Abstract
Tumor cells accumulate high level of reactive oxygen species (ROS) because they are metabolically more active than normal cells. Elevated ROS levels increase tumorigenecity but also render cancer cells more vulnerable to oxidative stress than normal cells. The oncogenic transcription factor Forkhead Box M1 (FOXM1), which is overexpressed in a wide range of human cancers, was reported to protect cancer cells from the adverse effects of oxidative stress by up regulating the expression of scavenger enzymes. We therefore hypothesized that the combination of FOXM1 ablation and ROS inducers could selectively eradicate cancer cells. We show that RNA interference–mediated knockdown of FOXM1 further elevates intracellular ROS levels and increases sensitivity of cancer cells to ROS-mediated cell death after treatment with ROS inducers. We also demonstrate that the combination of ROS inducers with FOXM1/proteasome inhibitors induces robust apoptosis in different human cancer cells. In addition, we show evidence that FOXM1/proteasome inhibitor bortezomib in combination with the ROS inducer β-phenylethyl isothiocyanate efficiently inhibits the growth of breast tumor xenografts in nude mice. We conclude that the combination of ROS inducers and FOXM1 inhibitors could be used as a therapeutic strategy to selectively eliminate cancer cells.
Reactive oxygen species (ROS) can be generated as by-products of oxidative phosphorylation and also after environmental stress by exogenous sources, such as ionizing radiation, UV light, and redox chemicals.1 ROS are highly reactive and are usually considered to be harmful because they can damage proteins, lipids, and DNA.1,2 Consequently, cells to protect themselves from the adverse effects of ROS have developed a complex antioxidant defense system.1 However, ROS have also been recognized to play an important role in many different physiologic processes, such as proliferation, cell signaling, metabolism, aging, cell death, and cancer.2 Oxidative stress occurs when the balance between ROS production and detoxification is compromised and the generation of ROS overcomes the antioxidant defense system of the cell.1,3
In cancer treatment it is a daunting challenge to selectively eradicate cancer cells but spare normal cells. An alternative approach to achieve this goal is to take advantage of the biochemical alterations in cancer cells instead of targeting one specific oncogene.4 One common biochemical alteration in cancer cells is that they accumulate higher level of ROS due to their increased metabolic activity.5 Elevated ROS levels accelerate protumorigenic signaling pathways and increase mutation rates, thereby enhancing tumorigenesis. However, the high levels of ROS in cancer cells also render them more prone to oxidative stress and more dependent on their antioxidant system.4 Studies have reported that such an abnormal increase in ROS could be exploited to preferentially kill cancer cells by inducing oxidative stress.4,6
Mammalian, oncogenic transcription factor Forkhead Box M1 (FOXM1) has a well-defined role in cell proliferation and cell cycle progression.7 Expression of FOXM1 is excluded in resting or differentiated cells, but its level is highly elevated in proliferating and malignant cells, and also in different human cancers.7 In recent years FOXM1 has been implicated in diverse cellular processes,8 including oxidative stress.9 FOXM1 was identified as a pivotal regulator of oncogene-induced ROS in cycling cells. FOXM1, by directly regulating the expression of scavenger enzymes, reduces intracellular ROS levels, thus protecting tumor cells from oxidative stress and allowing their proliferation.9 Because FOXM1 is so abundantly expressed in human cancers, the authors of the study postulated that cancer cells become accustomed to elevated ROS levels by the overexpression of FOXM1.9
Recently, we reported that repression of FOXM1 sensitizes human cancer cells to DNA damage.10 In this study, we examined the combinatorial effect of FOXM1 suppression in conjunction with oxidative stress on cell death in vitro and xenograft tumor growth in vivo. We found that RNA interference (RNAi)–mediated knockdown of FOXM1 leads to the elevation of intracellular ROS levels and increases sensitivity to ROS-mediated cell death after treatment with ROS inducers, including β-phenylethyl isothiocyanate (PEITC) and 2-methoxyestradiaol (2-ME) in different human cancer cells. Moreover, we found that the combination of ROS inducers with proteasome inhibitors that target FOXM1 induces robust apoptosis and efficiently inhibits the growth of breast tumor xenografts in nude mice.
Materials and Methods
Cell Culture and Chemical Compounds
The MIA PaCa-2 pancreatic (ATCC, Manassas, VA) and the HepG2 liver (ATCC) human cancer cell lines were grown in Dulbecco's modified Eagle's medium (DMEM; Invitrogen, Carlsbad, CA). The MDA-MB-231 (ATCC) breast human cancer cell line was grown in RPMI medium (Invitrogen). The media were supplemented with 10% fetal bovine serum (FBS; Atlanta Biologicals, Atlanta, GA) and 1% penicillin-streptomycin (Gibco, Grand Island, NY). BJ primary human foreskin fibroblasts (ATCC) and BJ oncogenic cells (Dr. William Hahn, Harvard Medical School, Boston, MA) were grown in knockout DMEM and medium 199 (Invitrogen) supplemented with 15% FBS (Atlanta Biologicals), 2 mmol/L l-glutamine (Gibco), and 1% penicillin-streptomycin (Gibco). All of the cells were maintained at 37°C in 5% CO2. Stable cell lines using the ATCC-obtained parental cells were generated by transduction of control and FOXM1 shRNA lentiviral particles (Sigma, St. Louis, MO) followed by selection with puromycin (Sigma). PEITC (Sigma), 2-ME (Sigma), thiostrepton (Sigma), and bortezomib (Velcade; Millenium Pharmaceuticals, St. Louis, MO) were dissolved in dimethyl sulfoxide (DMSO; Fisher Scientific, Chicago, IL), H2O2 (Fisher Scientific) in culture media, N-acetyl-l-cysteine (NAC) (Sigma), and glutathione (Sigma) in deionized water.
Immunoblot Analysis
Treated cells and tumor tissues were harvested and lysed by using IP buffer [20 mmol/L HEPES, 1% Triton X-100, 150 mmol/L NaCl, 1 mmol/L EDTA, 1 mmol/L EGTA, 100 mmol/L NaF, 10 mmol/L Na4P2O7, 1 mmol/L sodium orthovanadate, and 0.2 mmol/L phenylmethylsulfonyl fluoride supplemented with protease inhibitor tablet (Roche Applied Sciences, Indianapolis, IN)]. Protein concentration was determined by the Bio-Rad protein assay reagent (Bio-Rad, Hercules, CA). Isolated proteins were separated on SDS-PAGE and transferred to polyvinylidene difluoride membrane (PVDF; Millipore, Billerica, MA). Immunoblotting was performed with antibodies specific for FOXM1 (the rabbit polyclonal antibody against FOXM1 was described previously11), cleaved caspase-3 (Cell Signaling, Danvers, MA), poly (ADP-ribose) polymerase (PARP) 1/2 (Santa Cruz Biotechnology, Santa Cruz, CA), catalase (Abcam, Cambridge, MA), MnSOD (Stressgen, San Diego, CA), and β-actin (Sigma).
Transfection and siRNA
Control (5'-AACAGUCGCGUUUGCGACUGGUU-3') siRNA and siRNA specific to FOXM1 (5'-GGACCACUUUCCCUACUUUUU-3') were synthesized by Sigma. Fifty nanomolar siRNA duplexes were transfected into cells using Lipofectamine 2000 (Invitrogen) according to the manufacturer's recommendation. Cells were treated as indicated 48 or 72 hours after transfection.
Adenoviral Infection
Human cancer cells were infected with adenoviral particles (Gene Transfer Vector Core, University of Iowa, Ames, IA) at MOI 100 in FBS free media for 1 hour. Then the FBS concentration was brought up to 10% and additional media was added. Forty-eight hours after infection the cells were treated as indicated.
Flow Cytometry
Propidium Iodide Staining
Cells were treated as indicated and harvested by trypsinization. Then cells were washed in PBS and fixed in ice-cold 95% ethanol. After fixation, cells were stained with 50 μg/mL of propidium iodide (Invitrogen) in PBS/RNase A/Triton X-100 for 30 minutes at room temperature and analyzed by flow cytometry.
Annexin V-PE/7-AAD Staining
Cells were stained using the Annexin V–phycoerythrin (PE) apoptosis detection kit (Enzo Life Sciences, Farmingdale, NY). Harvested cells were washed in PBS and resuspended in binding buffer, and 5 μL of Annexin V–PE and 5 μL of 7-AAD were added. After 15 minute incubation in the dark at room temperature, the samples were analyzed by flow cytometry.
ROS Measurement
Cells were incubated with 10 μmol/L H2DCF-DA dye (Molecular Probes) for 30 minutes. Then cells were washed with PBS, trypsinized, and analyzed for intracellular ROS production by flow cytometry.
CI Measurement by the Chou-Talalay Median-Effect Method
Serial dilutions of each drug were made and combined from the lowest to the highest concentration, while measuring the cell fraction affected. In our experiments, IC20 to IC95 (fractional effect 0.2 to 0.95) values (drug concentrations needed to cause 20% to 95% reduction in cell viability) were used for measurements. The percentage of viable cells was assessed by the standard MTT assay. Combination index (CI) values were calculated with the formula described in the article by Chou.12 CI values of <1 indicate synergy, a value of 1 additive effects, and values of >1 antagonism.
Anchorage-Dependent Growth Assay (Colony-Forming Assay)
A total of 1 × 105 cells were plated on 100 mm dishes in duplicate and were treated as indicated for 24 hours. Colonies were allowed to form for 10 days, and then cells were stained with crystal violet. Quantification of colonies was performed with ImageJ (NIH).
Xenograft Animal Experiment
Four-week-old, male, athymic, nude mice were purchased from Taconic (Petersburgh, NY). Bilaterally, 2 × 106 MDA-MB-231 cells per site in a 100 μL mixture of 70% Matrigel (BD Biosciences, San Jose, CA) and 30% PBS were injected s.c. in the flank region. After tumors became palpable, tumor size was measured once a week using a Vernier caliper, and tumor volume was calculated with the following formula: (length × width × height)/2. When tumors reached 70 mm3 in size, mice were divided into the following treatment groups: control (untreated), PEITC (25 mg/kg), bortezomib (0.4 mg/kg), and the combination of PEITC and bortezomib. PEITC was dissolved in a solvent that consisted of ethanol, Cremophor-EL (Sigma), and PBS in a ratio of 1:1:8 and bortezomib in saline. All drugs were administered i.p. 4 to 5 times per week up to 25 injections. At the completion of the study, mice were sacrificed by CO2 inhalation followed by cervical dislocation, and tumors were excised. All of the procedures that involved animals were in accordance with and approved by the Animal Care and Use Committee of the University of Illinois at Chicago.
Results
Knockdown of FOXM1 Sensitizes Human Cancer Cells to Oxidative Stress–Induced Cell Death
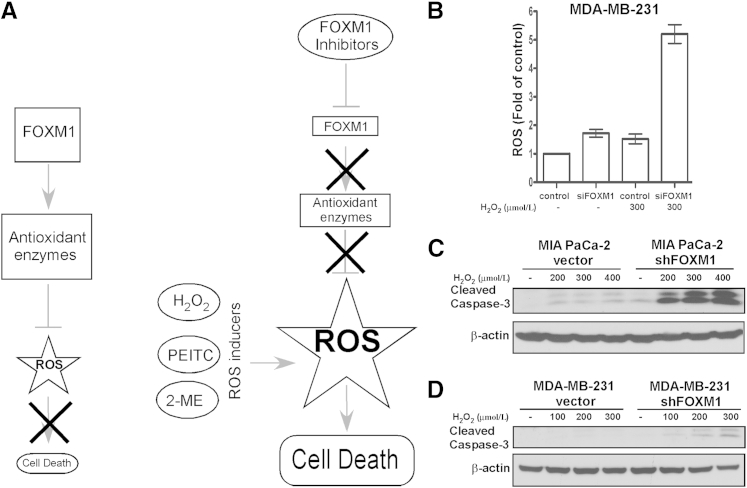
In general, cancer cells exhibit higher level of ROS as a result of their increased metabolic activity.5 It has been suggested that malignant cells might adapt to elevated ROS levels by overexpressing FOXM1. FOXM1 down regulates ROS levels via direct activation of the transcription of antioxidant enzymes, thus protecting tumor cells from oxidative stress9 (Figure 1A). Consequently, suppression of FOXM1 would counteract its inhibitory action on ROS production, and the combination of ROS inducers with FOXM1 inhibition could elevate ROS beyond threshold level, leading to the death of malignant cells (Figure 1A). Because normal cells express very low level of FOXM1 and are not as dependent on their antioxidant system as tumor cells, this treatment strategy would primarily target cancer cells and spare normal cells.
Figure 1.
Suppression of FOXM1 by RNAi sensitizes human cancer cells to H2O2-induced oxidative stress. A: Treatment strategy. FOXM1 reduces intracellular ROS levels by inducing the expression of ROS scavengers. Co-treatment with ROS inducers and FOXM1 inhibitors elevates the levels of ROS, leading to cell death. B: Breast cancer cells MDA-MB-231 were transfected with control and FOXM1 siRNA for 72 hours, then treated with 300 μmol/L H2O2 and 10 μmol/L H2DCF-DA dye for 30 minutes. Intracellular ROS production was determined by flow cytometry. Graph shows quantification as fold of ROS induction compared with control cells, means ± SD of a representative triplicate experiment. C: MIA PaCa-2 vector control and FOXM1 knockdown pancreatic cancer cells were treated with the indicated concentrations of H2O2. Twenty-four hours after treatment, cells were harvested and immunoblotting was performed with antibodies against cleaved caspase-3. β-Actin was used as the loading control. D: MDA-MB-231 vector control and FOXM1 knockdown breast cancer cells were treated with H2O2 as indicated. Twenty-four hours after treatment, cells were harvested and immunoblotting was performed for cleaved caspase-3 and β-actin as the loading control.
In agreement with earlier studies,9 we also observed that transient depletion of FOXM1 results in higher ROS levels compared with control cells as assessed by flow cytometry after incubation with dichlorodihydrofluorescein diacetate (H2DCF-DA) dye (Figure 1B). We also found that exposure to H2O2 notably increased intracellular ROS levels in FOXM1-deficient breast cancer cells relative to treated control cells (Figure 1B). In addition, cell death was detected by immunoblotting for cleaved caspase-3, a marker of apoptosis (Figure 1, C and D) after treatment with different concentrations of H2O2 in human cancer cells of different origin harboring stable knockdown of FOXM1. We found that knockdown of FOXM1 increased sensitivity of pancreatic and breast cancer cells to H2O2-mediated apoptosis. These data suggest that in FOXM1-depleted human cancer cells elevated ROS levels associate with increased cell death after exposure to oxidative stress.
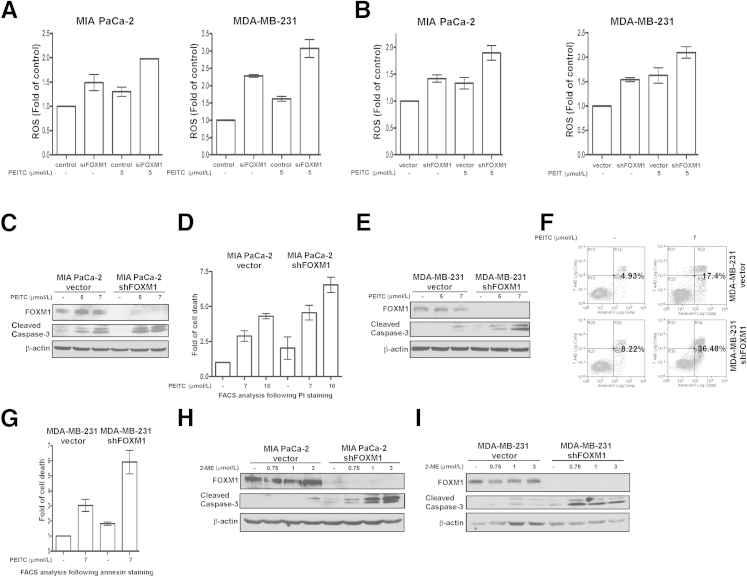
Next, we examined whether FOXM1 suppression sensitizes cancer cells to oxidative stress induced by other types of ROS inducers. To this end, we treated transient and stable FOXM1 knockdown pancreatic and breast cancer cells along with their corresponding control counterparts with PEITC. PEITC is a natural compound found in cruciferous vegetables with chemopreventive activity that has been found to increase ROS generation13 and to induce apoptosis in cancer cell lines.14,15 It is currently in clinical trials. We measured ROS levels by flow cytometry and found that treatment with PEITC also generated more ROS in FOXM1 knockdown cells compared with treated control cells (Figure 2, A and B). We also observed that cells with FOXM1 knockdown were more susceptible to apoptosis after PEITC treatment as detected by immunoblotting for cleaved caspase-3 (Figure 2, C and E) and by flow cytometry after propidium iodide (Figure 2D) or Annexin V staining (Figure 2, F and G). To confirm these effects, the pancreatic and breast cancer cells with stable FOXM1 knockdown were treated with 2-ME, another ROS inducer,16 and also in clinical trials. 2-ME treatment also elevated ROS levels in FOXM1 knockdown cells compared with their treated control counterparts (data not shown). In addition, Western blot analysis of cleaved caspase-3 demonstrated that FOXM1 knockdown pancreatic and breast cancer cells were more sensitive to 2-ME–induced apoptosis (Figure 2, H and I). Altogether, these data suggest that suppression of FOXM1 further sensitizes cancer cells to oxidative stress–mediated apoptosis induced by different types of ROS inducers.
Figure 2.
Suppression of FOXM1 by RNAi further elevates ROS levels and increases cell death after treatment with known ROS inducers. A: MIA PaCa-2 pancreatic and MDA-MB-231 breast cancer cells were transfected with control and FOXM1 siRNA for 72 hours, then treated with 5 μmol/L PEITC for 3 hours. After treatment, cells were incubated with 10 μmol/L H2DCF-DA dye for 30 minutes and analyzed for intracellular ROS production by flow cytometry. Graph shows quantification as fold of ROS induction compared with control cells, means ± SEM of two (left) or three (right) independent experiments, respectively. B: MIA PaCa-2 pancreatic and MDA-MB-231 breast vector control and FOXM1 knockdown cancer cells were treated with PEITC as indicated. Three hours after treatment, cells were incubated with 10 μmol/L H2DCF-DA dye for 30 minutes, and intracellular ROS production was measured by flow cytometry. Graph shows quantification as fold of ROS induction compared with control cells, means ± SEM of four (left) or two (right) independent experiments, respectively. C: MIA PaCa-2 vector control and FOXM1 knockdown pancreatic cancer cells were treated with the indicated concentrations of PEITC. Twenty-four hours after treatment, cell lysates were immunoblotted for FOXM1, cleaved caspase-3, and β-actin as the loading control. D: Degree of cell death was assessed by flow cytometry after propidium iodide staining in MIA PaCa-2 pancreatic vector control and FOXM1 knockdown cancer cells 6 hours after PEITC treatment. Graph shows quantification as fold of cell death induction compared with control cells, means ± SEM of two independent experiments. E: MDA-MB-231 vector control and FOXM1 knockdown breast cancer cells were treated with PEITC as indicated. Immunoblot analysis was performed for FOXM1, cleaved caspase-3, and β-actin as the loading control, 24 hours after treatment. F: MDA-MB-231 breast vector control and FOXM1 knockdown cancer cells were treated with 7 μmol/L PEITC for 24 hours. Cell death was determined by PE Annexin V/7-AAD assay. G: Graph shows quantification as fold of cell death induction compared with control cells, means ± SEM of three independent experiments under the same conditions as in F. H: Pancreatic MIA PaCa-2 vector control and FOXM1 knockdown cancer cells were treated with the indicated concentrations of 2-ME. Twenty-four hours after treatment, cell lysates were immunoblotted for FOXM1, cleaved caspase-3, and β-actin as the loading control. I: Breast MDA-MB-231 vector control and FOXM1 knockdown cancer cells were treated with 2-ME as indicated. Immunoblot analysis was performed for FOXM1, cleaved caspase-3, and β-actin as the loading control 24 hours after treatment.
PEITC-Induced Apoptosis in FOXM1 Knockdown Cells Is ROS Dependent
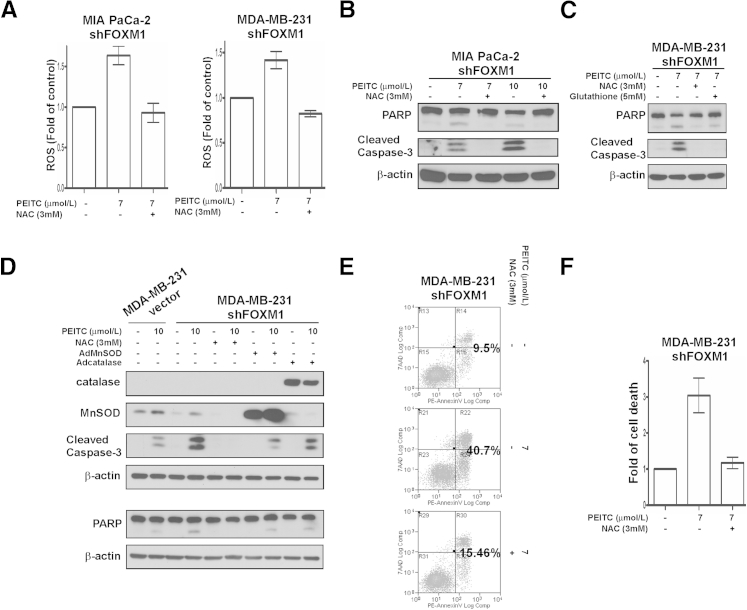
To verify that PEITC-induced cell death is ROS mediated in the cell lines tested, MIA PaCa-2 pancreatic and MDA-MB-231 breast FOXM1 knockdown cancer cells were treated with different concentrations of PEITC in the presence or absence of various antioxidants, including NAC, glutathione, catalase, and MnSOD. We determined ROS levels by flow cytometry and found that PEITC treatment elevated ROS levels and that the antioxidants NAC (Figure 3A), catalase, and MnSOD (data not shown) efficiently prevented PEITC-induced ROS accumulation. Next, apoptosis was determined by immunoblotting for cleaved caspase-3 or PARP (Figure 3, B–D) and by flow cytometry after Annexin V staining (Figure 3, E and F). We found that NAC and glutathione completely diminished cell death (Figure 3, B and C), whereas overexpression of FOXM1 direct transcriptional targets catalase and MnSOD9 partially protected against cell death (Figure 3D) in the PEITC-treated FOXM1 knockdown cells, suggesting that ROS is indeed involved in PEITC-induced cell death in pancreatic and breast cancer cells after FOXM1 suppression.
Figure 3.
PEITC induces ROS-dependent apoptosis in FOXM1 knockdown cells. A: MIA PaCa-2 pancreatic and MDA-MB-231 breast FOXM1 knockdown cancer cells were preincubated with ROS inhibitor NAC for 2 hours, after which they were treated with PEITC as indicated for 3 hours. Intracellular ROS production was determined by flow cytometry after incubating the cells with 10 μmol/L H2DCF-DA dye for 30 minutes. Graph shows quantification as fold of ROS induction compared with control cells, means ± SEM of two independent experiments. B: MIA PaCa-2 pancreatic FOXM1 knockdown cancer cells were preincubated with ROS inhibitor NAC for 2 hours then treated with the indicated concentrations of PEITC. Twenty-four hours after treatment, cell lysates were immunoblotted for PARP, cleaved caspase-3, and β-actin as the loading control. C: MDA-MB-231 breast FOXM1 knockdown cancer cells were preincubated with ROS inhibitors NAC and glutathione for 3 hours then treated with PEITC as indicated. Twenty-four hours after treatment, cell lysates were immunoblotted for PARP, cleaved caspase-3, and β-actin as the loading control. D: MDA-MB-231 breast FOXM1 knockdown cancer cells were infected with MnSOD or catalase adenoviral particles for 48 hours or were preincubated with NAC for 1 hour. Then the vector control and FOXM1 knockdown breast cancer cells were treated with PEITC as indicated for 10 hours. Immunoblotting was performed with antibodies specific for anti-catalase, MnSOD, cleaved caspase-3, and PARP. β-Actin was used as the loading control. E: MDA-MB-231 breast FOXM1 knockdown cancer cells were preincubated with NAC for 2 hours and then treated with 7 μmol/L PEITC for 24 hours. Cell death was determined by PE Annexin V/7-AAD assay. F: Graph shows quantification as fold of cell death induction compared with control cells, means ± SD of a representative duplicate experiment under the same conditions as in E.
Treatment With Proteasome Inhibitors Sensitizes Human Cancer Cells to Apoptosis Induced by Oxidative Stress
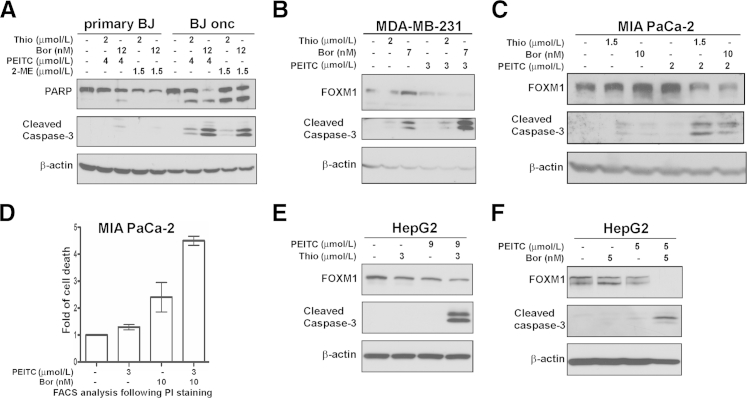
We previously identified the thiazole antibiotics thiostrepton and Siomycin A as potent FOXM1 inhibitors17-20 and found that they also exhibit proteasome inhibitory activity.21 More importantly, we found that bona fide proteasome inhibitors such as bortezomib (Velcade) and MG132 also inhibit FOXM1.21 To test FOXM1/proteasome inhibitors together with ROS inducers, we examined whether this combinatorial treatment has specificity toward transformed cells compared with normal cells. We observed that treatment of isogenic human foreskin fibroblast cells with thiostrepton or bortezomib in combination with PEITC or 2-ME induced apoptosis in the Ras-transformed oncogenic cells but not in the primary fibroblasts as detected by the cleavage of caspase-3 and PARP (Figure 4A). This observation suggests that combination of FOXM1/proteasome inhibitors and ROS inducers might specifically kill cancer cells and spare normal ones, thus reducing their potential toxicity in the treatment of human cancer.
Figure 4.
Combination of FOXM1/proteasome inhibitors and ROS inducers sensitizes human cancer cells to programmed cell death. A: Primary BJ fibroblasts and BJ oncogenic cells were treated with thiostrepton (thio) or bortezomib (bor) in combination with PEITC or 2-ME for 24 hours. Immunoblotting was performed with antibodies specific for anti-PARP and cleaved caspase-3. β-Actin was used as the loading control. B: MDA-MB-231 breast cancer cell line was treated with PEITC in combination with thio or bor as indicated for 24 hours. Cell lysates were analyzed by immunoblotting with antibodies specific for anti-FOXM1, cleaved caspase-3, and β-actin as the loading control. C: Pancreatic cancer cell line MIA PaCa-2 was treated with the indicated concentrations of PEITC, thio, and bor alone or in combination. Twenty-four hours after treatment, cell lysates were analyzed by immunoblotting with anti-FOXM1, cleaved caspase-3, and β-actin antibodies. D: Cell death was assessed by flow cytometry after propidium iodide staining in MIA PaCa-2 pancreatic cancer cells 24 hours after the indicated treatments. Graph shows quantification as fold of cell death induction compared with control cells, means ± SEM of two independent experiments. E: HepG2 liver cancer cells were treated with PEITC and thio alone or in combination as indicated for 24 hours. Immunoblot analysis was performed with anti-FOXM1, cleaved caspase-3, and β-actin antibodies. F: HepG2 liver cancer cells were treated with PEITC and bor alone or in combination with the indicated concentrations for 24 hours. Immunoblotting was performed with antibodies specific for anti-FOXM1, cleaved caspase-3, and β-actin as the loading control.
Next, we assessed the sensitivity of breast, pancreatic, and liver cancer cells to cell death induced by the combination of subapoptotic concentrations of proteasome inhibitors and ROS inducers. To this end, MDA-MB-231 breast, MIA PaCa-2 pancreatic, and HepG2 liver cancer cell lines were treated with the combination of PEITC and thiostrepton or bortezomib (Figure 4, B–F), respectively. We found that thiostrepton and bortezomib in conjunction with PEITC decreased FOXM1 expression (Figure 4, B, C, E, F) and induced cell death compared with single drug treatment in these particular cancer cells as depicted by cleaved caspase-3 (Figure 4, B, C, E, F) and by flow cytometry after propidium iodide staining (Figure 4D).
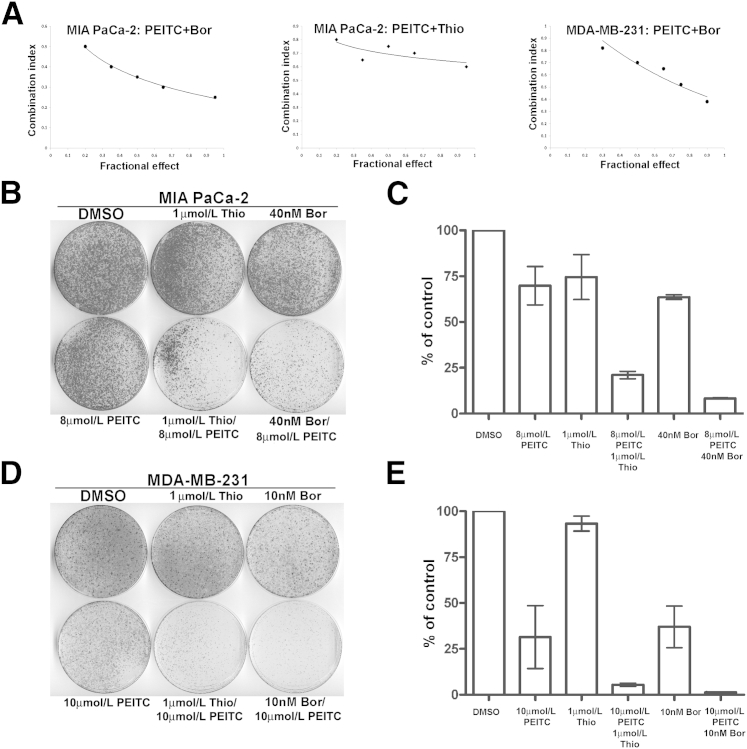
In addition, the synergistic nature of the interaction between PEITC and bortezomib or thiostrepton was quantitatively validated after measurement of cell viability after single and combination drug treatments using the Chou-Talalay median-effect equation method. CI values <1 indicate a synergistic antiproliferative effect. The CI values for the combined treatments with PEITC/bortezomib in MIA PaCa-2 pancreatic and MDA-MB-231 breast (Figure 5A) cancer cells range from 0.25 to 0.80 for fractional effect corresponding to 0.95 to 0.20, suggesting a strong synergistic effect. Similarly, the CI values for the combined treatments with PEITC/thiostrepton in MIA PaCa-2 cancer cells range from 0.60 to 0.80 for fractional effect, corresponding to 0.95 to 0.20 (Figure 5A).
Figure 5.
Combination of FOXM1/proteasome inhibitors and ROS inducer PEITC synergistically inhibits cell viability and long-term colony formation. A: Combination of PEITC with bortezomib (bor) or thiostrepton (thio), respectively, is synergistic in inhibiting proliferation of cancer cells. The CI chart for the PEITC/bor or PEITC/thio drug combinations was plotted with CI on the y axis and fractional effect on the x axis. Percentage inhibition of viability was determined after treatment with a single agent or the PEITC/bor and PEITC/thio combination, respectively. The CI values of the agents are plotted for MIA PaCa-2 pancreatic and MDA-MB-231 breast cancer cells. B: A total of 1 × 105 MIA PaCa-2 human pancreatic cancer cells were plated and treated as indicated for 24 hours. Ten days after treatment, cells were stained with crystal violet and representative plates are shown. C: Graph shows the quantification means ± SD of duplicate experiments. D: A total of 1 × 105 MDA-MB-231 human breast cancer cells were plated and treated as indicated for 24 hours. Ten days after treatment, cells were stained with crystal violet and representative plates are shown. E: Graph shows the quantification means ± SD of duplicate experiments.
Because the combination of FOXM1/proteasome inhibitors and ROS inducers efficiently increased cell death, their combinatorial effect on long-term survival was also tested by performing clonogenic assay. The colony-forming capacity of pancreatic and breast cancer cells was determined after treatment with PEITC in combination with thiostrepton or bortezomib (Figure 5, B–E). We observed that the combination of PEITC with FOXM1/proteasome inhibitors considerably reduced the number of colonies relative to control and single drug treatment. Taken together, these data suggest that combination of oxidative stress and FOXM1 suppression could result in an effective strategy to kill cancer cells.
Combination of Bortezomib and PEITC Inhibits Xenograft Tumor Growth
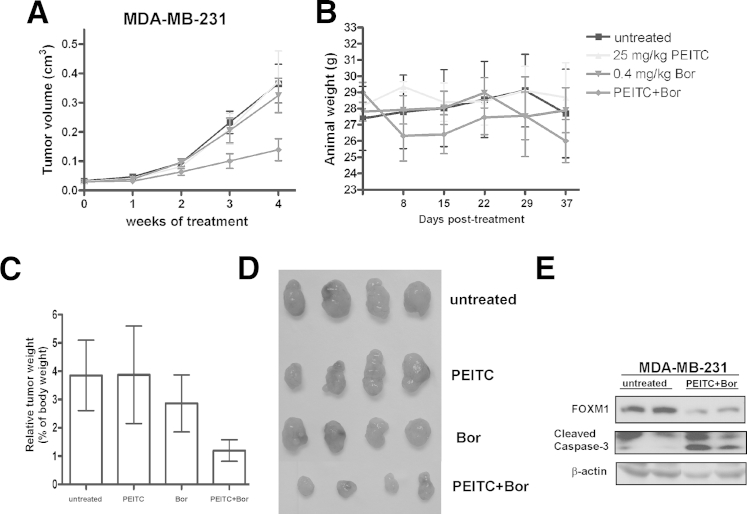
The potential anticancer activity of FOXM1/proteasome inhibitors in combination with ROS inducers was also evaluated in nude mice bearing breast tumor xenografts. Male athymic nude mice were injected s.c. with MDA-MB-231 human breast cancer cells in the flank region bilaterally to establish xenograft tumors. After tumors became palpable, their size was measured by caliper and the animals were randomized into the following treatment groups: (1) untreated, (2) PEITC (25 mg/kg), (3) bortezomib (0.4 mg/kg), and (4) PEITC and bortezomib. Animals were injected i.p. with the drugs 4 to 5 times per week. Tumor size was recorded weekly. Upon completion of the study, mice were sacrificed by CO2 inhalation and cervical dislocation, and their tumors were excised.
The mean of tumor volume was plotted against time in weeks to monitor growth of the breast tumor xenografts. During the study period, the combination of PEITC and bortezomib significantly inhibited the growth of MDA-MB-231 human breast tumor xenografts compared with untreated and single drug treatment (Figure 6A). We also found that the doses used for this study did not induce any undue toxic effects. As demonstrated, only the animals injected with the combination of PEITC and bortezomib lost weight (Figure 6B). However, any likely toxic effect was not lethal, and the weight loss reached a maximum of approximately 10%. The excised tumors were weighed. The tumors treated with the combination of PEITC and bortezomib weighed significantly less (Figure 6C) and were much smaller (Figure 6D) relative to untreated and single drug treated tumors. Harvested tumors were also examined for FOXM1 level by immunoblotting. FOXM1 expression was strongly suppressed in tumor samples treated with the combination of the drugs compared with untreated samples (Figure 6E). Furthermore, Western blot analysis for cleaved caspase-3 demonstrated that tumors were more sensitive to the combination of PEITC with bortezomib (Figure 6E), suggesting that the repression of tumor growth by the combination treatment might be a result of the induction of cell death in the tumor cells. Collectively, these data suggest that proteasome inhibitors in combination with ROS inducers could suppress tumor growth in human cancer xenograft models, implying that they could have potential anticancer activities in patients as well.
Figure 6.
Combination of FOXM1/proteasome inhibitor bortezomib (bor) and ROS inducer PEITC inhibits tumor growth in vivo. A: MDA-MB-231 breast cancer cells (2 × 106) were injected s.c. into the flank region on both sides of 4–week-old, male, nude mice. After tumors became palpable their size was recorded weekly by caliper measurement. Animals were dosed with the indicated concentrations of the drugs 4 to 5 times per week up to 25 injections in total. Graph demonstrates differences in rates of tumor growth during the study period. B: Graph shows the change in weight of the animals during the treatment period. C: Graph shows differences in weight of the excised tumors. D: Representative image of the excised tumors is shown. E: Homogenized tumor samples were used for immunoblotting to determine the level of FOXM1 and cleaved caspase-3. β-Actin was used as the loading control.
Discussion
The aim of this study was to explore the potential of the combination of oxidative stress with FOXM1 suppression in vitro and in vivo. We showed evidence that after treatment with ROS inducers FOXM1 suppression by RNAi further elevates intracellular ROS levels (Figure 1B and Figure 2, A and B) and sensitizes human cancer cells of different origin to apoptosis (Figure 1, C and D and Figure 2, C–I) that is ROS dependent (Figure 3, B–F). Moreover, overexpression of the FOXM1 target scavenger MnSOD partially reversed this effect (Figure 3D). These data confirm that FOXM1 exerts its antioxidant activity in part via the activation of MnSOD. We also found that treatment with FOXM1/proteasome inhibitors along with ROS inducers greatly increases the sensitivity of cancer cells to cell death (Figure 4, A–F). However, more importantly, we presented compelling data that the combination of FOXM1/proteasome inhibitors and ROS inducers suppresses tumor growth in a human breast cancer xenograft model (Figure 6, A, C, and D).
We have used RNAi-mediated knockdown of FOXM1 as a proof of principal to support the assumption that FOXM1 suppression in combination with ROS inducers would further sensitize cancer cells to cell death (Figure 1A). However, RNAi treatment in patients is not a plausible therapeutic approach at present. To circumvent this problem, we tested proteasome inhibitors that target FOXM121 in combination with ROS inducers. Proteasome inhibitors were found to be as efficient (Figure 4, B–F) in regard to induction of cell death as RNAi (Figure 2, C–I) with the combination of ROS inducers. More importantly, the combinatorial treatment of proteasome inhibitors and ROS inducers greatly inhibited tumor growth in vivo in nude mice (Figure 6, A, C, and D). Because proteasome inhibitor bortezomib is already in clinical practice and the ROS inducer PEITC is in clinical trials, our data highly support a feasible treatment strategy for clinical trials based on the induction of ROS and inhibition of FOXM1 in patients with tumors already exhibiting high levels of ROS. This novel combinatorial treatment is projected to be less toxic to normal cells and highly specific toward cancer cells (Figure 4A) because normal cells generally express very low level of FOXM1 and do not depend on their antioxidant system as much as tumor cells.
Overall, we have proposed and tested the combination of FOXM1 suppression with ROS induction in preclinical settings, which has never been tested. Our findings imply that targeting FOXM1 in combination with ROS inducers could offer an effective and applicable treatment strategy against different types of human cancer with high levels of ROS. We believe that this combinatorial treatment should be seriously considered as a feasible anticancer therapy to improve therapeutic response in the treatment of cancer.
Acknowledgments
We thank Dr. Jessica J. Gierut (Harvard Medical School) for proofreading the manuscript and for her valuable comments. We also thank Dr. William Hahn (Harvard Medical School) for the generous gift of BJ oncogenic cells.
Footnotes
Supported by NIH grant 1RO1CA1294414 (A.L.G.).
References
- 1.Curtin J.F., Donovan M., Cotter T.G. Regulation and measurement of oxidative stress in apoptosis. J Immunol Methods. 2002;265:49–72. doi: 10.1016/s0022-1759(02)00070-4. [DOI] [PubMed] [Google Scholar]
- 2.Azad M.B., Chen Y., Gibson S.B. Regulation of autophagy by reactive oxygen species (ROS): implications for cancer progression and treatment. Antioxid Redox Signal. 2009;11:777–790. doi: 10.1089/ars.2008.2270. [DOI] [PubMed] [Google Scholar]
- 3.Finkel T., Holbrook N.J. Oxidants, oxidative stress and the biology of ageing. Nature. 2000;408:239–247. doi: 10.1038/35041687. [DOI] [PubMed] [Google Scholar]
- 4.Trachootham D., Zhou Y., Zhang H., Demizu Y., Chen Z., Pelicano H., Chiao P.J., Achanta G., Arlinghaus R.B., Liu J., Huang P. Selective killing of oncogenically transformed cells through a ROS-mediated mechanism by beta-phenylethyl isothiocyanate. Cancer Cell. 2006;10:241–252. doi: 10.1016/j.ccr.2006.08.009. [DOI] [PubMed] [Google Scholar]
- 5.Szatrowski T.P., Nathan C.F. Production of large amounts of hydrogen peroxide by human tumor cells. Cancer Res. 1991;51:794–798. [PubMed] [Google Scholar]
- 6.Nogueira V., Park Y., Chen C.C., Xu P.Z., Chen M.L., Tonic I., Unterman T., Hay N. Akt determines replicative senescence and oxidative or oncogenic premature senescence and sensitizes cells to oxidative apoptosis. Cancer Cell. 2008;14:458–470. doi: 10.1016/j.ccr.2008.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Laoukili J., Stahl M., Medema R.H. FoxM1: at the crossroads of ageing and cancer. Biochim Biophys Acta. 2007;1775:92–102. doi: 10.1016/j.bbcan.2006.08.006. [DOI] [PubMed] [Google Scholar]
- 8.Wang Z., Ahmad A., Li Y., Banerjee S., Kong D., Sarkar F.H. Forkhead box M1 transcription factor: a novel target for cancer therapy. Cancer Treat Rev. 2010;36:151–156. doi: 10.1016/j.ctrv.2009.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Park H.J., Carr J.R., Wang Z., Nogueira V., Hay N., Tyner A.L., Lau L.F., Costa R.H., Raychaudhuri P. FoxM1, a critical regulator of oxidative stress during oncogenesis. EMBO J. 2009;28:2908–2918. doi: 10.1038/emboj.2009.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Halasi M., Gartel A.L. Suppression of FOXM1 sensitizes human cancer cells to cell death induced by DNA damage. PLoS One. 2012;7:e31761. doi: 10.1371/journal.pone.0031761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Major M.L., Lepe R., Costa R.H. Forkhead box M1B transcriptional activity requires binding of Cdk-cyclin complexes for phosphorylation-dependent recruitment of p300/CBP coactivators. Mol Cell Biol. 2004;24:2649–2661. doi: 10.1128/MCB.24.7.2649-2661.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chou T.C. Theoretical basis, experimental design, and computerized simulation of synergism and antagonism in drug combination studies. Pharmacol Rev. 2006;58:621–681. doi: 10.1124/pr.58.3.10. [DOI] [PubMed] [Google Scholar]
- 13.Yu R., Mandlekar S., Harvey K.J., Ucker D.S., Kong A.N. Chemopreventive isothiocyanates induce apoptosis and caspase-3-like protease activity. Cancer Res. 1998;58:402–408. [PubMed] [Google Scholar]
- 14.Rose P., Whiteman M., Huang S.H., Halliwell B., Ong C.N. beta-Phenylethyl isothiocyanate-mediated apoptosis in hepatoma HepG2 cells. Cell Mol Life Sci. 2003;60:1489–1503. doi: 10.1007/s00018-003-3150-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xiao D., Powolny A.A., Moura M.B., Kelley E.E., Bommareddy A., Kim S.H., Hahm E.R., Normolle D., Van Houten B., Singh S.V. Phenethyl isothiocyanate inhibits oxidative phosphorylation to trigger reactive oxygen species-mediated death of human prostate cancer cells. J Biol Chem. 2010;285:26558–26569. doi: 10.1074/jbc.M109.063255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hileman E.O., Liu J., Albitar M., Keating M.J., Huang P. Intrinsic oxidative stress in cancer cells: a biochemical basis for therapeutic selectivity. Cancer Chemother Pharmacol. 2004;53:209–219. doi: 10.1007/s00280-003-0726-5. [DOI] [PubMed] [Google Scholar]
- 17.Radhakrishnan S.K., Bhat U.G., Hughes D.E., Wang I.C., Costa R.H., Gartel A.L. Identification of a chemical inhibitor of the oncogenic transcription factor forkhead box M1. Cancer Res. 2006;66:9731–9735. doi: 10.1158/0008-5472.CAN-06-1576. [DOI] [PubMed] [Google Scholar]
- 18.Bhat U.G., Zipfel P.A., Tyler D.S., Gartel A.L. Novel anticancer compounds induce apoptosis in melanoma cells. Cell Cycle. 2008;7:1851–1855. doi: 10.4161/cc.7.12.6032. [DOI] [PubMed] [Google Scholar]
- 19.Bhat U.G., Halasi M., Gartel A.L. Thiazole antibiotics target FoxM1 and induce apoptosis in human cancer cells. PLoS ONE. 2009;4:e5592. doi: 10.1371/journal.pone.0005592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Halasi M., Zhao H., Dahari H., Bhat U.G., Gonzalez E.B., Lyubimov A.V., Tonetti D.A., Gartel A.L. Thiazole antibiotics against breast cancer. Cell Cycle. 2010;9:1214–1217. doi: 10.4161/cc.9.6.10955. [DOI] [PubMed] [Google Scholar]
- 21.Bhat U.G., Halasi M., Gartel A.L. FoxM1 is a general target for proteasome inhibitors. PLoS One. 2009;4:e6593. doi: 10.1371/journal.pone.0006593. [DOI] [PMC free article] [PubMed] [Google Scholar]