Abstract
Objective
Gadolinium enhanced coronary magnetic resonance angiography (MRA) at 3 Tesla appears to be superior to non-contrast methods. Gadofosveset is an intravascular contrast agent that may be well suited to this application. The purpose of this study was to perform an intra-individual comparison of gadofosveset and gadobenate for coronary MRA at 3 Tesla.
Materials and Methods
In this prospective randomized study, 22 study subjects [8 (36%) male; 27.9 ± 6 years; BMI = 22.8 ± 2 Kg/m2] underwent two studies using a contrast-enhanced inversion recovery three-dimensional fast low angle shot MRA at 3 Tesla. The order of contrast agent administration was varied randomly, separated by an average of 30 ± 5 days, using either gadobenate dimeglumine (Gd-BOPTA; Bracco, 0.1 mmol/Kg) or gadofosveset trisodium (MS-325; Lantheus Med, 0.03 mmol/Kg). Acquisition time, signal-to-noise ratio (SNR) of coronary vessels and contrast-to-noise ratio (CNR) were evaluated.
Results
Of 308 coronary arteries and veins segment analyzed, overall SNR of coronary arteries and veins segments were not different for the two contrast agents (132 ± 79 for gadofosveset vs 135 ± 78 for gadobenate, p=0.69). Coronary artery CNR was greater for gadofosveset in comparison to gadobenate (73.5 ± 46.9vs. 59.3 ± 75.7 respectively, p=0.03). Gadofosveset-enhanced MRA images displayed better image quality than gadobenate-enhanced MRA images (2.77 ± 0.61 for gadofosveset vs. 2.11 ± 0.51, P<.001). Inter- and intra-reader variability was excellent (ICC > 0.90) for both contrast agents.
Conclusion
Gadofosveset trisodium appears to show slightly better performance for coronary MRA at 3T compared to gadobenate.
Keywords: Gadofosveset trisodium, gadobenate dimeglumine, coronary MRA
INTRODUCTION
Coronary artery disease (CAD) is a leading cause of morbidity and mortality in the United States [1-3]. Coronary magnetic resonance angiography (MRA) offers a safer solution than computed tomography (CT) due to lack of radiation [4-6]. Theoretically, use of 3 Tesla scanners may result in doubling of the signal-to-noise (SNR) ratio in comparison to 1.5T, leading to enhanced spatial resolution and reduced imaging time [7-10]. However, multiple challenges exist when transferring the established techniques at 1.5T to 3.0T. For example, the steady-state free precession (SSFP) technique commonly used for 1.5T coronary MRA is associated with multiple off-resonance artifacts at 3.0T [11]. Currently, gadolinium-enhanced spoiled gradient-echo imaging is preferred to non-contrast SSFP methods at 3.0T due to reduced sensitivity from field inhomogeneity, diminished radiofrequency (rf) offset from tissue susceptibility variation, and lower specific absorption rates [10, 12]. Additionally, shorter repetition time (TR), parallel imaging, and contrast agents further improve spatial resolution, reduce imaging time and motion-related artifacts, and enhance SNR and CNR for qualitative and quantitative assessment for coronary MRA at 3.0T [8, 9, 13, 14].
Whole-heart coronary MRA usually requires prolonged acquisition time of many minutes due to navigator inefficiency. Thus, an intravascular contrast agent may have a advantage for this application compared to traditional extravascular agents. Dimeglumine gadobenate has high relaxivity due to a low level of albumin binding [11, 15, 16]. Gadofosveset trisodium is considered an “intravascular” contrast agent due to transient, reversible, and noncovalent binding to serum albumin [15-17]. The primary clinical application of gadofosveset to date has been for aortic magnetic resonance angiography (MRA) [18-20]. A recent study has used this intravascular contrast agent to better visualize coronary arterial segments [7]. The purpose of this study was to compare acquisition time, SNR, CNR, and image quality, for both coronary arteries and veins, using standard clinical doses of gadofosveset trisodium and gadobenate dimeglumine for whole-heart coronary MRA at 3.0T.
MATERIALS AND METHODS
Study population
This study was approved by our institutional review board. All study participants provided written informed consent and completed two CMR studies. From October 2011 to February 2012, 26 normal volunteers were enrolled. The inclusion criteria for study subjects were free of cardiovascular symptoms, no history of infection within 4 weeks before the CMR exam, no history of clinical cardiovascular risk factors including diabetes, smoking, hypertension, hyperlipidemia and cerebrovascular or peripheral arterial disease. The exclusion criteria were: contraindication to CMR studies such as claustrophobia, presence of metallic implants, inability to follow instructions for breath holding and presence of scar on late gadolinium enhancement (LGE) imaging.
Hematocrit, serum creatinine, and estimated glomerular filtration rate (eGFR) were obtained 24 hours before CMR studies. In addition, height, weight, heart rate, systolic and diastolic blood pressures (SBP and DBP), and contrast dose were also recorded.
CMR protocol
All studies were done in two separate exam sessions using a 3.0T scanner (Verio, Siemens Medical Solutions, Erlangen, Germany) and a 32-channel cardiac array coil (In Vivo, Orlando, FL). Whole-heart contrast MRA was performed with a previously reported respiratory-gated, electrocardiography-triggered, fat saturated, segmented 3D FLASH sequence [9]. Briefly, the navigator acceptance window was set up for ±3 mm, and sixty-four slices were acquired and interpolated to 128 slices. Parallel imaging (GRAPPA) was used in the phase-encoding direction with an acceleration factor of 2. The additional scan parameters were: TR/TE: 3.0/1.4 ms; TI: 300 ms; flip angle: 20°; bandwidth: 710 Hz/pixel; and voxel size: 1.1 × 1.1 × 1.3 mm3 interpolated to 0.55 × 0.55 × 0.65 mm3. The order of contrast administration was varied randomly, and either gadobenate dimeglumine (0.1 mmol/kg, Bracco Diagnostic Inc., Princeton, NJ, USA) or gadofosveset trisodium (0.03 mmol/kg, Lantheus Medical Imaging, North Billerica, MA, USA) was injected followed by a 20 ml saline bolus administered at a mean flow rate of 0.66 mL/s for both contrast agents. All study subjects underwent routine steady-state free precession cine-MR and phase sensitive inversion recovery LGE imaging after the MRA acquisition. Inversion times were individually adjusted to suppress normal myocardium. After a mean interval of 30 ± 5 days, the same study participants underwent the identical MR protocol with the second contrast agent.
Quantitative Analysis
One blinded observer (--) did the quantitative analysis of CMR data, while a second observer (--) performed reproducibility studies. Signal-to-noise ratio (SNR), contrast-to-noise ratio (CNR), and acquisition time were compared for each technique. Images were transferred via PACS to a 3D model-based software (Vitrea, Vital Images, Minnetonka, Minnesota), which was used to analyze all the 3D coronary artery images.
Both SNR and CNR values were derived from the raw images in the axial view and were calculated as follows:
SNR was determined for the blood within the coronary arterial and venous segments as well as the epicardial fat and myocardium. SNR was defined as the mean signal intensity of the coronary blood divided by the standard deviation of the air, or the background signal intensity (equation 1). Similarly, the CNR was calculated between the coronary arterial and venous segments blood and the surrounding myocardial or epicardial fat tissue [7]. CNR was defined as the mean value of the difference between the signal intensity of the coronary blood and the surrounding myocardium or fat tissue divided by the standard deviation of the background signal intensity (equations 2 and 3).
Circular regions of interest (ROI, area: 1.3-1.5 mm2) for the measurement of signal intensity were taken of the vessel lumen from the proximal segments of the following coronary arterial segments: left main stem (LMS), left anterior descending (LAD), left circumflex (LCX), and the right coronary artery (RCA). Similarly, ROI measurements (area: 1.3 mm2 to 1.5 mm2) were taken of the coronary veins at the proximal segments of the inferior cardiac vein and the coronary sinus and the distal segment of the great cardiac vein. Myocardium signal intensity (SImyo), used for CNRmyocardium calculations (equation 2), was measured in the interventricular septum at an ROI (area 1.5 mm2) located 25.0 mm from the center of the heart at the base of the interventricular groove. The epicardial fat signal intensity (SIfat), used for CNRfat calculations (equation 3), was measured at an ROI (area 1.4mm2) adjacent to the mid-segment of the RCA.
Blood pool signal intensity was measured by taking a large ROI (area approximately 500 mm2) within the left ventricular (LV) cavity. The mean standard deviation of the background noise (SDair) used for both SNR and CNR calculations was determined by taking a large ROI (area in the range from 50 to 100cm2) of the air outside of the chest wall and determining the mean signal intensity. The size of the ROI used to measure the standard deviation of the background noise varied depending on each specific case to avoid areas of phase ghosting. Thus, the most accurate standard deviation of the background signal intensity was measured. Ten out of the 22 images were analyzed independently by a second observer (--) for inter-observer reproducibility. In addition to SNR and CNR measurements, mean acquisition time was compared for all scans for the two contrast agents.
Qualitative Analysis
Two independent and blinded readers who were not involved in the MRI examinations conducted the qualitative analysis of the individual coronary arteries and the overall MRA image quality. The quality score of the LMS and the proximal, mid, and distal segments of the LAD, LCx, and RCA as well as the overall MRA image were evaluated according to the model of the American College of Cardiology/American Heart Association [18]. The following five-point scale was used: 0, not interpretable; 1, poor (severe artifacts); 2, good (mild to moderate artifacts); 3, very good (minimum to mild artifacts); 4, excellent (minimum or no artifacts). The overall image quality took into account both the quality score assigned to the individual vessels as well as general background artifacts.
Statistical Analysis
Statistical analysis was performed using MedCalc®, version 12.2.1 (MedCalc Software, Mariakerke, Belgium) and Excel plug-in (Daniel’s XL Toolbox, version 4.01, Boston, MA, USA). SNR, CNR, quality score, and acquisition time measurements were represented by their mean ± SD. All of these measurements were compared using a two-tailed Paired Student’s t-test where p-value of less than 0.05 indicated a significance difference between the contrast agents. For the quantitative measurements, all 22 subjects were analyzed twice by the first observer to assess intra-observer variability. For 45% (n=10) of randomly chosen subjects, quantitative measurements were performed by a second independent reader to assess inter-observer variability. Intra- and inter-observer agreement were displayed using intraclass correlation coefficient (ICC) with two-way random model (ICC<0.40 = poor; ICC≥0.40 to 0.75 = fair to good; ICC>0.75 = excellent agreement). Bland-Altman plots were used to describe the difference of CNR and SNR values between these repeated observations for each contrast agent. Additionally, for the qualitative assessment, a paired-sample Wilcoxon signed-rank test was used to evaluate the image quality comparison results between the two gadolinium chelates for each individual observer where an outcome of 0.05 or less indicated statistical significance.
RESULTS
Twenty-six individuals met inclusion criteria and were enrolled. Four participants were excluded: two had severely impaired image quality due to MRI scan artifacts and gating difficulty, one did not return for the second scan, and one was claustrophobic. Two had nausea after contrast administration, one with gadobenate dimeglumine and the other one with gadofosveset trisodium.
A total of 22 subjects (8 males; with 27.9 ± 6.7 years old) were included for analysis. The average total CMR study duration was 39 ± 6 minutes. The average acquisition time for the IR 3D FLASH sequence was 15 minutes for gadofosveset and 13 minutes for gadobenate studies. The average navigator efficiency was 33%. All volunteers had normal cardiac function with no history of heart disease. None of the volunteers showed myocardial scar on delayed enhancement images. There was no significant difference between clinical and imaging parameters between both study visits. However, the heart rates for gadofosveset studies were lower than for gadobenate (63 ± 10 bpm vs 69 ± 14 bpm, p=0.03). Participant characteristics are summarized in Table 1.
Table 1.
Participant characteristics
| Gadofosveset trisodium (exam 1) |
Gadobenate Dimeglumine (exam 2) |
p value* |
|
|---|---|---|---|
| Demographics | |||
| Age | 27.9±6.7 | 27.9±6.7 | N/A |
| Male | 8 (36) | 8 (36) | N/A |
| Height (cm) | 169.5±8.1 | 169.5±8.1 | N/A |
| Weight (kg) | 65.7±9.5 | 65.1±10.0 | 0.13 |
| Body mass index (Kg/m2) | 22.8±2.0 | 22.6±2.1 | 0.09 |
| Hematocrit (%) | 42.6±3.5 | 42.1±3.7 | 0.45 |
| Creatine (mg/dL) | 0.8±0.2 | 0.8±0.1 | 0.63 |
| eGFR (mL/min/1.73 m2) | 97.7±16.5 | 97.7±16.6 | 1.00 |
| Heart rate (bpm) | 63.1±10.4 | 69.7±14.4 | 0.03 |
| Systolic blood pressure (mmHg) | 121.6±12.5 | 125.2±12.8 | 0.18 |
| Diastolic blood pressure (mmHg) | 72.7±6.7 | 72.5±7.8 | 0.90 |
| LV Systolic function by CMRI | |||
| EDV (ml) | 131.4±29.6 | 132.8±24.5 | 0.50 |
| ESV (ml) | 45.6±13.6 | 45.2±13.1 | 0.73 |
| EF (%) | 65.4±5.0 | 66.4±5.7 | 0.19 |
| Mass (g) | 126.3±32.3 | 124.7±31.2 | 0.27 |
| Contrast administration | |||
| Dose (mL) | 7.9±1.1 | 13.1±2.2 | <0.001 |
| LGE by CMRI | |||
| Negative | 0 (0) | 0 (0) | N/A |
Note: p value, comparison between exam 1 vs exam 2. Mean and standard deviations or number and percentages as appropriate. LV, left ventricular; EDV, end-diastolic volume; ESV, endsystolic volume; EF, ejection fraction; LGE, late gadolinium enhancement; and N/A, not applicable.
SNR and CNR Measurements
A total of 308 coronary arterial and venous ROI measurements were obtained were obtained from the 22 healthy subjects, in addition to the 126 myocardial, epicardial fat, and background noise ROI measurements needed for SNR and CNR calculations.
The mean SNR values of both contrast agents are summarized in Table 2. Overall SNR was similar for both agents (132 ± 75 for gadofosveset vs. 136 ± 74 for gadobenate, p=0.97). Additionally, none of the individual arterial or venous segments displayed significant differences in SNR between contrast agents. Overall arterial and venous SNR values were not significantly different between contrast agents (i.e. 131.9 ± 74.5 for gadofosveset vs 135.8 ± 73.6 for gadobenate, p=.97. However, myocardial signal intensity values were significantly lower for gadofosveset compared to gadobenate (46.0 ± 41.7 and 64.0 ± 35.9, respectively with p=.043). However, no significant difference existed for epicardial fat between the two contrast agents (p=.48).
Table 2.
Mean signal to noise ratio (SNR) values of both contrast agents: Gadofosveset trisodium vs Gadobenate Dimeglumine
| Arterial segments | Gadofosveset trisodium |
Gadobenate Dimeglumine |
p-value |
|---|---|---|---|
| LMS | 126.5 ± 69.0 | 125.3 ± 69.4 | .95 |
| LADprox | 123.0 ± 69.8 | 125.8 ± 67.5 | .90 |
| LCXprox | 126.7 ± 79.3 | 125.8 ± 67.5 | .97 |
| RCAprox | 114.8 ± 71.9 | 112.4 ± 67.6 | 1.0 |
| Overall | 122.8 ± 70.5 | 123.0 ± 67.5 | .99 |
|
| |||
| Vein segments | |||
|
| |||
| Great cardiac | 132.0 ± 84.5 | 130.2 ± 72.3 | .94 |
| Middle cardiac | 143.4 ± 91.7 | 160.8 ± 99.4 | .59 |
| Coronary sinus | 157.2 ± 85.8 | 167.5 ± 92.3 | .74 |
| Overall | 144.2 ± 80.8 | 152.9 ± 84,8 | .94 |
|
| |||
| Others | |||
|
| |||
| Fat | 24.6 ± 17.2 | 28.1 ± 21.0 | .48 |
| Myocardium | 46.0 ± 41.7 | 64.0 ± 35.9 | .043 |
| Blood Pool | 124.4 ± 66.5 | 154.3 ± 80.1 | .070 |
| Overall (all vessels) | 131.9 ± 74.5 | 135.8 ± 73.6 | .97 |
Note: Data is presented as mean ± standard deviation. LMS, left main stem; LAD, left anterior descending coronary artery; LCX, left circumflex coronary artery; and RCA, right coronary artery. Prox indicates proximal coronary segment.
The mean CNR values of both contrast agents are summarized in Table 3. CNR between coronary arteries and myocardium was greater for gadofosveset in comparison to gadobenate; (73.5 ± 44.2 vs. 59.3 ± 40.1 respectively, p=.28). CNR between coronary veins and myocardium showed similar, non-significant differences (94.9 ± 49.8 vs. 89.0 ± 55.9, respectively, p=0. .73). Table 4 indicates the inter- and intra-observer variability of both contrast agents. For both gadofosveset and gadobenate, an excellent interobserver correlation was shown (ICC: 0.91 to 0.96 for gadofosveset and 0.90 to 0.96 for gadobenate). Intra-reader variability also had excellent correlation.
Table 3.
Mean contrast to noise ratio (CNR) values for Gadofosveset trisodium and Gadobenate Dimeglumine.
| CNRCOR-FAT |
CNRCOR-MYO |
|||||
|---|---|---|---|---|---|---|
| Arterial segments |
Gadofosveset trisodium |
Gadobenate Dimeglumine |
P value |
Gadofosveset trisodium |
Gadobenate Dimeglumine |
P value |
| LMS | 100.1 ± 58.7 | 98.2 ± 59.8 | .93 | 77.2 ± 43.4 | 61.4 ± 42.9 | .24 |
| LADprox | 96.6 ± 59.8 | 98.9 ± 64.5 | .91 | 73.7 ± 46.2 | 62.7 ± 41.1 | .38 |
| LCXprox | 100.3 ± 69.1 | 98.7 ± 56.9 | .94 | 77.4 ± 52.7 | 61.9 ± 42.2 | .29 |
| RCAprox | 88.4 ± 61.3 | 87.9 ± 61.5 | .98 | 65.5 ± 47.1 | 51.0 ± 46.5 | .32 |
| Overall | 96.4 ± 59.9 | 95.9 ± 61.6 | .73 | 73.5 ± 44.2 | 59.3 ± 40.1 | .28 |
|
| ||||||
|
Vein segments
| ||||||
| Great cardiac | 105.6 ± 73.1 | 103.1 ± 62.7 | .91 | 82.7 ± 64.4 | 66.3 ± 46.1 | .30 |
| Middle cardiac | 117.0 ± 80.9 | 133.7 ± 87.6 | .57 | 94.1 ± 61.0 | 96.9 ± 69.5 | .89 |
| Sinus | 130.8 ± 80.1 | 140.4 ± 80.2 | .73 | 107.9 ± 53.9 | 103.6 ± 59.6 | .83 |
| Overall | 117.8 ± 70.7 | 125.8 ± 75.1 | .75 | 94.9 ± 49.8 | 89.0 ± 55.9 | .73 |
|
| ||||||
|
All segments
| ||||||
| Overall | 105.5 ± 64.0 | 108.7 ± 64.7 | .73 | 82.6 ± 45.7 | 72.0 ± 45.2 | ..45 |
Note: Data is presented as mean ± standard deviation. CNRCOR-FAT, indicates CNR between coronary blood and myocardium; CNRCOR-MYO, indicates CNR between coronary blood and epicardial fat; LMS, left main stem; LAD, left anterior descending coronary artery; LCX, left circumflex coronary artery; and RCA, right coronary artery. Prox indicates proximal coronary segment.
Table 4.
Inter- and intra-observer variability of both contrast agents: Gadofosveset trisodium vs Gadobenate Dimeglumine
| Gadofosveset trisodium | |||
|---|---|---|---|
| Inter-observer | ICC | Bias | 95% Limits of agreement |
| SNR Coronary arteries | 0.96 | 8.3 | −41.9 to 58.5 |
| SNR Coronary veins | 0.77 | 8.2 | −125.2 to 141.6 |
| CNRCOR-FAT | 0.80 | 15.4 | −86.6 to 117.4 |
| CNRCOR-MYO | 0.72 | 3.5 | −92.5 to 99.6 |
|
| |||
|
Intra-observer
| |||
| SNR Coronary arteries | 0.96 | −4.9 | −43.3 to 33.5 |
| SNR Coronary veins | 0.93 | −6.9 | −68.7 to 55.0 |
| CNRCOR-FAT | 0.95 | −5.0 | −47.0 to 37.1 |
| CNRCOR-MYO | 0.91 | −3.5 | −47.1 to 40.1 |
|
| |||
|
Gadobenate Dimeglumine
| |||
| Inter-observer | ICC | Bias | 95% Limits of agreement |
|
| |||
| SNR Coronary arteries | 0.94 | −6.9 | −48.5 to 34.8 |
| SNR Coronary veins | 0.78 | −14.7 | −121.8 to 92.4 |
| CNRCOR-FAT | 0.72 | −14.3 | −102.6 to 74.0 |
| CNRCOR-MYO | 0.78 | −2.9 | −77.8 to 72.0 |
|
| |||
|
Intra-observer
| |||
| SNR Coronary arteries | 0.92 | −1.7 | −55.1 to 51.7 |
| SNR Coronary veins | 0.96 | −6.7 | −56.2 to 42.8 |
| CNRCOR-FAT | 0.91 | −4.4 | −59.8 to 51.1 |
| CNRCOR-MYO | 0.90 | −0.5 | −45.8 to 44.8 |
Note: ICC, intraclass correlation coefficient, and bias (95% limits of agreement) come from Bland-Altman analysis. SNR, indicates signal to noise ratio. CNRCOR-FAT, indicates contrast to noise ratio between coronary blood and myocardium; CNRCOR-MYO, indicates contrast to noise ratio between coronary blood and epicardial fat;
Image quality
Table 5 shows the results of the overall image quality as well as the arterial quality of the LMS and the proximal, mid, and distal segments of the other coronary arteries: LAD, LCX, and RCA. A total of 420 coronary segments as well as the overall image quality were assessed for each patient for both gadofosveset and gadobenate. Gadofosveset-enhanced MRA images displayed significantly greater image quality than gadobenate-enhanced MRA images (2.77 ± 0.61 for gadofosveset vs. 2.11 ± 0.51, P<.001). All coronary arterial segments except for the distal LAD and LCX showed higher image quality with gadofosveset over gadobenate. Both readers reported improved image quality for gadofosveset over gadobenate for these same arterial segments for both readers (Table 5). For venous segments, image quality was not significantly different between the two contrast agents (p>0.05).
Table 5.
Image quality results of both contrast agents: Gadofosveset trisodium vs Gadobenate Dimeglumine
| Wilcoxon Test |
Mean Image Quality |
||||
|---|---|---|---|---|---|
| Arterial | Gadofosveset | Gadobenate | |||
| Reader 1 | Reader 2 | p-value | |||
| segments | trisodium | Dimeglumine | |||
| LMS | 0.16 | 0.13 | 3.0 ± 0.6 | 2.7 ± 0.5 | .05 |
|
LADprox
LADmid LADdist |
0.013 0.040 0.24 |
0.002 0.020 0.78 |
2.8 ± 0.7 2.4 ± 0.8 1.7 ± 1.0 |
2.1 ± 0.6 1.8 ± 0.6 1.5 ± 0.7 |
<.001 .004 .51 |
|
LCXprox
LCXmid LCXdist |
0.041 0.003 0.063 |
0.41 0.047 0.81 |
2.3 ± 0.9 1.9 ± 0.9 0.8 ± 1.0 |
1.9 ± 0.7 1.3 ± 0.8 0.7 ± 0.8 |
.06 .003 .21 |
|
RCAprox
RCAmid RCAdist |
0.042 0.25 0.035 |
0.010 0.11 0.049 |
3.1 ± 0.6 2.4 ± 1.0 2.3 ± 1.4 |
2.5 ± 0.7 2.0 ± 0.9 1.6 ± 1.0 |
.009 .09 .02 |
|
| |||||
| Overall | 0.003 | 0.009 | 2.8 ± 0.6 | 2.1 ± 0.5 | <.001 |
Note: LMS, left main stem; LAD, left anterior descending coronary artery; LCX, left circumflex coronary artery; and RCA, right coronary artery. Prox indicates proximal coronary segment, mid indicates middle coronary segments, and dist indicates distal coronary segments.
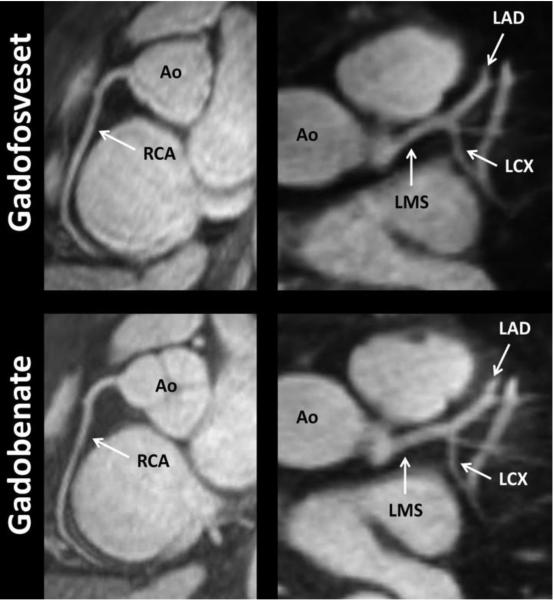
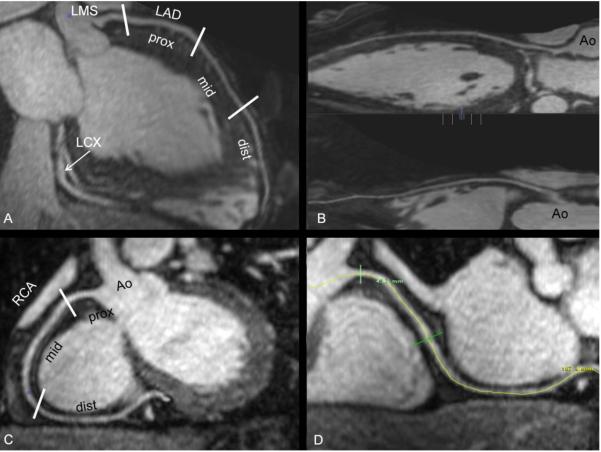
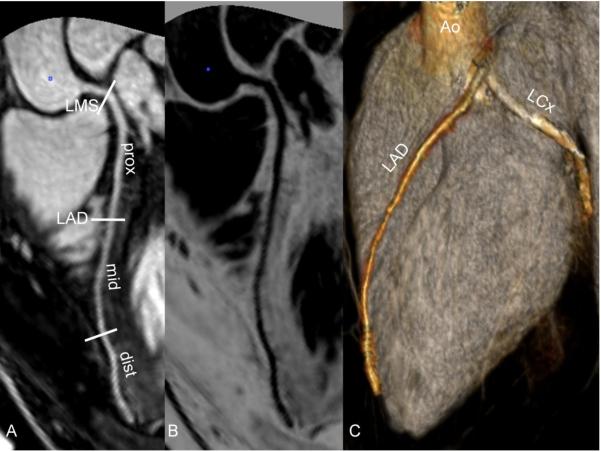
Figure 1 shows representative images from coronary MRA studies using each contrast agents (image quality of 4 for each). The arrows indicate the location of ROIs measured at the proximal sections of the various arterial segments. Figure 2 and Figure 3 show studies with gadofosveset and reformatted images through maximum intensity projection and volume rendering.
Fig. 1.
22-year-old male healthy volunteer. Good quality representation of same individual with both contrast agents. The arrows indicate the general locations of the ROIs that were measured at the proximal sections of the various arterial segments. Note – Ao, Aorta; LMS, left main stem; LAD, left anterior descending coronary artery; LCX, left circumflex coronary artery; and RCA, right coronary artery.
Fig. 2.
27 year-old woman healthy volunteer. Study done with Gadovosveset. (A and C) Segment analysis of LAD and RCA. (B and D) Reformatted images through curve maximum intensity projection (MIP) of LAD and RCA, respectively. Note – Ao, Aorta; LMS, left main stem; LAD, left anterior descending coronary artery; LCX, left circumflex coronary artery; and RCA, right coronary artery. Prox, mid and dist indicates proximal, middle and distal coronary segments, respectively.
Fig. 3.
28 year-old male healthy volunteer. (A) Segment analysis of LAD and RCA. (B) Inverted, “negative”, black and white image similar of (A). (C) Volume-rendered image of LAD and LCx providing nice display of coronary anatomy. Note – LMS, left main stem; LAD, left anterior descending coronary artery; LCX, left circumflex coronary artery; and RCA, right coronary artery. Prox, mid and dist indicates proximal, middle and distal coronary segments, respectively.
DISCUSSION
Gadolinium-enhanced methods are currently preferred for coronary MRA for 3.0T [9]. However, the quantitative and qualitative benefits of using intravascular contrast agents over extracellular chelates have not been studied to our knowledge. This study of coronary MRA at 3.0T was designed as an intra-individual comparison between gadofosveset trisodium, an intravascular contrast agent, and gadobenate dimeglumine, an extracellular chelate. In summary, gadofosveset had superior image quality and CNR compared with gadobenate for coronary MRA at 3T, corroborating with results seen in similar studies [19, 20]. Despite showing improvements, CNR between the coronary arteries and the myocardium was still not significantly greater for gadofosveset over gadobenate.
Unlike non-specific extracellular contrast agents that tend to be rapidly excreted, blood pool contrast agents tend to have prolonged intravascular enhancement, as shown by gadofosveset’s rapid distribution half-life of 0.48 ± 0.11 hours in comparison to gadobenate’s 0.084 ± 0.012 hours [21, 22]. Gadofosveset’s non-covalent binding to albumin not only contributes to slower renal excretion but also increases T1 relaxivity through a pharmacodynamic effect, with relaxivity levels six to 10 times as high as Gd-DTPA [17, 23-25]. However, even though gadofosveset use did demonstrate a 24% increase in CNR between the arteries and myocardium and better image quality than gadobenate, the differences between these two agents were not as large as expected. As CNR differences primarily resulted from lower myocardial enhancement for gadofosveset than gadobenate. This most likely resulted from the inherent intravascular condition of gadofosveset as the albumin-bound chelate is more likely to be retained in the blood pool over the surrounding myocardium. Additionally, differences in image quality between the two agents could have been resulted from significantly higher heart rate for study subjects with gadobenate than gadofosveset (69 vs 63 bpm, respectively), perhaps causing more motion-related artifacts. A beta blocker was not used for this study.
A lower molar dosage of gadofosveset (0.03mmol/kg) was used compared to gadobenate (0.1mmol/kg). These doses were chosen based on labeled clinical doses. This lower molar dose for gadofosveset could have mitigated its effectiveness. For gadofosveset, 79.8 to 87.4% is bound to plasma proteins resulting in high T1 relaxivity. It is important to note is that the fraction of gadofosveset that is bound to albumin is dosage dependent with secondary binding sites having less affinity than the initial sites. However, at the low dose of 0.03 mmol/kg, the percent of bound chelate should theoretically be at the upper end of this spectrum, and thus should not affect T1 relaxivity enhancement [17]. For Gd-BOPTA , there is weak, transient binding to albumin through the chelate’s aromatic ring structure [23, 26]. Thus, Gd-BOPTA displays a two-fold increase in T1 relaxivity compared to Gd-DTPA [27]. Recent studies have shown that even lower doses of Gd-BOPTA (0.05mmol/kg) can perform as well as standard 0.1mmol/kg of Gd-DTPA [27-29]. It is possible that the optimum coronary MRA dose of gadofosveset is closer to that of the dose of gadobenate, but we did not intend to perform a dose ranging study due to safety concerns.
Nephrogenic systemic fibrosis (NSF) has been associated with gadolinium-based contrast agents in patients with renal dysfunction [19, 30]. Metabolic acidosis, caused by disassociation of free Gd3+ ions, is known to be a significant cause of renal failure and NSF [31]. Gadolinium-based contrast agents with lower kinetic stability release more free Gd3+ ions into the bloodstream [22].
These free ions can participate in transmetallation as endogenous metal ions bind to vacancies on the chelate while the Gd3+ ions bind to other physiological substances, such as phosphate, citrate, and carbonate. This is postulated to lead to increased toxicity and fibrosis in the body. Both gadobenate and gadofosveset have relatively small dissociation constants, releasing insignificant amounts of free Gd3+ [32]. Thus, the risk of NSF for both chelates appears to be low compared to several other gadolinium based contrast agents.
Overall acquisition time for this study was reduced through an acceleration factor of 2 with parallel imaging, helping to reduce motion-related artifacts. Parallel imaging relies upon RF detector coil sensitivities to encode simultaneous spatial information to add to the intrinsic MR signal from the gradient pulses [12, 33]. Normally, a correction factor has to be used to adjust for reductions in SNR (and CNR) through the following formula:
In equation 4, “R” represents the acceleration factor and “g” is the geometric factors that results from the noise that may be amplified from the linear combinations used in parallel imaging reconstruction [33]. However, these correction factors were not necessary to be taken into account for this study as both contrast agent groups used identical parameters. Thus, the statistical significance of the differences would not be affected. However, the ROI of the background noise was chosen specifically for each case to avoid areas of phase ghosting. Several limitations could have affected the results. First, as parallel imaging was used in this study, absolute SNR was not achieved. However, results should represent relative SNR measurements and should not affect intraindividual analysis of means. Second, more than a three-fold molar increase in gadolinium injection of gadobenate over gadofosveset was used due to clinical dosage requirements. Equimolar dosages would have tested the true effectiveness of contrast agent; however, gadofosveset showed similar results despite less gadolinium. This should be important for specific clinical scenarios as chronic renal disease and in diabetes population. In addition, significant heart rate differences and different breathing patterns could have affected image quality. A larger sample size could compensate for intra-individual heart rate and breathing variability between scans.
In conclusion, the use of an intravascular contrast agent, gadofosveset performed as well as, or slightly better than, a primarily extravascular contrast, gadobenate dimeglumine, for coronary MRA at 3T. CNR between blood and myocardium was slightly higher for the intravascular contrast agent. Our results show the potential of an intravascular contrast agent to be used for coronary MRA at 3T.
Acknowledgments
This research was supported by the NIH intramural research program. Clinical trial registration information—URL: http://www.clinicaltrials.gov. Unique identifier: NCT01130545 (10-CC-0115).
Footnotes
Funding Sources Funded by the National Institutes of Health (NIH) Intramural program.
Competing interests The authors declare they have no competing interests.
Authors’ contribution Complete text on the cover letter.
REFERENCES
- 1.Banerjee A, Newman DR, Van den Bruel A, Heneghan C. Diagnostic accuracy of exercise stress testing for coronary artery disease: a systematic review and meta-analysis of prospective studies. Int J Clin Pract. 2012;66:477–492. doi: 10.1111/j.1742-1241.2012.02900.x. [DOI] [PubMed] [Google Scholar]
- 2.Shah DJ, Kim HW, Kim RJ. Evaluation of ischemic heart disease. Heart Fail Clin. 2009;5:315–332. v. doi: 10.1016/j.hfc.2009.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lemmert ME, de Vreede-Swagemakers JJ, Eurlings LW, et al. Electrocardiographic predictors of out-of-hospital sudden cardiac arrest in patients with coronary artery disease. Am J Cardiol. 2012;109:1278–1282. doi: 10.1016/j.amjcard.2011.12.020. [DOI] [PubMed] [Google Scholar]
- 4.Rhee CM, Bhan I, Alexander EK, Brunelli SM. Association between iodinated contrast media exposure and incident hyperthyroidism and hypothyroidism. Arch Intern Med. 2012;172:153–159. doi: 10.1001/archinternmed.2011.677. [DOI] [PubMed] [Google Scholar]
- 5.Mc Laughlin PD, O’Connor OJ, O’Neill SB, Shanahan F, Maher MM. Minimization of Radiation Exposure due to Computed Tomography in Inflammatory Bowel Disease. ISRN Gastroenterol. 2012;2012:790279. doi: 10.5402/2012/790279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Craig O, O’Neill S, O’Neill F, et al. Diagnostic Accuracy of Computed Tomography Using Lower Doses of Radiation for Patients With Crohn’s Disease. Clin Gastroenterol Hepatol. 2012 doi: 10.1016/j.cgh.2012.03.014. [DOI] [PubMed] [Google Scholar]
- 7.Prompona M, Cyran C, Nikolaou K, Bauner K, Reiser M, Huber A. Contrast-enhanced whole-heart MR coronary angiography at 3.0 T using the intravascular contrast agent gadofosveset. Invest Radiol. 2009;44:369–374. doi: 10.1097/rli.0b013e3181a40d1d. [DOI] [PubMed] [Google Scholar]
- 8.Gweon HM, Kim SJ, Lee SM, Hong YJ, Kim TH. 3D whole-heart coronary MR angiography at 1.5T in healthy volunteers: comparison between unenhanced SSFP and Gd-enhanced FLASH sequences. Korean J Radiol. 2011;12:679–685. doi: 10.3348/kjr.2011.12.6.679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu X, Bi X, Huang J, Jerecic R, Carr J, Li D. Contrast-enhanced whole-heart coronary magnetic resonance angiography at 3.0 T: comparison with steady-state free precession technique at 1.5 T. Invest Radiol. 2008;43:663–668. doi: 10.1097/RLI.0b013e31817ed1ff. [DOI] [PubMed] [Google Scholar]
- 10.Bi X, Deshpande V, Simonetti O, Laub G, Li D. Three-dimensional breathhold SSFP coronary MRA: a comparison between 1.5T and 3.0T. J Magn Reson Imaging. 2005;22:206–212. doi: 10.1002/jmri.20374. [DOI] [PubMed] [Google Scholar]
- 11.Bi X, Carr JC, Li D. Whole-heart coronary magnetic resonance angiography at 3 Tesla in 5 minutes with slow infusion of Gd-BOPTA, a high-relaxivity clinical contrast agent. Magn Reson Med. 2007;58:1–7. doi: 10.1002/mrm.21224. [DOI] [PubMed] [Google Scholar]
- 12.Kotys MS, Herzka DA, Vonken EJ, et al. Profile order and time-dependent artifacts in contrast-enhanced coronary MR angiography at 3T: origin and prevention. Magn Reson Med. 2009;62:292–299. doi: 10.1002/mrm.21997. [DOI] [PubMed] [Google Scholar]
- 13.Gharib AM, Herzka DA, Ustun AO, et al. Coronary MR angiography at 3T during diastole and systole. J Magn Reson Imaging. 2007;26:921–926. doi: 10.1002/jmri.21056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gharib AM, Abd-Elmoniem KZ, Ho VB, et al. The Feasibility of 350 mum Spatial Resolution Coronary Magnetic Resonance Angiography at 3 T in Humans. Invest Radiol. 2012;47:339–345. doi: 10.1097/RLI.0b013e3182479ec4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rohrer M, Bauer H, Mintorovitch J, Requardt M, Weinmann HJ. Comparison of magnetic properties of MRI contrast media solutions at different magnetic field strengths. Invest Radiol. 2005;40:715–724. doi: 10.1097/01.rli.0000184756.66360.d3. [DOI] [PubMed] [Google Scholar]
- 16.Bock M, Schulz J, Ueltzhoeffer S, Giesel F, Voth M, Essig M. Intravascular contrast agent T1 shortening: fast T1 relaxometry in a carotid volunteer study. Magma. 2008;21:363–368. doi: 10.1007/s10334-008-0134-2. [DOI] [PubMed] [Google Scholar]
- 17.Lauffer RB, Parmelee DJ, Dunham SU, et al. MS-325: albumin-targeted contrast agent for MR angiography. Radiology. 1998;207:529–538. doi: 10.1148/radiology.207.2.9577506. [DOI] [PubMed] [Google Scholar]
- 18.Scanlon PJ, Faxon DP, Audet AM, et al. ACC/AHA guidelines for coronary angiography: executive summary and recommendations. A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee on Coronary Angiography) developed in collaboration with the Society for Cardiac Angiography and Interventions. Circulation. 1999;99:2345–2357. doi: 10.1161/01.cir.99.17.2345. [DOI] [PubMed] [Google Scholar]
- 19.Prompona M, Cyran C, Nikolaou K, Bauner K, Reiser M, Huber A. Contrast-enhanced whole-heart coronary MRA using Gadofosveset 3.0 T versus 1.5 T. Acad Radiol. 2010;17:862–870. doi: 10.1016/j.acra.2010.02.009. [DOI] [PubMed] [Google Scholar]
- 20.Frydrychowicz A, Russe MF, Bock J, et al. Comparison of gadofosveset trisodium and gadobenate dimeglumine during time-resolved thoracic MR angiography at 3T. Acad Radiol. 2010;17:1394–1400. doi: 10.1016/j.acra.2010.05.022. [DOI] [PubMed] [Google Scholar]
- 21.Wagner M, Rief M, Asbach P, et al. Gadofosveset trisodium-enhanced magnetic resonance angiography of the left atrium--a feasibility study. Eur J Radiol. 2010;75:166–172. doi: 10.1016/j.ejrad.2009.04.052. [DOI] [PubMed] [Google Scholar]
- 22.Aime S, Caravan P. Biodistribution of gadolinium-based contrast agents, including gadolinium deposition. J Magn Reson Imaging. 2009;30:1259–1267. doi: 10.1002/jmri.21969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Caravan P. Protein-targeted gadolinium-based magnetic resonance imaging (MRI) contrast agents: design and mechanism of action. Acc Chem Res. 2009;42:851–862. doi: 10.1021/ar800220p. [DOI] [PubMed] [Google Scholar]
- 24.Nassenstein K, Waltering KU, Kelle S, et al. Magnetic resonance coronary angiography with Vasovist: in-vivo T1 estimation to improve image quality of navigator and breath-hold techniques. Eur Radiol. 2008;18:103–109. doi: 10.1007/s00330-007-0720-0. [DOI] [PubMed] [Google Scholar]
- 25.Kelle S, Thouet T, Tangcharoen T, et al. Whole-heart coronary magnetic resonance angiography with MS-325 (Gadofosveset) Med Sci Monit. 2007;13:CR469–474. [PubMed] [Google Scholar]
- 26.Knopp MV, Giesel FL, von Tengg-Kobligk H, et al. Contrast-enhanced MR angiography of the run-off vasculature: intraindividual comparison of gadobenate dimeglumine with gadopentetate dimeglumine. J Magn Reson Imaging. 2003;17:694–702. doi: 10.1002/jmri.10313. [DOI] [PubMed] [Google Scholar]
- 27.Schneider G, Maas R, Schultze Kool L, et al. Low-dose gadobenate dimeglumine versus standard dose gadopentetate dimeglumine for contrast-enhanced magnetic resonance imaging of the liver: an intra-individual crossover comparison. Invest Radiol. 2003;38:85–94. doi: 10.1097/00004424-200302000-00003. [DOI] [PubMed] [Google Scholar]
- 28.Kuwatsuru R, Kadoya M, Ohtomo K, et al. Comparison of gadobenate dimeglumine with gadopentetate dimeglumine for magnetic resonance imaging of liver tumors. Invest Radiol. 2001;36:632–641. doi: 10.1097/00004424-200111000-00002. [DOI] [PubMed] [Google Scholar]
- 29.Balci NC, Inan N, Anik Y, Erturk MS, Ural D, Demirci A. Low-dose gadobenate dimeglumine versus standard-dose gadopentate dimeglumine for delayed contrast-enhanced cardiac magnetic resonance imaging. Acad Radiol. 2006;13:833–839. doi: 10.1016/j.acra.2006.04.002. [DOI] [PubMed] [Google Scholar]
- 30.Grobner T. Gadolinium - a specific trigger for the development of nephrogenic fibrosing dermopathy and nephrogenic systemic fibrosis? Nephrology Dialysis Transplantation. 2005;21:1104–1108. doi: 10.1093/ndt/gfk062. [DOI] [PubMed] [Google Scholar]
- 31.Grobner T. Gadolinium--a specific trigger for the development of nephrogenic fibrosing dermopathy and nephrogenic systemic fibrosis? Nephrol Dial Transplant. 2006;21:1104–1108. doi: 10.1093/ndt/gfk062. [DOI] [PubMed] [Google Scholar]
- 32.Perazella MA, Reilly RF. Imaging patients with kidney disease: how do we approach contrast-related toxicity? Am J Med Sci. 2011;341:215–221. doi: 10.1097/MAJ.0b013e3181f016e6. [DOI] [PubMed] [Google Scholar]
- 33.Niendorf T, Sodickson DK. Parallel imaging in cardiovascular MRI: methods and applications. NMR Biomed. 2006;19:325–341. doi: 10.1002/nbm.1051. [DOI] [PubMed] [Google Scholar]