Abstract
Background
Wenxin Keli (WK), a Chinese herb extract, is reported to be effective in the treatment of atrial and ventricular cardiac arrhythmias. Recent studies suggest that WK inhibits the transient outward current (Ito).
Objective
The present study examines the effectiveness of WK, alone and in combination with quinidine, to suppress arrhythmogenesis in an experimental model of Brugada syndrome (BrS).
Methods and Results
Action potential and ECG recordings were obtained from epicardial and endocardial sites of coronary-perfused canine right ventricular wedge preparations. The Ito agonist NS5806 (10–15 μM) was used to pharmacologically mimic a genetic predisposition to BrS. The Ito agonist induced Phase 2 reentry (P2R) in 13/19 preparations and polymorphic ventricular tachycardia (pVT) in 11/19 wedge preparations. WK (10g/L) suppressed P2R and pVT in 100% (3/3) of preparations. A lower concentration of WK (5g/L) suppressed P2R in 60% (3/5) and pVT in 50% (2/4), but in combination with a low concentration of quinidine (5 μM) was 100% effective in suppressing P2R and pVT. Quinidine alone suppressed P2R and pVT in 60% (3/5) and 50% (2/4), respectively and in combination with WK (5g/L) suppressed P2R and pVT by 80% (4/5) and 75% (3/4), respectively. WK reduced Ito, ICa and contractility in single cardiomyocytes, but dose-dependently increased contractility in intact wedge preparations, an effect mimicked by tyramine.
Conclusions
Our data provide support for the hypothesis that Wenxin Keli, particularly in combination with quinidine, effectively suppresses arrhythmogenesis in an experimental model of BrS via inhibition of Ito and indirect adrenergic sympathomimetic effects.
Keywords: Transient outward potassium channel current, positive inotropic effect, cardiac arrhythmias, sudden cardiac death
Introduction
Wenxin Keli (WK), a Chinese herb extract comprised of 5 components (Nardostachys chinensis batal extract (NcBe), Codonopsis, Notoginseng, Amber, and Rhizoma polygonati), is reported to be effective for the treatment of cardiac arrhythmias and heart failure.1, 2 WK has been reported to block the transient outward potassium channel current (Ito), sodium current (INa) and L-type calcium current (ICa) in rat and rabbit ventricular cardiomyocytes.3–5 The mechanism by which WK improves cardiac function is not well established.
Brugada Syndrome (BrS) is an inherited cardiac disorder associated with a high incidence of sudden death due to the development of life-threatening polymorphic ventricular tachycardia (VT) and ventricular fibrillation (VF). Previous studies performed in the canine ventricular wedge preparation have demonstrated that the Brugada ECG-phenotype can be induced by an outward shift in the balance of currents active at the end of phase 1 of the ventricular epicardial (Epi) action potential (AP) via inhibition of INa, ICa or augmentation of Ito or IK-ATP.6–11 Inhibition of the transient outward current (Ito) was first identified by our group as a therapy for BrS over 13 years ago.6, 12
The present study evaluates the potency with which WK inhibits Ito as well as other currents contributing to the balance of current during the early phases of the cardiac action potential and test the hypothesis that WK can suppress the electrocardiographic and arrhythmic manifestations of BrS in an experimental model of BrS.
Methods
Wedge Preparations
Detailed methods for isolation, perfusion, and recording of transmembrane activity from coronary-perfused canine right ventricular wedge preparations have been reported previously7, 13 and are briefly describe in the Online Supplement.
Voltage-clamp studies using canine cardiomyocytes
Cardiomyocytes were isolated as previously described.14 Ito was measured in isolated left ventricular epicardial and M cells at 36.5 °C using whole-cell patch clamp techniques.15 Details of the protocol are presented in the Online Supplement.
Unloaded cell shortening in isolated myocytes
Myocytes were isolated from canine mid-myocardium as described above and unloaded cell shortening was recorded using a CCD camera and video-based edge detector as detailed in the Online Supplement.
Simulation of Ventricular Epicardial Action Potentials
Right ventricular (RV) epicardial action potentials were simulated using a LRII AP model, modified to include the Ito as previously described.16, 17 Details are provided in the Online Supplement.
Statistical approaches and drugs used are described in the Online Supplement.
Results
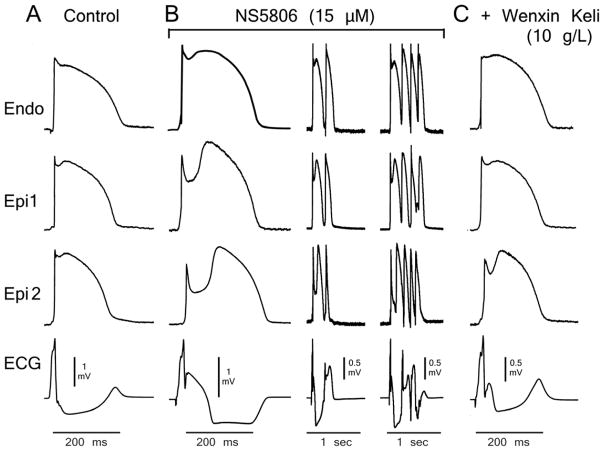
Figure 1 depicts APs and ECG recordings obtained from a RV coronary-perfused wedge preparation under control conditions (Fig. 1A), following addition of 15 μM NS5806 (Fig. 1B), and addition of 10 g/L WK to the perfusate (Fig. 1C). The Ito agonist NS5806 was used to mimic a presumed genetic predisposition to BrS. NS5806 caused loss of the AP dome in Epi2 but not Epi1. Phase 2 Reentry (P2R) developed as the Epi AP dome propagated from Epi1 to Epi2, leading to development of pVT. Addition of 10 g/L WK to the perfusate reduced the AP notch throughout the preparation, leading to recovery of the AP dome at Epi1, normalization of the ST segment and suppression of pVT in 3/3 preparations (Fig. 4A). Online Table 1 shows composite data of 5 RV wedge preparations. Neither NS5806 nor the combination of NS5806 plus WK affected transmural conduction time, as estimated by width of the R wave. NS5806 (10–15 μM) significantly prolonged Epi action potential duration (APD) measured at 90% repolarization (APD90) secondary to a dramatic increase in the Ito-mediated AP notch. Online Table 1 also shows that WK (10g/L) significantly shortened APD90 due to normalization of the Epi AP notch.
Figure 1.
Wenxin Keli (10g/L) suppression of NS5806-induced Brugada syndrome phenotype. Shown are transmembrane action potentials (APs) simultaneously recorded from two sites on the epicardial (Epi) surface and one site on the endocardial (Endo) surface of a coronary-perfused right ventricular wedge preparation. The bottom trace is the pseudo-electrocardiogram (ECG) recorded across the bath. A: Control. B: The transient outward potassium channel current agonist NS5806 (15 μM) accentuates the Epi AP notch and electrocardiographic J wave, leading to loss of the AP dome at Epi2 but not Epi1. Heterogeneous loss of the dome leads to the development of a closely–coupled phase 2 reentrant extrasystole which precipitates a polymorphic ventricular tachycardia. C: Wenxin Keli (10g/L) suppresses NS5806-induced phase 2 reentry and ventricular tachycardia/ventricular fibrillation and restores the AP dome, thus restoring homogeneity and aborting all arrhythmic activity. Basic cycle length=2000 ms.
Figure 4.
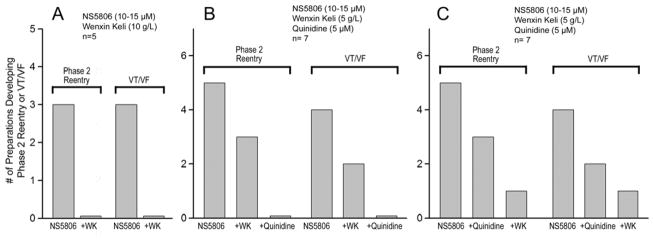
Incidence of Phase 2 Reentry or ventricular tachycardia/ventricular fibrillation (VF/VT) in response to the indicated treatments. WK=Wenxin Keli.
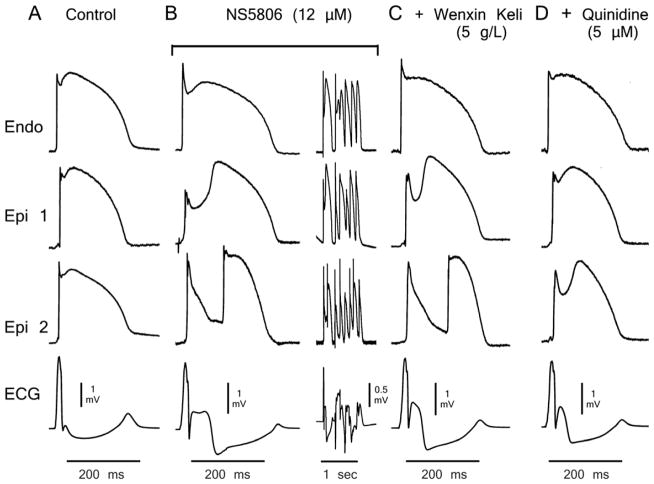
Figure 2 shows AP and ECG recordings obtained from a RV wedge preparation under control conditions (Fig. 2A), following perfusion with 12 μM NS5806 (Fig. 2B) and after addition of WK (5 g/L) (Fig. 2C). Figure 2D illustrates the effect of WK (5 g/L) in the presence of a relatively low concentration of quinidine (5 μM). In this example, WK (5 g/L) alone did not prevent P2R (Fig. 2C), although this arrhythmia mechanism was effectively suppressed when quinidine (5 μM) was added to the perfusate. The lower concentration of WK effectively suppressed P2R and pVT in 60% (3/5) and 50% (2/4) of preparations tested, respectively. The combination of a low concentrations of WK and quinidine suppressed P2R and pVT in 100% of cases (i.e. 5/5 and 4/4, respectively) as illustrated in Figure 4B. Online Table 1 shows composite data of RV wedge preparations following the protocol illustrated in Figure 2.
Figure 2.
Wenxin Keli (5g/L) and quinidine (5 μM) suppression of NS5806-induced Brugada syndrome phenotype. Shown are transmembrane action potentials (APs) simultaneously recorded from two sites on the epicardial (Epi) surface and one site on the endocardial (Endo) surface of a coronary-perfused right ventricular wedge preparation. The bottom trace is the pseudo-electrocardiogram (ECG) recorded across the bath. A: Control. B: The transient outward potassium channel current agonist NS5806 (12 μM) accentuates the Epi AP notch and electrocardiographic J wave, leading to loss of the AP dome at Epi1 but not Epi2. Heterogeneous loss of the dome leads to the development of a closely–coupled phase 2 reentrant extrasystole which precipitates a polymorphic ventricular tachycardia. C: Wenxin Keli (5g/L) suppressed ventricular fibrillation but did not prevent phase 2 reentry. D: The combination of Wenxin Keli (5g/L) and quinidine restored homogeneity and aborted all arrhythmic activity. Basic cycle length=2000 ms.
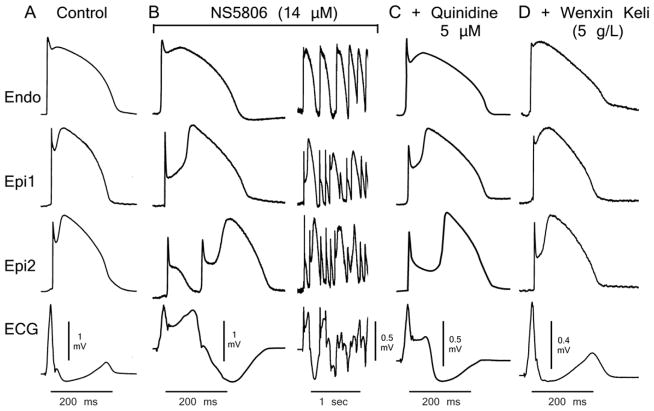
Figure 3 displays AP and ECG traces recorded from a RV preparation under control conditions (Fig. 3A), following perfusion with 14 μM NS5806 (Fig. 3B) and addition of quinidine (5 μM) (Fig. 3C). Figure 3D, illustrates the effect of quinidine in the presence of low concentrations of WK (5 g/L). In this experiment, quinidine alone prevented pVT, but not P2R. Addition of WK (5 g/L) suppressed all arrhythmic activity. A low concentration of quinidine effectively suppressed P2R and pVT in 60% (3/5) and 50% (2/4) of the preparations, respectively. The combination of 5 μM quinidine and 5 g/L WK suppressed P2R and pVT in 80% (4/5) and 75%, respectively (Fig. 4C). Online Table 1 shows composite data of RV wedge preparations based on the experimental protocol illustrated in Figure 3.
Figure 3.
Quinidine (5 μM) and Wenxin Keli (WK 5g/L) suppression of NS5806-induced Brugada syndrome phenotype. Shown are transmembrane action potentials (APs) simultaneously recorded from two sites on the epicardial (Epi) surface and one site on the endocardial (Endo) surface of a coronary-perfused right ventricular wedge preparation. The bottom trace is the pseudo-electrocardiogram (ECG) recorded across the bath. A: Control. B: The transient outward potassium channel current agonist NS5806 (14 μM) accentuates the Epi AP notch and electrocardiographic J wave, leading to loss of the AP dome at Epi1 but not Epi2. Heterogeneous loss of the dome leads to the development of a closely–coupled phase 2 reentrant extrasystole which precipitates a polymorphic ventricular tachycardia. C: Quinidine (5 μM) inhibited ventricular fibrillation but couldn’t inhibit phase 2 reentry. D: The combination of quinidine and WK (5g/L) restored homogeneity and aborted all arrhythmic activity. Basic cycle length=2000 ms.
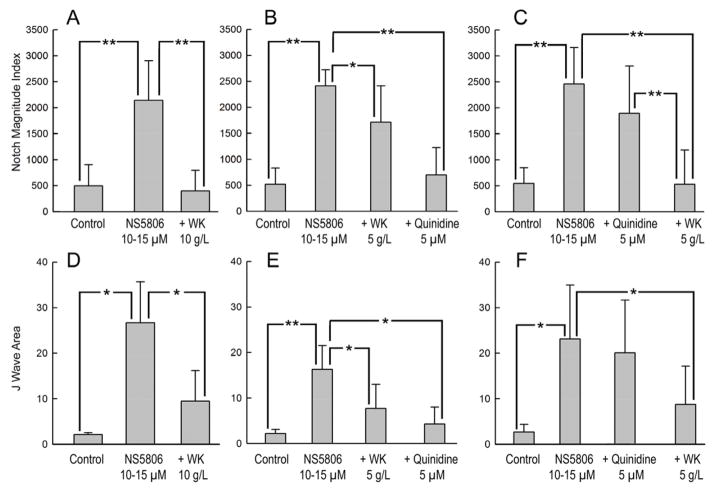
Figure 5 presents summary data of the effect of WK and quinidine to reduce the epicardial action potential notch magnitude and J wave area in the ECG. Notch magnitude index, an estimate of the epicardial action potential notch area and J wave area were significantly increased by NS5806 and dramatically reduced following exposure to WK (10g/L). WK (5 g/L) and quinidine (5 μM) produced more modest reductions in notch index and J wave area, but in combination produced a synergistic effect to dramatically reduce these electrophysiologic parameters.
Figure 5.
Effect of Wenxin Keli (WK) and quinidine on epicardial action potential notch magnitude and J wave area in the electrocardiogram. A and D: Notch magnitude index (NMI) and J wave area are significantly increased by NS5806 and reduced following addition of WK (10g/L) to the coronary perfusate (n=3–4). B and E: NMI and J wave area are significantly increased by NS5806 and significantly reduced by WK (5g/L) and further reduced by the combination of quinidine (5 μM) and WK (5 g/L).(n=5–6). C and F: The NS5806-induced increases in NMI and J wave area are significantly reduced by quinidine (5 μM) and further reduced by WK (5g/L). n=5–6. Values are mean±SD. * P<0.05, **P<0.001.
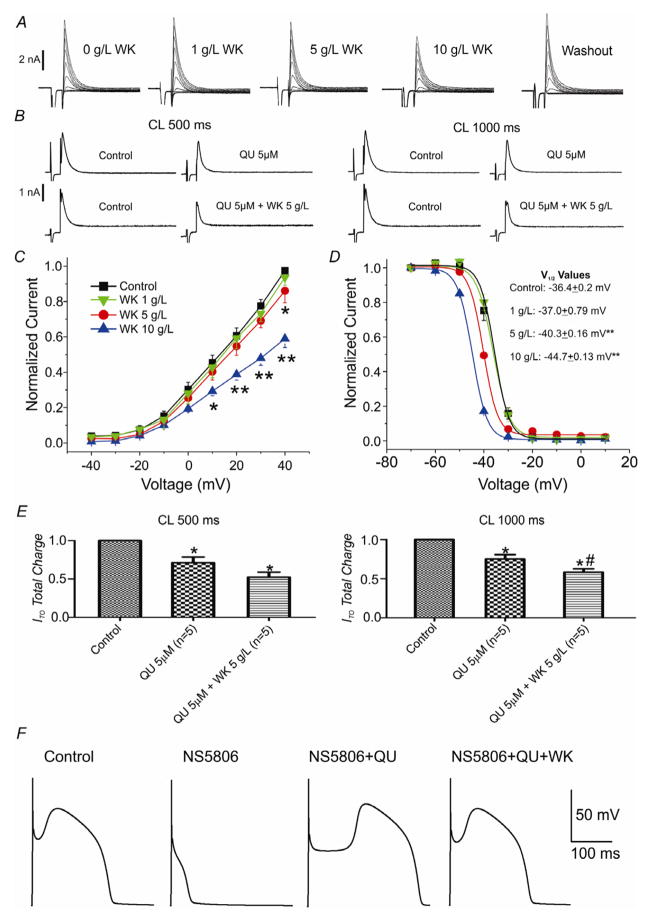
We next examined the effect of WK with and without quinidine on the two major currents contributing to the early phases of the epicardial AP, Ito and ICa. We evaluated these effects in canine left ventricular myocytes using patch clamp techniques (Fig. 6A and B). WK (10g/L) significantly inhibited Ito in a concentration-dependent manner (n=5, p<0.0001). In concentrations of 5g/L and 10g/L, WK caused a shift in steady-state voltage dependence of inactivation of Ito towards negative potentials (Fig. 6D). Quinidine (5 μM) reduced the total charge carried by Ito by 21 (Fig. 6E) (p<0.05), and the combination of WK (5g/L) and quinidine (5 μM) further reduced total charge by a total of 41% at a cycle lengths of 1 sec (Fig. 6E) (p<0.05). To better evaluate the combined effect of Ito block by quinidine and WK on the AP, we simulated APs from the RV using LRII model (see Methods) and incorporated our experimental findings. Figure 6F shows the effect of NS5806-induced augmentation to cause loss of the epicardial action potential dome and the Ito blocking effects of quinidine alone and together with WK to restore the action potential dome, thus normalizing the AP.
Figure 6.
Effect of Wenxin Keli (WK) and quinidine on the transient outward potassium current (Ito). A: Representative current traces recorded from ventricular cardiomyocytes under control conditions, following increasing concentrations of WK, and after washout. Also shown are Ito traces recorded after exposure to WK (5 g/L) as well as a combination of WK (5 g/L) and quinidine (5 μM) at CLs of 500 and 1000 ms. B: Representative current traces at cycle lengths (CL) of 500 and 1000 ms of Ito comparing effects of 5μM quinidine (QU) and QU+WK with controls.: C: Normalized averaged I-V plot for Ito at different concentration of WK (n=6–10). D: Effect of different concentrations of WK on average steady-state inactivation of Ito. Data where fit with a single Boltzmann function (n=6, 2, 3 and 4 for control, 1g/L, 5g/L and 10 g/L WK, respectively. E: The total charge carried by Ito (relative to control) following exposure to QU (5uM) alone and the combination of QU and WK (5 g/L) at cycle lengths (CL) of 500 and 1000 ms. p<0.05 vs. control. **, p<0.01 vs. control. #, p<0.05 vs. QU (5μM) alone.
F: Right ventricular action potentials simulated using Luo-Rudy II model incorporating normal Ito (1.2 mS/μF) representing baseline “control” conditions, enhanced Ito (2.2 mS/μF) simulating the effect of NS5806, partial block of enhanced Ito (1.6 mS/μF) simulating effect of 5 μM of QU, and further block of Ito (1.3 mS/μF) simulating the combined effect of 5 μM of QU and 5 g/L of WK.
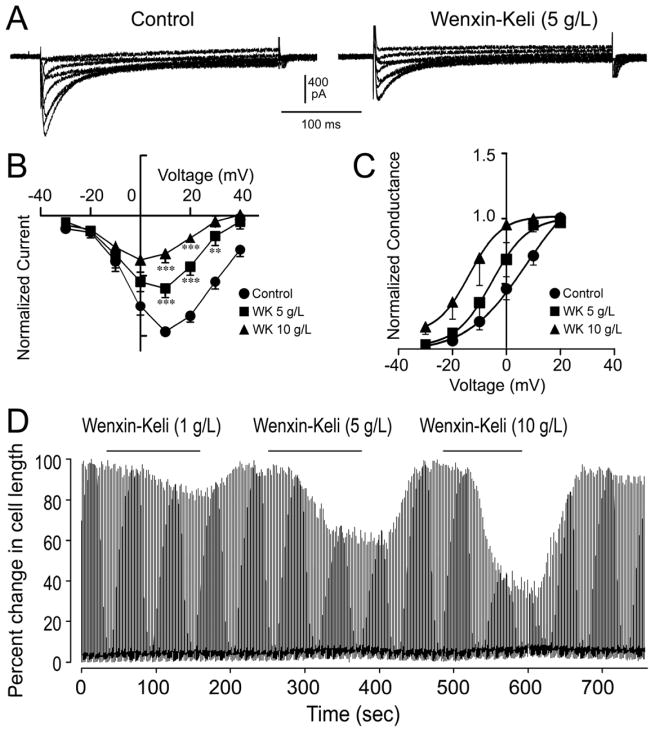
We also evaluated the effects WK on calcium currents. Interestingly, in concentrations of 5 and 10 g/L, WK reduced ICa by 40 % and 60 %, respectively (Fig. 7) and 10g/L WK caused a shift of ICa activation towards negative potentials (Fig. 7C). Consistent with this observation, WK also exhibited a concentration-dependent inhibition of unloaded cell shortening of myocytes. Cell shortening was inhibited by 20%, 35% and 50% in the presence of 1 g/L, 5 g/L and 10 g/L respectively (Fig. 7D).
Figure 7.
Effects of Wenxin Keli (WK) on L-type calcium channel current (ICa). A: Representative current traces in the presence and the absence of 5g/L WK. B: Normalized averaged I-V relationship for ICa at different concentrations of WK (n=4–9). C: Averaged steady-state activation plot for ICa at different concentrations of WK. The normalized conductance vs. voltage plots where fit with a single Boltzmann function (n=9, 5 and 4 for control, 5g/L and 10 g/L WK, respectively). D: Representative example of the inhibitory effects of WK on unloaded cell shortening in field stimulated isolated myocytes. WK (1 g/L, 5g/L, 10g/L) was added sequentially to the bath solution for 120 seconds each during continuous field stimulation at 0.5 Hz.
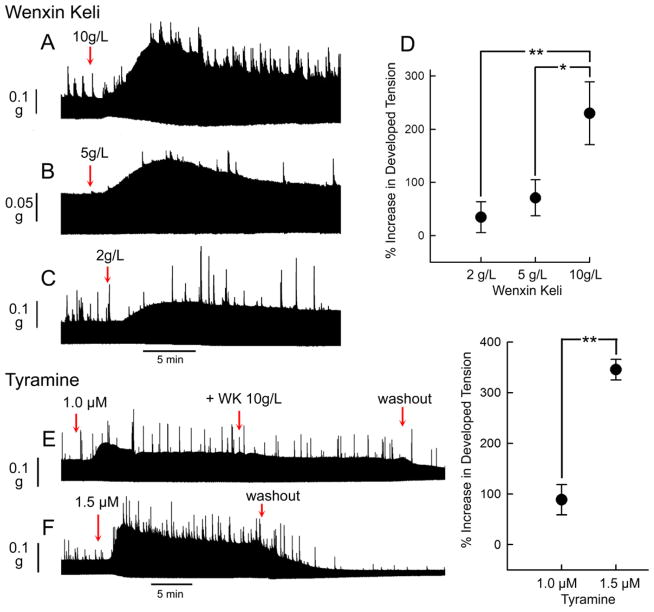
The potent effects of WK to inhibit ICa and to induce a negative inotropic effect in isolated myocytes are at odds with its beneficial effect in the treatment of heart failure. To assess its inotropic effect in the intact ventricular wall, we evaluated the effects of WK in coronary-perfused right ventricular wedge preparations. In sharp contrast to its negative inotropic effect in single myocytes, WK produced a concentration-dependent increase in contractile force in the wedge preparation. Figure 8 illustrates the effect of 2, 5 and 10 g/L of WK on isometric tension development. WK exerted an enduring positive inotropic effect that peaked within 5–10 min at all the concentrations tested. Similar responses to WK were observed in 12/12 preparations. WK produced an average increase in peak-contractile force of 34±29% at 2 g/L (n=3), 71±34% at 5 g/L (n=5) and 230±59% at 10 g/L (n=4; Fig. 8D).
Figure 8.
Effect of Wenxin Keli (WK) and tyramine on isometric tension development in a coronary-perfused right ventricular wedge preparation. A, B and C: WK caused a progressively greater positive inotropic effect at concentrations of 2, 5 and 10 g/L. D: Composite data of effect of 2, 5, and 10 g/L WK to increase developed tension. E and F: Concentration-dependent effect of tyramine (1 and 1.5 μM) to cause a positive inotropic effect. Addition of WK during exposure to tyramine did not produce a further increase in contractile force. * P<0.05, **P<0.001.
To explain the disparity between myocyte and wedge responses to WK, we considered the hypothesis that WK’s positive inotropic effect is due to its ability to release catecholamines from sympathetic nerve ending. As a test of this hypothesis, we exposed the perfused wedge preparations to tyramine, a compound known to induce release of catecholamines from the adrenergic nerve terminals. Tyramine (1.0–1.5 μM) produced a positive inotropic effect recapitulating the action of WK (Figs. 8E and F). The addition of 10 g/L WK during tyramine exposure failed to produce a further increase in contractile force (Fig. 8E; n= 3). Moreover, β adrenergic blockade using propranolol inhibited the positive inotropic effect of tyramine and WK (n=3).
Discussion
An outward shift of current active during the early phases of the AP is known to facilitate the development of the BrS phenotype and is the basis for the genetic predisposition to BrS. We have previously reported that an outward shift in the balance of current active during the early phases of the right ventricular Epi AP by the Ito agonist NS580611 can recapitulate the BrS phenotype in the canine right ventricular wedge preparation.6–8
The present study demonstrates the effect of WK to inhibit or totally suppress the electrocardiographic and arrhythmic manifestations of BrS in a coronary-perfused canine RV wedge model of the syndrome. Our findings suggest that WK’s actions to suppress Phase 2 Reentry and VT/VF involve inhibition of Ito as well as an indirect adrenergic stimulation via a tyramine-like effect. These ameliorative actions of WK are accompanied by normalization of the repolarization defects in the epicardial action potential as well as normalization of the ECG by a diminution of the accentuated J waves and ST segment elevation.
WK is comprised of 5 components including NcBe, Codonopsis, Notoginseng, Amber, and Rhizoma polygonati. Little data are available relative to the effect of the individual components on cardiac ion channel currents. Codonopsis has been reported to cause the inhibition of phosphodiesterase activity, leading to an increase in cAMP in rat myocytes.18 Notoginseng is reported to inhibit Na+/K+-ATPase activity.19 These effects may contribute to the positive inotropic effect of WK. Nordostachys has been reported to protect cardiomyocytes against oxidative injury.20 NcBe is reported to block Ito by 57.9%3 or 69.8%4 in rabbit ventricular myocytes at a concentration of 10 g/L. In our study involving canine ventricular myocytes, 10g/L WK inhibited peak Ito by 40%. Moreover, effect of WK (5 g/L) and quinidine (5 μM) to decrease the total charge carried by Ito was greater than that of quinidine alone (Fig. 6). This is consistent with the enhanced ability of these two drugs when combined to diminish the notch amplitude of the AP, as well as to suppress P2R and pVT. These experimental findings were corroborated by computer simulations of APs from the RV using the LRII model, showing that the combined effect of quinidine and WK is more effective in reducing the AP notch than quinidine alone (Fig. 6E).
In addition we also demonstrate an effect of WK to block ICa by 40 and 60 % at concentrations of 5 and 10 g/L, respectively. The potent effect of WK to block ICa is expected to contribute to an outward shift in the balance of currents active during the early phases of the action potential and thus to accentuate the BrS phenotype.21, 22 Moreover, this action of the drug, which is associated with a negative inotropic effect in isolated myocytes (Fig. 7), is at odds with the ameliorative effects of WK in the management of heart failure.2 Interestingly, we found that WK induces a concentration-dependent positive inotropic effect in the RV wedge preparation and provide evidence that this action of the drug is due to its tyramine-like effect. Tyramine is a naturally occurring monoamine compound that acts as a catecholamine releasing agent. It is taken up by the catecholamine reuptake transporter and thus leads to release of norepinephrine from sympathetic nerve endings. Thus, the indirect adrenergic effect of WK, together with the positive inotropic effects of the other components of WK, totally counters the negative inotropic effect of the drug and converts it into a positive inotropic effect. The positive inotropic effect is likely due principally to an increase in ICa secondary to release of catecholamines. The boost in ICa also contributes to restoration of the action potential dome and suppression of phase 2 reentry and pVT. We first reported the ability of tyramine to restore the AP dome in RV Epi tissue slices in 1990.23
Pharmacologic therapy for BrS is aimed at rebalancing the currents active during the early phases of the epicardial AP, either by increasing inward currents such as ICa or reducing outward currents such as Ito. WK exerts its ameliorative effects in the setting of BrS by reducing Ito as well as increasing ICa via indirect adrenergic stimulation.
Because of its action to inhibit Ito, quinidine was proposed for the management of BrS in 1999.24 Experimental studies have shown quinidine to be effective in restoring the Epi AP dome, thus normalizing the ST segment and preventing P2R and pVT in experimental models of the Brugada syndrome.6 Clinical evidence of the effectiveness of quinidine in normalizing ST segment elevation in patients with BrS has been reported.25–30 At the doses required to treat BrS (900–1500 mg/day), quinidine produces diarrhea in 40–50 % of patients.31
In another series of experiments, we tested the hypothesis that the combination of quinidine and WK, at relatively low concentrations at which adverse effects could be minimized, are effective in suppressing the electrocardiographic and arrhythmic manifestations of BrS. Either drug alone was effective in preventing the development of pVT, but not P2R. The combination of the two drugs effectively suppressed all arrhythmic activity and normalized the ECG.
Study Limitations
As with all data derived from experimental animal models, extrapolation to the clinic must be done with great caution. It is noteworthy that coronary-perfused models of BrS have been well characterized and have consistently been shown to recapitulate the clinical ECG and arrhythmic manifestations of BrS as well as the response to drugs. Extrapolation of the data from these in vitro models to the clinic must be done with care since a number of assumptions must be considered. Among these is the supposition that pretreatment with NS5806 is a reasonable surrogate for elevated levels of Ito that may be encountered physiologically or pathophysiologically in association with a genetic predisposition to BrS. Mutations in three genes associated with BrS have been shown to cause a gain of function in Ito and two others have been described in preliminary reports, suggesting that this mechanism of BrS is not a very rare one.
It is also important to point out that the degree to which the effectiveness of the combination of WK and quinidine depends on a BrS mechanism involving a gain of function of Ito is not known. However, it is noteworthy that in preliminary experiments, we have obtained qualitatively similar results with other models of BrS, including those involving inhibition of ICa (unpublished observation).
Finally, WK is a complex mixture of plant extracts and little is known about the effects of the individual components and their interaction with other medications. The precise concentration of active ingredients in solution is difficult to gauge since only a fraction of the herbal mixture makes it into solution with much of it filtered away. Consequently, it is difficult to reconcile the dose to which the experimental preparations are exposed with clinical dose or with plasma levels that are achieved clinically.
Supplementary Material
Acknowledgments
Funding Sources: This study was supported by a grant from the Buchang group as well as grant HL47678 from NHLBI, NRSA fellowship grant #F32-HL107029, NYSTEM grant #C026424, Grant-in-Aid (10GRNT4210016) from the American Heart Association and the Masons of New York, Florida, Massachusetts and Connecticut.
We gratefully acknowledge the technical assistance of Judy Hefferon and Robert Goodrow.
Abbreviations
- APD
action potential duration
- APD90
action potential duration at 90% duration
- AP
action potential
- BrS
Brugada syndrome
- Epi
epicardial
- ICa
L-type calcium current
- INa
transient outward sodium current
- Ito
transient outward potassium current
- LRII
Luo-Rudy II
- NcBe
nardostachy chinenis batal extract
- P2R
phase 2 reentry
- pVT
polymorphic ventricular tachycardia
- RV
right ventricle
- VF
ventricular fibrillation
- VT
ventricular tachycardia
- WK
Wenxin Keli
Footnotes
Disclosures: This study was in part supported by Buchang Group, who markets Wenxin Keli..
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Burashnikov A, Petroski A, Hu D, Barajas-Martinez H, Antzelevitch C. Atrial-selective inhibition of sodium channel current by Wenxin Keli is effective in suppressing atrial fibrillation. Heart Rhythm. 2012;9:125–31. doi: 10.1016/j.hrthm.2011.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhou F, Hu SJ, Mu Y. Protection effect of Wenxin Keli on isoproterenol induced heart failure in rats. Zhongguo Zhong Yao Za Zhi. 2007;32:1676–9. [PubMed] [Google Scholar]
- 3.Liu YW, Guo JH, Zhang P, Li C. The effects of nardostachys chinensis beta 1 extract on the sodium current and transient outward potassium current of rat ventricular myocytes. Chin J Cardiac Paceing Electrophysiol. 2009;23:533–5. [Google Scholar]
- 4.Tang QZ. The effect of NcBe from Buchangwenxin on potassium channels in single rabbit ventricular myocytes. Chin Physician Tribune. 2008;9:56–7. [Google Scholar]
- 5.Wang X, Wang X, Gu Y, Wang T, Huang C. Wenxin Keli attenuates ischemia-induced ventricular arrhythmias in rats: Involvement of Ltype calcium and transient outward potassium currents. Mol Med Report. 2013;7:519–24. doi: 10.3892/mmr.2012.1195. [DOI] [PubMed] [Google Scholar]
- 6.Yan GX, Antzelevitch C. Cellular basis for the Brugada syndrome and other mechanisms of arrhythmogenesis associated with ST segment elevation. Circulation. 1999;100:1660–6. doi: 10.1161/01.cir.100.15.1660. [DOI] [PubMed] [Google Scholar]
- 7.Fish JM, Welchons DR, Kim YS, Lee SH, Ho WK, Antzelevitch C. Dimethyl lithospermate B, an extract of danshen, suppresses arrhythmogenesis associated with the Brugada syndrome. Circulation. 2006;113:1393–400. doi: 10.1161/CIRCULATIONAHA.105.601690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Minoura Y, Di Diego JM, Barajas-Martinez H, et al. Ionic and cellular mechanisms underlying the development of acquired Brugada syndrome in patients treated with antidepressants. J Cardiovasc Electrophysiol. 2012;23:423–32. doi: 10.1111/j.1540-8167.2011.02196.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Di Diego JM, Antzelevitch C. Pinacidil-induced electrical heterogeneity and extrasystolic activity in canine ventricular tissues. Does activation of ATP-regulated potassium current promote phase 2 reentry? Circulation. 1993;88:1177–89. doi: 10.1161/01.cir.88.3.1177. [DOI] [PubMed] [Google Scholar]
- 10.Barajas-Martinez H, Hu D, Ferrer T, et al. Molecular genetic and functional association of Bugada and early repolarization syndromes with S422L missense mutation in KCNJ8. Heart Rhythm. 2012;9:548–55. doi: 10.1016/j.hrthm.2011.10.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Calloe K, Cordeiro JM, Di Diego JM, et al. A transient outward potassium current activator recapitulates the electrocardiographic manifestations of Brugada syndrome. Cardiovasc Res. 2009;81:686–94. doi: 10.1093/cvr/cvn339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Antzelevitch C, Yan GX. J wave syndromes. Heart Rhythm. 2010;7:549–58. doi: 10.1016/j.hrthm.2009.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Di Diego JM, Sicouri S, Myles RC, Burton FL, Smith GL, Antzelevitch C. Optical and electrical recordings from isolated coronary-perfused ventricular wedge preparations. J Mol Cell Cardiol. 2013;54:53–64. doi: 10.1016/j.yjmcc.2012.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zygmunt AC, Goodrow RJ, Antzelevitch C. Sodium effects on 4-aminopyridine-sensitive transient outward current in canine ventricular cells. Am J Physiol. 1997;272:H1–H11. doi: 10.1152/ajpheart.1997.272.1.H1. [DOI] [PubMed] [Google Scholar]
- 15.Hamill OP, Marty A, Neher E, Sakmann B, Sigworth FJ. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981;391:85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- 16.Dumaine R, Towbin JA, Brugada P, et al. Ionic mechanisms responsible for the electrocardiographic phenotype of the Brugada syndrome are temperature dependent. Circ Res. 1999;85:803–9. doi: 10.1161/01.res.85.9.803. [DOI] [PubMed] [Google Scholar]
- 17.Giudicessi JR, Ye D, Tester DJ, et al. Transient outward current (Ito) gain-of-function mutations in the KCND3-encoded Kv4. 3 potassium channel and Brugada syndrome. Heart Rhythm. 2011;8:1024–32. doi: 10.1016/j.hrthm.2011.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Qin LM, Yan YF, Wang ZC. Experimental study on the cardiotonic action of extract from Codonopsis pilosula (Franch)Nannf. Zhongguo Zhong Yao Za Zhi. 1994;19:238–40. [PubMed] [Google Scholar]
- 19.Chen RJ, Chung TY, Li FY, Lin NH, Tzen JT. Effect of sugar positions in ginsenosides and their inhibitory potency on Na+/K+-ATPase activity. Acta Pharmacol Sin. 2009;30:61–9. doi: 10.1038/aps.2008.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Subashini R, Yogeeta S, Gnanapragasam A, Devaki T. Protective effect of Nardostachys jatamansi on oxidative injury and cellular abnormalities during doxorubicin-induced cardiac damage in rats. J Pharm Pharmacol. 2006;58:257–62. doi: 10.1211/jpp.58.2.0014. [DOI] [PubMed] [Google Scholar]
- 21.Fish JM, Antzelevitch C. Role of sodium and calcium channel block in unmasking the Brugada syndrome. Heart Rhythm. 2004;1:210–7. doi: 10.1016/j.hrthm.2004.03.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fish JM, Antzelevitch C. Cellular mechanism and arrhythmogenic potential of T-wave alternans in the Brugada syndrome. J Cardiovasc Electrophysiol. 2008;19:301–8. doi: 10.1111/j.1540-8167.2007.01025.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Litovsky SH, Antzelevitch C. Differences in the electrophysiological response of canine ventricular subendocardium and subepicardium to acetylcholine and isoproterenol. A direct effect of acetylcholine in ventricular myocardium. Circ Res. 1990;67:615–27. doi: 10.1161/01.res.67.3.615. [DOI] [PubMed] [Google Scholar]
- 24.Antzelevitch C, Brugada P, Brugada J, Brugada R, Nademanee K, Towbin J. Clinical approaches to tachyarrhythmias. Armonk NY: Futura Publishing Company; 1999. The Brugada syndrome. [Google Scholar]
- 25.Belhassen B, Viskin S, Antzelevitch C. The Brugada syndrome: is an implantable cardioverter defibrillator the only therapeutic option? Pacing Clin Electrophysiol. 2002;25:1634–40. doi: 10.1046/j.1460-9592.2002.01634.x. [DOI] [PubMed] [Google Scholar]
- 26.Alings M, Dekker L, Sadee A, Wilde A. Quinidine induced electrocardiographic normalization in two patients with Brugada syndrome. Pacing Clin Electrophysiol. 2001;24:1420–2. doi: 10.1046/j.1460-9592.2001.01420.x. [DOI] [PubMed] [Google Scholar]
- 27.Belhassen B, Viskin S. Pharmacologic approach to therapy of Brugada syndrome: quinidine as an alternative to ICD therapy? In: Antzelevitch C, Brugada P, Brugada J, et al., editors. The Brugada Syndrome: From Bench to Bedside. Oxford: Blackwell Futura; 2004. pp. 202–211. [Google Scholar]
- 28.Belhassen B, Viskin S, Fish R, Glick A, Setbon I, Eldar M. Effects of electrophysiologic-guided therapy with Class IA antiarrhythmic drugs on the long-term outcome of patients with idiopathic ventricular fibrillation with or without the Brugada syndrome. J Cardiovasc Electrophysiol. 1999;10:1301–12. doi: 10.1111/j.1540-8167.1999.tb00183.x. [DOI] [PubMed] [Google Scholar]
- 29.Hermida JS, Denjoy I, Clerc J, et al. Hydroquinidine therapy in Brugada syndrome. J Am Coll Cardiol. 2004;43:1853–60. doi: 10.1016/j.jacc.2003.12.046. [DOI] [PubMed] [Google Scholar]
- 30.Mok NS, Chan NY, Chi-Suen CA. Successful use of quinidine in treatment of electrical storm in Brugada syndrome. Pacing Clin Electrophysiol. 2004;27:821–3. doi: 10.1111/j.1540-8159.2004.00537.x. [DOI] [PubMed] [Google Scholar]
- 31.Mizusawa Y, Sakurada H, Nishizaki M, Hiraoka M. Effects of low-dose quinidine on ventricular tachyarrhythmias in patients with Brugada syndrome: low-dose quinidine therapy as an adjunctive treatment. J Cardiovasc Pharmacol. 2006;47:359–64. doi: 10.1097/01.fjc.0000206437.27854.65. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.