Abstract
It has been increasingly recognized that tumor microenvironment plays an important role in carcinogenesis. Inflammatory component is present and contributes to tumor proliferation, angiogenesis, metastasis, and resistance to hormonal and chemotherapy. This review highlights the role of inflammation in the tumor metastasis. We focus on the function of proinflammatory factors, particularly cytokines during tumor metastasis. Understanding of the mechanisms by which inflammation contributes to metastasis will lead to innovative approach for treating cancer.
Keywords: Inflammation, Epithelial-mesenchymal transition, metastasis
How tumor spread remains an enigma and has received great attention in recent years, as metastasis is the major cause of cancer mortality. The complex and highly selective metastatic cascade not only depends on the intrinsic properties of tumor cells but also the microenvironment that they derive from. An inflammatory milieu consisting of infiltrated immune cells and their secretory cytokines, chemokines, and growth factors contribute significantly to the invasive and metastatic traits of cancer cells. Here, we review new insights into the molecular pathways that link inflammation in the tumor microenvironment to metastasis.
Cancer and chronic inflammation
Nearly 150 years ago, Rudolf Virchow speculated that cancer is similar with chronic inflammatory process of wound healing as he noticed the presence of large amount of leukocyte infiltration in tumor tissues1. Epidemiologic and clinical studies support his notion and show that approximately 25% of all human cancers in adults result from chronic inflammation2. For example, chronic viral or bacterial infection in stomach, liver, and cervical, repeated solar irradiation of the skin, and prolonged exposure to tobacco smoke or other environmental chemicals in lung, contribute significantly to the chronic tissue injury and subsequently oncogenesis of these organs3. During this oncogenic event, persistent tissue injury result in changes in the cellular architecture of the epithelium and the surrounding stromal elements and promote genetic mutations and epigenetic alternations of epithelia cells. This change in tissue homeostasis can in turn lead to a chronic inflammatory response that never fades away, which then further promotes tumor growth, angiogenesis, invasion, and metastasis through the activation of surrounding stromal cells and recruitment of different inflammatory cells. The tight correlation of inflammation and cancer is further supported by population-based studies demonstrated that the use of anti-inflammatory agents is associated with a reduced incidence of colorectal, breast, pancreatic, and gastric cancers4. Wound healing and cancer shared a number of phenotypic similarities in cellular behavior, signaling molecules, and gene expression. Both processes involve cell proliferation, survival, and migration that controlled by growth factors, cytokines, as well as inflammatory and angiogenic signals that derived from the microenvironment of wound and cancer. The similarities between of these two processes were subsequently led to the description of cancer as wound that does not heal by Dvorak5. The crucial function of inflammation in wound healing is to protect against pathogens by destroying infectious agents, to gather intelligence for the immune system by initiating specific and long-term immunity, and to repair damaged tissue. This acute inflammation is a rapid self-limiting process; the signals and regulatory systems that normally initiate and sustain are eventually shut down during the wound healing process. In cancer, inflammation becomes a persistent and chronic process and “failure to heal” tumors. Although the association of inflammation and cancer is clear and has been recognized as the “seventh hallmark of cancer”6, 7, the mechanism underlying the persistent and chronic inflammation in cancer remains unclear. There are still many questions remain elusive, for example, how does chronic inflammation promote tumor cell migration, invasion, and metastasis? Here we review and summarize many recent studies from us and others on the mechanisms and signaling pathways underlying inflammation-mediated metastasis.
EMT and metastasis
Metastasis is an exceedingly complex process involving tumor cell motility, intravasation, circulation in the blood or lymph system, extravasation, and growth in new tissues and organs. The initiation of tumor cell migration is a prerequisite for the metastatic cascade. The increased motility and invasiveness of metastatic tumor cells are reminiscent of the events at the epithelial-mesenchymal transition (EMT), which is a characteristic of three physiological and pathophysiological events during embryonic development and morphogenesis, cancer progression and metastasis, and chronic degeneration and fibrosis of mature organs8–10. In these EMT processes, epithelial cells acquire fibroblast-like properties and exhibit reduced intercellular adhesion and increased motility. Activation of this normally latent EMT program confers cancer cells a distinct advantage for invasion and metastasis to distant organs or tissues. Although EMT is not the sole mechanism responsible for metastasis, EMT is the most important mechanism behind the initiation of cancer metastasis. The hallmark of EMT is the loss of E-cadherin expression, an important caretaker of the epithelial phenotype. E-cadherin is a cell-cell adhesion molecule that participates in homotypic, calcium-dependent interactions to form epithelial adherent junctions11, 12. Loss of E-cadherin expression is often correlated with the tumor grade and stage because it results in the disruption of the cell-cell adhesion and an increase in the nuclear β-catenin11, 12. Several transcription factors have been implicated in the regulation of EMT, including zinc finger proteins of the Snail/Slug family, the basic helix-loop-helix factor Twist, E12/E47, Goosecoid, δEF1/ZEB1, and SIP18, 13, 14. These factors act as a molecular switch of EMT program by repressing a subset of common genes that encode cadherins, claudins, integrins, mucins, plakophilin, occludin, and ZO1 to induce EMT. For example, Snail, a transcriptional repressor, was identified in Drosophila as a suppressor of the transcription of shotgun in controlling the large-scale cell movement during the formation of mesoderm and neural crest8, 10. Absent of Snail is lethal because of severe defects at the gastrula stage during development15. The expression of Snail is associated with E-cadherin repression in metastasis and correlates with tumor recurrence and poor prognosis in various cancers16–18.
Recent work has indicated that EMT is a dynamic process that is controlled by signals that cells receive from their microenvironment. By adopting a mesenchymal phenotype through EMT, individual carcinoma cells can infiltrate adjacent tissues, cross endothelial barriers, and enter the circulation through blood and lymphatic vessels. Once the cancer cells reach their secondary tissues or organs, they no longer encounter the signals that they experienced in the primary tumor, and they can revert to an epithelial state via a mesenchymal-epithelial transition (MET). Consistent with this notion, EMT is commonly occurred at the invasive front (tumor-stromal boundary) of many invasive carcinomas19, 20. These observations indicate that EMT is triggered by cellular signals from microenvironment. The tumor microenvironment consist of extracellular matrix (ECM), such as collagen and hyaluronic acid, stromal cells, such as fibroblasts/myofibroblasts, endothelial cells, pericytes, and smooth-muscle cells, and infiltrated inflammatory cells, such as neutrophils, lymphocytes, macrophages, and myeloid-derived suppressor cells (MDSC). These infiltrated immune cells secrete cytokines, chemokines, and growth factors, such as TNFα, TGFβ, IL-6, fibroblast growth factor (FGF), epidermal growth factor (EGF), and HGF21 and play an essential role for supporting tumor progression and metastasis. In analogous with the role of inflammation in mediating wound healing, we hypothesize that the migratory and invasive ability of tumor cells at the invasive front is initiated and propelled by an inflammatory microenvironment through the induction of EMT.
The function of inflammatory cells in metastasis
Consistent with our hypothesis, a high content of inflammatory cells, particularly tumor-associated macrophages, is commonly found at the invasive fronts of advanced carcinoma22. Macrophages are key cells in chronic inflammation. M1 macrophages are involved in Type 1 reactions and are classically activated by microbial products, killing microorganisms and producing reactive oxygen and nitrogen intermediates. In contrast, M2 cells (tumor associated macrophage; TAM) are important component of infiltrated leukocytes in most malignant tumors. They involve in Type 2 reactions, tune inflammation and adaptive immunity, promote cell proliferation by producing growth factors and enhancing angiogenesis, tissue remodeling, and repair. Macrophage directly influences the behavior and function of tumor cells and has been regarded as an “obligate partner for tumor-cell migration, invasion and metastasis”22. Clinical studies indicate a correlation between TAM density and poor prognosis23. For example, in PyMT-induced mammary tumors, macrophages are present in the areas of basement membrane breakdown during the development of “early-stage” metastatic lesions and systemic depletion of macrophages results in reduced formation of lung metastasis24. TAMs produce a wide variety of growth factors (such as FGF, HGF, EGF, PDGF and TGFβ) and cytokines (such as TNFα, interleukin-6, interleukin-1, and interferons) to stimulate the growth, motility, and invasiveness of tumor cells. TAMs also produce many proteases, ranging from uPA to a variety of matrix metalloproteinases to degrade the basement membrane for creating a channel for tumor cell invasion. In our recent study, we found that the invasiveness of tumor cells was dramatically enhanced when they were co-cultured with macrophages or macrophage conditioned medium25. We showed that this effect was mainly mediated by the secretion of TNFα from macrophages as neutralization of TNFα by TNFα antibody greatly suppressed macrophage-mediated tumor cell invasion25. Consistent with our finding, Hagemann et al found that co-culturing macrophages with tumor cells enhanced their invasive ability in a manner dependent on TNFα and matrix metalloproteinases (MMP)26. Interestingly, expression of Snail in the non-metastatic breast cancer cell lines MCF7 and T47D, which contain little endogenous Snail, greatly increased the invasiveness of these cells by inflammation, indicating that Snail, through the induction of EMT program, is a critical for mediating inflammation-induced invasion of breast cancer cells. Knockdown of Snail expression significantly inhibited cell migration and invasion induced by inflammatory cytokines and suppressed inflammation-mediated breast cancer metastasis in animal model. Thus, macrophages, the major inflammatory component of the stroma in malignancies, facilitate angiogenesis, extracellular matrix breakdown, invasion, and metastasis through multiple mechanisms.
In addition to macrophages, fibroblasts/myofibroblasts comprise as another major component of tumor stroma. These cancer-associated fibroblasts (CAF) shared a lot of characteristics with activated fibroblasts in wound healing and promote tumor progression. Recent studies have demonstrated that CAF are important in tumor cell migration and invasion. CAF isolated from metastatic breast cancer produce elevated levels of IL-6 and enhance cancer cell invasiveness27. In agreement with this, De Wever et al found that myofibroblasts could stimulate the invasive growth of breast and colon cancer cells using myofibroblasts isolated from surgical colon cancer specimens28. In addition, CAF in pancreatic ductal adenocarcinoma are responsible for a poorly vascularized architecture that imposes a barrier for drug delivery and spurs metastasis29. Furthermore, fibroblast promotes tumor cell proliferation and metastasis through the production of several growth factors, cytokines, chemokines, and matrix metalloproteinases (MMPs). MMPs derived from tumor cells and stromal components are regarded as major players in assisting the metastasis of tumor cells. For example, transgenic expression of MMP3 stimulates expression of Snail through the increased cellular reactive oxygen species and thus induces down-regulation of E-cadherin and increased tumor progression30.
Myeloid-derived suppressor cells (MDSC) is present in many cancer patients and mice with transplanted or spontaneous tumors31, 32. MDSC, characterized as CD11b+ Gr-1+ in mice, can be recruited and activated by multiple factors, such as VEGF, IL-1β and IL-6, many of which are associated with chronic inflammation33. Recruitment of MDSCs in turn further produces these pro-inflammatory factors, resulting in the amplification of the pro-inflammatory response. MDSCs not only suppress the adaptive immune responses but also regulate innate immune responses by modulating the cytokine production of macrophages34 and thus directly facilitate metastasis. Recent studies have shown a close correlation between the level of MDSCs and cancer stage, metastatic tumor burden, and responsiveness to chemotherapy35. MDSCs from mammary carcinoma can promote tumor invasion and metastasis36. In Tgfbr2-decificent mice, MDSCs are concentrated at the invasive tumor front and facilitate tumor cell invasion and metastasis through chemokine receptors CXCR2 and CXCR437.
Neutrophils are also noted as important cells at the tumor inflammatory microenvironment. CXCR2 can induce the expression of matrix metalloproteinase 9 (MMP9) and vascular endothelial growth factor (VEGF) to recruit neutrophils38. This subsequently leads to endothelial cell invasion and blood vessel formation. Taken together, all of these infiltrated inflammatory cells secret different cytokines, chemokines, and other factors to promote the tumor cell migration and invasion and contribute to inflammation-mediated metastasis.
Hypoxia and inflammatory cytokines in metastasis
Hypoxic condition has been detected in many human solid malignancies and it provides a strong selective advantage for tumor growth and survival by enhancing angiogenesis and metastasis. Under hypoxic conditions, cells respond by stabilizing hypoxia-inducible factor (HIF1α) that, in turn, forms the transcriptional complex with HIF1β and activates the transcription of downstream target genes through direct binding to the hypoxic response element located in the promoter of those genes that control glucose metabolism, angiogenesis, survival, and invasion. The well-recognized mechanism involved in hypoxia-induced metastasis is the activation of VEGF by HIF1α to promote angiogenesis and facilitate tumor metastasis. In addition, HIF1α can induce the expression of the chemokine receptor CXCR4 in renal cell carcinoma to promote organ specific metastatic dissemination39. Although hypoxia and EMT are considered as crucial events favoring invasion and metastasis of many cancer cells, two processes are long thought to involve few common molecular mechanisms. The link between hypoxia and EMT has been established recently as hypoxia induces the expression and coordinates the interplay of several EMT regulators. For example, HIF1α can regulate the expression of several EMT inducers such as Snail, Slug, Twist, ZEB1, and SIP1 directly or indirectly and thus leads to the induction of EMT and enhances metastasis under hypoxic condition40–44. Interestingly, tumor hypoxia (measured by the expression of HIF1α) significantly correlated with both Snail and Twist expression and co-expression of these molecules correlated with the highest probability of metastasis and the worst prognosis in head and neck cancer44. Furthermore, hypoxia also induces the expression of lysyl oxidas (LOX), which promotes Snail stabilization and repressor activity45, 46. LOX is required for the maturation of newly synthesized collagen fibrils and promotes metastasis through changes in focal adhesion kinase activity. These results indicate that Snail not only can repress E-cadherin but also cooperates with LOX to induce EMT under hypoxic condition. Interestingly, EMT mainly occurs at the tumor invasion front (tumor-stroma boundary) while hypoxia mainly operates in the intratumoral areas where the pO2 level is below 10 mmHg, how these two events communicate with each other in vivo remain further investigated. We speculate that cytokines and other factors generated from the inflammatory tumor microenvironment play essential roles in orchestrate these two processes.
In fact, under hypoxic and inflammatory conditions, tumor microenvironment generates and sustains a tumor-promoting cytokine network for facilitating tumor growth and metastasis. For example, the production of TGFβ from myeloid cells, mesenchymal cells, and cancer cells is significantly enhanced in hypoxic or inflammatory state. TGFβ is a multifunctional growth factor with a complicated dual role in tumorigenesis47. At the early stages of tumor formation, TGFβ acts as a tumor suppressor by inhibiting proliferation and inducing apoptosis of tumor cells. At the later stages of tumorigenesis, TGFβ functions as a tumor promoter by increasing tumor growth, survival, motility, and invasion. TGFβ has also been showed to induce EMT in normal mammary epithelial cells and breast cancer cell lines48, 49. Constitutive activation of Raf enhances the function of TGFβ in inducing EMT in MDCK cells50. It is interesting to note that SMAD3 and SMAD4 interact and form a complex with Snail and target to the promoters of CAR (a tight-junction protein) and E-cadherin during TGFβ-inducing EMT in breast epithelial cells51. Bos et al identified that TGFβ primed cancer cells for metastasis to the lung through angiopoietin-like 4 (ANGPTL4) via Smad signaling pathway52. On contrast, inhibition of TGFβ or TGFβ receptor reduces the invasive and metastatic activities of cancer cells. In addition, TGFβ can cooperate with other oncogenic pathway such as Ras, Notch, Wnt/β-catenin, and NF-κB to maintain the mesenchymal phenotype of invasive/metastatic tumor cells53–55.
TNFα, another key inflammatory cytokines, plays a central role in the tumor progression. Constitutive expression of the TNFα from tumor microenvironment is a characteristic of many malignant tumors and its presence is often associated with poor prognosis. Several lines of evidence point to the tumor-promoting effects of TNFα in inflammation-driven tumorigenesis. First, overexpression of TNFα confers migratory and invasive properties of many tumor cell lines56. Second, TNFα and TNFα receptor 1 (TNFR1) knock-out mice are resistant to chemical induced-carcinogenesis in skin and liver metastasis in experimental colon cancer model57, 58. Third, various tumor-promoting effects of TNFα are further confirmed in enhancing tumor cell motility, activating oncogenic pathways, and triggering EMT. TNFα can also promote breast cancer cell migration through up-regulation LOX59. Endogenous TNFα contributes to the growth and invasiveness of primary pancreatic ductal adenocarcinoma and anti-TNFα inhibit metastasis of these tumors60. Using RNA interference technology, Kulbe et al demonstrated that tumor growth and dissemination were significantly inhibited when TNFα production was blocked61. In addition, TNFα can up-regulate SELECTIN and VCAM1 on endothelial cells that promote tumor cell adhesion and migration62, 63. Furthermore, TNFα enhances the invasive property of cancer cells by inducing EMT through Snail or ZEB1/ZEB264, 65. In our recent study, we found that inflammatory cytokine TNFα is the major signal to induce Snail stabilization and EMT induction25. We showed that TNFα greatly enhanced the migration and invasion of tumor cells by inducing EMT program through NF-κB-mediated Snail stabilization. Knockdown of Snail expression not only inhibits TNFα-induced cancer cell migration and invasion in vitro but also suppresses LPS-mediated metastasis in vivo. Furthermore, knockdown of Snail expression not only blocks metastasis that is intrinsic to the metastatic breast cancer cells but also greatly suppresses inflammation-accelerated metastasis. Collectively, our study indicates that Snail stabilization and EMT induction mediated by the inflammatory cytokine TNFα are critical for metastasis. Our study provides a plausible molecular mechanism for tumor cell dissemination and invasion at the tumor invasive front.
IL-6 is another important inflammatory cytokine linking inflammation and cancer. IL-6 transmits its signal through a common signaling receptor, gp130, expressed on many cell types. IL-6 binds to the sIL-6R receptor (gp80, present either on the cell surface or in solution), which then induces dimerization of gp130 chains resulting in activation of the associated Janus kinases (JAKs). JAKs phosphorylate gp130, leading to the recruitment and activation of the STAT3 and STAT1 transcription factors as well as other molecules (SHP2, Ras-MAPK, and PI3K)66. The role of IL-6 in accelerating tumorigenesis is becoming clear as exogenous administration of IL-6 to mice during tumor initiation resulted in an increase in tumor burden and multiplicity67. IL-6 also enhances tumor proliferation in tumor-initiating intestinal epithelial cells (IECs) through NF-κB-IL-6-STAT3 cascade67–69. IL-6 can also act as an inducer of EMT in breast cancer cells. Ectopic expressing of IL-6 in breast adenocarcinoma cells exhibits an EMT phenotype characterized by suppressing E-cadherin expression and inducting vimentin, N-cadherin, Snail and Twist70. In addition, IL-6 also synergizes with EGF in inducing EMT through the activation JNK2/STAT3 in ovarian carcinomas71.
The interleukin-1 (IL-1) also promotes inflammatory processes and augments metastasis. There are two forms of IL-1 protein, IL-1α and IL-1β, and one antagonistic protein IL-1 receptor antagonist (IL-1ra). IL-1β is active solely in its secreted form, whereas IL-1α is active mainly as an intracellular precursor. IL-1 is abundant at tumor sites, where it affects the process of carcinogenesis, tumor growth and invasiveness, and the patterns of tumor-host interactions72. Genetic ablation of IL-1β in mice results in the absence of metastatic tumors in vivo73. Liver metastasis almost completely inhibited in mice with deletion of interleukin-1β converting enzyme, which is required for the processing of IL-1β74. IL-1β also directly induces uPA expression and NF-κB activation, which results in the migration of A549 cells75.
Central regulators of tumor metastasis facilitated by inflammation
In the panoply of molecular signaling pathways connecting inflammation and EMT, nuclear factor-κB (NF-κB) and STAT3 are emerging as two key players involved in inflammation-induced metastasis. NF-κB is a central regulator of tumor development in inflammation-induced cancer76. Activation of NF-κB plays a central role in the activation of numerous proinflammatory cytokines in multiple cell types, including macrophages, T cells, and epithelial cells. NF-κB promotes breast cancer cell migration and metastasis by inducing the expression of the chemokine receptor CXCR477. Wang et al. demonstrated that increased NF-κB level induced expression of Bcl-2 to suppress apoptosis and induce EMT78. NF-κB activity is required in EpH4 model of EMT79. In addition, TGFβ-dependent EMT induction depends at least in part on NF-κB activity. There is unequivocal evidence that NF-κB activation has been associated with the induction of many transcription factors involved in EMT, such as Slug, Snail, Twist, and ZEB1/ZEB280. NF-κB is also responsible for the activation of mesenchymal marker vimentin and MMPs, such as MMP2 and MMP981. In our recent study, we revealed a new mechanism of Snail regulation by NF-κB in inflammation-induced EMT and cancer metastasis25. We showed that TNFα-mediated Snail stabilization required COP9 signalosome through the activation of NF-κB pathway. Activation of NF-κB correlated with the level of Snail in breast cancer cell lines and tumor samples. In addition, there are increasing evidence of crosstalk between the NF-κB and HIF1α that links inflammation and hypoxia in metastasis.
Signal transducer and activator of transcription 3 (STAT3) is also one of the most recognized signal molecules and represents as a central regulator of tumor metastasis. Although STAT3 can be activated by various cytokines, growth factors, and oncogenic molecules82, constitutive activation of STAT3 is often found at the invasive front of tumors adjacent to infiltrated immune cells. The mechanism of constitutive STAT3 activation is mainly due to autocrine and paracrine production of IL-6 from tumor inflammatory environment leading to STAT3 phosphorylation. Activation of STAT3 protects cells from apoptosis and promotes cell-cycle progression and metastasis83. Consistent with this notion, colitis-associated colorectal cancer is markedly reduced in mice deficient for IL-6 or in mice lacking STAT3 in intestinal epithelial cells67. Furthermore, gp130Y757F/Y757F mice, which contain a mutant gp130 receptor that activates STAT3, enhance the growth of colitis-associated colorectal cancer69. In addition, activation of STAT3 facilitates metastasis in vivo. Wei et al demonstrated that ectopic expression of dominant-negative STAT3 significantly blocked the activation of STAT3 and suppressed VEGF expression and liver metastasis in a pancreatic tumor model84. In agreement with this, blockade of activated STAT3 inhibits metastasis through reducing the expression of MMP-2 in a mouse melanoma model while enforcing the expression of a constitutively activated STAT3 converts poorly metastatic melanoma cells into highly metastatic tumor cells85. Furthermore, STAT3 transcriptionally activates the target genes that promote tumor cell migration, such as MMP-1, MMP-2, and MMP-10 in bladder cancer cells86 and induces MMP-9 in transformed human mammary epithelial cells87. STAT3 can also directly interact with microtubules, focal adhesion kinase, and paxillin, and subsequently modulates the invasiveness of tumor cells88. Taken together, constitutive activation of STAT3 can influence multiple steps of metastasis, including invasion, cell survival, self-renewal, angiogenesis, and tumor-cell immune evasion89.
Summary
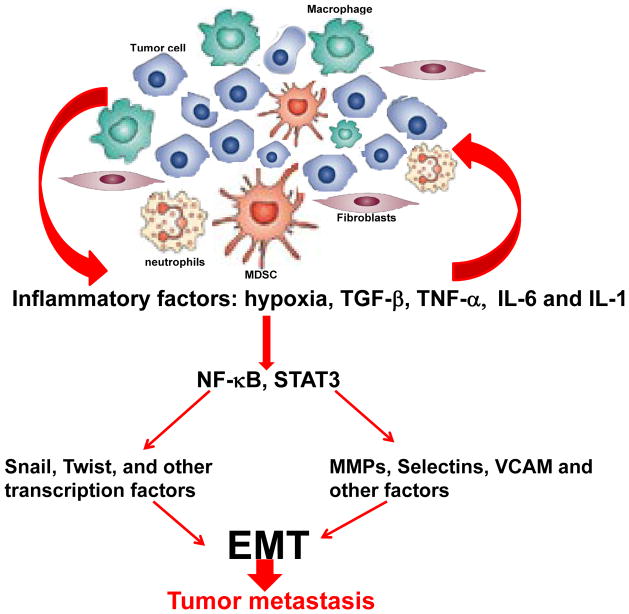
Here we have summarized and outlined the recent progress underlying the dysregulated signaling network between inflammation and metastasis (Figure 1). Understanding how inflammatory microenvironment is maintained and how it contributes to the tumor progression and metastasis will be crucial for understanding tumor biology as well as the development of new therapeutic interventions. Although significant advances have been generated in our understanding of the effect of inflammation in tumor progression, challenges are remained to translate these basic findings into clinical practice and arrive at novel treatment strategies that can target the inflammatory tumor microenvironment to achieve more efficient and selective killing of cancer cells.
Figure 1.
Highly simplified diagram showing the signaling network between the inflammation and metastasis. Cancer cell in primary tumors are surrounded by numerous cells including fibroblasts, macrophages, myeloid-derived suppressors cells (MDSC) and neutrophils. These inflammatory cells produce pro-inflammatory factors, such as hypoxia, TGF-β, TNF-α, IL-6 and IL-1. These inflammatory factors activate NF-κB and STAT3 to induce epithelial-to-mesenchymal transition (EMT) and tumor metastasis through regulation the expression of transcription factors and proteases.
Acknowledgments
We apologize to the many contributors to this field whose work are important while we were able to cite here. Our study is supported by the grants from NIH (RO1CA125454), the Susan G Komen Foundation (KG081310), and the Mary Kay Ash Foundation (to B.P. Zhou).
References
- 1.Balkwill F, Mantovani A. Inflammation and cancer: back to Virchow? Lancet. 2001;357 (9255):539–545. doi: 10.1016/S0140-6736(00)04046-0. [DOI] [PubMed] [Google Scholar]
- 2.Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420 (6917):860–867. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hussain SP, Harris CC. Inflammation and cancer: an ancient link with novel potentials. Int J Cancer. 2007;121 (11):2373–2380. doi: 10.1002/ijc.23173. [DOI] [PubMed] [Google Scholar]
- 4.Ulrich CM, Bigler J, Potter JD. Non-steroidal anti-inflammatory drugs for cancer prevention: promise, perils and pharmacogenetics. Nat Rev Cancer. 2006;6 (2):130–140. doi: 10.1038/nrc1801. [DOI] [PubMed] [Google Scholar]
- 5.Dvorak HF. Tumors: wounds that do not heal. Similarities between tumor stroma generation and wound healing. N Engl J Med. 1986;315 (26):1650–1659. doi: 10.1056/NEJM198612253152606. [DOI] [PubMed] [Google Scholar]
- 6.Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature. 2008;454 (7203):436–444. doi: 10.1038/nature07205. [DOI] [PubMed] [Google Scholar]
- 7.Mantovani A. Cancer: Inflaming metastasis. Nature. 2009;457 (7225):36–37. doi: 10.1038/457036b. [DOI] [PubMed] [Google Scholar]
- 8.Nieto MA. The snail superfamily of zinc-finger transcription factors. Nat Rev Mol Cell Biol. 2002;3 (3):155–166. doi: 10.1038/nrm757. [DOI] [PubMed] [Google Scholar]
- 9.Peinado H, Olmeda D, Cano A. Snail, Zeb and bHLH factors in tumour progression: an alliance against the epithelial phenotype? Nat Rev Cancer. 2007;7 (6):415–428. doi: 10.1038/nrc2131. [DOI] [PubMed] [Google Scholar]
- 10.Thiery JP, Sleeman JP. Complex networks orchestrate epithelial-mesenchymal transitions. Nat Rev Mol Cell Biol. 2006;7 (2):131–142. doi: 10.1038/nrm1835. [DOI] [PubMed] [Google Scholar]
- 11.Cowin P, Rowlands TM, Hatsell SJ. Cadherins and catenins in breast cancer. Curr Opin Cell Biol. 2005;17 (5):499–508. doi: 10.1016/j.ceb.2005.08.014. [DOI] [PubMed] [Google Scholar]
- 12.Junghans D, Haas IG, Kemler R. Mammalian cadherins and protocadherins: about cell death, synapses and processing. Curr Opin Cell Biol. 2005;17 (5):446–452. doi: 10.1016/j.ceb.2005.08.008. [DOI] [PubMed] [Google Scholar]
- 13.Hartwell KA, Muir B, Reinhardt F, et al. The Spemann organizer gene, Goosecoid, promotes tumor metastasis. Proc Natl Acad Sci U S A. 2006;103 (50):18969–18974. doi: 10.1073/pnas.0608636103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yang J, Mani SA, Donaher JL, et al. Twist, a master regulator of morphogenesis, plays an essential role in tumor metastasis. Cell. 2004;117 (7):927–939. doi: 10.1016/j.cell.2004.06.006. [DOI] [PubMed] [Google Scholar]
- 15.Carver EA, Jiang R, Lan Y, Oram KF, Gridley T. The mouse snail gene encodes a key regulator of the epithelial-mesenchymal transition. Mol Cell Biol. 2001;21 (23):8184–8188. doi: 10.1128/MCB.21.23.8184-8188.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moody SE, Perez D, Pan TC, et al. The transcriptional repressor Snail promotes mammary tumor recurrence. Cancer Cell. 2005;8 (3):197–209. doi: 10.1016/j.ccr.2005.07.009. [DOI] [PubMed] [Google Scholar]
- 17.Elloul S, Elstrand MB, Nesland JM, et al. Snail, Slug, and Smad-interacting protein 1 as novel parameters of disease aggressiveness in metastatic ovarian and breast carcinoma. Cancer. 2005;103 (8):1631–1643. doi: 10.1002/cncr.20946. [DOI] [PubMed] [Google Scholar]
- 18.Bruyere F, Namdarian B, Corcoran NM, et al. Snail expression is an independent predictor of tumor recurrence in superficial bladder cancers. Urol Oncol. 2009 doi: 10.1016/j.urolonc.2008.11.005. [DOI] [PubMed] [Google Scholar]
- 19.Franci C, Takkunen M, Dave N, et al. Expression of Snail protein in tumor-stroma interface. Oncogene. 2006;25 (37):5134–5144. doi: 10.1038/sj.onc.1209519. [DOI] [PubMed] [Google Scholar]
- 20.Christofori G. New signals from the invasive front. Nature. 2006;441 (7092):444–450. doi: 10.1038/nature04872. [DOI] [PubMed] [Google Scholar]
- 21.Joyce JA, Pollard JW. Microenvironmental regulation of metastasis. Nat Rev Cancer. 2009;9 (4):239–252. doi: 10.1038/nrc2618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Condeelis J, Pollard JW. Macrophages: obligate partners for tumor cell migration, invasion, and metastasis. Cell. 2006;124 (2):263–266. doi: 10.1016/j.cell.2006.01.007. [DOI] [PubMed] [Google Scholar]
- 23.Pollard JW. Tumour-educated macrophages promote tumour progression and metastasis. Nat Rev Cancer. 2004;4 (1):71–78. doi: 10.1038/nrc1256. [DOI] [PubMed] [Google Scholar]
- 24.Lin EY, Nguyen AV, Russell RG, Pollard JW. Colony-stimulating factor 1 promotes progression of mammary tumors to malignancy. J Exp Med. 2001;193 (6):727–740. doi: 10.1084/jem.193.6.727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wu Y, Deng J, Rychahou PG, et al. Stabilization of snail by NF-kappaB is required for inflammation-induced cell migration and invasion. Cancer Cell. 2009;15 (5):416–428. doi: 10.1016/j.ccr.2009.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hagemann T, Wilson J, Kulbe H, et al. Macrophages induce invasiveness of epithelial cancer cells via NF-kappa B and JNK. J Immunol. 2005;175 (2):1197–1205. doi: 10.4049/jimmunol.175.2.1197. [DOI] [PubMed] [Google Scholar]
- 27.Studebaker AW, Storci G, Werbeck JL, et al. Fibroblasts isolated from common sites of breast cancer metastasis enhance cancer cell growth rates and invasiveness in an interleukin-6-dependent manner. Cancer Res. 2008;68 (21):9087–9095. doi: 10.1158/0008-5472.CAN-08-0400. [DOI] [PubMed] [Google Scholar]
- 28.De Wever O, Westbroek W, Verloes A, et al. Critical role of N-cadherin in myofibroblast invasion and migration in vitro stimulated by colon-cancer-cell-derived TGF-beta or wounding. J Cell Sci. 2004;117 (Pt 20):4691–4703. doi: 10.1242/jcs.01322. [DOI] [PubMed] [Google Scholar]
- 29.Olive KP, Jacobetz MA, Davidson CJ, et al. Inhibition of Hedgehog signaling enhances delivery of chemotherapy in a mouse model of pancreatic cancer. Science. 2009;324 (5933):1457–1461. doi: 10.1126/science.1171362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Radisky DC, Levy DD, Littlepage LE, et al. Rac1b and reactive oxygen species mediate MMP-3-induced EMT and genomic instability. Nature. 2005;436 (7047):123–127. doi: 10.1038/nature03688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Almand B, Clark JI, Nikitina E, et al. Increased production of immature myeloid cells in cancer patients: a mechanism of immunosuppression in cancer. J Immunol. 2001;166 (1):678–689. doi: 10.4049/jimmunol.166.1.678. [DOI] [PubMed] [Google Scholar]
- 32.Young MR, Lathers DM. Myeloid progenitor cells mediate immune suppression in patients with head and neck cancers. Int J Immunopharmacol. 1999;21 (4):241–252. doi: 10.1016/s0192-0561(99)00008-9. [DOI] [PubMed] [Google Scholar]
- 33.Gabrilovich DI, Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nat Rev Immunol. 2009;9 (3):162–174. doi: 10.1038/nri2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sinha P, Clements VK, Bunt SK, Albelda SM, Ostrand-Rosenberg S. Cross-talk between myeloid-derived suppressor cells and macrophages subverts tumor immunity toward a type 2 response. J Immunol. 2007;179 (2):977–983. doi: 10.4049/jimmunol.179.2.977. [DOI] [PubMed] [Google Scholar]
- 35.Diaz-Montero CM, Salem ML, Nishimura MI, et al. Increased circulating myeloid-derived suppressor cells correlate with clinical cancer stage, metastatic tumor burden, and doxorubicin-cyclophosphamide chemotherapy. Cancer Immunol Immunother. 2009;58 (1):49–59. doi: 10.1007/s00262-008-0523-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bunt SK, Yang L, Sinha P, et al. Reduced inflammation in the tumor microenvironment delays the accumulation of myeloid-derived suppressor cells and limits tumor progression. Cancer Res. 2007;67 (20):10019–10026. doi: 10.1158/0008-5472.CAN-07-2354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yang L, Huang J, Ren X, et al. Abrogation of TGF beta signaling in mammary carcinomas recruits Gr-1+CD11b+ myeloid cells that promote metastasis. Cancer Cell. 2008;13 (1):23–35. doi: 10.1016/j.ccr.2007.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Albini A, Mirisola V, Pfeffer U. Metastasis signatures: genes regulating tumor-microenvironment interactions predict metastatic behavior. Cancer Metastasis Rev. 2008;27 (1):75–83. doi: 10.1007/s10555-007-9111-x. [DOI] [PubMed] [Google Scholar]
- 39.Staller P, Sulitkova J, Lisztwan J, et al. Chemokine receptor CXCR4 downregulated by von Hippel-Lindau tumour suppressor pVHL. Nature. 2003;425 (6955):307–311. doi: 10.1038/nature01874. [DOI] [PubMed] [Google Scholar]
- 40.Krishnamachary B, Berg-Dixon S, Kelly B, et al. Regulation of colon carcinoma cell invasion by hypoxia-inducible factor 1. Cancer Res. 2003;63 (5):1138–1143. [PubMed] [Google Scholar]
- 41.Hotz B, Arndt M, Dullat S, et al. Epithelial to mesenchymal transition: expression of the regulators snail, slug, and twist in pancreatic cancer. Clin Cancer Res. 2007;13 (16):4769–4776. doi: 10.1158/1078-0432.CCR-06-2926. [DOI] [PubMed] [Google Scholar]
- 42.Imai T, Horiuchi A, Wang C, et al. Hypoxia attenuates the expression of E-cadherin via up-regulation of SNAIL in ovarian carcinoma cells. Am J Pathol. 2003;163 (4):1437–1447. doi: 10.1016/S0002-9440(10)63501-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Davidson NE, Sukumar S. Of Snail, mice, and women. Cancer Cell. 2005;8 (3):173–174. doi: 10.1016/j.ccr.2005.08.006. [DOI] [PubMed] [Google Scholar]
- 44.Yang MH, Wu MZ, Chiou SH, et al. Direct regulation of TWIST by HIF-1alpha promotes metastasis. Nat Cell Biol. 2008;10 (3):295–305. doi: 10.1038/ncb1691. [DOI] [PubMed] [Google Scholar]
- 45.Peinado H, Del Carmen Iglesias-de la Cruz M, Olmeda D, et al. A molecular role for lysyl oxidase-like 2 enzyme in snail regulation and tumor progression. EMBO J. 2005;24 (19):3446–3458. doi: 10.1038/sj.emboj.7600781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Peinado H, Portillo F, Cano A. Switching on-off Snail: LOXL2 versus GSK3beta. Cell Cycle. 2005;4 (12):1749–1752. doi: 10.4161/cc.4.12.2224. [DOI] [PubMed] [Google Scholar]
- 47.Leivonen SK, Kahari VM. Transforming growth factor-beta signaling in cancer invasion and metastasis. Int J Cancer. 2007;121 (10):2119–2124. doi: 10.1002/ijc.23113. [DOI] [PubMed] [Google Scholar]
- 48.Miettinen PJ, Ebner R, Lopez AR, Derynck R. TGF-beta induced transdifferentiation of mammary epithelial cells to mesenchymal cells: involvement of type I receptors. J Cell Biol. 1994;127 (6 Pt 2):2021–2036. doi: 10.1083/jcb.127.6.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Forrester E, Chytil A, Bierie B, et al. Effect of conditional knockout of the type II TGF-beta receptor gene in mammary epithelia on mammary gland development and polyomavirus middle T antigen induced tumor formation and metastasis. Cancer Res. 2005;65 (6):2296–2302. doi: 10.1158/0008-5472.CAN-04-3272. [DOI] [PubMed] [Google Scholar]
- 50.Janda E, Lehmann K, Killisch I, et al. Ras and TGF[beta] cooperatively regulate epithelial cell plasticity and metastasis: dissection of Ras signaling pathways. J Cell Biol. 2002;156 (2):299–313. doi: 10.1083/jcb.200109037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Vincent T, Neve EP, Johnson JR, et al. A SNAIL1-SMAD3/4 transcriptional repressor complex promotes TGF-beta mediated epithelial-mesenchymal transition. Nat Cell Biol. 2009 doi: 10.1038/ncb1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bos PD, Zhang XH, Nadal C, et al. Genes that mediate breast cancer metastasis to the brain. Nature. 2009;459 (7249):1005–1009. doi: 10.1038/nature08021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nawshad A, Lagamba D, Polad A, Hay ED. Transforming growth factor-beta signaling during epithelial-mesenchymal transformation: implications for embryogenesis and tumor metastasis. Cells Tissues Organs. 2005;179 (1–2):11–23. doi: 10.1159/000084505. [DOI] [PubMed] [Google Scholar]
- 54.Zavadil J, Bottinger EP. TGF-beta and epithelial-to-mesenchymal transitions. Oncogene. 2005;24 (37):5764–5774. doi: 10.1038/sj.onc.1208927. [DOI] [PubMed] [Google Scholar]
- 55.Neth P, Ries C, Karow M, et al. The Wnt signal transduction pathway in stem cells and cancer cells: influence on cellular invasion. Stem Cell Rev. 2007;3 (1):18–29. doi: 10.1007/s12015-007-0001-y. [DOI] [PubMed] [Google Scholar]
- 56.Rosen EM, Goldberg ID, Liu D, et al. Tumor necrosis factor stimulates epithelial tumor cell motility. Cancer Res. 1991;51 (19):5315–5321. [PubMed] [Google Scholar]
- 57.Arnott CH, Scott KA, Moore RJ, et al. Expression of both TNF-alpha receptor subtypes is essential for optimal skin tumour development. Oncogene. 2004;23 (10):1902–1910. doi: 10.1038/sj.onc.1207317. [DOI] [PubMed] [Google Scholar]
- 58.Knight B, Yeoh GC, Husk KL, et al. Impaired preneoplastic changes and liver tumor formation in tumor necrosis factor receptor type 1 knockout mice. J Exp Med. 2000;192 (12):1809–1818. doi: 10.1084/jem.192.12.1809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Liang M, Zhang P, Fu J. Up-regulation of LOX-1 expression by TNF-alpha promotes trans-endothelial migration of MDA-MB-231 breast cancer cells. Cancer Lett. 2007;258 (1):31–37. doi: 10.1016/j.canlet.2007.08.003. [DOI] [PubMed] [Google Scholar]
- 60.Egberts JH, Cloosters V, Noack A, et al. Anti-tumor necrosis factor therapy inhibits pancreatic tumor growth and metastasis. Cancer Res. 2008;68 (5):1443–1450. doi: 10.1158/0008-5472.CAN-07-5704. [DOI] [PubMed] [Google Scholar]
- 61.Kulbe H, Thompson R, Wilson JL, et al. The inflammatory cytokine tumor necrosis factor-alpha generates an autocrine tumor-promoting network in epithelial ovarian cancer cells. Cancer Res. 2007;67 (2):585–592. doi: 10.1158/0008-5472.CAN-06-2941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Stoelcker B, Hafner M, Orosz P, Nieswandt B, Mannel DN. Role of adhesion molecules and platelets in TNF-induced adhesion of tumor cells to endothelial cells: implications for experimental metastasis. J Inflamm. 1995;46 (3):155–167. [PubMed] [Google Scholar]
- 63.Mannel DN, Orosz P, Hafner M, Falk W. Mechanisms involved in metastasis enhanced by inflammatory mediators. Circ Shock. 1994;44 (1):9–13. [PubMed] [Google Scholar]
- 64.Chuang MJ, Sun KH, Tang SJ, et al. Tumor-derived tumor necrosis factor-alpha promotes progression and epithelial-mesenchymal transition in renal cell carcinoma cells. Cancer Sci. 2008;99 (5):905–913. doi: 10.1111/j.1349-7006.2008.00756.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Chua HL, Bhat-Nakshatri P, Clare SE, et al. NF-kappaB represses E-cadherin expression and enhances epithelial to mesenchymal transition of mammary epithelial cells: potential involvement of ZEB-1 and ZEB-2. Oncogene. 2007;26 (5):711–724. doi: 10.1038/sj.onc.1209808. [DOI] [PubMed] [Google Scholar]
- 66.Mumm JB, Oft M. Cytokine-based transformation of immune surveillance into tumor-promoting inflammation. Oncogene. 2008;27 (45):5913–5919. doi: 10.1038/onc.2008.275. [DOI] [PubMed] [Google Scholar]
- 67.Grivennikov S, Karin E, Terzic J, et al. IL-6 and Stat3 are required for survival of intestinal epithelial cells and development of colitis-associated cancer. Cancer Cell. 2009;15 (2):103–113. doi: 10.1016/j.ccr.2009.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bromberg J, Wang TC. Inflammation and cancer: IL-6 and STAT3 complete the link. Cancer Cell. 2009;15 (2):79–80. doi: 10.1016/j.ccr.2009.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bollrath J, Phesse TJ, von Burstin VA, et al. gp130-mediated Stat3 activation in enterocytes regulates cell survival and cell-cycle progression during colitis-associated tumorigenesis. Cancer Cell. 2009;15 (2):91–102. doi: 10.1016/j.ccr.2009.01.002. [DOI] [PubMed] [Google Scholar]
- 70.Sullivan NJ, Sasser AK, Axel AE, et al. Interleukin-6 induces an epithelial-mesenchymal transition phenotype in human breast cancer cells. Oncogene. 2009 doi: 10.1038/onc.2009.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Colomiere M, Ward AC, Riley C, et al. Cross talk of signals between EGFR and IL-6R through JAK2/STAT3 mediate epithelial-mesenchymal transition in ovarian carcinomas. Br J Cancer. 2009;100 (1):134–144. doi: 10.1038/sj.bjc.6604794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Apte RN, Krelin Y, Song X, et al. Effects of micro-environment- and malignant cell-derived interleukin-1 in carcinogenesis, tumour invasiveness and tumour-host interactions. Eur J Cancer. 2006;42 (6):751–759. doi: 10.1016/j.ejca.2006.01.010. [DOI] [PubMed] [Google Scholar]
- 73.Voronov E, Shouval DS, Krelin Y, et al. IL-1 is required for tumor invasiveness and angiogenesis. Proc Natl Acad Sci U S A. 2003;100 (5):2645–2650. doi: 10.1073/pnas.0437939100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Vidal-Vanaclocha F, Fantuzzi G, Mendoza L, et al. IL-18 regulates IL-1beta-dependent hepatic melanoma metastasis via vascular cell adhesion molecule-1. Proc Natl Acad Sci U S A. 2000;97 (2):734–739. doi: 10.1073/pnas.97.2.734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Cheng CY, Hsieh HL, Sun CC, et al. IL-1 beta induces urokinase-plasminogen activator expression and cell migration through PKC alpha, JNK1/2, and NF-kappaB in A549 cells. J Cell Physiol. 2009;219 (1):183–193. doi: 10.1002/jcp.21669. [DOI] [PubMed] [Google Scholar]
- 76.Karin M. Nuclear factor-kappaB in cancer development and progression. Nature. 2006;441 (7092):431–436. doi: 10.1038/nature04870. [DOI] [PubMed] [Google Scholar]
- 77.Helbig G, Christopherson KW, 2nd, Bhat-Nakshatri P, et al. NF-kappaB promotes breast cancer cell migration and metastasis by inducing the expression of the chemokine receptor CXCR4. J Biol Chem. 2003;278 (24):21631–21638. doi: 10.1074/jbc.M300609200. [DOI] [PubMed] [Google Scholar]
- 78.Wang X, Belguise K, Kersual N, et al. Oestrogen signalling inhibits invasive phenotype by repressing RelB and its target BCL2. Nat Cell Biol. 2007;9 (4):470–478. doi: 10.1038/ncb1559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Huber MA, Azoitei N, Baumann B, et al. NF-kappaB is essential for epithelial-mesenchymal transition and metastasis in a model of breast cancer progression. J Clin Invest. 2004;114 (4):569–581. doi: 10.1172/JCI21358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Min C, Eddy SF, Sherr DH, Sonenshein GE. NF-kappaB and epithelial to mesenchymal transition of cancer. J Cell Biochem. 2008;104 (3):733–744. doi: 10.1002/jcb.21695. [DOI] [PubMed] [Google Scholar]
- 81.Vincenti MP, Brinckerhoff CE. Signal transduction and cell-type specific regulation of matrix metalloproteinase gene expression: can MMPs be good for you? J Cell Physiol. 2007;213 (2):355–364. doi: 10.1002/jcp.21208. [DOI] [PubMed] [Google Scholar]
- 82.Groner B, Lucks P, Borghouts C. The function of Stat3 in tumor cells and their microenvironment. Semin Cell Dev Biol. 2008;19 (4):341–350. doi: 10.1016/j.semcdb.2008.06.005. [DOI] [PubMed] [Google Scholar]
- 83.Chan IT, Kutok JL, Williams IR, et al. Conditional expression of oncogenic K-ras from its endogenous promoter induces a myeloproliferative disease. J Clin Invest. 2004;113 (4):528–538. doi: 10.1172/JCI20476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wei D, Le X, Zheng L, et al. Stat3 activation regulates the expression of vascular endothelial growth factor and human pancreatic cancer angiogenesis and metastasis. Oncogene. 2003;22 (3):319–329. doi: 10.1038/sj.onc.1206122. [DOI] [PubMed] [Google Scholar]
- 85.Xie TX, Wei D, Liu M, et al. Stat3 activation regulates the expression of matrix metalloproteinase-2 and tumor invasion and metastasis. Oncogene. 2004;23 (20):3550–3560. doi: 10.1038/sj.onc.1207383. [DOI] [PubMed] [Google Scholar]
- 86.Itoh M, Murata T, Suzuki T, et al. Requirement of STAT3 activation for maximal collagenase-1 (MMP-1) induction by epidermal growth factor and malignant characteristics in T24 bladder cancer cells. Oncogene. 2006;25 (8):1195–1204. doi: 10.1038/sj.onc.1209149. [DOI] [PubMed] [Google Scholar]
- 87.Dechow TN, Pedranzini L, Leitch A, et al. Requirement of matrix metalloproteinase-9 for the transformation of human mammary epithelial cells by Stat3-C. Proc Natl Acad Sci U S A. 2004;101 (29):10602–10607. doi: 10.1073/pnas.0404100101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Silver DL, Naora H, Liu J, Cheng W, Montell DJ. Activated signal transducer and activator of transcription (STAT) 3: localization in focal adhesions and function in ovarian cancer cell motility. Cancer Res. 2004;64 (10):3550–3558. doi: 10.1158/0008-5472.CAN-03-3959. [DOI] [PubMed] [Google Scholar]
- 89.Kortylewski M, Jove R, Yu H. Targeting STAT3 affects melanoma on multiple fronts. Cancer Metastasis Rev. 2005;24 (2):315–327. doi: 10.1007/s10555-005-1580-1. [DOI] [PubMed] [Google Scholar]