Abstract
TNFα, produced by most malignant cells, orchestrates the interplay between malignant cells and myeloid cells, which have been linked to tumor growth and metastasis. Although TNFα can exist as one of two isoforms, a 26-kDa membrane tethered form (mTNFα) or a soluble 17-kDa cytokine (sTNFα), the vast majority of published studies have only investigated the biological effects of the soluble form. We demonstrate for the first time that membrane and soluble isoforms have diametrically opposing effects on both tumor growth and myeloid content. Mouse lung and melanoma tumor lines expressing mTNFα, generated small tumors devoid of monocytes versus respective control lines or lines expressing sTNFα. The lack of myeloid cells was due to a direct effect of mTNFα on myeloid survival via induction of cell necrosis by increasing reactive oxygen species. Human NSCLCs expressed varying levels of both soluble and membrane TNFα, and gene expression patterns favoring mTNFα were predictive of improved lung cancer survival. These data suggest that there are significant differences in the role of different TNFα isoforms in tumor progression and the bioavailability of each isoform may distinctly regulate tumor progression. This insight is critical for effective intervention in cancer therapy with the available TNFα inhibitors, which can block both TNFα isoforms.
Keywords: Membrane-Tumor Necrosis Factor-alpha, Tumor Associated Myeloid Cells, ROS, TACE, NSCLC
INTRODUCTION
TNFα is a major inflammatory cytokine expressed within the tumor microenvironment. TNFα is not normally detected in the serum of healthy individuals, but elevated levels have been detected in patients with prostate, pancreatic, renal cell, hematopoietic and metastatic breast cancers (1–6). The role of TNFα in cancer progression is conflicting. Multiple studies have demonstrated a pro-tumorogenic role of TNFα in vivo, in part by inhibiting necrosis of and by stimulating a proangiogenic myeloid phenotype (7, 8). Despite the growing body of evidence showing that TNFα can function as a tumor promoter, there remain conflicting findings. Several case reports describe a temporal relationship between development of skin malignancies and lymphoma and the use of TNFα inhibitors (9, 10). Moreover, the use of infliximab, which prevents binding of TNFα to its receptors, does not improve clinical outcome in renal cell carcinoma (11). Collectively, these data demonstrate the complexity of TNFα in cancer pathogenesis.
The majority of studies to date focus on the 17-kDa soluble moiety of TNFα, which is released after proteolytic cleavage of the 26-kDa type II transmembrane isoform by TNFα-converting enzyme (TACE; ADAM-17) (12). The role of membrane form of TNFα and its expression pattern in different tissue is poorly understood. Cardiac-restricted expression of membrane versus soluble TNFα isoform has been shown to have adverse effect in cardiac remodeling (13, 14). Expression of sTNFα in cardiomyocytes can cause dilation of left ventricle in mice whereas the mTNFα results in a concentric hypertrophic cardiac phenotype. Increased mTNFα expression on T-cells is shown to modulate monocytes IL10 production (15). In spite of these findings, the role of mTNFα in tumor biology is unknown (13). Thus far it is not known whether tumors can express both isoforms. In addition there is little understanding of the difference in the mechanism of action of sTNFα versus mTNFα in regulating tumor behavior or impact on the tumor inflammatory stroma.
The goal of the current study was to assess if the conflicting data regarding the association of TNFα with tumor progression was due to distinct effects of tumor expression of membrane vs. soluble isoforms. We found that whereas sTNFα expression promoted tumor growth, mTNFα-expressing tumors exhibited reduced growth and were largely devoid of myeloid cells. Our study demonstrated distinct TNFα isoform-dependent effects on myeloid cell survival. Importantly, we showed for the first time that human non-small cell lung cancer (NSCLC) tissues exhibit differential expression of membrane versus soluble TNFα. Moreover, patients with lung tumors predicted by the molecular signature to have higher mTNFα had better survival when compared to patients with tumors with higher soluble form of TNFα expression. Together, these data demonstrate that opposing functions of membrane versus soluble TNFα significantly impact tumor progression for several cancer types, including NSCLC, one of the most commonly diagnosed cancer in the United States and one of the most difficult to treat (16). Strategies for molecularly targeted anti-TNFα agents must include screening patients for expression of each isoform and tailoring inhibitors to specifically target the soluble isoform.
MATERIALS AND METHODS
Mice and cell lines
Wild-type C57Bl/6 (WT) mice were purchased from Jackson Laboratory. Homozygous mutants for TNFR1 and R2 knockout (TNFR-DKO) on a C57Bl/6 background were a generous gift from Dr. D. Polk. Lewis Lung Carcinoma (LLC), B16F10 melanoma, and RAW 264.7 cells were purchased from American Type Culture Collection (ATCC) and maintained in DMEM supplemented with glucose (4.5 g/l) along with penicillin (10 U/L), streptomycin (10 μg/ml), plasmocin (25 μg/ml), amphotericin-B (2.5 μg/ml). H520, HCC95, SW900, H157, HCC15, and A549 were provided by Dr. P. P. Massion and were maintained in RPMI 1640 medium (Gibco). A549 cells were maintained in Ham’s F-12K medium (Gibco). All cell lines were supplied with 10% (v/v) fetal bovine serum and incubated at 37°C in 5% CO2.
Constructs and retroviral transductions
Secretable TNFα was generated by replacing amino acids −76 to −1 containing the cytoplasmic signal-anchor for type II membrane protein and a short extracellular region with a sequence coding for the IL-2 signal peptide (IL2sp, amino acids −20 to −1) that directs the transport of TNFα to the outer cellular space and produces a solely secretable form of 17-kDa TNFα This was done by amplifying mouse wild-type TNFα cDNA using forward primers flanked by BamH1-IL2sp. The amplified fragment was isolated and purified by gel electrophoresis. The restriction sites at each end allowed ligation of the IL2sp-TNFα fragment into the BamH1-EcoR1 site of LZRS-IRES-Neo retroviral vector, conferring neomycin resistance. The mTNFΔ1–9, K11E sequence encoding a mutant transmembrane TNFα molecule with a deletion at the cleavage site between presequnce and mature membrane TNFα (BCCM/LMBP plasmid collection, Ghent University) was also cloned into LZRS. This mutation prevents cleavage of the 26-kDa membrane TNFα into secretory TNFα isoform. An empty LZRS vector was used as a control vector.
Surface expression of TNFα
Cells were detached from tissue culture plate and incubated with anti-TNFα antibody (1 μl/2.5 × 104 cells, Southern Biotech) for 30 minutes on ice without permeabilization. PE conjugated secondary antibody (0.125 μg/106 cells/100 μl) was added for 30 min on ice. Surface expression of TNFα was measured using flow cytometry. Data are presented as percent of viable cells.
In vivo murine tumor model
Control, IL2spTNFα, and mTNFα cell lines (106 cells in 100 μl of PBS) were implanted subcutaneously into WT, TNFR-DKO or TNFR-DKO-BMT mice. Mice were sacrificed 15 days post-implantation, tumors were excised and the volume was calculated by multiplying tumor length by width by height.
BrdU assay
The BrdU ELISA was performed according to the manufacturer’s instructions. Briefly, 1000 cells/well were seeded in triplicate in a 96-well plate. Cells were allowed to attach for 8 hours. BrdU label was added to each well and incubated for an additional 24 hours. Absorbance was analyzed at 450–540 nm.
Cell viability assay
Cell viability was measured by seeding 5000 cells/well in a 96-well plate for 48 hours. Cells were labeled with 100 μl of PBS containing 0.5 mg/mL of 3-[4,5-dimethylthiazol-2-yl] 2,5,-diphenyltetrazolium bromide (MTT) (Sigma). After 2 hours of incubation at 37°C, cells were lysed with 0.1 ml DMSO. Photometric measurement was carried out at 540 nm.
Leukocyte quantification in tumors
Tumor tissues were finely minced and incubated in 5 mL dissociation solution (RPMI medium supplemented with 5% FBS, and 1 mg/mL of Collagenase type IV (Worthington)) for 30 min at 37°C. To obtain a single-cell suspension, cells were passed through 70-μm nylon cell strainer (Becton Dickinson, NJ). Cells were washed with FACS buffer (PBS, 2 mM EDTA, 0.5% BSA) and incubated for 5 min in RBC lysis buffer solution (155 mM NH4Cl, 12 mM NaHCO3, 0.1 mM EDTA). Cells were washed twice in FACS buffer and incubated with anti-CD3 (Biolegend), -Ly6G (BD Pharminogen), -F480 (eBioscience) and -CD11b+ (BD Pharminogen). After two washes, labeled cells were resuspended in vital dye 7-AAD (BD Pharminogen) and subjected to flow cytometry on LSRFortessa flow cytometer (Becton, Dickinson and Company, Franklin Lakes, NJ) and analyzed by using FlowJo software (TreeStar, Ashland, OR).
Myeloid cell-trafficking to tumor
Control vector or mTNFα-expressing tumor cells were injected subcutaneously into wild-type mice. After 12 days, freshly isolated myeloid cells were labeled with 5(6)-Carboxyfluorescein N-hydroxysuccinimidyl ester (CFSE) fluorescent tracking dyes and injected into retro-orbital space (5 × 106 cells/animal). Eighteen hours later, tumors were harvested and single-cell suspensions were made. CFSE labeled cells were detected using flow cytometry.
Caspase 3/7 activity
Freshly isolated CD11b+ (105/96-well) were cocultured with fixed control, control+recTNFα (100 U/mL), and mTNFα B16F10 cells at CD11b+/tumor cells ratio of 1:10 for 5 hours. Apoptosis was quantified in the form of caspase-3/7 activation using the Apo-One fluorometric assay system from Promega Corporation (Madison, WI) according to the manufacturer’s protocol.
Measurement of intracellular ROS
The oxidant-sensing probe CM-H2DCFDA (Invitrogen) was used to detect intracellular reactive oxygen species (ROS). Freshly isolated CD11b+ cells were loaded with 10 μM CM-H2DCFDA, and cocultured with fixed B16F10 control, control+100 U/ml of recombinant TNFα, mTNFα or mTNFα+2 mM N-acetyl-cysteine (NAC) for 8 hours. Fluorescence was determined using a luminescence spectrophotometer (Spectra max, Molecular Devices) with an excitation wavelength of 429 nm and emission wavelength of 517 nm.
Survival analysis
A cohort published by Shedden et al. was analyzed for disease-free survival (17). The data set included gene-expression profiles for 442 lung adenocarcinomas with high-quality gene-expression data, pathological data and clinical information. The association between gene expression and the survival was examined using the Cox proportional hazard model. Kaplan-Meier curves were generated to visualize the survival pattern by dichotomizing the gene-expression. The subgroup analysis of mTNFα and TACE was done by dividing the cohort into 4 groups of high TNFα with high/low TACE or low TNFα with high/low TACE. Log-rank overall tests were performed for the 4 groups. All statistical analyses were performed using R (www.r-project.org).
Statistical analysis
The statistical significance between experimental and control groups was determined by Student’s t-test or ANOVA followed by Tukey’s post-test using Prism software (Graphpad, San Diego, CA). A P-value of <0.05 was considered statistically significant.
RESULTS
mTNFα isoform reduces tumor growth
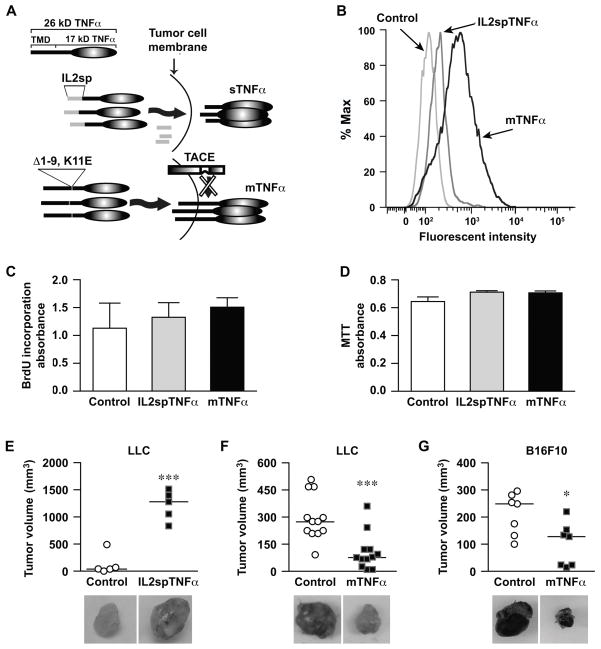
In this study we investigated the effects of different TNFα isoforms on malignant tumor phenotypes using murine Lewis Lung Carcinoma (LLC) sublines expressing either sTNFα (LZRS-IRES-IL2spTNFα) or mTNFα (LZRS-IRES-mTNFΔ1–9) by retroviral transduction (Fig. 1A). Cells transduced with empty vector (LZRS-IRES-Neo) were used as control. Untransduced LLC cells exhibited undetectable levels of TNFα expression as determined by ELISA. The relative expression of sTNFα and cell surface expression of TNFα by transduced cells were confirmed by ELISA and flow cytometry respectively. sTNFα expression was detected in IL2spTNFα-expressing cells at 5 ng/ml. Surface expression of TNFα was not detected in control and IL2spTNFα tumor cells (mean fluorescent intensity of 150 and 202 respectively). In contrast, mTNFα-expressing LLC cells displayed 7.1- and 5.3-fold increase in surface TNFα as compared with control and IL2spTNFα, respectively (Fig. 1B).
Figure 1. mTNFα-expressing tumor cells demonstrate delayed tumor growth.
(A) Schematic representation of TNFα mutant which has the region coding for the TNFα transmembrane domain (TMD) replaced with region coding for interleukin-2 (IL-2) signal peptide to generate soluble TNFα (sTNFα) and TNFα lacking TACE cleavage site (Δ1–9, K11E) to generate membrane TNFα (mTNFα). (B) Expression of transmembrane TNFα on the surface of LLC tumor cells transduced with empty vector (control), IL2spTNFα or mTNFα vectors was analyzed by flow cytometery. (C and D) Proliferation rate and the viability of transduced tumor cells were determined by BrdU and MTT labeling assays respectively. All cell lines showed no significant difference in proliferation or viability. Data is representative of three independent experiments expressed as the mean±SEM. (E–G) In vivo growth of tumor cells transduced with control vector was compared to LLC lines expressing IL2spTNFα (E), or mTNFα (F), and B16F10 expressing mTNFα (G), by subcutaneous implantation in WT bl/6 mice for 14 days. Each point represents an individual animal and the horizontal bar is the Mean. *P<0.05, ***P<0.0005, Student’s t-test. Photomicrograph of each tumor is shown with each experimental group.
To evaluate whether overexpressing various TNFα isoforms affected growth or survival of tumor cells, we assessed in vitro proliferation and viability using the BrdU incorporation and MTT assay, respectively. Both IL2spTNFα and mTNFα-expressing cells exhibited similar in vitro growth rates compared with control LLC lines (P>0.05; Fig. 1C). IL2spTNFα and mTNFα LLC cell lines tested for viability also displayed similar levels of survival rate compared to control (P>0.05; Fig. 1D). To gain additional evidence that the membrane isoform did not reduce survival or viability and that these observations were not cell specific, we transduced B16F10-melanoma cell lines with retroviral constructs, containing mTNFα or an empty construct as control cells. Similar to LLC lines, the proliferation rate (P>0.05) and viability (P>0.05) were not affected in mTNFα-expressing B16F10 cells compared to control cells (Supplementary Fig. S1A and B). These findings are consistent with earlier studies in which we showed that overexpressing the wild-type TNFα in both LLC and B16F10 melanoma did not alter in vitro growth (8).
LLC cell lines expressing soluble (IL2spTNFα) or membrane (mTNFα) isoforms were implanted subcutaneously into the flank of wild-type C57Bl/6 mice (WT). The same number of cells transduced with empty vector was implanted as a control. After 14 days, LLC tumors expressing IL2spTNFα were ~7 fold (1214±122 mm3) larger compared to control tumors (124.4±92 mm3; n=5, P<0.0005; Fig. 1E). By contrast, tumors expressing mTNFα exhibited 65% reduction in tumor volume (105.8±29.3 mm3) compared to control tumor (294.1±35.9 mm3) (n=12, P<0.0005; Fig. 1F). Similar growth reduction was observed with mTNFα-expressing B16F10 tumors cell line when compared with matched controls (n=7, P<0.05; Fig. 1G). In addition, tumor weight measurement followed similar pattern to tumor volume as presented in Supplementary Fig. S2A–C. These data suggested that different TNFα isoforms have opposing effects on tumor size.
Expression of mTNFα does not affect tumor proliferation or vascularity in vivo
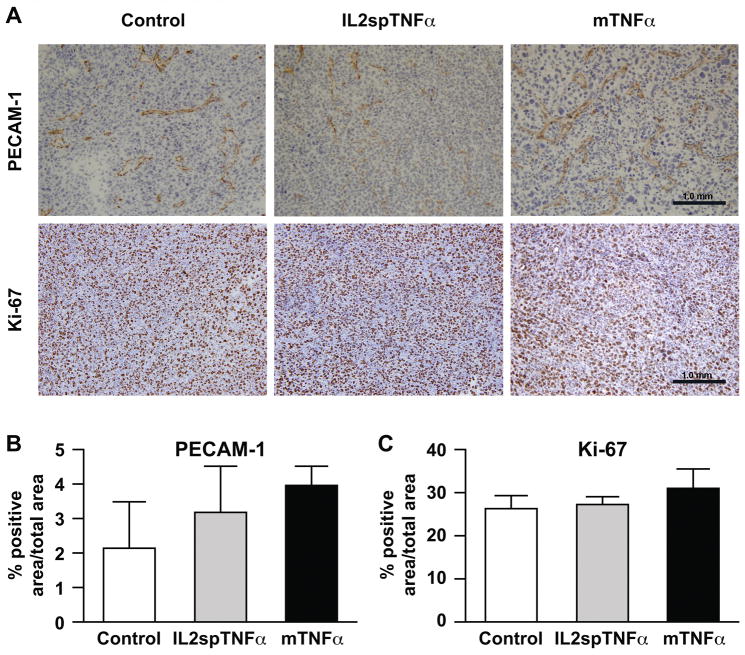
To evaluated tumor vascular density and tumor cell proliferation rate, histological sections from control, IL2spTNFα and mTNFα LLC tumor were immunostained with platelet/endothelial cell adhesion molecule-1 (PECAM-1) antibody to evaluate microvessel density, and anti-Ki67 to assess tumor cell proliferation (Fig. 2A). Histomorphometry of PECAM-1-positive areas showed no difference in vascular density amongst the tumors expressing different TNFα isoforms versus control (P>0.05; Fig. 2B). Furthermore, IL2spTNFα and mTNFα-expressing LLC tumors showed no significant difference in immunoreactivity with the Ki-67 antibody compared to control tumors (P>0.05; Fig. 2C).
Figure 2. Expression of mTNFα does not affect tumor proliferation or vascularity in vivo.
(A) Representative sections of LLC tumors transduced with control, IL2spTNFα and mTNFα constructs were analyzed by immunohistochemistry for PECAM-1 or Ki-67 staining to define vascularity or proliferation, respectively. (B and C) Percentage of PECAM-1-positive (B) or Ki-67-positive area (C) in control, IL2spTNFα and mTNFα in LLC tumors was quantitated. There was no significant difference between the cohorts for either PECAM-1-positive or Ki-67-positive cells (P>0.05), 1-way ANOVA with Tukey’s post-test.
Vascular density and cell proliferation analyses were also performed between B16F10 melanoma-derived tumors expressing mTNFα versus control (Supplementary Fig. S3A). Similar to LLC tumors, no significant difference was observed in microvessel density and in vitro proliferation between the control and mTNFα-expressing melanoma tumors (P>0.05; Supplementary Fig. S3B and C).
mTNFα-expressing tumors are devoid of tumor associated myeloid cells
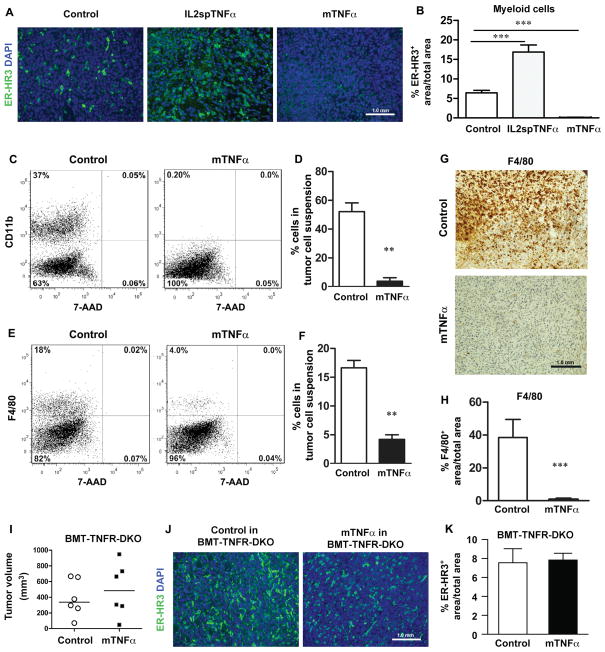
It is often assumed that TNFα-mediated tumor promotion is secondary to TNFα-mediated inflammation. To determine whether mTNFα-expressing tumors had altered composition of inflammatory cells, we quantified LLC tumor-associated T-cells (anti-CD3), B-cells (anti-B220b), neutrophils (anti-Ly6G) and myeloid-monocytic lineage (anti-ER-HR3, CD11b, F4/80) using immunohistochemistry staining and flow cytometric analysis of single-cell suspension of tumors. Both immunostaining and flow cytometric analysis showed no significant difference in T-cell content among control, IL2sp TNFα and mTNFα-expressing tumor cells (Supplementary Fig. S4A–C). Anti-B220b staining revealed only rare, infiltrating B-cells for all tumor groups (data not shown). Flow cytometric analysis of anti-Ly6G showed no significant difference in neutrophils population within control and mTNFα-expressing tumors (P>0.05; Supplementary Fig. S4D). Interestingly when we evaluated tumor-associated myeloid cell population a significant difference was observed in mTNFα-expressing tumors compared to control and IL2spTNFα tumors. Histological staining of mTNFα-expressing LLC tumors for ER-HR3, a myeloid marker reactive to ~70% of circulating monocytes and a subset of mature tissue macrophages (18), had fewer number of infiltrated ERHR3+ cells (0.04±0.02%) versus control (6.4±0.37%) or IL2sp-TNFα tumors (16±1.03%) (P>0.005; Fig. 3C and D). Further evaluation of single-cell suspension of tumors showed significantly lower number of CD11b+ myeloid cells (52.17±6.1%; P>0.005) and F4/80 macrophages (3.75±2.4%; P>0.005) in mTNFα-expressing tumors as compared to control (Fig. 3A–F). Anti-F4/80 staining of tumor sections further confirmed the significant reduction of F4/80-positive macrophages in mTNFα expressing tumors (Fig. 3G and H).
Figure 3. mTNFα-expressing tumors are devoid of tumor-associated myeloid cells.
(A) ER-HR3 staining of LLC tumor cells, expressing various TNFα isoforms. Control (left), IL2spTNFα (middle), and mTNFα (right) LLC tumor sections from wild-type bl/6 were stained with ER-HR3 myeloid markers (green). (B) Percentage of ER-HR3-positive cells in LLC tumors transduced with control or different of TNFα isoforms was quantitated. There was a significant decrease in the number of ER-HR3-positive cells in LLC tumors expressing mTNFα isoform compared to control tumors. (C and E) Representative flow cytometric analysis of CD11b- and F4/80-postitive population in LLC tumor cell suspension. Dot plots show CD11b/7-AAD (C) and F4/80/7-AAD (E) from one representative animal for each group. (D and F) Percentage of CD11b- and F4/80-positive cells were quantitated in control and mTNFα tumor cell suspensions. (G) Representative sections of control and mTNFα-transfected LLC tumors were analyzed by immunohistochemistry for F4/80+ macrophages. (H) Number of F4/80-postivie cells in control and mTNFα in LLC tumors was quantitated. (I) Control and mTNFα-expressing LLC tumor cells were implanted subcutaneously in WT bl/6 mice received bone marrow (BM) transplant from TNFα receptors1/2 knockout donor (BMT-TNFR-DKO mice) for 14 days. The mean is shown for each group (n=6 animals). (J) Representative ER-HR3 immunofluorescence staining from control and mTNFα-transduced tumors. (K) Percentage of ER-HR3-positive cells in control and mTNFα-expressing LLC tumors from WT mice with BMT from DRKO donor was quantitated. There was no significant difference between the cohorts for ER-HR3-positive cells (n=3). Data are presented as mean±SEM, **P<0.005, ***P<0.0005; Student’s t-test.
To test whether the tumor inhibitory effects of mTNFα required the presence of TNFα receptors, tumor growth was assessed in TNFα receptors deficient mice (TNFα-R1 and TNFα-R2 double knockout, TNFR-DKO). Mice were implanted with LLC cell line expressing various TNFα isoforms. LLC lines expressing mTNFα isoform did not generate significantly smaller tumors (110.5±17.6 mm3) in compared with their paired control tumors (161.7±29.6 mm3, n=5) (P>0.05; Supplementary Fig. S5A). In addition implantation of mTNFα-expressing LLC tumor cells in TNFR-DKO mice restored the ER-HR3+ myeloid population in mTNFα-expressing tumors (0.91±0.16% ER-HR positive area/total area in mTNFα vs. 0.67%±0.16% in control; P>0.05; Supplementary Fig. S5B). Similar results were observed in B16F10 line (data not shown).
Restoration of myeloid cell population in mTNFα-expressing LLC tumors in TNFR-DKO host prompted us to further evaluate the requirement of TNFR signaling in inflammatory cells (i.e bone marrow-derived cells). Therefore, tumor growth was assessed in WT mice receiving bone marrow (BM) transplants from TNFR-DKO mice (referred to as BMT-TNFR-DKO mice). Similar to experiments in TNFR-DKO host, LLC line expressing mTNFα isoform, implanted into BMT-TNFR-DKO, did not generate smaller tumors in mice engrafted with TNFR-deficient BM (497.2±137.6 mm3) as compared with control tumors (387.9±95.94 mm3) (P>0.05; Fig. 3E). Furthermore, we quantified tumor associated myeloid cell populations in these tumors isolated from BMT-TNFRDKO. The overall percentage of myeloid populations in mTNFα-expressing LLC tumors (7.8±0.4%) was similar to control tumors (7.5±1.4%) (P>0.05; Fig. 3F and G). These data suggested that tumor derived mTNFα significantly reduced myeloid population within the tumor microenvironment. Since this effect was abrogated in WT mice transplanted with TNFα receptor deficient BM, it was concluded that intact TNFα signaling through its receptor in bone marrow derived cells was required for this effect, and that this effect was not mediated by secondary factors from the tumor cells.
mTNFα-derived soluble factors do not affect CD11b+ myeloid cell migration/recruitment
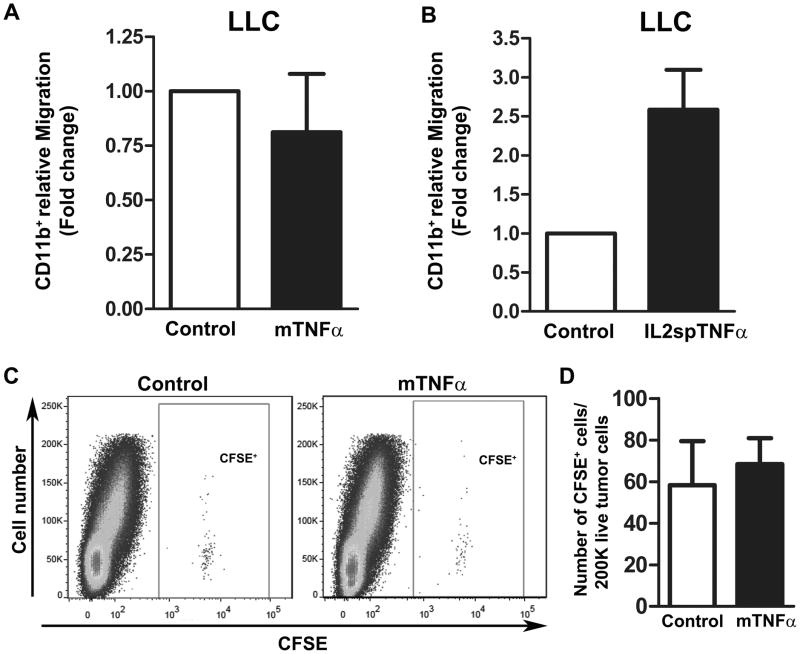
One possible explanation for the reduction of myeloid cells observed in mTNFα-expressing tumors was that such tumors exhibited reduced expression of necessary signals for myeloid recruitment. Using a modified Boyden chamber assay, we evaluated the ability of conditioned media (CM) from LLC and B16F10 melanoma cell lines expressing various forms of TNFα to promote migration (i.e. recruitment) of primary murine CD11b+ myeloid cells. CM from mTNFα did not inhibit migration of CD11b+ as compared to control-CM in both LLC and B16F10 melanoma line (Fig. 4A and Supplementary Fig. S6A). An increase in CD11b+ myeloid cells migration was observed in CM derived from both IL2spTNFα-expressing LLC (~1.5-fold; Fig. 4B) and B16F10 line (~4-fold; Supplementary Fig. S6B). This may be attributed to the presence of TNFα itself, which is known to induce chemotactic response (19, 20). These results suggested that the relative paucity of myeloid cells in mTNFα-expressing tumors was likely not due to reduced expression of key cytokines, necessary for myeloid extravasation and migration into the tumor.
Figure 4. Soluble factors derived from mTNFα do not affect the rate of CD11b+ myeloid cell migration compared to control.
(A and B) Transwell migration assay of primary CD11b+ cells treated with conditioned media derived from LLC tumor cells transduced with control/mTNFα (A) or cotnrol/IL2spTNFα (B) constructs. Data presents the mean±SEM. (C) Representative flow cytometric analysis of CFSE-positive cells presented in LLC tumor suspension expressing either control (left) or mTNFα (right) isoform. (D) Quantification of CFSE-positive cells detected in a given number of tumor suspension (n=3 for each tumor type). Data are presented as mean±SEM, P>0.05.
We further evaluated the ability of control and mTNFα-expressing LLC tumors to effectively recruit myeloid cell in vivo by adoptive transfer of CFSE labeled CD11b+ into tumor bearing mice. After 18 hours post injection CFSE-positive myeloid cells were quantified in each tumor type by flow analysis of single-cell suspension of tumor digests. The overall number of CFSE-positive cells in mTNFα-expressing LLC tumors (58±21) was similar to control tumors (68±12.57) (P>0.05; Fig. 4C and D). These data demonstrate that reduced myeloid cells in mTNFα-expressing tumors was not due to impaired recruitment of circulating cells.
mTNFα induces cell death through apoptosis-independent pathway
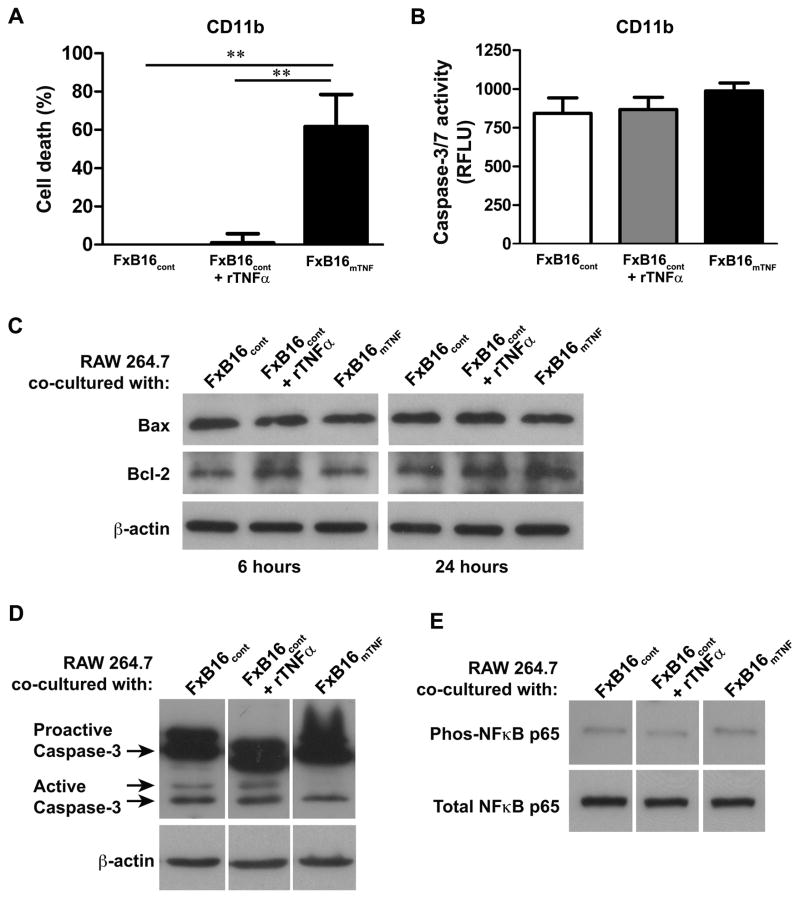
To investigated if the distinct TNFα isoforms exerted cytotoxic effects on myeloid cells, freshly isolated CD11b+ cells (target) were mixed with 1% paraformaldehyde-fixed B16F10 melanoma cells (effector) expressing empty vector with or without 100 U/ml recombinant murine TNFα, (FxB16cont or FxB16cont+rTNFα) or fixed mTNFα-expressing B16F10 cells (FxB16mTNF) at an effector:target ratio of 10:1. As measured by the MTT assay (Fig. 5A), FxB16mTNF resulted in more than 60±29% cytotoxicity of CD11b+ myeloid cells after 48 hours of incubation, as compared to CD11b+ cells incubated with FxB16cont (P<0.005). CD11b+ in the presence of FxB16cont+rTNFα showed less than 1% cytotoxicity in compared to control. We assessed the activation of apoptotic pathway as the mechanism of mTNFα-induced myeloid cell death by determining the caspase-3/7 enzymatic activity in CD11b+ cells. Compared to control, CD11b+ myeloid cells cocultured with FxB16cont+rTNFα or FxB16mTNF did not show any significant increase in the level of caspase 3/7 activity (P>0.05, Fig. 5B).
Figure 5. mTNFα induces cell death through apoptosis-independent pathway.
(A) Cytotoxic effect of sTNFα and mTNFα on CD11b+ cells measured by MTT assay. (B) Caspase-3/7 activity in CD11b+ cocultured with Paraformaldehyde-fixed control (FxB16cont), control+rTNFα (FxB16cont+TNFα), or mTNFα (FxB16mTNF). (C–E) Kinetics of Bax/Bcl-2, caspase-3 and NF-κB activation in RAW 264.7 after incubation with cancer cells transduced with various TNFα constructs. FxB16cont, FxB16cont+TNFα, or FxB16mTNF was added for indicated incubation period. RAW 264.7 cells were harvested and total cellular protein was analyzed for Bax/Bcl-2 ratio (C), activated caspase-3 (D), and total and phopho- NF-κB p65 (E). Immunoblot analysis showed no differences in Bax/Bcl-2 ratio or caspase-3 and NF-κB pathway activation with different TNFα isoforms compared to control. **P<0.05, 1-way ANOVA with Tukey’s post-test.
We further assessed the activation of apoptotic pathway in RAW 264.7 cells by determining the level of Bax/Bcl-2of and cleavage/activation of caspase-3 proteins in RAW 264.7. The data suggested that the Bax/Bcl-2 ratio (Fig. 5C) and active-caspase-3 (Fig. 5D) proteins in RAW 264.7 cells treated with both soluble and membrane TNFα had no significant changes when compared to control. Together these findings are indicative of another death pathway independent of apoptosis.
NF-κB is a critical factor in the determination of cell death versus survival and proliferation (21, 22). In cases of failed NF-κB activation, TNFα can induce either programmed cell death or necrosis through complex signal transduction cascades (23). To evaluate whether soluble versus mTNFα isoforms induced distinct cellular responses via regulation of NF-κB, we tested NF-κB activity in RAW 264.7 cells co-cultured with FxB16cont, FxB16cont+rTNFα, or FxB16mTNF. As shown in Figure 5E, the level of NF-κB p65 phosphorylation activity in RAW 264.7 cells stimulated with both FxB16cont+rTNFα and FxB16mTNF was similar to FxB16cont, suggesting that mTNFα isoform did not affect the activity of NF-κB p65 compared to sTNFα.
mTNFα-induced cell death occurs through induction of ROS
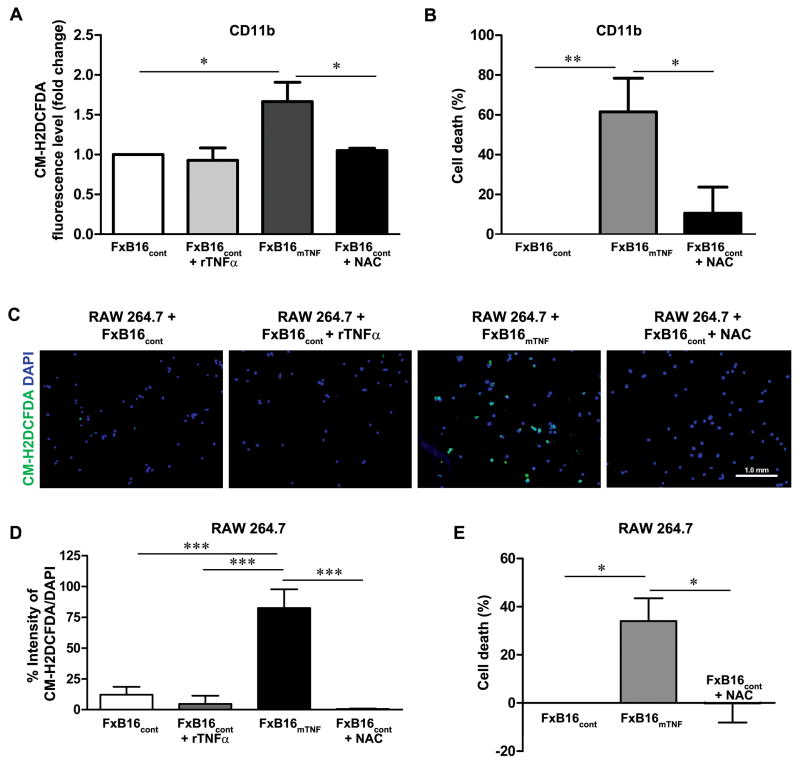
TNFα can induce cell death by induction of intracellular reactive oxygen spices (ROS)(24, 25). To test the possibility that soluble versus membrane TNFα isoforms induced distinct cellular responses via regulation of intracellular ROS, we evaluated ROS levels in CD11b+ myeloid cells incubated with different TNF isoform by measuring CM-H2DCFDA fluoresce. In CD11b+ cell incubated with FxB16cont+rTNFα, the CM-H2DCFDA fluorescence did not differ from control, whereas in cells with FxB16mTNF a 1.6-fold increase was observed after 8 hours of incubation (P<0.05; Fig. 6A). Addition of N-acetyl-cysteine into cells cultured with FxB16mTNF significantly decreased the intensity of CM-H2DCFDA fluorescence indicating decrease in the level of ROS (P>0.05). NAC treatment of FxB16mTNF treated CD11b+ reduced mTNFα induced cytotoxicity (FxB16mTNF, 61.57±29.12% cytotoxicity; FxB16mTNF+NAC, 10.64±29.17% cytotoxicity; P<0.05; Fig. 6B).
Figure 6. mTNFα-induced cell death occurs through induction of ROS.
(A) Effects of various TNFα isoforms on intracellular ROS generation in CD11b+ cells. Cells were labeled with ROS detection reagent, CM-H2DCFDA, and incubated with FxB16cont, FxB16cont+TNFα, or FxB16mTNF, FxB16mTNF+N-acetyl-cysteine (NAC, 2mM) and then ROS level was quantitatively analyzed. (B) Cytotoxic effect of mTNFα on CD11b+ cells decreased in the presence of ROS scavenger NAC. (C) Fluorescent micrographs of RAW 264.7 (blue: DAPI nuclear staining) incubated for 8 hours with FxB16cont, FxB16cont+TNFα, or FxB16mTNF, FxB16mTNF+NAC (2mM) and subsequently treated with ROS detection reagent, CM-H2DCFDA (green). (D) Intensity of CM-H2DCFDA was quantitated. Increase in number of ROS generating cell was detected in RAW 264.7 cocultured with mTNFα-expressing B16F10 cells which was reversed by addition of NAC. (E) Cytotoxic effect of mTNFα on RAW 264.7. *P<0.05, **P<0.005 and ***P<0.0005, 1-way ANOVA with Tukey’s post-test.
In addition we evaluated ROS generation in individual RAW 264.7 by CM-H2DCFDA fluorescent staining assay (26). The fluorescent intensity in RAW 264.7 co-cultured with FxB16cont+rTNFα was similar to the basal level (4.6±3%). However in the presence of FxB16mTNF, we observed an induction of ROS intensity (82.5±15%) as detected by the presence of green fluorescence staining (Fig. 6C and D). Treatment of RAW 264.7 cocultured with FxB16mTNF cells supplied with the ROS scavenger N-acetyl-cysteine (FxB16mTNF+NAC) diminished mTNFα-induced accumulation of intracellular ROS in RAW 264.7(0.5±0.4%; Fig. 6C and D) and led to abolition of mTNFα induced cytotoxicity (FxB16mTNF, 34±9% cytotoxicity; FxB16mTNF+NAC, 0.1±7% proliferation; P<0.05; Fig. 6E).
Relative expression pattern of TNFα/TACE correlates with survival probability in lung cancer patients
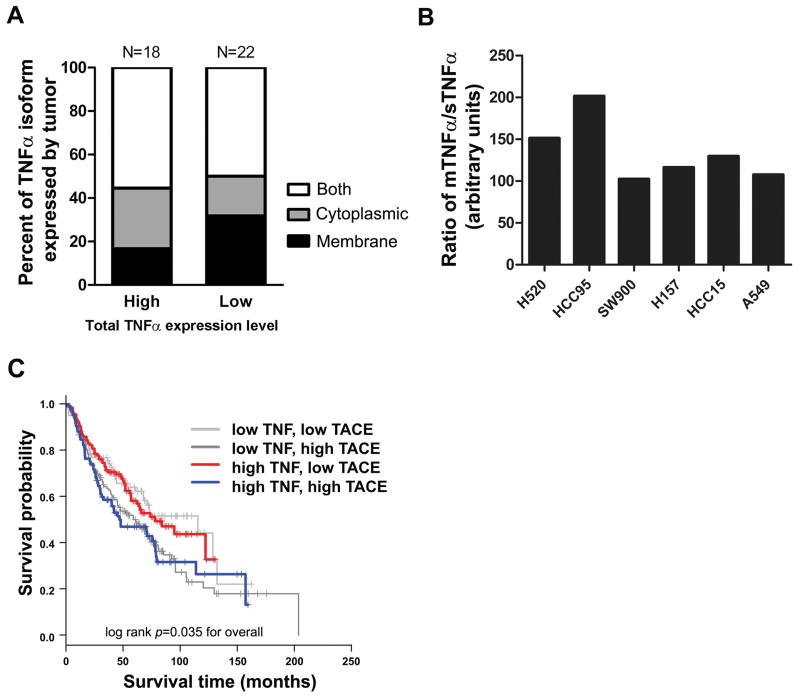
In most studies, the role of TNFα in cancer has only been investigated in murine models or in modified cell lines and to our knowledge the expression level of mTNFα has not been evaluated in human tumors. To extend the relevance of our findings from the murine model to human cancer, we examined 62 tissue cores available in a single human non-small cell lung carcinoma (NSCLC) tissue array containing squamous cell carcinoma, large cell carcinoma, and adenocarcinoma. Using co-immunofluorescent staining analysis with anti-TNFα and carcinoma-enriched membrane antigen (EMA) we evaluated the distribution of both degree (high- or low-expressors) and localization (membrane, cytoplamic or both) of TNFα staining. Forty of 62 individual tumors were positive for TNFα expression. Among 40 tumors, expressing either high or low TNFα, we detected tumors presenting high levels of membrane-localized TNFα, tumors presenting high cytoplasmic TNFα (i.e. tumors with higher expression of sTNFα) or tumors with cytoplasmic and membrane-localized TNFα. Eighteen of the 40 tumors (45%) were high expresser with 27.8% cytoplasmic localization, 16.7% localized on the membrane and 55.5% were positive for both membrane and cytoplasmic TNFα. Twenty two (65%) showed low expression of TNFα with 18.3% cytoplasmic, 31.8% membrane and 50% with both membrane and cytoplasmic (Fig. 7A). These data are the first evidence for the existence of mTNFα in human tumors and that its level varies significantly from patient to patient. Moreover, its level and localization varies significantly from patient to patient, which is likely an important consideration in predicted therapeutic response to anti-TNF agents based on our pre-clinical observations.
Figure 7. Relative expression pattern of TNFα/TACE correlates with survival probability in lung cancer patients.
(A) Analysis of TNFα expression from 40 human non-small cell lung carcinoma (NSCLC) tissue array. Graph displays the distribution of both degree and localization of TNFα staining 40 NSCLC patient samples. (B) Expression pattern of sTNFα and mTNFα in human NSCLC cell lines measured by ELISA and immunoblotting methods, respectively. A significant variation was observed in the ratio of mTNFα to sTNFα expressed by each cell line. (C) Association between TNFα and TACE co-expression pattern and survival probability in patients with NSCLC. Analysis of the publicly available data from the Shedden cohort (17) was used to correlate TNFα/TACE expression pattern with survival probability (n=442, log rank P=0.035).
To provide further evidence that human tumors exhibit varying expression of TNFα isoforms, the expression of sTNFα was determined by ELISA of CM and the expression of mTNFα was evaluated by immunoblot analysis of cell membrane fraction. The analysis showed significant variation in the relative expression of membrane versus soluble TNFα among different human lung cancer-derived cell lines (Fig. 7B).
Next we investigated the possible association of sTNFα versus mTNFα ratio with patient outcome (17). It has been shown that there is a positive correlation between TACE surface expression and TNFα cleavage. Upregulation of TACE protein has also been shown to be associated with a decline in mTNFα level and increased soluble level and vice versa (27). Using publicly accessible NSCLC microarray database (n=442 patients), we divided the gene expression data into four groups. The first two groups featured low TNFα gene expression and low or high TACE. The third and fourth groups demonstrated high TNFα with either low or high TACE. Over all higher TACE level was significantly correlated to lower survival probability. Expression of high TNFα/low TACE — representing tumors with high mTNFα:sTNFα relative expression — was associated with longer survival than expression of high TNFα/high TACE — representing tumors with low mTNFα:sTNFα relative expression (log rank P=0.035; Fig. 7C).
DISCUSSION
Data from both experimental and human cancers have identified TNFα as key cytokine modulating tumor progression, yet its effects are incompletely understood. In murine models, deletion or inhibition of TNFα reduces the incidence of cancer formation and even increases resistance to chemically induced carcinogenesis of the skin (28, 29). Consistent with this, there was a positive correlation between level of TNFα expression and tumor grade in ovarian tumors (30). On the other hand, anti-tumorigenic properties of TNFα are also well-documented. In a study by Boldrini et. al, assessment of TNFα expression in 61 NSCLC samples demonstrated expression of TNFα in 45.9% of cases and directly correlated with a better clinical outcome (31).
Many soluble proteins such as TNFα are originally expressed as a membrane-bound form and then processed to a secretory form through proteolytic cleavage. Some of these proteins such as Fas ligand, a member of TNFα super family, have been described to have distinct biological effects on disease process as a membrane isoform when compared to the soluble isoform (32). In this study we sought to better understand the role of different TNFα isoforms in the modulation of tumor progression and to determine if some of the reported opposing effects can be attributed to distinct effects of these isoforms.
We generated tumor cell lines expressing either uncleavable mTNFα or sTNFα. Subcutaneous implantation of mTNFα expressing LLC and B16F10 cancer cell lines resulted in significantly smaller tumors which was not the result of impaired angiogenesis or reduced tumor cell proliferation but was driven by components of the host derived cells. This idea was further strengthened by significant reduction of tumor associated myeloid cell content in mTNFα-expressing tumors which were restored in tumor cells transplanted in TNFR-DKO mice. Numerous studies have shown critical roles for tumor-associated stromal cells, specifically, bone marrow-derived myeloid cells, in tumor growth (33, 34). Upon activation by cancer cells, tumor-associated macrophages can release growth factors, cytokines and inflammatory mediators that may facilitate cancer cell invasion, migration, angiogenesis, tumor progression or metastasis (35–37). Furthermore, systemic depletion (38) or inhibition (39) of tumor associated myeloid cells migration into the tumor has shown to significantly reduce tumor growth. In light of our findings, it would be of great interest to determine the precise role of myeloid cells in mTNFa-mediated tumor growth.
Our study revealed that tumor cell expression of the membrane isoform of TNFα resulted in tumor associated myeloid cell death through increased ROS production. It has been shown that TNFα has the ability to induce necrotic cell death by utilizing death domain-containing adaptor proteins such as RIP1, TRADD and FADD upon TNFR activation. Once recruited to the TNFR death domain further downstream events lead to ROS generation and cell death (40–42). Multiple pathways have been shown to lead to ROS generation upon TNFR activation (24, 43). Necrotic cell death induced by TNFR has been associated with generation of ROS derived from either mitochondrial or non-mitochondrial sources (43, 44). Mitochondrial complex I-mediated generation of ROS has been linked to direct activation by TNFR and ceramide mediated activation (40, 41). In a study by Kim et al., TNFR was reported as an activator of Nox1 NADPH oxidase complex in a TRADD-and RIP1-dependent recruitment (45). Our findings suggest that the membrane form of TNFα is very efficient at stimulating ROS generation and initiating necrotic cell death. This could be due to the ability of mTNF to recruit death domain-containing adaptor proteins more efficiently or mTNFα activates a pathway that is more efficient in ROS generation. The mechanistic pathway(s) which leads to mTNFα induced ROS generation requires further investigation.
To our knowledge there has not been any study evaluating the relative expression of soluble and membrane TNFα during tumor progression in human cancer, including analyses designed to determine if there is any correlation between the level of sTNFα versus mTNFα and the cancer outcome. Here, in this study our in vivo tissue array staining and in vitro assessment of soluble and membrane TNFα expression in human NSCLC cell lines showed that the ratio of soluble to membrane TNFα varies among different tumor cell types. Furthermore, we verified this by immunofluorescence staining of tumor section for TNFα. The fact that mTNFα is present in tumor and at different levels and subcellular localization may provides important clues to divergent outcomes seen in TNFα positive tumor phenotype seen in different patients.
As it previously described, in order to generate sTNFα, the membrane associated TNFα is cleaved through proteolytic activity of TACE. Although it has been suggested that other proteinases are capable of TNFα cleavage it has been shown that TACE has the highest affinity for TNFα ectodomain shedding among the other known substrate (27). Level of TACE present on the surface of the membrane has been inversely correlated with the level of membrane associated TNFα and inhibition of TACE by MMP inhibitors has demonstrated a transient increase in mTNFα surface expression (27, 46). These studies suggest that the regulation of TACE activity and subsequent alteration of the sTNFα to mTNFα ratio could have a great impact on tumor growth. The association between higher TACE and higher TNFα gene expression in NSCLC and decreased survival further confirms the importance of different TNFα isoforms availability on tumor regulation.
Recently several Phase I/II clinical trials have been undertaken with TNFα antagonists in cancer patients (47–49). In these clinical trials, TNFα antagonist treatment resulted in a period of disease stabilization in only 20% of the patients with advanced cancer. It is suggested that in order to take this forward, we need to identify those patients who are most likely to benefit from TNFα antagonist treatment. Perhaps determining the predominant form of TNFα expressed by tumor in these patients would be beneficial for a more effective treatment with TNFα inhibitors which can block both soluble and membrane isoforms.
In summary, we demonstrate that TNFα membrane versus soluble isoforms have opposing effects on cancer growth. Expression of both forms of TNFα in NSCLCs indicates that this finding is relevant to human malignancies and that isoform analysis should be applied to identify candidates for which anti-TNFα agents are likely to be beneficial versus detrimental.
Supplementary Material
Acknowledgments
Financial support: This work was supported by funding from the NIH (R01-HL088424) to PPY, Veterans Affairs Merit Award to PPY, Vanderbilt University CTSA Grant (UL1 RR024975-01 from NCRR/NIH) to PPY, SPORE in lung cancer (NCI CA 90949) to PPM, and IBVS training grant (5 T32 HL069765) to SA.
GRANT SUPPORT
This work was supported by funding from the NIH (R01-HL088424) to PPY, Veterans Affairs Merit Award to PPY, Vanderbilt University CTSA Grant (UL1 RR024975-01 from NCRR/NIH) to PPY, SPORE in lung cancer (NCI CA 90949) to PPM, and IBVS training grant (5 T32 HL069765) to SA.
Footnotes
Conflict of Interest: The authors declare no conflict of interest.
References
- 1.Bozcuk H, Uslu G, Samur M, et al. Tumour necrosis factor-alpha, interleukin-6, and fasting serum insulin correlate with clinical outcome in metastatic breast cancer patients treated with chemotherapy. Cytokine. 2004;27:58–65. doi: 10.1016/j.cyto.2004.04.002. [DOI] [PubMed] [Google Scholar]
- 2.Ferrajoli A, Keating MJ, Manshouri T, et al. The clinical significance of tumor necrosis factor-alpha plasma level in patients having chronic lymphocytic leukemia. Blood. 2002;100:1215–9. [PubMed] [Google Scholar]
- 3.Michalaki V, Syrigos K, Charles P, Waxman J. Serum levels of IL-6 and TNF-alpha correlate with clinicopathological features and patient survival in patients with prostate cancer. Br J Cancer. 2004;90:2312–6. doi: 10.1038/sj.bjc.6601814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pfitzenmaier J, Vessella R, Higano CS, Noteboom JL, Wallace D, Jr, Corey E. Elevation of cytokine levels in cachectic patients with prostate carcinoma. Cancer. 2003;97:1211–6. doi: 10.1002/cncr.11178. [DOI] [PubMed] [Google Scholar]
- 5.Yoshida N, Ikemoto S, Narita K, et al. Interleukin-6, tumour necrosis factor alpha and interleukin-1beta in patients with renal cell carcinoma. Br J Cancer. 2002;86:1396–400. doi: 10.1038/sj.bjc.6600257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Balkwill F. TNF-alpha in promotion and progression of cancer. Cancer Metastasis Rev. 2006;25:409–16. doi: 10.1007/s10555-006-9005-3. [DOI] [PubMed] [Google Scholar]
- 7.Balkwill F, Charles KA, Mantovani A. Smoldering and polarized inflammation in the initiation and promotion of malignant disease. Cancer Cell. 2005;7:211–7. doi: 10.1016/j.ccr.2005.02.013. [DOI] [PubMed] [Google Scholar]
- 8.Li B, Vincent A, Cates J, Brantley-Sieders DM, Polk DB, Young PP. Low levels of tumor necrosis factor alpha increase tumor growth by inducing an endothelial phenotype of monocytes recruited to the tumor site. Cancer Res. 2009;69:338–48. doi: 10.1158/0008-5472.CAN-08-1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chakravarty EF, Michaud K, Wolfe F. Skin cancer, rheumatoid arthritis, and tumor necrosis factor inhibitors. J Rheumatol. 2005;32:2130–5. [PubMed] [Google Scholar]
- 10.Brown SL, Greene MH, Gershon SK, Edwards ET, Braun MM. Tumor necrosis factor antagonist therapy and lymphoma development: twenty-six cases reported to the Food and Drug Administration. Arthritis Rheum. 2002;46:3151–8. doi: 10.1002/art.10679. [DOI] [PubMed] [Google Scholar]
- 11.Larkin JM, Ferguson TR, Pickering LM, et al. A phase I/II trial of sorafenib and infliximab in advanced renal cell carcinoma. Br J Cancer. 103:1149–53. doi: 10.1038/sj.bjc.6605889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kriegler M, Perez C, DeFay K, Albert I, Lu SD. A novel form of TNF/cachectin is a cell surface cytotoxic transmembrane protein: ramifications for the complex physiology of TNF. Cell. 1988;53:45–53. doi: 10.1016/0092-8674(88)90486-2. [DOI] [PubMed] [Google Scholar]
- 13.Diwan A, Dibbs Z, Nemoto S, et al. Targeted overexpression of noncleavable and secreted forms of tumor necrosis factor provokes disparate cardiac phenotypes. Circulation. 2004;109:262–8. doi: 10.1161/01.CIR.0000109642.27985.FA. [DOI] [PubMed] [Google Scholar]
- 14.Dibbs ZI, Diwan A, Nemoto S, et al. Targeted overexpression of transmembrane tumor necrosis factor provokes a concentric cardiac hypertrophic phenotype. Circulation. 2003;108:1002–8. doi: 10.1161/01.CIR.0000085203.46621.F4. [DOI] [PubMed] [Google Scholar]
- 15.Parry SL, Sebbag M, Feldmann M, Brennan FM. Contact with T cells modulates monocyte IL-10 production: role of T cell membrane TNF-alpha. J Immunol. 1997;158:3673–81. [PubMed] [Google Scholar]
- 16.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012;62:10–29. doi: 10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]
- 17.Shedden K, Taylor JM, Enkemann SA, et al. Gene expression-based survival prediction in lung adenocarcinoma: a multi-site, blinded validation study. Nat Med. 2008;14:822–7. doi: 10.1038/nm.1790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.de Jong JP, Voerman JS, van der Sluijs-Gelling AJ, Willemsen R, Ploemacher RE. A monoclonal antibody (ER-HR3) against murine macrophages. I. Ontogeny, distribution and enzyme histochemical characterization of ER-HR3-positive cells. Cell Tissue Res. 1994;275:567–76. doi: 10.1007/BF00318825. [DOI] [PubMed] [Google Scholar]
- 19.de Jong AL, Green DM, Trial JA, Birdsall HH. Focal effects of mononuclear leukocyte transendothelial migration: TNF-alpha production by migrating monocytes promotes subsequent migration of lymphocytes. J Leukoc Biol. 1996;60:129–36. doi: 10.1002/jlb.60.1.129. [DOI] [PubMed] [Google Scholar]
- 20.Torrente Y, El Fahime E, Caron NJ, et al. Tumor necrosis factor-alpha (TNF-alpha) stimulates chemotactic response in mouse myogenic cells. Cell Transplant. 2003;12:91–100. doi: 10.3727/000000003783985115. [DOI] [PubMed] [Google Scholar]
- 21.Senftleben U, Karin M. The IKK/NF-kappaB pathway. Crit Care Med. 2002;30:S18–S26. [PubMed] [Google Scholar]
- 22.Karin M, Lin A. NF-kappaB at the crossroads of life and death. Nat Immunol. 2002;3:221–7. doi: 10.1038/ni0302-221. [DOI] [PubMed] [Google Scholar]
- 23.Gupta S. A decision between life and death during TNF-alpha-induced signaling. J Clin Immunol. 2002;22:185–94. doi: 10.1023/a:1016089607548. [DOI] [PubMed] [Google Scholar]
- 24.Corda S, Laplace C, Vicaut E, Duranteau J. Rapid reactive oxygen species production by mitochondria in endothelial cells exposed to tumor necrosis factor-alpha is mediated by ceramide. Am J Respir Cell Mol Biol. 2001;24:762–8. doi: 10.1165/ajrcmb.24.6.4228. [DOI] [PubMed] [Google Scholar]
- 25.Deshpande SS, Angkeow P, Huang J, Ozaki M, Irani K. Rac1 inhibits TNF-alpha-induced endothelial cell apoptosis: dual regulation by reactive oxygen species. FASEB J. 2000;14:1705–14. doi: 10.1096/fj.99-0910com. [DOI] [PubMed] [Google Scholar]
- 26.Kim JH, Lee BC, Sim GS, et al. The isolation and antioxidative effects of vitexin from Acer palmatum. Arch Pharm Res. 2005;28:195–202. doi: 10.1007/BF02977715. [DOI] [PubMed] [Google Scholar]
- 27.Armstrong L, Godinho SI, Uppington KM, Whittington HA, Millar AB. Contribution of TNF-alpha converting enzyme and proteinase-3 to TNF-alpha processing in human alveolar macrophages. Am J Respir Cell Mol Biol. 2006;34:219–25. doi: 10.1165/rcmb.2005-0087OC. [DOI] [PubMed] [Google Scholar]
- 28.Moore RJ, Owens DM, Stamp G, et al. Mice deficient in tumor necrosis factor-alpha are resistant to skin carcinogenesis. Nat Med. 1999;5:828–31. doi: 10.1038/10552. [DOI] [PubMed] [Google Scholar]
- 29.Arnott CH, Scott KA, Moore RJ, Robinson SC, Thompson RG, Balkwill FR. Expression of both TNF-alpha receptor subtypes is essential for optimal skin tumour development. Oncogene. 2004;23:1902–10. doi: 10.1038/sj.onc.1207317. [DOI] [PubMed] [Google Scholar]
- 30.Naylor MS, Stamp GW, Foulkes WD, Eccles D, Balkwill FR. Tumor necrosis factor and its receptors in human ovarian cancer. Potential role in disease progression. J Clin Invest. 1993;91:2194–206. doi: 10.1172/JCI116446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Boldrini L, Calcinai A, Samaritani E, et al. Tumour necrosis factor-alpha and transforming growth factor-beta are significantly associated with better prognosis in non-small cell lung carcinoma: putative relation with BCL-2-mediated neovascularization. Br J Cancer. 2000;83:480–6. doi: 10.1054/bjoc.2000.1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.LAOR, Tai L, Lee L, et al. Membrane-bound Fas ligand only is essential for Fas-induced apoptosis. Nature. 2009;461:659–63. doi: 10.1038/nature08402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.De Palma M, Venneri MA, Galli R, et al. Tie2 identifies a hematopoietic lineage of proangiogenic monocytes required for tumor vessel formation and a mesenchymal population of pericyte progenitors. Cancer Cell. 2005;8:211–26. doi: 10.1016/j.ccr.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 34.Shojaei F, Zhong C, Wu X, Yu L, Ferrara N. Role of myeloid cells in tumor angiogenesis and growth. Trends Cell Biol. 2008;18:372–8. doi: 10.1016/j.tcb.2008.06.003. [DOI] [PubMed] [Google Scholar]
- 35.Condeelis J, Pollard JW. Macrophages: obligate partners for tumor cell migration, invasion, and metastasis. Cell. 2006;124:263–6. doi: 10.1016/j.cell.2006.01.007. [DOI] [PubMed] [Google Scholar]
- 36.White ES, Strom SR, Wys NL, Arenberg DA. Non-small cell lung cancer cells induce monocytes to increase expression of angiogenic activity. J Immunol. 2001;166:7549–55. doi: 10.4049/jimmunol.166.12.7549. [DOI] [PubMed] [Google Scholar]
- 37.Wang FQ, So J, Reierstad S, Fishman DA. Matrilysin (MMP-7) promotes invasion of ovarian cancer cells by activation of progelatinase. Int J Cancer. 2005;114:19–31. doi: 10.1002/ijc.20697. [DOI] [PubMed] [Google Scholar]
- 38.Zeisberger SM, Odermatt B, Marty C, Zehnder-Fjallman AHM, Ballmer-Hofer K, Schwendener RA. Clodronate-liposome-mediated depletion of tumour-associated macrophages: a new and highly effective antiangiogenic therapy approach. British Journal of Cancer. 2006;95:272–81. doi: 10.1038/sj.bjc.6603240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Allavena P, Signorelli M, Chieppa M, et al. Anti-inflammatory properties of the novel antitumor agent yondelis (Trabectedin): Inhibition of macrophage differentiation and cytokine production. Cancer Research. 2005;65:2964–71. doi: 10.1158/0008-5472.CAN-04-4037. [DOI] [PubMed] [Google Scholar]
- 40.Festjens N, Vanden Berghe T, Vandenabeele P. Necrosis, a well-orchestrated form of cell demise: signalling cascades, important mediators and concomitant immune response. Biochim Biophys Acta. 2006;1757:1371–87. doi: 10.1016/j.bbabio.2006.06.014. [DOI] [PubMed] [Google Scholar]
- 41.Morgan MJ, Kim YS, Liu ZG. TNFalpha and reactive oxygen species in necrotic cell death. Cell Res. 2008;18:343–9. doi: 10.1038/cr.2008.31. [DOI] [PubMed] [Google Scholar]
- 42.Lin Y, Choksi S, Shen HM, et al. Tumor necrosis factor-induced nonapoptotic cell death requires receptor-interacting protein-mediated cellular reactive oxygen species accumulation. J Biol Chem. 2004;279:10822–8. doi: 10.1074/jbc.M313141200. [DOI] [PubMed] [Google Scholar]
- 43.Vanden Berghe T, Declercq W, Vandenabeele P. NADPH oxidases: new players in TNF-induced necrotic cell death. Mol Cell. 2007;26:769–71. doi: 10.1016/j.molcel.2007.06.002. [DOI] [PubMed] [Google Scholar]
- 44.Schwandner R, Wiegmann K, Bernardo K, Kreder D, Kronke M. TNF receptor death domain-associated proteins TRADD and FADD signal activation of acid sphingomyelinase. J Biol Chem. 1998;273:5916–22. doi: 10.1074/jbc.273.10.5916. [DOI] [PubMed] [Google Scholar]
- 45.Kim S, Takahashi H, Lin W-W, et al. Carcinoma-produced factors activate myeloid cells through TLR2 to stimulate metastasis. Nature. 2009;457:102–6. doi: 10.1038/nature07623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Solomon KA, Covington MB, DeCicco CP, Newton RC. The fate of pro-TNF-alpha following inhibition of metalloprotease-dependent processing to soluble TNF-alpha in human monocytes. J Immunol. 1997;159:4524–31. [PubMed] [Google Scholar]
- 47.Brown ER, Charles KA, Hoare SA, et al. A clinical study assessing the tolerability and biological effects of infliximab, a TNF-alpha inhibitor, in patients with advanced cancer. Ann Oncol. 2008;19:1340–6. doi: 10.1093/annonc/mdn054. [DOI] [PubMed] [Google Scholar]
- 48.Harrison ML, Obermueller E, Maisey NR, et al. Tumor necrosis factor alpha as a new target for renal cell carcinoma: two sequential phase II trials of infliximab at standard and high dose. J Clin Oncol. 2007;25:4542–9. doi: 10.1200/JCO.2007.11.2136. [DOI] [PubMed] [Google Scholar]
- 49.Madhusudan S, Foster M, Muthuramalingam SR, et al. A phase II study of etanercept (Enbrel), a tumor necrosis factor alpha inhibitor in patients with metastatic breast cancer. Clin Cancer Res. 2004;10:6528–34. doi: 10.1158/1078-0432.CCR-04-0730. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.