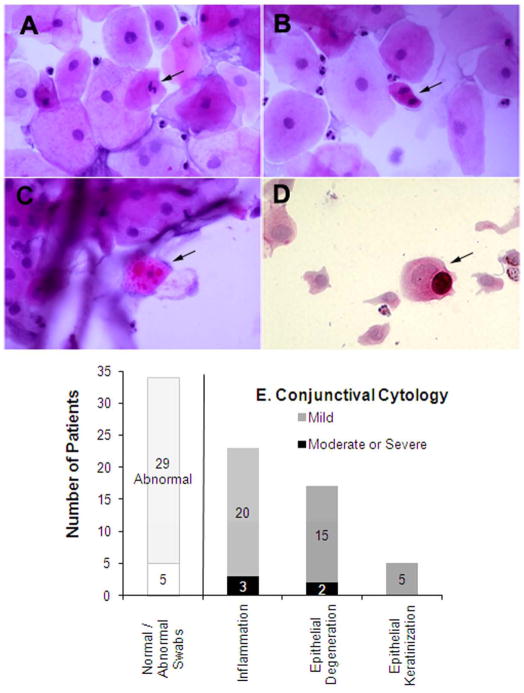
Starting in late 2003, we also evaluated the state of the ocular surface in patients by cytological analysis of conjunctival surface swabs (Figure 6).Thirty-four patients underwent conjunctival swabs, and they were graded for inflammation, epithelial keratinisation, and degeneration. Only 5 patients were graded as being cytologically normal. Cytological abnormalities included the following: inflammation e.g. lymphocytes and neutrophils; epithelial degeneration e.g. epithelial cells with folded angular cytoplasm, condensed nuclei, irregular and/or pyknotic nuclei, occasional binuclear cells, degenerated goblet cells, and fibrous strands; and keratinisation e.g. epithelial cells with prominent cytokeratin filaments and/decreased number of mitochondria. Figure 6 (Panels A-C) show examples of this abnormal pathology in 39 year-old Case 3 (Figure 1C). Her conjunctival cytology was unique in that mitotic figures (Figure 6B) were present. Because cytology specimens likely sample the epithelial surface and not the basal layers of conjunctiva--where mitosis normally occurs--this may be a sign of pre-malignancy.
Figure 6. Conjunctival and corneal surface swabs show severity of ocular surface disease in Xeroderma pigmentosum (XP)-affected patients.
Panels A–C show conjunctival swab cytology from Case 3, a 39 year old XP-C patient. Exam discloses many edematous epithelial cells and few acute inflammatory cells. A few epithelial cells are pyknotic. A) A degenerative epithelial cell with a pyknotic nucleus (arrow). B) A mitotic epithelial cell (arrow), C) A degenerative epithelial cell with many cytoplasmic inclusions (arrow). D) Cytology of cells obtained by corneal swab from Case 7 at age 19 (also shown in Figure 3). Degenerative and folded epithelial cells are evident, as well as a mucin-containing goblet cell (arrow), indicative of limbal stem cell deficiency. E) Summary of cytology findings.
Figure 6E shows the cytological characteristics of the group of patients tested. Twenty-three (68%) of the patients evaluated showed at least mild inflammation, and 3 of those 23 were graded as having moderate or severe levels of inflammation. Fifteen patients showed epithelial cell degeneration, prominent cytokeratin filaments and a decreased number of mitochondria, 2 were severe. A few patients presented with further abnormalities, i.e. conjunctival metaplasia or early epithelial dysplasia.
Three African patients (aged 9, 11, and 19) showed goblet cells in corneal swabs; signs of breakdown of the barrier function of the limbus and limbal stem cell deficiency.40 Figure 6D shows a corneal swab containing a mucin containing goblet cell from Case 6 (Figure 6).
Corneal Endothelial Cell Densities and Central Corneal Thickness
Since corneal endothelial cells are post-mitotic cells exposed to UV radiation in daily activities, we examined whether XP patients have premature loss of corneal endothelial cells and secondary changes in central corneal thickness (n=11 tested). Endothelial cell densities (ECDs) have been shown to start at birth between 3,500–6,000 cells/μm2 41 and decrease very gradually through early adulthood, where values stabilize between 2,500–3,000 cells/μm2 for the remaining lifespan.42 Central corneal thickness in children typically increases over time to reach adult thickness of approximately 550 μm43 by 9 years of age.44 Seven patients, ranging in age from 7 to 21 with one 59 year-old, had abnormally low ECDs, ranging from borderline (~2,400 cells/μm2) to extremely (~1120 cells/μm2) low values. Four of these patients with low ECDs also had abnormally high central corneal thickness, ranging from 641 to 808 μm (ages 13 to18, and 59). Three additional patients (ages 8 to 17, including case 7) had abnormally thick corneas as well; ECDs were unreported in these patients.
Discussion
Our study reports the ocular status of the largest group of XP patients systematically examined at one facility over an extended period of time. The size of the patient group in this current study additionally allowed us to start delineating the ocular phenotypes of patients with and without acute burning skin phenotypes. Of the 87 patients, 91% presented with some degree of ophthalmic abnormality.
A1987 literature review of 830 XP case reports found a 40% prevalence of ocular involvement and a median age of onset of ocular symptoms of age 4. However ocular status was not recorded in 59% of the recorded cases.2 A 1994 report of 10 XP patients from India identified ocular symptoms in all 10, although 2 patients had lid freckling and atrophic skin changes only.23 The 2008 prospective study of 16 XP-A, C, and G patients from Finland identified ocular symptoms in 80% of patients, with the most severe manifestations, including uni-lateral and bi-lateral blindness in the XP-C patients.24 A case series of 27 Saudi Arabian patients was described in 2011.26 Ramkumar et al comprehensively reviewed the English language literature from 1965 to 2010 for reported ocular malignancies, finding a total of 37 XP patients with malignancies of the conjunctiva, limbus, cornea, and iris.25
Ocular manifestations of XP are primarily ophthalmohelioses -- those that have been linked to UV exposure of the eyelids, cornea, or lens.45–47 Ocular and non-ocular morbidity in the form of atrophic skin changes, carcinoma, melanoma, lid freckles, and photophobia tends to appear fairly early in XP patients. In this study, 85% of patients whose first visit was at age 10 or under already had some degree of ocular morbidity.
Surgical removal of skin lesions from on or around the eyelids often affect lid functionality in XP patients, and 25% presented with ectropion. Tear films and tear production are often abnormal as well (Figure 5). Entropion, ectropion and lid margin keratinization all can lead to meibomian gland dysfunction, which may result in alteration of the lipid layer of the tear film. Normally, individuals under age 45 have longer tear film breakup time than do individuals over 45.48–50 However in our XP patients, tear abnormalities were just as likely to be present in younger XP patients as older. Therefore, both the structural and functional conditions that lead to exposure of the ocular surface – entropion, ectropion, lagophthalmos, or rapid tear film breakup time and reduced baseline tear production -- appear to be present early in the course of the disease. These abnormalities may be interpreted as evidence of premature photoaging of the eyes of XP patients related to their DNA repair deficiency.
XPA-deficient mice (but not wild-type mice) exposed to low daily doses of UV-B radiation for 14 days (cumulative dose of 22 kJ m2 at 250±400 nm) first develop photophobia, red, irritated eyelid margins and conjunctivas, followed by corneal opacification, and finally squamous cell carcinoma.51 Progression into more severe stages of disease appeared to continue in these mice after the UV-B exposure had stopped.51 In humans, UV exposure to the eye induces oxidative stress and epidermal growth factor receptor activation: this leads to the production of inflammatory effector molecules, an influx of inflammatory cells, angiogenesis and fibrosis.47 In UV-repair-deficient XP patients this process is exacerbated by the absence of DNA repair mechanisms. This leads to a vicious cycle of local damage and inflammation which can exceed that normally induced by UV exposure alone. We see confirmation of this as XP patients can show ocular surface damage or lesions in areas not exposed to UV (Cases 4–6; Figure 1D, 2A, and 3B).
XP patients in this study experienced high rates of pterygium and neovascularization (Table 2), and additionally, 10 patients ranging in age from 13 to 59 showed either a beaten metal appearance or guttae of the cornea or a poorly defined limbus. We hypothesize that UV radiation may be inducing damage in limbal stem cells. Although we have no way to accurately estimate the overall ocular UV exposure of these patients because terrain, season, cloud cover, time of day, and geometrical factors related to lid opening all impact exposure levels,52 the “Coreno hypothesis47 proposes that because little direct UV strikes the ocular surface, reflected light is focused by the anterior eye, and peak light intensity at the distal limbus is approximately 20 times the incident light intensity. This may subject the corneal stem cell niche to damage from focused light even though superficial limbal cells normally absorb directly incident light.53 The spilling over of melanosis from the conjunctiva onto the cornea which we saw in three patients ages 8 to 17 (Case 6, Figure 4D, available at http://aaojournal.org; XP337DC; XP422BE) is uncommon in such young patients and may represent an early manifestation of altered limbal physiology.
In this study, 20% of the patients presented with conjunctival melanosis (Table 2) -- hyperpigmented spots which are clones of individual melanocytes altered by mutations induced by ultraviolet radiation.54 Melanosis was present in African/African American (5), Hispanic (2), Indian (1), and Native American (1) patients as well as non-Hispanic White (8) patients. Rarely in XP patients (although at a much greater rate than the general population), hyperpigmented conjunctival melanoisis can develop into malignant melanoma 2, 55 and should be carefully monitored. One 7 year old patient in this study (XP422BE) presented with a pigmented lesion at the limbus OD, which was excised and shown to be malignant melanoma. This patient also had multiple skin melanomas and died at age 11 of malignant melanoma.
Eleven percent of the patients had either a history of ocular surface cancer or cancerous lesions (melanoma, squamous cell carcinoma, or basal cell carcinoma) that occurred during the study. Median age of onset for these cancers identified during the study was 16 years old. In contrast the median age of onset for eye and orbit cancers in the general population is 60 years old.56 In two XP patients, squamous cell carcinoma of the eye/orbit was invasive and spread into the brain. Ocular squamous cell carcinomas as early as age 3 years have been reported in darkly pigmented patients with little or no shielding from ultraviolet exposure.57
Recently, Bradford et al showed that XP patients with pronounced skin burning upon minimal exposure were less likely to develop skin cancer compared to patients with a normal burning phenotype.28 Burning phenotype patients in this current analysis were also significantly less likely to develop ectropion and conjunctival melanosis than non-burning phenotype patients (Table 2). These paradoxical findings may be explained by a greater tendency to avoid sun exposure by the patients who experience acute burning on minimal sun exposure. Although sun exposure even among normal populations in the same geographic location is highly variable,58 burning may provide an additional incentive to follow aggressive UV protection (see Figure 3 results for detailed description of the protective measures [available at http://aaojournal.org]).
Although macular degeneration is sometimes considered among the ophthalmohelioses,45–47 none of the XP patients in this study showed clinically apparent retinal degeneration. Ocular histopathology of two XP patients (XP12BE and XP18BE) in this study who died at ages 44 and 45 from complications of neurodegeneration showed mild to moderate peripheral retinal pigmentary degeneration in one patient and retinal gliosis in both.25 Neither of these conditions was clinically apparent at the patients’ last ophthalmic exam. Optic nerve atrophy was seen in one patient with advanced neurologic degeneration, and patients with neurological involvement should be assessed for optic pallor.25
Ocular manifestations of XP are severe with a >1,000 fold increased risk of developing lid and ocular surface neoplasms,2, 5 and a spectrum of manifestations of ophthalmohelioses. Regular ophthalmologic evaluation is important to clarify the diagnosis and help distinguish between XP, XP/CS and XP/TTD phenotypes, to identify progression of disease, to reinforce protective measures, to manage symptoms of dry eye, and to identify malignancy at its earliest stages. Strict sun protection measures, including use of fully shielding eyewear is important for all patients to prevent a spectrum of ophthalmologic abnormalities.
Supplementary Material
Footnotes
Conflict of Interest(s): The authors have no conflicts of interest to declare.
Financial disclosure(s): This study was supported by the Intramural Research program of the Center for Cancer Research, National Cancer Institute and of the National Eye Institute, National Institutes of Health, Bethesda, MD.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kraemer KH, Patronas NJ, Schiffmann R, et al. Xeroderma pigmentosum, trichothiodystrophy and Cockayne syndrome: a complex genotype-phenotype relationship. Neuroscience. 2007;145:1388–96. doi: 10.1016/j.neuroscience.2006.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kraemer KH, Lee MM, Scotto J. Xeroderma pigmentosum. Cutaneous, ocular, and neurologic abnormalities in 830 published cases. Arch Dermatol. 1987;123:241–50. doi: 10.1001/archderm.123.2.241. [DOI] [PubMed] [Google Scholar]
- 3.Kleijer WJ, Laugel V, Berneburg M, et al. Incidence of DNA repair deficiency disorders in western Europe: xeroderma pigmentosum, Cockayne syndrome and trichothiodystrophy. DNA Repair (Amst) 2008;7:744–50. doi: 10.1016/j.dnarep.2008.01.014. [DOI] [PubMed] [Google Scholar]
- 4.Neel JV, Kodani M, Brewer R, Anderson RC. The incidence of consanguineous matings in Japan, with remarks on the estimation of comparative gene frequencies and the expected rate of appearance of induced recessive mutations. Am J Hum Genet. 1949;1:156–78. [PMC free article] [PubMed] [Google Scholar]
- 5.Kraemer KH, Lee MM, Andrews AD, Lambert WC. The role of sunlight and DNA repair in melanoma and nonmelanoma skin cancer. The xeroderma pigmentosum paradigm. Arch Dermatol. 1994;130:1018–21. [PubMed] [Google Scholar]
- 6.Robbins JH. Xeroderma pigmentosum. Defective DNA repair causes skin cancer and neurodegeneration. JAMA. 1988;260:384–8. doi: 10.1001/jama.260.3.384. [DOI] [PubMed] [Google Scholar]
- 7.Robbins JH, Brumback RA, Mendiones M, et al. Neurological disease in xeroderma pigmentosum. Documentation of a late onset type of the juvenile onset form. Brain. 1991;114:1335–61. doi: 10.1093/brain/114.3.1335. [DOI] [PubMed] [Google Scholar]
- 8.Stary A, Sarasin A. The genetics of the hereditary xeroderma pigmentosum syndrome. Biochimie. 2002;84:49–60. doi: 10.1016/s0300-9084(01)01358-x. [DOI] [PubMed] [Google Scholar]
- 9.Kraemer KH, Slor H. Xeroderma pigmentosum. Clin Dermatol. 1985;3:33–69. doi: 10.1016/0738-081x(85)90096-3. [DOI] [PubMed] [Google Scholar]
- 10.Vermeulen W, Jaeken J, Jaspers NG, et al. Xeroderma pigmentosum complementation group G associated with Cockayne syndrome. Am J Hum Genet. 1993;53:185–92. [PMC free article] [PubMed] [Google Scholar]
- 11.Hamel BC, Raams A, Schuitema-Dijkstra AR, et al. Xeroderma pigmentosum--Cockayne syndrome complex: a further case. J Med Genet. 1996;33:607–10. doi: 10.1136/jmg.33.7.607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Norris PG, Hawk JL, Avery JA, Giannelli F. Xeroderma pigmentosum complementation group G--report of two cases. Br J Dermatol. 1987;116:861–6. doi: 10.1111/j.1365-2133.1987.tb04906.x. [DOI] [PubMed] [Google Scholar]
- 13.Scott RJ, Itin P, Kleijer WJ, et al. Xeroderma pigmentosum-Cockayne syndrome complex in two patients: absence of skin tumors despite severe deficiency of DNA excision repair. J Am Acad Dermatol. 1993;29:883–9. doi: 10.1016/0190-9622(93)70263-s. [DOI] [PubMed] [Google Scholar]
- 14.Oh KS, Khan SG, Jaspers NG, et al. Phenotypic heterogeneity in the XPB DNA helicase gene (ERCC3): xeroderma pigmentosum without and with Cockayne syndrome. Hum Mutat. 2006;27:1092–103. doi: 10.1002/humu.20392. [DOI] [PubMed] [Google Scholar]
- 15.Weeda G, van Ham RC, Vermeulen W, et al. A presumed DNA helicase encoded by ERCC-3 is involved in the human repair disorders xeroderma pigmentosum and Cockayne’s syndrome. Cell. 1990;62:777–91. doi: 10.1016/0092-8674(90)90122-u. [DOI] [PubMed] [Google Scholar]
- 16.Broughton BC, Thompson AF, Harcourt SA, et al. Molecular and cellular analysis of the DNA repair defect in a patient in xeroderma pigmentosum complementation group D who has the clinical features of xeroderma pigmentosum and Cockayne syndrome. Am J Hum Genet. 1995;56:167–74. [PMC free article] [PubMed] [Google Scholar]
- 17.Takayama K, Salazar EP, Lehmann A, et al. Defects in the DNA repair and transcription gene ERCC2 in the cancer-prone disorder xeroderma pigmentosum group D. Cancer Res. 1995;55:5656–63. [PubMed] [Google Scholar]
- 18.Broughton BC, Berneburg M, Fawcett H, et al. Two individuals with features of both xeroderma pigmentosum and trichothiodystrophy highlight the complexity of the clinical outcomes of mutations in the XPD gene. Hum Mol Genet. 2001;10:2539–47. doi: 10.1093/hmg/10.22.2539. [DOI] [PubMed] [Google Scholar]
- 19.Brooks BP, Thompson AH, Clayton JA, et al. Ocular manifestations of trichothiodystrophy. Ophthalmology. 2011;118:2335–42. doi: 10.1016/j.ophtha.2011.05.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lehmann AR. The xeroderma pigmentosum group D (XPD) gene: one gene, two functions, three diseases. Genes Dev. 2001;15:15–23. doi: 10.1101/gad.859501. [DOI] [PubMed] [Google Scholar]
- 21.Hebra F, Kaposi M, Tay W, translators and editors. On Diseases of the Skin, Including the Exanthemata. Vol. 3. London: The New Sydenham Society; 1874. [Accessed November 1, 2012.]. pp. 252–8. Available at: http://archive.org/details/ondiseasesskini00hebrgoog. [Google Scholar]
- 22.Marshall J. Radiation and the ageing eye. Ophthalmic Physiol Opt. 1985;5:241–63. [PubMed] [Google Scholar]
- 23.Goyal JL, Rao VA, Srinivasan R, Agrawal K. Oculocutaneous manifestations in xeroderma pigmentosa. Br J Ophthalmol. 1994;78:295–7. doi: 10.1136/bjo.78.4.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Anttinen A, Koulu L, Nikoskelainen E, et al. Neurological symptoms and natural course of xeroderma pigmentosum. Brain. 2008;131:1979–89. doi: 10.1093/brain/awn126. [DOI] [PubMed] [Google Scholar]
- 25.Ramkumar HL, Brooks BP, Cao X, et al. Ophthalmic manifestations and histopathology of xeroderma pigmentosum: two clinicopathological cases and a review of the literature. Surv Ophthalmol. 2011;56:348–61. doi: 10.1016/j.survophthal.2011.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Alfawaz AM, Al-Hussain HM. Ocular manifestations of xeroderma pigmentosum at a tertiary eye care center in Saudi Arabia. Ophthal Plast Reconstr Surg. 2011;27:401–4. doi: 10.1097/IOP.0b013e31821c7323. [DOI] [PubMed] [Google Scholar]
- 27.Boyle J, Ueda T, Oh KS, et al. Persistence of repair proteins at unrepaired DNA damage distinguishes diseases with ERCC2 (XPD) mutations: cancer-prone xeroderma pigmentosum vs. non-cancer-prone trichothiodystrophy. Hum Mutat. 2008;29:1194–208. doi: 10.1002/humu.20768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bradford PT, Goldstein AM, Tamura D, et al. Cancer and neurologic degeneration in xeroderma pigmentosum: long term follow-up characterises the role of DNA repair. J Med Genet. 2011;48:168–76. doi: 10.1136/jmg.2010.083022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bron AJ, Evans VE, Smith JA. Grading of corneal and conjunctival staining in the context of other dry eye tests. Cornea. 2003;22:640–50. doi: 10.1097/00003226-200310000-00008. [DOI] [PubMed] [Google Scholar]
- 30.Jones LT. The lacrimal secretory system and its treatment. Am J Ophthalmol. 1966;62:47–60. doi: 10.1016/0002-9394(66)91676-x. [DOI] [PubMed] [Google Scholar]
- 31.Abelson MB, Ousler GW, III, Nally LA, et al. Alternative reference values for tear film break up time in normal and dry eye populations. Adv Exp Med Biol. 2002;506:1121–5. doi: 10.1007/978-1-4615-0717-8_157. [DOI] [PubMed] [Google Scholar]
- 32.Tu EY, Rheinstrom S. Dry eye. In: Yanoff M, Duker JS, Goldstein MH, editors. Ophthalmology. 3. Edinburgh, UK: Mosby Elsevier; 2009. pp. 326–7. [Google Scholar]
- 33.Robbins JH, Kraemer KH, Merchant SN, Brumback RA. Adult-onset xeroderma pigmentosum neurological disease--observations in an autopsy case. Clin Neuropathol. 2002;21:18–23. [PubMed] [Google Scholar]
- 34.Robbins JH, Kraemer KH, Lutzner MA, et al. Xeroderma pigmentosum. An inherited disease with sun sensitivity, multiple cutaneous neoplasms, and abnormal DNA repair. Ann Intern Med. 1974;80:221–48. doi: 10.7326/0003-4819-80-2-221. [DOI] [PubMed] [Google Scholar]
- 35.Robbins JH, Brumback RA, Moshell AN. Clinically asymptomatic xeroderma pigmentosum neurological disease in an adult: evidence for a neurodegeneration in later life caused by defective DNA repair. Eur Neurol. 1993;33:188–90. doi: 10.1159/000116932. [DOI] [PubMed] [Google Scholar]
- 36.Gaasterland DE, Rodrigues MM, Moshell AN. Ocular involvement in xeroderma pigmentosum. Ophthalmology. 1982;89:980–6. doi: 10.1016/s0161-6420(82)34692-8. [DOI] [PubMed] [Google Scholar]
- 37.Mahindra P, DiGiovanna JJ, Tamura D, et al. Skin cancers, blindness, and anterior tongue mass in African brothers. J Am Acad Dermatol. 2008;59:881–6. doi: 10.1016/j.jaad.2008.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Faghri S, Tamura D, Kraemer KH, Digiovanna JJ. Trichothiodystrophy: a systematic review of 112 published cases characterises a wide spectrum of clinical manifestations. J Med Genet. 2008;45:609–21. doi: 10.1136/jmg.2008.058743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.McCarty CA, Bansal AK, Livingston PM, et al. The epidemiology of dry eye in Melbourne, Australia. Ophthalmology. 1998;105:1114–9. doi: 10.1016/S0161-6420(98)96016-X. [DOI] [PubMed] [Google Scholar]
- 40.Puangsricharern V, Tseng SC. Cytologic evidence of corneal diseases with limbal stem cell deficiency. Ophthalmology. 1995;102:1476–85. doi: 10.1016/s0161-6420(95)30842-1. [DOI] [PubMed] [Google Scholar]
- 41.Muller A, Doughty MJ, Wright L. Reassessment of the corneal endothelial cell organisation in children. Br J Ophthalmol. 2000;84:692–6. doi: 10.1136/bjo.84.7.692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wilson RS, Roper-Hall MJ. Effect of age on the endothelial cell count in the normal eye. Br J Ophthalmol. 1982;66:513–5. doi: 10.1136/bjo.66.8.513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Price FW, Jr, Koller DL, Price MO. Central corneal pachymetry in patients undergoing laser in situ keratomileusis. Ophthalmology. 1999;106:2216–20. doi: 10.1016/S0161-6420(99)90508-0. [DOI] [PubMed] [Google Scholar]
- 44.Hussein MAW, Paysse EA, Bell NP, et al. Corneal thickness in children. Am J Ophthalmol. 2004;138:744–8. doi: 10.1016/j.ajo.2004.06.030. [DOI] [PubMed] [Google Scholar]
- 45.Norval M, Cullen AP, de Gruijl FR, et al. The effects on human health from stratospheric ozone depletion and its interactions with climate change. Photochem Photobiol Sci. 2007;6:232–51. doi: 10.1039/b700018a. [DOI] [PubMed] [Google Scholar]
- 46.Taylor HR. Ultraviolet radiation and the eye: an epidemiologic study. Trans Am Ophthalmol Soc. 1989;87:802–53. [PMC free article] [PubMed] [Google Scholar]
- 47.Coroneo M. Ultraviolet radiation and the anterior eye. Eye Contact Lens. 2011;37:214–24. doi: 10.1097/ICL.0b013e318223394e. [DOI] [PubMed] [Google Scholar]
- 48.Maïssa C, Guillon M. Tear film dynamics and lipid layer characteristics--effect of age and gender. Cont Lens Anterior Eye. 2010;33:176–82. doi: 10.1016/j.clae.2010.02.003. [DOI] [PubMed] [Google Scholar]
- 49.Ozdemir M, Temizdemir H. Age- and gender-related tear function changes in normal population. Eye (Lond) 2010;24:79–83. doi: 10.1038/eye.2009.21. [DOI] [PubMed] [Google Scholar]
- 50.Patel S, Boyd KE, Burns J. Age, stability of the precorneal tear film and the refractive index of tears. Cont Lens Anterior Eye. 2000;23:44–7. doi: 10.1016/s1367-0484(00)80024-7. [DOI] [PubMed] [Google Scholar]
- 51.De Vries A, Gorgels TG, Berg RJ, et al. Ultraviolet-B induced hyperplasia and squamous cell carcinomas in the cornea of XPA-deficient mice. Exp Eye Res. 1998;67:53–9. doi: 10.1006/exer.1998.0485. [DOI] [PubMed] [Google Scholar]
- 52.Sliney DH. Geometrical assessment of ocular exposure to environmental UV radiation--implications for ophthalmic epidemiology. J Epidemiol. 1999;9(suppl):S22–32. doi: 10.2188/jea.9.6sup_22. [DOI] [PubMed] [Google Scholar]
- 53.Podskochy A. Protective role of corneal epithelium against ultraviolet radiation damage. Acta Ophthalmol Scand. 2004;82:714–7. doi: 10.1111/j.1600-0420.2004.00369.x. [DOI] [PubMed] [Google Scholar]
- 54.Robbins JH. Significance of repair of human DNA: evidence from studies of xeroderma pigmentosum. J Natl Cancer Inst. 1978;61:645–56. [PubMed] [Google Scholar]
- 55.Paridaens AD, McCartney AC, Hungerford JL. Premalignant melanosis of the conjunctiva and the cornea in xeroderma pigmentosum. Br J Ophthalmol. 1992;76:120–2. doi: 10.1136/bjo.76.2.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Surveillance Epidemiology and End Results. Table 1.11: Mean Age of Cancer Patients at Diagnosis, 2005–2009 by primary cancer site, race and sex. Bethesda, MD: National Cancer Institute; [Accessed September 19, 2012.]. SEER Cancer Statistics Review, 1975–2009 (Vintage 2009 populations). Updated August 20, 2012. Available at: http://seer.cancer.gov/csr/1975_2009_pops09/results_merged/topic_med_age.pdf. [Google Scholar]
- 57.Jacyk WK. Xeroderma pigmentosum in black South Africans. Int J Dermatol. 1999;38:511–4. doi: 10.1046/j.1365-4362.1999.00724.x. [DOI] [PubMed] [Google Scholar]
- 58.Diffey BL, Gies HP. The confounding influence of sun exposure in melanoma [letter] Lancet. 1998;351:1101–2. doi: 10.1016/s0140-6736(05)79381-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.