Abstract
Goblet cell numbers decrease within the conjunctival epithelium in drying and cicatrizing ocular surface diseases. Factors regulating goblet cell differentiation in conjunctival epithelium are unknown. Recent data indicate that the transcription factor SAM-pointed domain epithelial-specific transcription factor (Spdef) is essential for goblet cell differentiation in tracheobronchial and gastrointestinal epithelium of mice. Using Spdef−/− mice, we determined that Spdef is required for conjunctival goblet cell differentiation and that Spdef−/− mice, which lack conjunctival goblet cells, have significantly increased corneal surface fluorescein staining and tear volume, a phenotype consistent with dry eye. Microarray analysis of conjunctival epithelium in Spdef−/− mice revealed down-regulation of goblet cell–specific genes (Muc5ac, Tff1, Gcnt3). Up-regulated genes included epithelial cell differentiation/keratinization genes (Sprr2h, Tgm1) and proinflammatory genes (Il1-α, Il-1β, Tnf-α), all of which are up-regulated in dry eye. Interestingly, four Wnt pathway genes were down-regulated. SPDEF expression was significantly decreased in the conjunctival epithelium of Sjögren syndrome patients with dry eye and decreased goblet cell mucin expression. These data demonstrate that Spdef is required for conjunctival goblet cell differentiation and down-regulation of SPDEF may play a role in human dry eye with goblet cell loss. Spdef−/− mice have an ocular surface phenotype similar to that in moderate dry eye, providing a new, more convenient model for the disease.
Conjunctival goblet cells secrete hydrophilic glycoproteins, termed mucins, which are believed to maintain fluid on the ocular surface and to trap and remove surface debris through movement over the ocular surface by blinking. In humans, the conjunctival goblet cells secrete the mucin MUC5AC; in mice, an additional mucin, Muc5b (by convention, human mucins are designated MUC and mouse mucins, Muc) is also secreted, albeit at lower levels.1 It is currently thought that mucin secretion by conjunctival goblet cells is necessary for the maintenance of a healthy ocular surface, because there is a well-documented decrease in goblet cell numbers within the conjunctiva in cicatrizing diseases including Stevens-Johnson syndrome and ocular cicatricial pemphigoid, as well as in dry eye of several etiologies, including Sjögren syndrome, meibomian gland disease, and keratoconjunctivitis sicca of undefined cause.2 Approximately 4.8 million people are affected by dry eye in the United States alone.2 In addition to loss of goblet cells, these dry eye diseases also feature changes in the ocular surface epithelium, including increased corneal surface fluorescein staining, inflammation of the ocular surface tissues, changes in tear volume and composition, alterations in corneal epithelial barrier function, increases in conjunctival epithelial proliferation, and alterations in cell surface and secreted mucins as well as keratinization-related proteins.2,3 Currently, there are relatively few effective treatments for these diseases and few convenient animal models in which drying and cicatrizing diseases can be studied.4 The most commonly used method to create dry eye syndrome in mice involves repeated daily injections of scopolamine to inhibit production of aqueous tears in conjunction with exposure to environmental desiccating stress.5–8
Although it is known that goblet cell dropout commonly occurs in drying and cicatrizing diseases, to date, little is known about goblet cell differentiation in the conjunctiva. Early studies have shown that conjunctival epithelial cells and corneal-limbal epithelial cells are from two separate cell lineages that are intrinsically divergent.9 To date, no definitive goblet cell precursors have been identified, although it is known that goblet cells and differentiated conjunctival epithelial cells (keratinocytes) share a common progenitor.10,11 Identification of the factors required to induce goblet cell differentiation may be useful in understanding the mechanisms of dry eye pathology and may provide potential therapeutic treatments for replacement of goblet cells lost during dry eye.
Recent studies have demonstrated that the transcription factor sterile α motif pointed domain epithelial specific transcription factor (Spdef), is involved in the induction of goblet cell differentiation from precursor cells in the tracheobronchial epithelium. In respiratory epithelia, expression of Spdef in Clara cells (a goblet cell precursor cell) creates goblet cell hyperplasia by inducing their differentiation into goblet cells.12,13 Furthermore, studies from intestinal epithelia have shown that Spdef also plays an important role in regulating intestinal epithelial cell homeostasis and differentiation. Loss of Spdef severely impairs maturation of goblet and Paneth cells in the intestine14 and expression of Spdef promotes goblet cell differentiation in the intestinal epithelium at the expense of absorptive, Paneth, and enteroendocrine cell types.15
The purpose of this study was to determine whether, as in the tracheobronchial and gastrointestinal epithelium, the transcription factor Spdef regulates goblet cell differentiation in the conjunctiva, and if so, to determine the effect of loss of goblet cells on ocular surface function and phenotype. To address this, we characterized the ocular surface phenotype of mice null for the Spdef gene, and conducted microarray and real-time quantitative RT-PCR (real-time RT-qPCR) analyses to identify changes in expression patterns in inflammatory mediators and genes associated with epithelial cell stress and differentiation that have been shown to be altered in dry eye syndrome. Spdef null mice were also challenged with desiccating environmental stress. To determine the potential role of SPDEF in human dry eye disease, we assayed the levels of SPDEF in conjunctival epithelia derived from patients with Sjögren syndrome dry eye known to have diminution of expression of the goblet cell mucin MUC5AC. Our results indicate that Spdef is critical for goblet cell differentiation in the conjunctiva, that SPDEF is down-regulated in the conjunctival epithelium of patients with Sjögren dry eye, and that the Spdef null mouse serves as an animal model to study the effects of dry eye disease.
Materials and Methods
Mouse Models
Mice null for the transcription factor (Spdef−/−) were developed by Alex Gregorieff in the laboratory of Hans Clevers (Netherlands Institute of Developmental Biology)14 and were generously provided by Jeffrey Whitsett (Children’s Hospital, Cincinnati, OH). Spdef−/− mice, as obtained from Dr. Whitsett, were on a mixed background of C57BL/6 and 129. Spdef+/+ mice, also on a C57BL/6-129 mixed background were backcrossed with C57BL/6 mice to expand the colony. Young adult 8-week-old Spdef−/− mice (n = 14) and their wild-type control Spdef+/+ mice (n = 13), as well as aged adult animals >8-month-old Spdef−/− mice (n = 8) and Spdef+/+ mice (n = 10) were used. All animal protocols were approved by the Schepens Eye Research Institute Institutional Animal Care and Use Committee (IACUC).
Tissue Collection and Histology
Animals were euthanized by CO2, and eyes with intact lids were excised and fixed in 10% formalin, embedded in methacrylate, sectioned, and stained with PAS stain or H&E to determine presence of goblet and inflammatory cells, or they were embedded in optimal cutting temperature compound, frozen on dry ice, and stored at −80°C until use for laser capture microdissection (LCM) and immunofluorescence microscopy.
Inflammatory Cell Counts
Inflammatory cells within the conjunctival epithelium were counted either in H&E-stained sections from Spdef+/+ and Spdef−/− mice. Linear measurements of conjunctival epithelial basal lamina were made using Spot RT software version 3.1 (Spot Diagnostic Instruments, Sterling Heights, MI). Results are expressed as the number of inflammatory cells per 1-mm linear length of basal lamina. Inflammatory cell counts were done in a blind manner, with the genotype (+/+ or −/−) unknown to two independent observers (I.K.G. and Sandra Spurr-Michaud). Counts from the two blind observers were averaged for data analysis.
CD45 Immunohistochemistry and Cell Counts
CD45-positive cells within the conjunctival epithelium of frozen sections from Spdef+/+ and Spdef−/− mice were identified and quantified using immunofluorescence microscopy. Sections were incubated with either Alexa Fluor 488 anti-mouse CD45 antibody (dilution 1:250; BioLegend, San Diego, CA) or the isotype control antibody Alexa Fluor 488 rat IgG2b (dilution 1:100; BioLegend) for 1.5 hours at room temperature and coverslipped in Vectashield mounting medium with propidium iodide (Vector Laboratories, Burlingame, CA).16 CD45-positive cells were counted in a blind manner (genotype unknown) by two independent observers (I.K.G. and Ann Tisdale) on a Zeiss Photoscope III fluorescent microscope at ×25. Linear measurements of conjunctival epithelium basal lamina were made from ×10 images using ImageJ version 1.42Q (NIH, Bethesda, MD). Results are expressed as the number of CD45-positive cells per 1-mm linear length of basal lamina. Counts from the two blind observers were averaged together for data analysis.
Fluorescein Staining and Tear Volume Measurements
Spdef−/− mice and their wild-type controls were examined for gross ocular surface and/or eyelid phenotype, and then assayed for corneal fluorescein staining and tear volume. Corneal fluorescein staining was imaged with a Topcon SL-07 slit lamp biomicroscope (Topcon Corporation, Tokyo, Japan) using a cobalt blue filter 3 minutes after application of 1 μL of 2.5% sodium fluorescein (Sigma-Aldrich, St. Louis, MO) in sterile saline.17 Fluorescein staining was assayed daily for 5 days, and images were scored using a standardized National Eye Institute grading system. The cornea is divided into five areas: superior, inferior, temporal, nasal, and central; punctate fluorescein staining in each area was graded on a scale of 0 to 3, and the scores for all five areas were summed for a total score (0 to 15).3,17,18 Fluorescein scoring was performed in a blind manner, with the age (8 weeks or >8 months) and genotype (+/+ or −/−) unknown to the two independent scorers (C.K.M. and Sandra Spurr-Michaud). Scores were averaged for a final score used in all subsequent data analysis. Aqueous tear volume was measured using the phenol red thread test (Zone-Quick; Lacrimedics, Eastsound, WA).5 Fine forceps were used to place the thread into the lateral canthus of the conjunctival fornix, and the thread was held in place for 30 seconds. Wetting of the thread was measured in millimeters using the scale on the thread box under a light microscope. Tear volume measurements were assayed twice daily (AM and PM) for 3 consecutive days.
Laser Capture Microscopy
Conjunctival tissue from Spdef+/+ and Spdef−/− mice was cryostat-sectioned at −20°C. Sections (6 μm thick) were collected on Arcturus PEN Membrane glass slides (Applied Biosystems, Carlsbad, CA) and stained immediately or stored overnight at −80°C. Before laser microdissection, sections were fixed in 70% ethanol, washed in Nuclease-Free water (Ambion, Austin, TX), stained with Mayer’s Hematoxylin Solution (Sigma-Aldrich), and alcoholic Eosin Y solution (Sigma-Aldrich), dehydrated in 95% and 100% ethanol and xylene, and air dried. For microarray analysis and real-time RT-qPCR, 40% of the conjunctival epithelium, as measured from the deepest point of the fornix cul-de-sac, was captured using a laser microdissection microscope (Model AS LMD; Leica, Wetzlar, Germany). In studies investigating the location of Frzb and Spdef mRNA, multiple clusters of goblet cells and regions of stratified epithelial cells (where no goblet cells were present) were collected into two separate samples by LCM.
RNA Microarray Analysis
Total RNA was isolated from laser-captured sections of conjunctival epithelium using an Arcturus PicoPure Isolation Kit (Applied Biosystems). RNA integrity (RIN number) and concentration was determined using an Agilent 2100 Bioanalyzer (Agilent Technologies, Santa Clara, CA). The RNA samples were analyzed on Affymetrix murine genome MOE430 chips (Affymetrix, Santa Clara, CA), which have approximately 45,000 genes for array. Additional assessment of RNA quality and quantity, as well as probe preparation, labeling, hybridization, and image scans were performed by the Dana Farber Cancer Institute Microarray Core Facilities (Boston, MA). Three replicate arrays were performed for Spdef+/+ and Spdef−/− mice. One eye from one male and one female animal were pooled into a single sample. Microarray data analysis was performed using dChip software (Cheng Li Lab, Dana Farber Cancer Institute and Harvard School of Public Health, Boston, MA; http://www.hsph.harvard.edu/cli/complab/dchip, last accessed January 20, 2011), and differences in gene expression between Spdef+/+ and Spdef−/− mice were considered significant if fold changes were greater than 3.0 and P < 0.01. Gene ontology (GO) analysis was performed using DAVID Bioinformatics Resources 6.7 (http://david.abcc.ncifcrf.gov, last accessed January 26, 2011),19,20 and genes were sorted by functional annotation clustering with the KEGG: Kyoto Encyclopedia of Genes and Genomes (http://www.genome.ad.jp/kegg, last accessed; also accessible from within the DAVID website). Microarray data are available at the National Center for Biotechnology Information Gene Expression Omnibus (http://www.ncbi.nlm.nih.gov/projects/geo; accession number GSE44101).
Real-Time RT-qPCR
Real-time RT-qPCR was performed to validate expression levels of five genes selected from the microarray data: Sprr2h, Tgm1, K17, Frzb, and Wnt5b. Additionally, real-time RT-qPCR was performed to assess changes in Il-1α, Il-1β, and Tnf-α gene expression levels in the conjunctival epithelium of Spdef−/− mice compared to wild-type controls. Frzb and Spdef expression in conjunctival goblet cells, as compared to stratified conjunctival epithelium from Spdef+/+ mice, and SPDEF expression in human subjects with Sjögren syndrome dry eye, as compared to normal control subjects, were also analyzed. RNA was isolated using a Qiagen RNeasy Micro Isolation Kit (Qiagen, Valencia, CA). RNA integrity and concentration was determined using the NanoDrop 2000 Spectrophotometer (Thermo Scientific, Waltham, MA) before and after the pooling of one eye of one male and one female animal into a single sample. Real-time RT-qPCR was performed as previously described21,22 using the Roche LightCycler 480 (Roche Applied Science, Indianapolis, IN), with either TaqMan chemistry (SPDEF human data) or RT2 SYBR Green qPCR Mastermix chemistry (mouse microarray confirmation; Frzb and Spdef expression). Prevalidated primer sets (SABiosciences, Frederick, MD) were used. In mouse samples, 18S RNA was used as the endogenous control gene; GAPDH mRNA was used for human samples. Relative levels of mRNA were calculated using the ΔΔCt method described in the Qiagen RT2 qPCR Primer Assay Handbook with the mean of the Spdef+/+ (mouse), mean of the goblet cell (Frzb and Spdef localization), or normal control (human) samples as the calibrator.
Immunohistochemistry
Frzb and SPDEF proteins were localized in mouse conjunctival goblet cells and human conjunctival epithelium, respectively, using immunofluorescence microscopy of frozen sections of mouse and human conjunctiva. Sections were incubated with either anti–sFRP-3 (Frzb) goat polyclonal primary antibody (dilution 1:50; R&D Systems, Minneapolis, MN) overnight at 4°C or anti-Pdef (SPDEF) mouse monoclonal primary antibody (dilution 1:50; Santa Cruz Biotechnology, Santa Cruz, CA) for 1 hour at room temperature, followed by incubation with either fluorescein isothiocyanate donkey anti-goat IgG or fluorescein isothiocyanate donkey anti-mouse IgG secondary antibody (dilution 1:50; Jackson ImmunoResearch Laboratories, West Grove, PA) for 1 hour at room temperature. Sections were coverslipped in Vectashield mounting medium with propidium iodide (Vector Laboratories) and then viewed under a fluorescence microscope.
Exposure to Desiccating Stress in the Controlled-Environment Chamber
For controlled-environment chamber (CEC)-exposure experiments, 4- to 5-month-old Spdef+/+ (n = 10) and Spdef−/− (n = 8) mice were exposed to desiccating environmental stress in a CEC (XDry Corporation, Las Vegas, NV) with an average temperature of 20.4 ± 0.5°C and an average relative humidity of 13.4 ± 2.9% for 15 days. Corneal fluorescein staining and tear volume measurements were collected and analyzed as described above before entering the CEC and subsequently every 3 days. Animals were euthanized after 15 days in the CEC (experimental day 16), and eyes with intact eyelids were excised.
Human Subjects and Sample Collection
Conjunctival tissue used for immunolocalization of SPDEF and for measurement of SPDEF mRNA levels was archived material from a previously reported study.23 Subject selection, as well as tear fluid and conjunctival epithelium sample collection, was performed as described.23 Tear fluid and conjunctival epithelium from normal subjects and patients with Sjögren syndrome dry eye were collected to determine MUC5AC protein levels and mRNA expression, respectively. Remnant cDNA samples from the study were used in the current study to determine SPDEF mRNA expression levels in normal subjects (n = 6) and in patients with Sjögren syndrome dry eye (n = 5). The original 2002 study, from which the archived material was used, was conducted in compliance with good clinical practice, institutional review board regulations, informed-consent regulations, and the tenets of the Declaration of Helsinki.
Data Analysis and Statistics
Statistics were performed using GraphPad InStat version 3.1a (GraphPad Software, La Jolla, CA). U-tests were used to evaluate differences in tear volume, fluorescein staining, and the number of inflammatory cells and CD45-positive cells between two groups of mice based on age, genotype, or time in the CEC, with P < 0.05 considered statistically significant. Student t-tests were used to evaluate differences in relative expression of the genes of interest in all real-time RT-qPCR experiments. P < 0.05 was considered statistically significant.
Results
Conjunctival Goblet Cells Are Absent in Mice Lacking Spdef
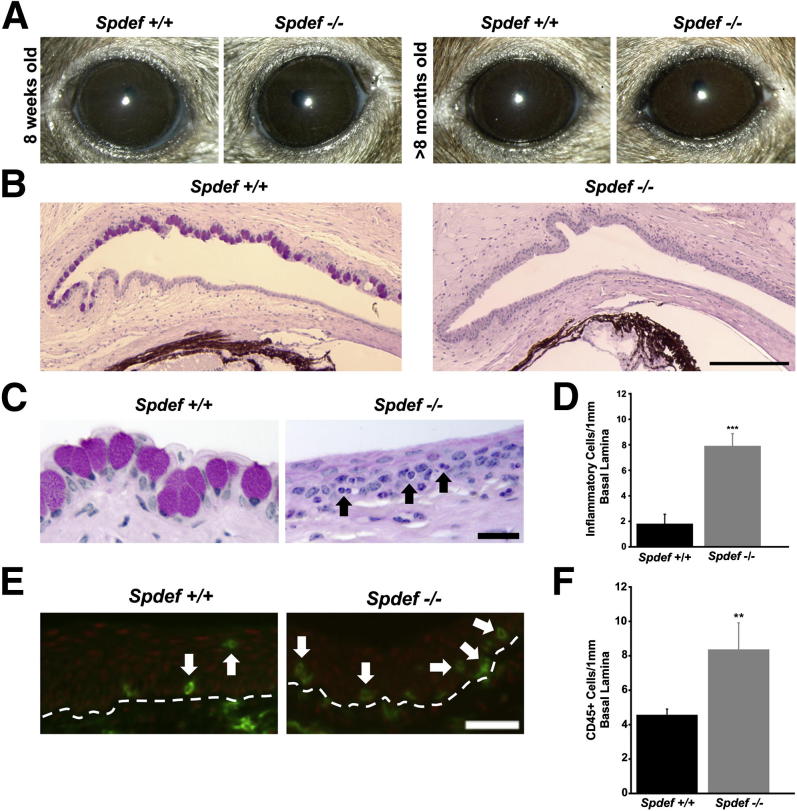
Assessment of the gross appearance of the eye and eyelids of Spdef−/− mice showed no significant changes, as they were indistinguishable from eyes of wild-type mice (Figure 1A). In addition, there were no changes in gross appearance with aging, as the eyes and eyelids of 8-week-old Spdef+/+ (wild-type) and Spdef−/− (null) mice (Figure 1A) were indistinguishable from those of Spdef+/+ and Spdef−/− mice that were >8 months of age (Figure 1A). Despite a normal exterior appearance, Spdef−/− mice lack goblet cells in the conjunctival epithelium (Figure 1B). Other than the lack of goblet cells, Spdef−/− mice do not appear to have any additional major detectable histological defects. Comparison of the number of epithelial cell layers and thickness of the conjunctiva and corneal epithelium in Spdef−/− mice compared to wild-type controls were not statistically significant, although a trend toward increased cell layers and thickness in the Spdef−/− mice was found (data not shown). However, inflammatory cells, identified both by morphology (Figure 1C) and by the inflammatory cell marker CD45 (Figure 1E), were observed within both the conjunctival epithelium and subjacent connective tissue in Spdef−/− mice. Counts of inflammatory cells and CD45-positive cells per 1 mm of conjunctival epithelium basal lamina were both significantly higher in the conjunctival epithelium of Spdef−/− mice compared to that of wild-type mice (Figure 1, D and F).
Figure 1.
Ocular surface phenotype in Spdef−/− mice. A: Despite the absence of goblet cells in the conjunctival epithelium, there were no obvious changes or defects in the gross appearance of the cornea or eyelids in 8-week-old or >8-month-old Spdef−/− mice as compared to age-matched Spdef+/+ mice. B: Sections of Spdef+/+ and Spdef−/− conjunctival epithelium stained with Periodic acid-Schiff (PAS) stain demonstrate goblet cells in Spdef+/+ conjunctival epithelium (left panel), and total lack of goblet cells in the conjunctival epithelium of Spdef−/− mice (right panel). C: Light micrographs of the conjunctival epithelium of Spdef+/+ and Spdef−/− mice stained with PAS demonstrate the presence of inflammatory cells within the conjunctival epithelium of Spdef−/− mice (black arrows). D: Significant increases in the number of inflammatory cells within the conjunctival epithelium were observed in Spdef−/− mice compared to Spdef+/+ mice. Error bars represent means ± SEM. ∗∗∗P < 0.001. E: The inflammatory cell marker CD45 (green) was localized by immunofluorescence within the conjunctival epithelium of Spdef+/+ and Spdef−/− mice (white arrows). The dashed line represents the basal lamina border between the upper stratified epithelium and conjunctival stroma. F: Significant increases in the number of CD45-positive cells within the conjunctival epithelium were observed in Spdef−/− mice compared to Spdef+/+ mice. Error bars represent means ± SEM. ∗∗P < 0.01. Scale bars: 200 μm (B); 20 μm (C and E).
Spdef−/− Mice Exhibit Increased Corneal Fluorescein Staining and Increased Tear Volume
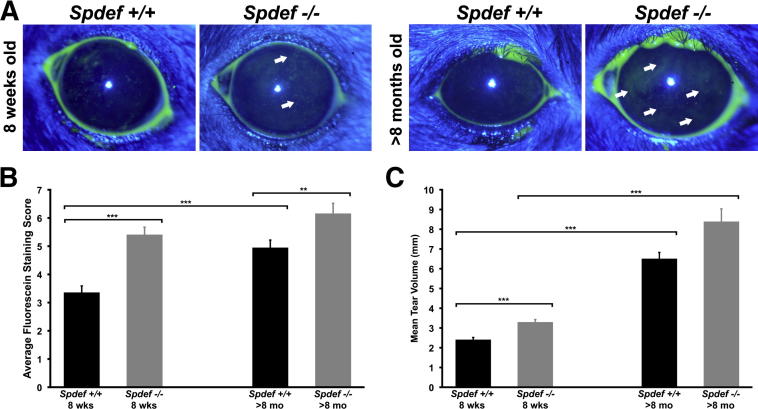
A solution of sodium fluorescein was applied to the ocular surface in Spdef+/+ and Spdef−/− mice to assess damage to the ocular surface (corneal) epithelium. Increased fluorescein dye uptake is a hallmark of human dry eye disease, and is often used in the clinic to diagnose dry eye syndrome2,3 (Figure 2A). Minimal to no corneal fluorescein staining was observed in 8-week-old wild-type mice, whereas scattered punctate fluorescein staining was observed in 8-week-old Spdef−/− mice (Figure 2A). In >8-month-old Spdef−/− mice, patches of punctate and diffuse corneal fluorescein staining were observed (Figure 2A). Scoring of the amount of fluorescein staining showed a significant increase in Spdef−/− mice at 8 weeks of age and >8 months of age, as compared to age-matched Spdef+/+ animals (Figure 2B). A significant increase in fluorescein staining was seen in aged wild-type mice (>8 months old) as compared to 8-week-old wild-type mice, consistent with data previously reported with aging in humans.24,25 The increase in fluorescein staining with age in wild-type mice was not observed in Spdef−/− mice, as there was no statistical difference in the fluorescein staining scores of 8-week-old Spdef−/− mice and >8-month-old Spdef−/− mice.
Figure 2.
Spdef−/− mice show signs of early/moderate dry eye. A: Fluorescein staining of the ocular surface (arrows) showed damaged areas on the surface of the corneal epithelium in Spdef+/+ and Spdef−/− mice at 8 weeks of age (left panels) and at >8 months of age (right panels). B: Scores of the amount of fluorescein staining for 8-week-old and >8-month-old Spdef+/+ mice and for 8-week-old and >8 month-old Spdef−/− mice showed significant increases in 8-week-old Spdef−/− mice as compared to 8-week-old Spdef+/+ mice, in >8-month-old Spdef+/+ mice as compared to 8-week-old Spdef+/+ mice, and in >8-month-old Spdef−/− mice compared to >8-month-old Spdef+/+ mice. Fluorescein staining was not significantly different between 8-week-old Spdef−/− mice and >8-month-old Spdef−/− mice. C: Tear volume measurements taken for 8-week-old and >8-month-old Spdef+/+ mice and for 8-week-old and >8-month-old Spdef−/− mice showed significant increases in 8-week-old Spdef−/− mice as compared to 8-week-old Spdef+/+ mice, in >8-month-old Spdef+/+ mice as compared to 8-week-old Spdef+/+ mice, and in >8-month-old Spdef+/+ mice as compared to 8-week-old Spdef−/− mice. No significant differences in tear volume were seen between >8-month-old Spdef+/+ mice and >8-month-old Spdef−/− mice. Error bars represent means ± SEM. ∗∗P < 0.01, ∗∗∗P < 0.001.
As alterations in tear volume have been commonly reported to occur in dry eye disease, aqueous tear volume was compared in Spdef−/− and wild-type mice at 8 weeks of age and at >8 months of age (Figure 2C). Tear volumes of Spdef−/− mice at 8 weeks were significantly higher than those of age-matched Spdef wild-type mice. Alterations in tear volume also occurred with aging, as significant increases in mean tear volume was seen in both Spdef−/− and wild-type mice >8 months of age compared to 8-week-old mice. There was no statistically significant difference in tear volume between Spdef−/− mice and wild-type mice at >8 months of age. Although decreases in tear volume are normally associated with both dry eye disease and aging,26 increases in lacrimal gland secretion have been reported to occur in response to ocular surface irritation,27 and reduction of tear volume in mouse models of dry eye occurs only on scopolamine treatment.4 Taken together, our data demonstrate an ocular surface phenotype in the Spdef−/− mouse similar to that observed in both human2 and mouse models5,17 of early/moderate dry eye.
Spdef−/− Mice Exhibit Alterations in Gene Expression in the Conjunctival Epithelium
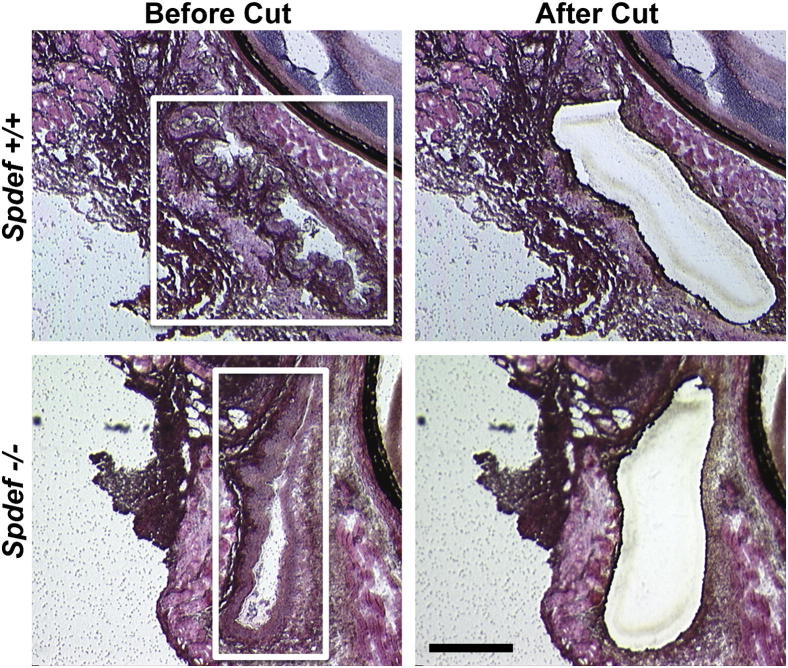
Comparative microarray analysis was performed on RNA isolated from conjunctival epithelium isolated from cryostat sections of Spdef+/+ and Spdef−/− fornicial tissue using LCM (Figure 3). Using the Affymetrix MOE43 murine chip, the array data identified 43 significantly up-regulated genes and 110 significantly down-regulated genes in the conjunctival epithelium of Spdef−/− mice compared to that of Spdef+/+ control mice (threefold change; P < 0.01; for a complete list, see Supplemental Tables S1 and S2).
Figure 3.
Collection of fornicial epithelial samples by LCM. Sections of conjunctival fornix cul-de-sac from 8-week-old Spdef+/+ mice and Spdef−/− mice were collected then stained with H&E, and 40% of the fornicial epithelium, as measured from the deepest point of the fornix cul-de-sac, was collected for RNA isolation and microarray analysis. Conjunctival epithelium from one eye of one male and one female animal were pooled into a single sample. Images show fornicial tissue before (left panels; boxed areas) and after (right panels) laser microdissection. Scale bar = 200 μm.
Up-regulated genes of particular interest included those involved in epithelial cell stress and differentiation, namely, small proline-rich protein 2h (Sprr2h; +39-fold), transglutaminase 1 (Tgm1; +4.46-fold), and keratin 17 (K17; +4.87-fold) (Table 1). Sprr2h and Tgm1 have been associated with cornified cell envelope formation, barrier function, and modulation of epithelial stress and have been shown to be up-regulated both in dry eye28–30 and in response to stress from UVB31 and hyperosmolarity.32 K17 has been demonstrated to play a role in epithelial cell growth33 and promotion of epithelial proliferation.34
Table 1.
Changes in Expression of Genes of Interest in Conjunctival Epithelium of Spdef −/− Mice Compared to Spdef+/+ Mice
| Description | Accession number∗ | Gene symbol | Fold change | Levels in DED | Reference |
|---|---|---|---|---|---|
| Epithelial stress, differentiation, and keratinization† | |||||
| Small proline-rich protein 2h | NM_011474 | Sprr2h | 39.16 | ↑ | 29, 30, 35 |
| Keratin 17 | NM_010663 | Krt17 | 4.87 | Unknown | |
| Transglutaminase 1 | NM_001161714 | Tgm1 | 4.46 | ↑ | 28, 30 |
| Goblet cell differentiation† | |||||
| Mucin 5, subtypes a and c | NM_010844 | Muc5ac | −1240.99 | ↓ | 23, 36 |
| Forkhead box a3 | NM_008260 | Foxa3 | −59.00 | Unknown | |
| Trefoil factor 1 | NM_009362 | Tff1 | −35.55 | Unknown | |
| Glucosaminyl (N-acetyl) transferase 3, mucin type | NM_028087 | Gcnt3 | −16.66 | ↓ | 37 |
| WNT signaling pathway† | |||||
| Frizzled-related protein | NM_011356 | Frzb | −115.67 | Unknown | |
| DIX domain containing 1 | NM_178118 | Dixdc1 | −11.28 | Unknown | |
| Wingless-related MMTV integration site 5b | NM_009525 | Wnt5b | −4.75 | Unknown | |
| Wingless-related MMTV integration site 11 | NM_009519 | Wnt11 | −3.74 | Unknown | |
| Inflammation | |||||
| Interleukin-1 beta | NM_008361 | Il-1β | 11.32 | ↑ | 26, 35, 38 |
| Interleukin-1 alpha | NM_010554 | Il-1α | 4.73 | ↑ | 26, 35, 38, 39 |
| Tumor necrosis factor alpha | NM_013693 | Tnf-α | 2.42 | ↑ | 26, 39 |
DED, dry eye disease.
Accession numbers correspond to mRNA sequences deposited in the NCBI database.
P < 0.01.
On the other hand, goblet cell–specific genes, such as the mucin 5ac (Muc5ac; −1240.99-fold), forkhead box a3 (Foxa3; −59-fold), trefoil factor 1 (Tff1; −35.55-fold), and the mucin-specific glucosaminyl (N-acetyl) transferase (Gcnt3; −16.66-fold), were all highly down-regulated in the conjunctival epithelium of Spdef−/− mice (Table 1). Muc5b was also highly down-regulated (−427 fold); however, it was not included in the list of 110 genes significantly down-regulated greater than threefold with P < 0.01, as its significance level was P < 0.05. Since both Muc5ac and Tff1 are known secretory products of conjunctival goblet cells,40,41 it is not surprising that mRNA expression of these genes is significantly down-regulated in Spdef−/− mice that lack conjunctival goblet cells. A significant decrease in Muc5ac production is well characterized in both animal models of dry eye and in several etiologies of human dry eye, where conjunctival goblet cell dropout is common.5,23,36,42 Interestingly, several Wnt pathway genes (Wnt5b, Wnt11, and the secreted Wnt inhibitor frizzled motif associated with bone development, termed Frzb) were also significantly down-regulated in Spdef−/− mice, with Frzb down-regulation approximately −115 fold (Table 1). The large 115-fold down-regulation of the Wnt pathway inhibitor indicated that Frzb may be expressed specifically by goblet cells.
Additional analysis of our microarray data identified an up-regulation in a number of proinflammatory cytokines (Table 1), such as interleukin-1 β (Il-1β; +11.32-fold), interleukin-1 α (Il-1α; +4.73-fold), and tumor necrosis factor α (Tnf-α; +2.42-fold). Although these proinflammatory mediators were not within the 43 genes significantly up-regulated greater than threefold with P < 0.01 (P > 0.05 for all three genes), a study by Pflugfelder et al39 examining the alteration of cytokines in conjunctival epithelium specimens from patients with Sjögren syndrome dry eye reported fold increases in IL-1α (+4.9-fold) and TNFα (+3.46-fold) that were very similar to the fold changes seen in our microarray analysis. As a number of studies have shown Il-1α, Il-β, and Tnf-α to be increased in both animal models of dry eye syndrome and in human dry eye disease,2,35,38,39 the elevated expression levels of these proinflammatory cytokines observed in Spdef−/− mice indicate a dry eye phenotype.
Real-time RT-qPCR validated the expression levels of select genes from the microarray analysis of gene expression in Spdef−/− mice compared to wild-type mice. Similar to that seen in the microarray analysis, Spdef−/− mice have significantly increased expression levels of epithelial stress and differentiation genes, including Sprr2h, Tgm1, and K17 (Figure 4A), as well as increased expression of the proinflammatory mediators Il-1α, Il-1β, and Tnf-α (Figure 4B) compared to Spdef+/+, suggesting that Spdef−/− mice may have early/moderate dry eye without scopolamine treatment or before exposure to desiccating stress. Furthermore, Wnt signaling pathway members Frzb and Wnt5b were significantly down-regulated in Spdef−/− mice, confirming microarray results (Figure 4C).
Figure 4.
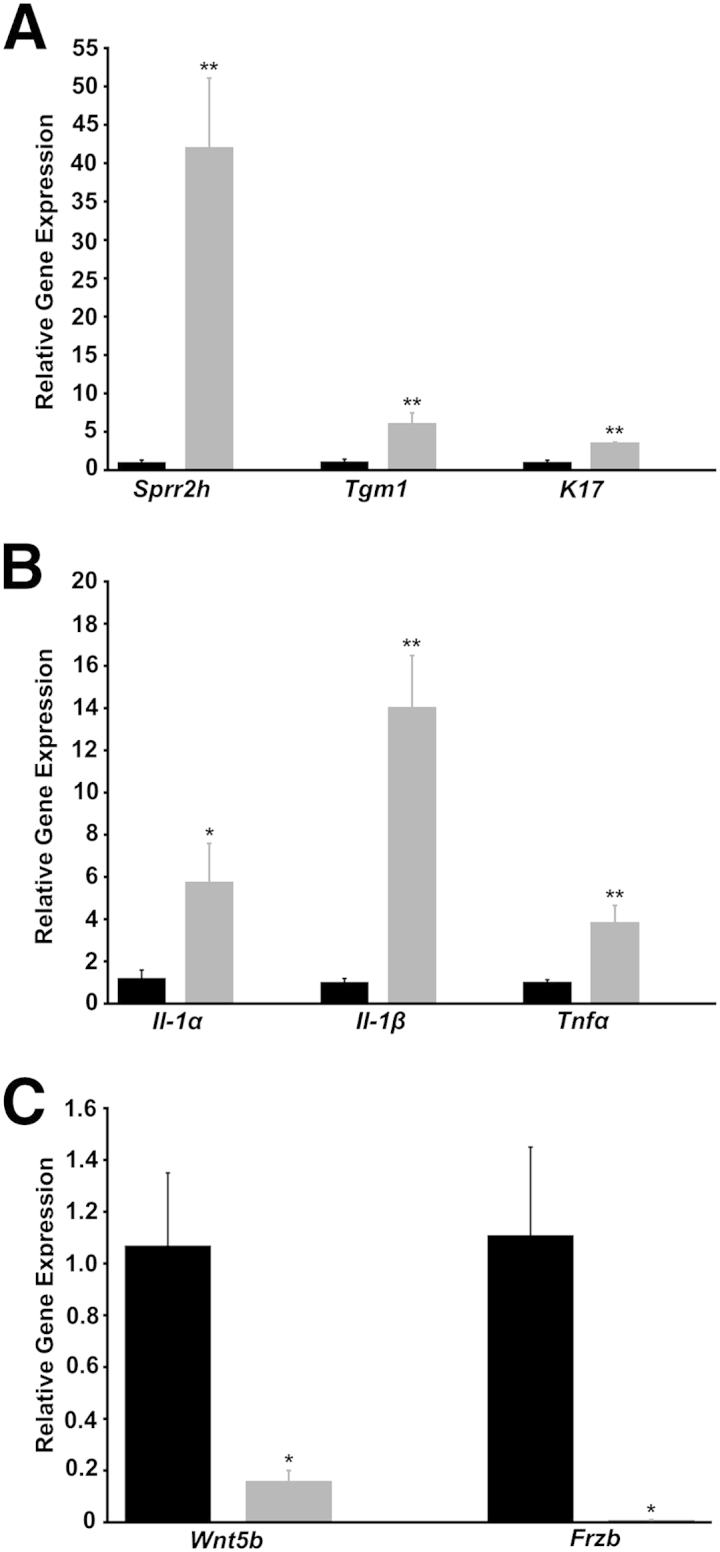
Validation of microarray analysis and assessment of changes in gene expression levels in Spdef−/− mice. Spdef−/− mice (grey bars) have significantly increased expression of the epithelial stress, differentiation, and keratinization genes Sprr2h, Tgm1, and K17 (A), as well as of the proinflammatory mediators Il-1α, Il-1β, and Tnf-α (B), compared to Spdef+/+ mice (black bars). C: Expression levels of the Wnt pathway genes Wnt5b and Frzb are significantly down-regulated in Spdef−/− mice. Error bars represent means ± SEM. ∗P < 0.05, ∗∗P < 0.01.
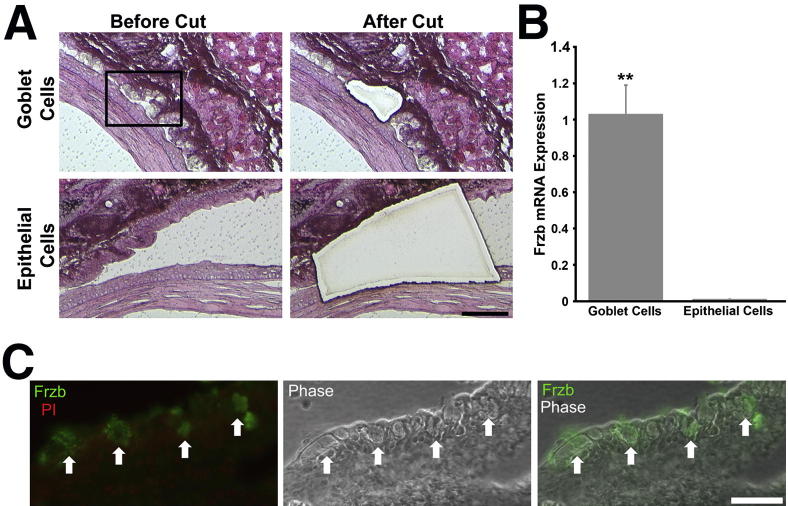
Frzb mRNA and Protein Expression Is Specific to Conjunctival Goblet Cells
After microarray data analysis showed a highly significant down-regulation (−115 fold) of Frzb in the conjunctival epithelium of Spdef−/− mice, we sought to determine whether the Wnt inhibitor is specifically expressed by goblet cells in the conjunctival epithelium. LCM was used to collect individual clusters of goblet cells, and as control, regions of stratified epithelium where goblet cells were not present in Spdef+/+ mice. Figure 5A shows light micrographs of H&E-stained conjunctival sections of goblet cells and stratified epithelium from wild-type mice collected by LCM. RNA isolated from the collected goblet cells and from the stratified epithelium was used to determine levels of Frzb mRNA expression by real-time RT-qPCR. The amount of Frzb mRNA in the stratified epithelium was 100-fold less than that in the conjunctival goblet cells, indicating that Frzb mRNA is a product of the goblet cells of the conjunctival epithelium, not the stratified epithelium (Figure 5B). These data were corroborated by immunohistochemistry that demonstrated the Frzb protein was localized to goblet cells in the conjunctival epithelium of the mice (Figure 5C). The function of the Wnt inhibitor Frzb in the conjunctival epithelium is currently not known, but its presence in goblet cells indicates a function for the cells heretofore unrecognized.
Figure 5.
The secreted Wnt inhibitor Frzb is specific to the goblet cells in the conjunctival epithelium. A: Multiple clusters of goblet cells (top panels) or areas of stratified epithelium without goblet cells (bottom panels) from Spdef+/+ mice were collected, separately, by LCM. Images show tissue stained with H&E before and after laser microdissection. B: Frzb mRNA was detected only in goblet cell samples and not in the stratified epithelial samples. Error bars represent means ± SEM. ∗∗P < 0.01. C: Frzb protein (green) was localized by immunofluorescence to goblet cells within the conjunctival epithelium (arrows; left panel). Phase contrast image (middle panel) allows for better visualization of epithelial and goblet cell (arrows) morphology, and the merged immunofluorescent and phase image (right panel) demonstrates localization of Frzb to goblet cells within the conjunctival epithelium (arrows). Scale bars: = 100 μm (A); 50 μm (B).
Exposure of Spdef−/− Mice to Desiccating Environmental Stress Does Not Enhance Moderate Dry Eye Phenotype
Spdef−/− and age-matched wild-type mice were exposed to environmental desiccating stress in a CEC for 15 days. Before entering the CEC (day 0), Spdef−/− mice had a significantly higher fluorescein staining score compared to Spdef+/+ mice, mirroring the fluorescein staining observed in 8-week-old and >8-month-old Spdef−/− mice (Figure 2A). After 3 days in the CEC, a significant increase in fluorescein staining was observed in Spdef−/− mice, as compared to Spdef+/+ mice (Figure 6A). By CEC day 6, and at each day afterward, corneal fluorescein staining scores in Spdef+/+ and Spdef−/− mice were not significantly different. These data suggest that although Spdef−/− mice show an earlier increase in fluorescein staining at the onset of exposure to desiccating environmental stress, CEC-exposed wild-type mice eventually also show increased fluorescein staining similar to that of the Spdef−/− mice.
Figure 6.
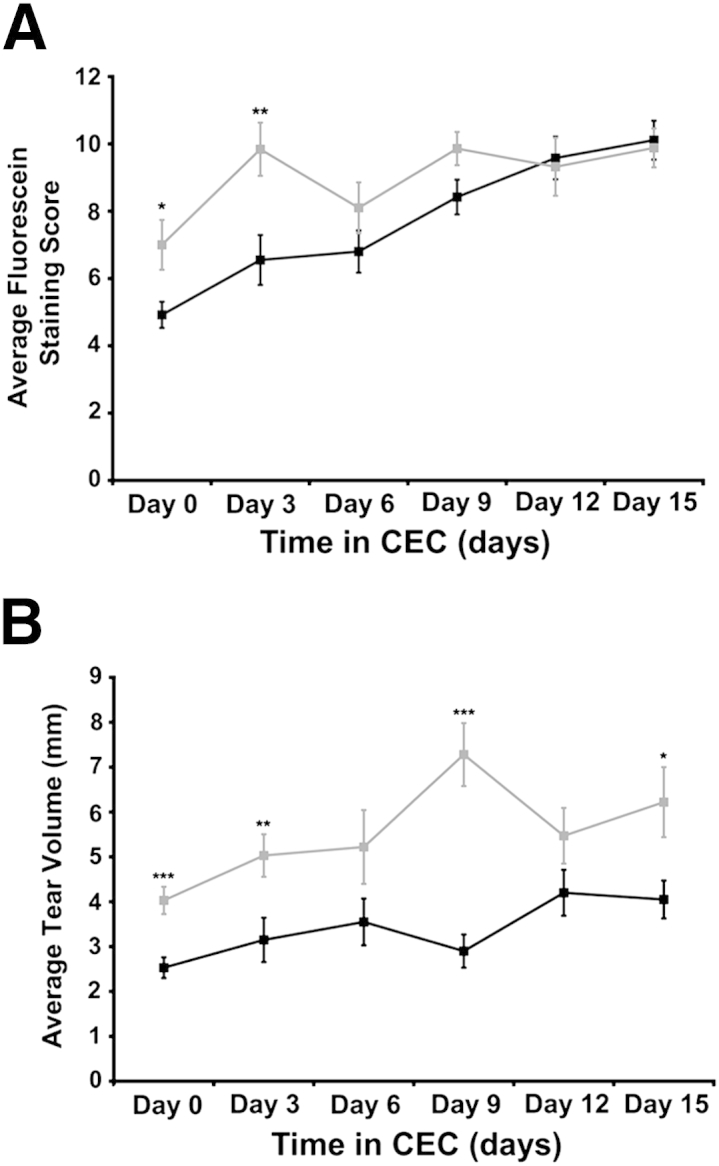
Phenotype of Spdef−/− mice after exposure to desiccating environmental stress. A: Fluorescein staining scores were significantly higher in Spdef−/− mice (grey lines) before entering the controlled environmental chamber (CEC; day 0) and after 3 days in the CEC; however, Spdef+/+ mice (black lines) also showed an increase in fluorescein staining scores over time in the CEC. B: Tear volume in CEC Spdef−/− mice is significantly increased at day 0 and at 3, 9, and 15 days of CEC exposure. CEC-exposed Spdef+/+ mice consistently had lower tear volumes compared to CEC-exposed Spdef−/− mice. ∗P < 0.05, ∗∗P < 0.01, and ∗∗∗P < 0.001.
Aqueous tear production was also assessed in Spdef+/+ and Spdef−/− mice over the course of exposure to environmental desiccating stress in the CEC (Figure 6B). Spdef−/− mice had significantly higher tear volumes before entry into (day 0) and throughout (days 3 through 15) CEC exposure, as compared to Spdef+/+ mice. Moreover, tear volume in CEC-exposed Spdef−/− mice increased significantly with exposure time (day 0 compared to day 15). In contrast, CEC-exposed Spdef+/+ mice consistently had lower tear volumes. Together, these data indicate that exposure to a dry environment caused earlier increases in corneal fluorescein staining and tear volume in Spdef−/− mice compared to wild-type mice.
Interestingly, expression levels of some genes increased following CEC exposure, as CEC-exposed Spdef−/− mice had significant increases in Il-1α, Il-1β, Sprr2h, and K17 compared to CEC-exposed Spdef+/+ mice. However, exposure of Spdef−/− mice to desiccating stress did not further increase the number of inflammatory cells within the conjunctival epithelium or increase gene expression levels of the epithelial stress and differentiation genes or in the proinflammatory mediators, because no significant changes were observed in CEC-exposed Spdef−/− mice compared to unexposed Spdef−/− mice (data not shown). Thus, it appears that although some measures of dry eye, such as fluorescein staining and tear volume, are enhanced by desiccating environmental stress in Spdef−/− mice, exposure to the CEC does not exacerbate the early-to-moderate inflammation phenotype observed in the unexposed Spdef−/− mice.
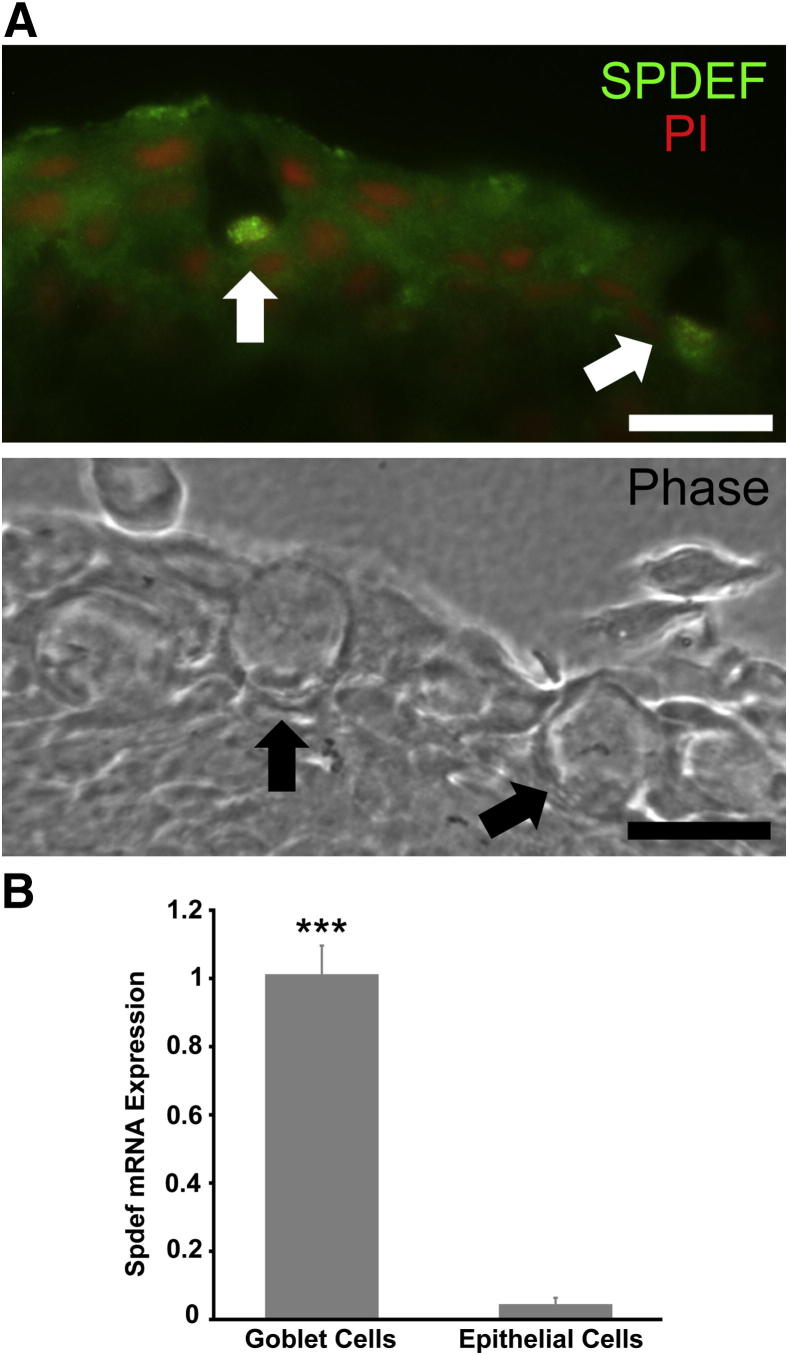
SPDEF Is Expressed in Human and Mouse Conjunctival Epithelium and Is Reduced in Sjögren Syndrome Dry Eye Patients
Since our data indicated that the transcription factor Spdef plays an important role in conjunctival goblet cell differentiation in the mouse, we first determined whether SPDEF is expressed by human conjunctival goblet cells and also localized Spdef expression to conjunctival goblet cells in the mouse. Levels of SPDEF mRNA expression in conjunctival epithelial samples from patients with Sjögren syndrome dry eye were then compared to normal age- and sex-matched controls. Immunohistochemistry on human conjunctival samples showed localization of SPDEF protein to the nucleus of conjunctival goblet cells (Figure 7A). The LCM-collected samples of individual goblet cell clusters and regions of stratified epithelium where no goblet cells were present, which were used to localize Frzb mRNA expression to goblet cells in Spdef+/+ mice (Figure 5, A and B), were used here to determine whether Spdef is also specifically expressed by goblet cells in the conjunctival epithelium. The expression level of Spdef in the stratified epithelium is below the range of real-time RT-qPCR detection (38.80 ± 0.9 cycles), compared to the Spdef expression levels in the conjunctival goblet cells (33.24 ± 1.2 cycles). Thus, Spdef is expressed within the goblet cells of the conjunctival epithelium and not in stratified epithelial cells (Figure 7B), indicating that loss of the Spdef gene has a direct effect on goblet cells and not a secondary effect on non-goblet cell epithelia. Real-time RT-qPCR was performed on samples of human conjunctival epithelium from normal subjects and those with Sjögren syndrome dry eye (previously used in a study examining MUC5AC protein levels and mRNA expression23). A significant decrease in SPDEF mRNA expression was seen in the subjects with Sjögren syndrome dry eye, as compared to normal control subjects (Figure 8). The data correlate with the previous findings that MUC5AC mRNA expression and corresponding protein levels were significantly decreased in these patients with Sjögren syndrome dry eye.23
Figure 7.
SPDEF is localized in human and mouse conjunctival goblet cells. A: Immunofluorescence micrographs demonstrated localization of SPDEF protein (green) in the nucleus of human conjunctival goblet cells (arrows). Phase contrast image from an adjacent section demonstrates goblet cells within the conjunctival epithelium (arrows). Scale bars = 20 μm. B: Spdef mRNA was detected only in goblet cell samples and not in stratified epithelial cell samples collected from Spdef+/+ mice, separately, by LCM. Error bars represent means ± SEM. ∗∗∗P < 0.001.
Figure 8.
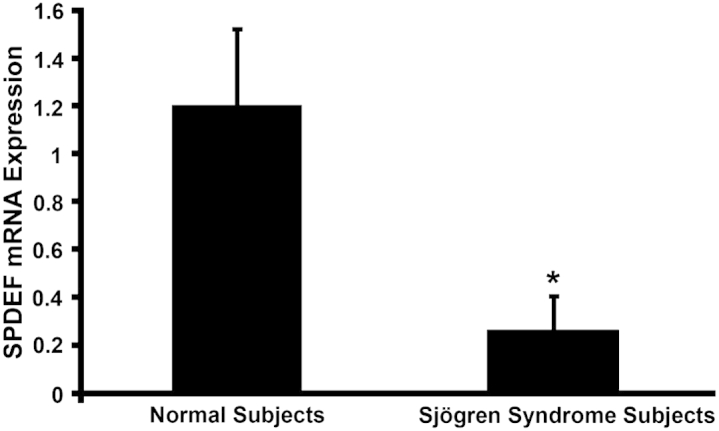
SPDEF mRNA levels are significantly decreased in dry eye disease. Real-time RT-qPCR showed a significant decrease in SPDEF mRNA in conjunctival epithelial samples from patients with dry eye resulting from Sjögren syndrome as compared to samples from normal patients. Error bars represent means ± SEM. ∗P < 0.05.
Discussion
Our finding that the transcription factor Spdef is required for goblet cell differentiation in the conjunctival epithelium, correlates well with previous studies that reported that Spdef expression is necessary for goblet cell differentiation and maturation in the respiratory and intestinal epithelium. In the epithelium of the lung, induced expression of Spdef in Clara cells (identified as the progenitor of goblet cells) caused inhibition of Clara cell differentiation and induction of goblet cell differentiation, resulting in increased mucus production.13 In the intestinal epithelium, induction of Spdef expression in crypts of the distal ileum and colon promoted goblet cell differentiation over other epithelial cell types (enteroendocrine, Paneth, and absorptive enterocytes) and caused cell cycle arrest in intestinal progenitor cells.15 Conversely, knockout of Spdef resulted in major defects in the maturation of goblet and Paneth cells, and led to an accumulation of secretory progenitor cells in the crypts of Spdef−/− mice.14 Although the goblet cell progenitor has been identified in both lung and intestinal epithelia (Clara cell and common goblet cell/Paneth cell precursor, respectively), the goblet cell progenitor in the conjunctiva is still unknown. It is possible that the goblet cell progenitor in the conjunctival epithelium may simply be the keratinocyte, as suggested by Pellegrini et al11 or that the goblet cell itself may divide and give rise to new goblet cells, as described in Wei et al.43
Whatever the goblet cell progenitor may be in the conjunctiva, it appears that Spdef is a common factor required for goblet cell differentiation in pulmonary, intestinal, and conjunctival epithelia; however, some tissue-specific regulation of goblet cell differentiation occurs. Goblet cells, although present in the submucosal glands, are not abundant in the conducting airway in the absence of inflammation.13 However, goblet cell differentiation can be induced through allergens, mediated primarily by the TH2-associated cytokines IL-4 and IL-13.44,45 Conversely, in the conjunctival epithelium, there is an abundance of goblet cells in the normal state and that number is increased by administration of exogenous IL-13.46 IL-13 null mice only demonstrate a 15% reduction in goblet cell numbers in the conjunctival epithelium,46 not a complete lack of goblet cells as observed in the Spdef null mice. These data suggest that not only does regulation of goblet cells differ in the conjunctival epithelium, but that although IL-13 may indeed play a role in the induction of conjunctival goblet cell differentiation, other growth/transcription factors or cytokines are also important for goblet cell differentiation and homeostasis in the conjunctiva, because IL-13 null mice still have a majority of their conjunctival goblet cells.
Recent studies have identified the transcription factors Klf4 and Klf5, members of the Krüppel-like family of transcription factors, as important in goblet cell differentiation and ocular surface development.1,47,48 Conditional knockout of Klf4 resulted in ocular surface epithelial fragility, stromal edema, and loss of conjunctival goblet cells,47 and conditional deletion of Klf5 resulted in formation of defective eyelids with malformed meibomian glands, abnormal cornea, and loss of conjunctival goblet cells.48 Although deletion of Klf4 or Klf5 caused loss of conjunctival goblet cells, as seen in the Spdef null mice, loss of these genes creates a whole host of other ocular surface defects not seen in our study. This suggests that although Klf4 and Klf5 are important regulators of goblet cell differentiation, they are most likely upstream of Spdef. In fact, microarray data from conditional Klf4 knockout mice showed a significant decrease in Spdef expression compared to wild-type animals.1 Decreases in Klf4 or Klf5 were not noted in our microarray analysis.
In addition to the role Spdef plays in goblet cell differentiation in the mouse conjunctiva, data from our study suggest that the transcription factor SPDEF may also play a role in human conjunctival goblet cell differentiation and dry eye disease with goblet cell loss. Immunohistochemistry demonstrated localization of SPDEF protein to the nucleus of human conjunctival goblet cells and a significant decrease in SPDEF mRNA was observed in samples from patients with Sjögren syndrome dry eye known to have a decrease in the goblet cell product MUC5AC. It is well known that there is a loss of goblet cells in human dry eye disease2; however, the pathological mechanisms of goblet cell loss are unknown. Whether the observed decrease in SPDEF expression in patients with Sjögren syndrome dry eye is the cause or effect of goblet cell loss in dry eye syndrome is still to be determined. Nevertheless, our data indicate that perhaps SPDEF also regulates goblet cell differentiation in the human conjunctiva and that loss of SPDEF may play a role in goblet cell dropout in human dry eye disease.
In addition to loss of goblet cells, decreased production of MUC5AC, increased proliferation of conjunctival epithelium and expression of epithelial stress, differentiation, and keratinization-related proteins, as well as expression of inflammatory cytokines, have also been reported to occur in both the human dry eye condition and in animal models of dry eye.23,28–30,35,38,39,49 Increased corneal fluorescein staining and decreased tear volume are often concomitant with these cellular changes on the ocular surface. Lack of the Spdef gene in mice appears to induce a phenotype characteristic of dry eye disease. First, Spdef−/− mice lack conjunctival goblet cells, echoing the loss of conjunctival goblet cells seen in various etiologies of dry eye syndrome. Second, Spdef−/− mice 8 weeks and >8 months of age have increased fluorescein staining compared to age-matched wild-type controls. This increase in fluorescein staining was also observed in Spdef−/− mice as they age. Third, alterations in aqueous tear volume were observed in Spdef−/− mice 8 weeks of age and >8 months of age. However, unlike in human dry eye disease or the scopolamine mouse model of dry eye, where a decrease in aqueous tear volume was seen, we found an increase in the tear volume of Spdef−/− mice. Because scopolamine is known to inhibit the production of aqueous tears in mice,4,5 it is not surprising that a reduction in tear volume is seen using this methodology. However, because the deletion of the Spdef gene affects goblet cells and goblet cell products, but not aqueous tear production, the increase in tear volume observed in our study may be a compensatory mechanism for the loss of the secreted mucins Muc5ac and Muc5b. Finally, our microarray data and real-time RT-qPCR analysis of gene expression from the conjunctival epithelium of Spdef−/− mice showed patterns similar to that seen in dry eye syndrome. Genes associated with epithelial cell stress, differentiation, and keratinization (Sprr2h, Tgm1, and K17) were significantly up-regulated, suggesting that perhaps deletion of the Spdef gene causes epithelial stress resulting in an overproduction and/or accumulation of differentiated stratified epithelial cells in the conjunctiva. Spdef−/− mice also have a significant increase in the number of inflammatory and CD45-positive cells, as well as in the proinflammatory mediators Il-1α, Il-1β, and Tnf-α, all of which have been shown to be up-regulated in dry eye.2,26,35,38,39 Down-regulated genes included those associated with goblet cell products, such as Muc5ac and Tff1, both of which express known components of the tear film on the ocular surface. The Spdef−/− mouse has an ocular surface phenotype (goblet cell loss and increased fluorescein staining) and changes in gene expression (up-regulation of epithelial stress, differentiation, and keratinization genes and genes associated with inflammation; down-regulation in goblet cell products) similar to those seen in human dry eye conditions. Thus, this mouse provides a new, more convenient animal model for the study of early dry eye syndrome as multiple daily scopolamine injections and/or exposure to desiccating environmental stress are not needed to induce a dry eye phenotype.
Although the Spdef−/− mouse does show signs of early dry eye, the lack of a more severe dry eye phenotype in Spdef−/− mice is curious. In a variety of ocular surface diseases including Stevens-Johnson syndrome, ocular cicatricial pemphigoid, chemical injury, dry eye, Sjögren syndrome, and vitamin A deficiency, the nonkeratinized stratified epithelium of the eye can transition into nonsecretory keratinized epithelium (a process termed squamous metaplasia).28,29 Squamous metaplasia involves abnormal epithelial differentiation, and is accompanied by loss of goblet cells, increases in cellular stratification, and enlargement of superficial cells, and keratinization.28 Nakamura et al28 suggest that Tgm1 and other keratinization-related proteins may be expressed because of inflammatory activity, resulting in conjunctival keratinization in severe ocular surface disease. We do not observe squamous metaplasia in Spdef−/− mice; in fact, even after exposure to desiccating environmental stress in the CEC, the corneal surface in Spdef−/− mice appears relatively normal. Lack of progression from an early to moderate dry eye phenotype into a severe phenotype in Spdef−/− mice on exposure to desiccating stress is not unexpected, as a recent study suggests that most patients with early to moderate dry eye disease do not experience worsening over time.50 Nonetheless, Spdef−/− mice show biochemical and immunohistochemical evidence of both inflammation and epithelial stress and keratinization. Real-time RT-qPCR shows an up-regulation in expression levels of the inflammatory mediators Il-1α, Il-1β, and Tnf-α, as well as an up-regulation in Sprr2h, Tgm1, and K17 in the conjunctival epithelium of Spdef−/− mice. Interestingly, Tgm1 is normally expressed, not in ocular surface epithelia, but rather during terminal differentiation of keratinocytes, where it helps to form a cornified cell envelope.28 It is not clear whether the up-regulation in the epithelial cell differentiation and keratinization genes found in our microarray analysis is a response to epithelial stress caused by lack of goblet cells or simply due to the additional keratinocytes produced to fill space in the conjunctival epithelium created by lost goblet cells. However, mice with induced dry eye syndrome,29 human samples with chronic dry eye disease,28 and human corneal epithelial cells exposed to UVB31 or hyperosmolarity32 have an up-regulation in Sprr2h and Tgm1 expression, suggesting that the increase in Sprr2h and Tgm1 seen in Spdef−/− mice may indeed be due to epithelial stress, not simply from addition of epithelial cells.
It has long been thought that the primary function of goblet cells, mucin secretion, is vital for ocular surface health. Spdef−/− mice lack conjunctival goblet cells and expression levels of Muc5ac and Muc5b are extremely down-regulated, yet the ocular surface of Spdef−/− mice appears to be relatively normal and healthy. One alteration noted was the consistent and significant increase in tear volume in Spdef−/− mice compared to wild-type mice. Perhaps when the secreted mucins (which are hydrophilic) are absent, more aqueous tears are produced to compensate for the loss of tear fluid to evaporation, as unlike humans, mice have additional intraorbital glands (harderian and lacrimal), which may compensate for goblet cell loss. Alternatively, the increase in tear volume could be caused by alterations in the tear drainage system or increased leakage of serum from inflamed conjunctival blood vessels and a more permeable conjunctival epithelium. On the other hand, perhaps the secreted mucins may not be as critical for maintenance of a healthy ocular surface as previously thought, with the membrane-tethered mucins being more important in maintaining ocular surface integrity. Studies have shown that the membrane-tethered mucins, MUCs 1, 4, and 16, form a protective barrier on the ocular surface epithelium and that increased expression levels of MUC1 and MUC16 can be observed in postmenopausal women with non-Sjögren dry eye.51–53 In Spdef−/− mice, the expression levels of Muc1 and Muc4 are unchanged compared to wild-type controls (data not shown). This normal expression of the membrane-tethered mucins in Spdef−/− mice, coupled with increased tear production, may prevent the development of a more severe dry eye ocular surface phenotype.
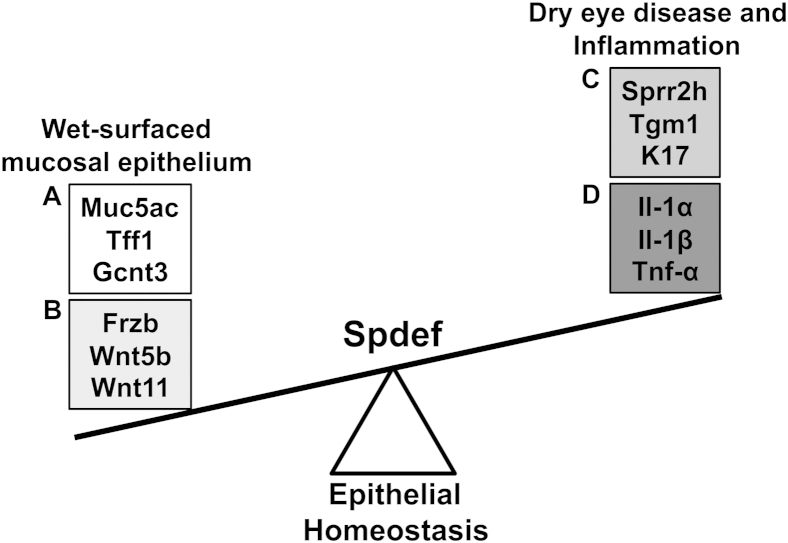
Although the secretion of mucin may not be as critical for ocular surface health as previously thought, it is possible that conjunctival goblet cells secrete other products that are involved in maintenance of epithelial homeostasis on the ocular surface (Figure 9). Our microarray data demonstrated that several members of the Wnt signaling pathway (Wnt5b, Wnt11, and Frzb) were significantly down-regulated in the conjunctival epithelium of Spdef−/− mice. This down-regulation of Wnt genes was unexpected, as Wnt signaling has been shown to play an important role in progenitor cell differentiation and epithelial homeostasis. In the intestinal epithelium, the Notch and Wnt signaling pathways are responsible for lineage commitment and differentiation of progenitor cells within the crypts of the intestine. Notch signaling is required for enterocyte development, whereas Wnt signaling is necessary for the formation of the goblet, Paneth, and enteroendocrine cells.14 Blockage of the Notch pathway results in conversion of progenitor cells into goblet cells,54 whereas inactivation of Wnt signaling in progenitor cells causes their conversion into differentiated enterocytes and a reduction in secretory cell types.55 Wnt signaling also plays a role in epithelial cell fate decisions during the development of the ocular surface, as Dickkopf2, a secreted Wnt inhibitor, has been shown to be a key regulator of corneal versus epidermal fate of the ocular surface epithelium. Dickkopf2 works to repress the Wnt signaling pathway to promote differentiation of corneal epithelial progenitor cells into a nonkeratinizing stratified epithelium during corneal morphogenesis.56,57 Interestingly, we found another secreted Wnt antagonist, Frzb, to be highly down-regulated (−115-fold) in the conjunctivae of Spdef−/− mice. Frzb (also called Sfrp3) is a member of the secreted Frizzled-related proteins (Sfrp) class of Wnt antagonists. Like Wnts, Frzb (and other Sfrp members) is a secreted glycoprotein that structurally mimics the Frizzled receptors. Frzb lacks the transmembrane domain of the Frizzled receptor, but it can prevent Wnt association to its receptors.58 Frzb contains a characteristic cysteine-rich domain that shares homology with the cysteine-rich domain of the Frizzled receptors, providing a binding site for Wnts.58,59 Thus, it was proposed that Frzb can act as a Wnt antagonist, and through the binding of Wnt proteins, modulate the Wnt signaling pathway. Also, since the expression of Frzb and other Sfrps are altered in several disease states, it is likely that their activity is fundamental for tissue homeostasis (reviewed by Bovolenta et al60). By immunofluorescence and real-time RT-qPCR, we localized Frzb protein and mRNA expression to goblet cells in the conjunctival epithelium of Spdef+/+ mice. Along with our data that Frzb and two Wnts (Wnt5b and Wnt11) are down-regulated in the conjunctival epithelium of Spdef−/− mice (which do not produce goblet cells), we propose two possibilities for the actions of these molecules on the conjunctiva. First, because Frzb is produced and secreted in conjunctival goblet cells, it is possible that Frzb modulates Wnt signaling to maintain homeostasis in the ocular surface. On secretion with mucins from the goblet cell, Frzb could be moved over the conjunctival and corneal epithelia where it acts as a Wnt antagonist and promotes epithelial homeostasis. In the Spdef−/− mice where goblet cells are absent, Frzb is not produced, and conjunctival epithelial homeostasis is disrupted, as evidenced by increases in Sprr2h, K17, and Tgm1. A second possibility is that the Wnt proteins play a role in goblet cell differentiation. Studies have shown that Wnt signaling in the adult intestine promotes proliferation of progenitor cells and drives differentiation of goblet, Paneth, and enteroendocrine cells.61 Although the exact precursor for the conjunctival epithelial cell has not been identified, Wnt signaling could be responsible for the maintenance and proliferation of the conjunctival epithelial cell precursor and differentiation of conjunctival goblet cells, similar to that seen in the intestinal epithelium.
Figure 9.
Model for Spdef in goblet cell maintenance of conjunctival epithelial homeostasis. Spdef is required for goblet cell differentiation in the conjunctival epithelium. When goblet cells are absent (through knockout of Spdef), expression of genes associated with goblet cell products (A) and the Wnt pathway (B) are highly down-regulated. This, in turn, promotes epithelial stress and imbalance within the conjunctiva, and the scale is tipped in favor of genes associated with epithelial stress, differentiation, and keratinization (C), as well as inflammation (D).
Conclusions
In summary, our data indicate that the transcription factor Spdef is critical for conjunctival goblet cell differentiation and may play a role in human drying and cicatrizing diseases such as Sjögren syndrome. Mice null for the Spdef gene lack conjunctival goblet cells, exhibit increased fluorescein staining and tear volume, show an increase in inflammatory and CD45-positive cells within the conjunctival epithelium, and have an up-regulation in genes associated with inflammation (Il-1α, Il-1β, and Tnf-α) and epithelial cell stress, differentiation, and keratinization (Sprr2h, Tgm1, and K17) and a down-regulation in genes expressing goblet cell products (Muc5ac and Tff1), all of which are characteristics seen in early/moderate dry eye disease. Exposure of the Spdef−/− mouse to desiccating environmental stress increases some measures of dry eye, such as fluorescein staining and tear volume; however, exposure to the desiccating stress does not exacerbate the early/moderate inflammatory components of the dry eye phenotype observed in Spdef−/− mice. Thus, with lack of conjunctival goblet cells, increased fluorescein staining and tear volume, increased number of inflammatory cells within the conjunctival epithelium, up-regulation of expression in genes associated with inflammation and epithelial stress and differentiation, and down-regulation in expression of goblet cell gene products, the Spdef−/− mouse may serve as a new, more convenient dry eye model than the currently used model in which repeated scopolamine injections and exposure to a desiccating environment are required. However, it remains to be determined whether the ocular surface disease that develops in the Spdef−/− strain of mice is modifiable like that observed in the scopolamine and desiccating environmental stress dry eye model.6–8
Acknowledgments
We thank Sandra Spurr-Michaud and Ann Tisdale for expert technical assistance, Albert Alhatem for assistance with tissue collection, and John Ubels for training in laser capture microscope methods.
Footnotes
Supported by NIH grants R01 EY03306 and R01 EY03306-S1 (AARA) (I.K.G).
Supplemental Data
References
- 1.Gupta D., Harvey S.A., Kaminski N., Swamynathan S.K. Mouse conjunctival forniceal gene expression during postnatal development and its regulation by Kruppel-like factor 4. Invest Ophthalmol Vis Sci. 2011;52:4951–4962. doi: 10.1167/iovs.10-7068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.DEWS Research in dry eye: report of the Research Subcommittee of the International Dry Eye WorkShop (2007) Ocul Surf. 2007;5:179–193. doi: 10.1016/s1542-0124(12)70086-1. [DOI] [PubMed] [Google Scholar]
- 3.Lemp M.A. Report of the National Eye Institute/Industry workshop on Clinical Trials in Dry Eyes. Clao J. 1995;21:221–232. [PubMed] [Google Scholar]
- 4.Barabino S., Dana M.R. Animal models of dry eye: a critical assessment of opportunities and limitations. Invest Ophthalmol Vis Sci. 2004;45:1641–1646. doi: 10.1167/iovs.03-1055. [DOI] [PubMed] [Google Scholar]
- 5.Dursun D., Wang M., Monroy D., Li D.Q., Lokeshwar B.L., Stern M.E., Pflugfelder S.C. A mouse model of keratoconjunctivitis sicca. Invest Ophthalmol Vis Sci. 2002;43:632–638. [PubMed] [Google Scholar]
- 6.de Paiva C.S., Schwartz C.E., Gjorstrup P., Pflugfelder S.C. Resolvin E1 (RX-10001) reduces corneal epithelial barrier disruption and protects against goblet cell loss in a murine model of dry eye. Cornea. 2012;31:1299–1303. doi: 10.1097/ICO.0b013e31823f789e. [DOI] [PubMed] [Google Scholar]
- 7.Okanobo A., Chauhan S.K., Dastjerdi M.H., Kodati S., Dana R. Efficacy of topical blockade of interleukin-1 in experimental dry eye disease. Am J Ophthalmol. 2012;154:63–71. doi: 10.1016/j.ajo.2012.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sadrai Z., Stevenson W., Okanobo A., Chen Y., Dohlman T.H., Hua J., Amparo F., Chauhan S.K., Dana R. PDE4 inhibition suppresses IL-17-associated immunity in dry eye disease. Invest Ophthalmol Vis Sci. 2012;53:3584–3591. doi: 10.1167/iovs.11-9110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wei Z.G., Wu R.L., Lavker R.M., Sun T.T. In vitro growth and differentiation of rabbit bulbar, fornix, and palpebral conjunctival epithelia. Implications on conjunctival epithelial transdifferentiation and stem cells. Invest Ophthalmol Vis Sci. 1993;34:1814–1828. [PubMed] [Google Scholar]
- 10.Wei Z.G., Lin T., Sun T.T., Lavker R.M. Clonal analysis of the in vivo differentiation potential of keratinocytes. Invest Ophthalmol Vis Sci. 1997;38:753–761. [PubMed] [Google Scholar]
- 11.Pellegrini G., Golisano O., Paterna P., Lambiase A., Bonini S., Rama P., De Luca M. Location and clonal analysis of stem cells and their differentiated progeny in the human ocular surface. J Cell Biol. 1999;145:769–782. doi: 10.1083/jcb.145.4.769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Park K.S., Korfhagen T.R., Bruno M.D., Kitzmiller J.A., Wan H., Wert S.E., Khurana Hershey G.K., Chen G., Whitsett J.A. SPDEF regulates goblet cell hyperplasia in the airway epithelium. J Clin Invest. 2007;117:978–988. doi: 10.1172/JCI29176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen G., Korfhagen T.R., Xu Y., Kitzmiller J., Wert S.E., Maeda Y., Gregorieff A., Clevers H., Whitsett J.A. SPDEF is required for mouse pulmonary goblet cell differentiation and regulates a network of genes associated with mucus production. J Clin Invest. 2009;119:2914–2924. doi: 10.1172/JCI39731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gregorieff A., Stange D.E., Kujala P., Begthel H., van den Born M., Korving J., Peters P.J., Clevers H. The ets-domain transcription factor Spdef promotes maturation of goblet and paneth cells in the intestinal epithelium. Gastroenterology. 2009;137:1333–1345. doi: 10.1053/j.gastro.2009.06.044. e1331–1333. [DOI] [PubMed] [Google Scholar]
- 15.Noah T.K., Kazanjian A., Whitsett J., Shroyer N.F. SAM pointed domain ETS factor (SPDEF) regulates terminal differentiation and maturation of intestinal goblet cells. Exp Cell Res. 2010;316:452–465. doi: 10.1016/j.yexcr.2009.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang X., Shen L., Jin Y., Saban D.R., Chauhan S.K., Dana R. Depletion of passenger leukocytes from corneal grafts: an effective means of promoting transplant survival? Invest Ophthalmol Vis Sci. 2009;50:3137–3144. doi: 10.1167/iovs.08-1899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Barabino S., Shen L., Chen L., Rashid S., Rolando M., Dana M.R. The controlled-environment chamber: a new mouse model of dry eye. Invest Ophthalmol Vis Sci. 2005;46:2766–2771. doi: 10.1167/iovs.04-1326. [DOI] [PubMed] [Google Scholar]
- 18.Barabino S., Rolando M., Chen L., Dana M.R. Exposure to a dry environment induces strain-specific responses in mice. Exp Eye Res. 2007;84:973–977. doi: 10.1016/j.exer.2007.02.003. [DOI] [PubMed] [Google Scholar]
- 19.Huang da W., Sherman B.T., Lempicki R.A. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat Protoc. 2009;4:44–57. doi: 10.1038/nprot.2008.211. [DOI] [PubMed] [Google Scholar]
- 20.Huang da W., Sherman B.T., Lempicki R.A. Bioinformatics enrichment tools: paths toward the comprehensive functional analysis of large gene lists. Nucleic Acids Res. 2009;37:1–13. doi: 10.1093/nar/gkn923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kunert K.S., Keane-Myers A.M., Spurr-Michaud S., Tisdale A.S., Gipson I.K. Alteration in goblet cell numbers and mucin gene expression in a mouse model of allergic conjunctivitis. Invest Ophthalmol Vis Sci. 2001;42:2483–2489. [PubMed] [Google Scholar]
- 22.Gipson I.K., Spurr-Michaud S., Argueso P., Tisdale A., Ng T.F., Russo C.L. Mucin gene expression in immortalized human corneal-limbal and conjunctival epithelial cell lines. Invest Ophthalmol Vis Sci. 2003;44:2496–2506. doi: 10.1167/iovs.02-0851. [DOI] [PubMed] [Google Scholar]
- 23.Argueso P., Balaram M., Spurr-Michaud S., Keutmann H.T., Dana M.R., Gipson I.K. Decreased levels of the goblet cell mucin MUC5AC in tears of patients with Sjogren syndrome. Invest Ophthalmol Vis Sci. 2002;43:1004–1011. [PubMed] [Google Scholar]
- 24.Carlson K.H., Bourne W.M., McLaren J.W., Brubaker R.F. Variations in human corneal endothelial cell morphology and permeability to fluorescein with age. Exp Eye Res. 1988;47:27–41. doi: 10.1016/0014-4835(88)90021-8. [DOI] [PubMed] [Google Scholar]
- 25.Chang S.W., Hu F.R. Changes in corneal autofluorescence and corneal epithelial barrier function with aging. Cornea. 1993;12:493–499. doi: 10.1097/00003226-199311000-00006. [DOI] [PubMed] [Google Scholar]
- 26.Barabino S., Chen Y., Chauhan S., Dana R. Ocular surface immunity: homeostatic mechanisms and their disruption in dry eye disease. Prog Retin Eye Res. 2012;31:271–285. doi: 10.1016/j.preteyeres.2012.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rolando M., Zierhut M. The ocular surface and tear film and their dysfunction in dry eye disease. Surv Ophthalmol. 2001;45(Suppl 2):S203–S210. doi: 10.1016/s0039-6257(00)00203-4. [DOI] [PubMed] [Google Scholar]
- 28.Nakamura T., Nishida K., Dota A., Matsuki M., Yamanishi K., Kinoshita S. Elevated expression of transglutaminase 1 and keratinization-related proteins in conjunctiva in severe ocular surface disease. Invest Ophthalmol Vis Sci. 2001;42:549–556. [PubMed] [Google Scholar]
- 29.De Paiva C.S., Villarreal A.L., Corrales R.M., Rahman H.T., Chang V.Y., Farley W.J., Stern M.E., Niederkorn J.Y., Li D.Q., Pflugfelder S.C. Dry eye-induced conjunctival epithelial squamous metaplasia is modulated by interferon-gamma. Invest Ophthalmol Vis Sci. 2007;48:2553–2560. doi: 10.1167/iovs.07-0069. [DOI] [PubMed] [Google Scholar]
- 30.Corrales R.M., de Paiva C.S., Li D.Q., Farley W.J., Henriksson J.T., Bergmanson J.P., Pflugfelder S.C. Entrapment of conjunctival goblet cells by desiccation-induced cornification. Invest Ophthalmol Vis Sci. 2011;52:3492–3499. doi: 10.1167/iovs.10-5782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tong L., Corrales R.M., Chen Z., Villarreal A.L., De Paiva C.S., Beuerman R., Li D.Q., Pflugfelder S.C. Expression and regulation of cornified envelope proteins in human corneal epithelium. Invest Ophthalmol Vis Sci. 2006;47:1938–1946. doi: 10.1167/iovs.05-1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen Z., Tong L., Li Z., Yoon K.C., Qi H., Farley W., Li D.Q., Pflugfelder S.C. Hyperosmolarity-induced cornification of human corneal epithelial cells is regulated by JNK MAPK. Invest Ophthalmol Vis Sci. 2008;49:539–549. doi: 10.1167/iovs.07-0569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kim S., Wong P., Coulombe P.A. A keratin cytoskeletal protein regulates protein synthesis and epithelial cell growth. Nature. 2006;441:362–365. doi: 10.1038/nature04659. [DOI] [PubMed] [Google Scholar]
- 34.Depianto D., Kerns M.L., Dlugosz A.A., Coulombe P.A. Keratin 17 promotes epithelial proliferation and tumor growth by polarizing the immune response in skin. Nat Genet. 2010;42:910–914. doi: 10.1038/ng.665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pelegrino F.S., Pflugfelder S.C., De Paiva C.S. Low humidity environmental challenge causes barrier disruption and cornification of the mouse corneal epithelium via a c-jun N-terminal kinase 2 (JNK2) pathway. Exp Eye Res. 2012;94:150–156. doi: 10.1016/j.exer.2011.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhao H., Jumblatt J.E., Wood T.O., Jumblatt M.M. Quantification of MUC5AC protein in human tears. Cornea. 2001;20:873–877. doi: 10.1097/00003226-200111000-00019. [DOI] [PubMed] [Google Scholar]
- 37.Argueso P., Tisdale A., Mandel U., Letko E., Foster C.S., Gipson I.K. The cell-layer- and cell-type-specific distribution of GalNAc-transferases in the ocular surface epithelia is altered during keratinization. Invest Ophthalmol Vis Sci. 2003;44:86–92. doi: 10.1167/iovs.02-0181. [DOI] [PubMed] [Google Scholar]
- 38.Solomon A., Dursun D., Liu Z., Xie Y., Macri A., Pflugfelder S.C. Pro- and anti-inflammatory forms of interleukin-1 in the tear fluid and conjunctiva of patients with dry-eye disease. Invest Ophthalmol Vis Sci. 2001;42:2283–2292. [PubMed] [Google Scholar]
- 39.Pflugfelder S.C., Jones D., Ji Z., Afonso A., Monroy D. Altered cytokine balance in the tear fluid and conjunctiva of patients with Sjogren’s syndrome keratoconjunctivitis sicca. Curr Eye Res. 1999;19:201–211. doi: 10.1076/ceyr.19.3.201.5309. [DOI] [PubMed] [Google Scholar]
- 40.Buron N., Guery L., Creuzot-Garcher C., Lafontaine P.O., Bron A., Rio M.C., Solary E. Trefoil factor TFF1-induced protection of conjunctival cells from apoptosis at premitochondrial and postmitochondrial levels. Invest Ophthalmol Vis Sci. 2008;49:3790–3798. doi: 10.1167/iovs.07-1270. [DOI] [PubMed] [Google Scholar]
- 41.Govindarajan B., Gipson I.K. Membrane-tethered mucins have multiple functions on the ocular surface. Exp Eye Res. 2010;90:655–663. doi: 10.1016/j.exer.2010.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Song X.J., Li D.Q., Farley W., Luo L.H., Heuckeroth R.O., Milbrandt J., Pflugfelder S.C. Neurturin-deficient mice develop dry eye and keratoconjunctivitis sicca. Invest Ophthalmol Vis Sci. 2003;44:4223–4229. doi: 10.1167/iovs.02-1319. [DOI] [PubMed] [Google Scholar]
- 43.Wei Z.G., Cotsarelis G., Sun T.T., Lavker R.M. Label-retaining cells are preferentially located in fornical epithelium: implications on conjunctival epithelial homeostasis. Invest Ophthalmol Vis Sci. 1995;36:236–246. [PubMed] [Google Scholar]
- 44.Kondo M., Tamaoki J., Takeyama K., Nakata J., Nagai A. Interleukin-13 induces goblet cell differentiation in primary cell culture from Guinea pig tracheal epithelium. Am J Respir Cell Mol Biol. 2002;27:536–541. doi: 10.1165/rcmb.4682. [DOI] [PubMed] [Google Scholar]
- 45.Zhen G., Park S.W., Nguyenvu L.T., Rodriguez M.W., Barbeau R., Paquet A.C., Erle D.J. IL-13 and epidermal growth factor receptor have critical but distinct roles in epithelial cell mucin production. Am J Respir Cell Mol Biol. 2007;36:244–253. doi: 10.1165/rcmb.2006-0180OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.De Paiva C.S., Raince J.K., McClellan A.J., Shanmugam K.P., Pangelinan S.B., Volpe E.A., Corrales R.M., Farley W.J., Corry D.B., Li D.Q., Pflugfelder S.C. Homeostatic control of conjunctival mucosal goblet cells by NKT-derived IL-13. Mucosal Immunol. 2011;4:397–408. doi: 10.1038/mi.2010.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Swamynathan S.K., Katz J.P., Kaestner K.H., Ashery-Padan R., Crawford M.A., Piatigorsky J. Conditional deletion of the mouse Klf4 gene results in corneal epithelial fragility, stromal edema, and loss of conjunctival goblet cells. Mol Cell Biol. 2007;27:182–194. doi: 10.1128/MCB.00846-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kenchegowda D., Swamynathan S., Gupta D., Wan H., Whitsett J., Swamynathan S.K. Conditional disruption of mouse Klf5 results in defective eyelids with malformed meibomian glands, abnormal cornea and loss of conjunctival goblet cells. Dev Biol. 2011;356:5–18. doi: 10.1016/j.ydbio.2011.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fabiani C., Barabino S., Rashid S., Dana M.R. Corneal epithelial proliferation and thickness in a mouse model of dry eye. Exp Eye Res. 2009;89:166–171. doi: 10.1016/j.exer.2009.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tarko L, Li JZ, Dartt DA, Shaumberg DA: The natural history of dry eye disease from the patient’s perspective. Invest Ophthalmol Vis Sci 2012, 53:E-Abstract 5442
- 51.Blalock T.D., Spurr-Michaud S.J., Tisdale A.S., Heimer S.R., Gilmore M.S., Ramesh V., Gipson I.K. Functions of MUC16 in corneal epithelial cells. Invest Ophthalmol Vis Sci. 2007;48:4509–4518. doi: 10.1167/iovs.07-0430. [DOI] [PubMed] [Google Scholar]
- 52.Govindarajan B., Menon B.B., Spurr-Michaud S., Rastogi K., Gilmore M.S., Argueso P., Gipson I.K. A metalloproteinase secreted by Streptococcus pneumoniae removes membrane mucin MUC16 from the epithelial glycocalyx barrier. PLoS One. 2012;7:e32418. doi: 10.1371/journal.pone.0032418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gipson I.K., Spurr-Michaud S.J., Senchyna M., Ritter R., 3rd, Schaumberg D. Comparison of mucin levels at the ocular surface of postmenopausal women with and without a history of dry eye. Cornea. 2011;30:1346–1352. doi: 10.1097/ICO.0b013e31820d852a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.van Es J.H., Jay P., Gregorieff A., van Gijn M.E., Jonkheer S., Hatzis P., Thiele A., van den Born M., Begthel H., Brabletz T., Taketo M.M., Clevers H. Wnt signalling induces maturation of Paneth cells in intestinal crypts. Nat Cell Biol. 2005;7:381–386. doi: 10.1038/ncb1240. [DOI] [PubMed] [Google Scholar]
- 55.Pinto D., Gregorieff A., Begthel H., Clevers H. Canonical Wnt signals are essential for homeostasis of the intestinal epithelium. Genes Dev. 2003;17:1709–1713. doi: 10.1101/gad.267103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mukhopadhyay M., Gorivodsky M., Shtrom S., Grinberg A., Niehrs C., Morasso M.I., Westphal H. Dkk2 plays an essential role in the corneal fate of the ocular surface epithelium. Development. 2006;133:2149–2154. doi: 10.1242/dev.02381. [DOI] [PubMed] [Google Scholar]
- 57.Gage P.J., Qian M., Wu D., Rosenberg K.I. The canonical Wnt signaling antagonist DKK2 is an essential effector of PITX2 function during normal eye development. Dev Biol. 2008;317:310–324. doi: 10.1016/j.ydbio.2008.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Jones S.E., Jomary C. Secreted Frizzled-related proteins: searching for relationships and patterns. Bioessays. 2002;24:811–820. doi: 10.1002/bies.10136. [DOI] [PubMed] [Google Scholar]
- 59.Kawano Y., Kypta R. Secreted antagonists of the Wnt signalling pathway. J Cell Sci. 2003;116:2627–2634. doi: 10.1242/jcs.00623. [DOI] [PubMed] [Google Scholar]
- 60.Bovolenta P., Esteve P., Ruiz J.M., Cisneros E., Lopez-Rios J. Beyond Wnt inhibition: new functions of secreted Frizzled-related proteins in development and disease. J Cell Sci. 2008;121:737–746. doi: 10.1242/jcs.026096. [DOI] [PubMed] [Google Scholar]
- 61.Gregorieff A., Clevers H. Wnt signaling in the intestinal epithelium: from endoderm to cancer. Genes Dev. 2005;19:877–890. doi: 10.1101/gad.1295405. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.