Abstract
Clinical studies and animal experimentation have shown that colonic inflammation is associated with an increased number and reactivity of platelets, coagulation abnormalities, and enhanced thrombus formation. The objective of this study was to define the contribution of IL-6 to the thrombocytosis, exaggerated agonist-induced platelet aggregation, and enhanced extra-intestinal thrombosis that occur during experimental colitis. The number of mature and immature platelets, platelet life span, thrombin-induced platelet aggregation response, and light/dye-induced thrombus formation in cremaster muscle arterioles were measured in wild-type (WT) and IL-6–deficient (IL-6−/−) mice with dextran sodium sulfate (DSS)-induced colitis. DSS colitis in WT mice was associated with thrombocytosis with an elevated number of both mature and immature platelets and no change in platelet life span. The thrombocytosis response was absent in IL-6−/− mice. DSS treatment also enhanced the platelet aggregation response to thrombin and accelerated thrombus development in WT mice, but not in IL-6−/− mice. Exogenous IL-6 administered to WT mice elicited a dose-dependent enhancement of thrombus formation. These findings indicate that IL-6 mediates the thrombocytosis, platelet hyperreactivity, and accelerated thrombus development associated with experimental colitis. The IL-6–dependent colitis-induced thrombocytosis appears to result from an enhancement of thrombopoiesis because platelet life span is unchanged.
Inflammatory bowel diseases (IBDs) are associated with an increased risk of thrombus formation both within the inflamed bowel and in extra-intestinal tissues, such as lung and skeletal muscles.1,2 The accelerated thrombus development during colonic inflammation is evidenced in large arteries and veins, as well as in the microvasculature.2,3 The prothrombogenic phenotype in IBD is often accompanied by increases in the blood count (reactive thrombocytosis)4 and reactivity of platelets3 and by an imbalance between procoagulant and anticoagulant mechanisms.3 Although a 50% to 100% increase in blood platelet count is commonly reported for patients with active IBD5–7 and the association of thrombocytosis with active IBD has led to the proposal that platelet count is a useful marker of disease activity,8 it remains unclear whether the elevated platelet count is mechanistically linked to the enhanced platelet reactivity and accelerated thrombus development in IBD. Such a link appears tenable because accelerated thrombopoiesis would result in an increased percentage of circulating platelets that are young and more hemostatically active than older platelets.9,10
Enhanced thrombus formation and the other extra-intestinal manifestations (eg, platelet abnormalities, inflammation of eyes, joints, liver) of IBD2,11 suggest that cellular (eg, activated leukocytes or platelets) and/or chemical signals are liberated from the inflamed bowel to elicit these responses in distant vascular beds and hematopoietic tissue. Several pro-inflammatory cytokines [eg, IL-1β, tumor necrosis factor-α (TNF-α), IL-6] produced and released by the inflamed colon in human and experimental IBD12,13 have been shown to target components of the coagulation cascade and to alter platelet function in a manner that would predispose the vasculature to thrombus development.14–16 Of the cytokines studied to date, only IL-6 exhibits the ability to mediate all of the platelet responses that are characteristic of colonic inflammation, that is, thrombocytosis,17 platelet hyperreactivity,18,19 and accelerated thrombus formation.20 For example, animals treated with IL-6 exhibit an increased platelet count, and the platelets are more sensitive to activation by thrombin and other platelet agonists.18 IL-6 has been shown to act directly on megakaryocytes to increase platelet production,18 and it acts on hepatocytes to increase the production and release of thrombopoietin (TPO), a potent stimulant of platelet production.6 Although IL-6 has been implicated in the thrombocytosis of IBD on the basis of clinical reports that describe an association between the elevated plasma concentrations of TPO and IL-6 with blood platelet count,6 the contribution of IL-6 to colitis-induced thrombocytosis has not been directly addressed in either the clinical or experimental setting.
The overall aim of this study was to assess the contribution of IL-6 to the platelet abnormalities and accelerated extra-intestinal thrombosis that are observed in a murine model of colonic inflammation. We evaluated the role of IL-6 in mediating the increased platelet count, whether IL-6 is responsible for the appearance of immature platelets, and if the life span of platelets is altered in an IL-6–dependent manner in experimental IBD. The role of IL-6 in colitis-induced platelet hyperreactivity to thrombin was also examined as well as the contribution of membrane-bound and soluble IL-6 receptors to thrombin-mediated aggregation of platelets from colitic mice. Finally, the contribution of IL-6 to colitis-enhanced extra-intestinal thrombosis was evaluated with immunoblockade or genetic deficiency of IL-6. Our findings indicate that IL-6 plays a major role in the enhanced production of highly reactive platelets and accelerated thrombus development in extra-intestinal tissue during colonic inflammation.
Materials and Methods
Animals
Male C57BL/6J mice and IL-6–deficient (IL-6−/−) mice (B6.129S6-Il6tm1Kopf, C57BL/6J background) were purchased from The Jackson Laboratory (Bar Harbor, ME). The mice were housed under specific pathogen-free conditions in standard cages and fed standard laboratory chow and water until the desired age (6 to 8 weeks). All animal procedures were reviewed and approved by the Institutional Animal Care and Use Committee of Louisiana State University Health Sciences Center and were performed according to the criteria outlined by the National Institutes of Health.
Acute Colitis Model
Acute colitis was induced with 3% dextran sodium sulfate (DSS; 40,000 molecular weight; MP Biomedicals, Solon, OH) dissolved in filter-purified drinking water for 7 days, as previously described.14,21 Control mice received regular drinking water without DSS.
Histology
Histological examination was performed on samples of distal colon, which were fixed in 10% formalin before embedding in paraffin and staining with hematoxylin and eosin. The histological examination was performed in a blinded manner (S.Z.) with the use of a previously validated scoring system,22 in which severity of inflammation (0 to 3 scale), depth of injury (0 to 3 scale), and crypt damage (0 to 4 scale) are multiplied by an integer from 1 through 4, representing the percentage of involvement of the colonic wall (1, 0% to 25%; 2, 26% to 50%; 3, 51% to 75%; 4, 76% to 100%), for a maximum possible score of 40.
In Vivo Biotinylation Method for Platelet Life Span Measurement
Flow cytometry was used to quantify the relative numbers and life spans of mature and immature platelets in blood of control mice and colitic mice. The in vivo biotinylation method of Ault et al9 was used to measure platelet life span. After intravenous administration (1.2 mg dissolved in 300 μL of saline), biotin (Sulfo-NHS-LC-biotin; ProteoChem, Denver, CO) covalently binds to free amino groups on the surface of all blood cells. Biotin-positive cells were detected in tail vein blood samples (10 to 15 μL, drawn daily over 5 days) with the use of flow cytometry by binding with streptavidin conjugated with phycoerythrin (eBioscience, San Diego, CA). Platelets were distinguished from other biotin-positive blood cells by staining with CD41-allophycocyanin (isotype control rat IgG1, κ; eBioscience). Newly released (immature) platelets are biotin negative, whereas mature platelets remain biotin positive for the remainder of their life span. Hence, the entire platelet population becomes biotin negative as the platelet population ages. Young reticulated platelets were identified by staining with thiazole orange (TO; Sigma-Aldrich, St. Louis, MO), which binds to nucleic acids (DNA and RNA). Fresh blood samples were incubated with 1 μg/mL TO (dissolved in PBS) for 15 minutes at room temperature.23 Formaldehyde (1%; Polyscience Inc., Warrington, PA) was used for cell fixation. An analysis of the staining pattern of platelets (CD41+) (detected over several days after TO and biotin administration) for TO+ and streptavidin-phycoerythrin+ was used to show the numbers and life spans of both mature and immature platelets, as previously described.9,24 The three-color analysis was performed on an LSRII flow cytometer (BD Biosciences, San Jose, CA) and analyzed with FACSDiva software version 6.1.3 (BD Biosciences), with the gate set around CD41-allophycocyanin+ cells, and 10,000 to 20,000 events were collected. The absolute blood platelet count was determined manually with the use of a hemocytometer. Platelet life span and immature and mature platelet counts were determined in the following groups of mice: i) WT-control (n = 5), ii) WT-DSS (n = 7), iii) IL-6−/−-DSS (n = 5), and iv) IL-6−/− bone marrow chimera (IL-6−/−⇒WT) chimera-DSS (n = 5).
Platelet Aggregation
Arterial blood from control and colitic mice was drawn into a syringe that contained acid citrate dextrose with 1 mmol/L EDTA and apyrase (grade VII; Sigma-Aldrich). Sample processing was started immediately after blood collection. Aggregation was induced with thrombin 0.63 U/mL (EC50), and platelet aggregation velocity was measured as previously described.14,25,26 Platelet aggregation velocity was measured from platelet-rich plasma derived from the following groups of mice: i) WT-control (n = 7), ii) WT-DSS (n = 7), iii) IL-6−/−-DSS mice (n = 7), iv) IL-6−/−⇒WT chimera-DSS (n = 6), v) WT-DSS mice treated with a rat anti–IL-6rα (CD126) blocking antibody (Angio-Proteomic, Boston, MA; 100 μg/mouse in 0.2 mL of normal saline administered 24 hour before the experiment; n = 5), and vi) WT-DSS mice treated with an anti-gp130 blocking antibody (R&D System Inc., Minneapolis, MN; 20 μg/mouse in 0.2 normal saline administered 24 hours before the experiment; n = 6).
Intravital Microscopy and Light/Dye-Induced Thrombosis
The mouse cremaster muscle was prepared for microscopic observation as previously described.14,27 Light and fluorescent microscopic images from an upright microscope (BX51WI; Olympus, Tokyo, Japan) with a 40× water immersion objective lens (LUMPlan FI/IR 40×/0.80×; Olympus) were projected onto a monitor (Trinitron PVM-2030; Sony, Tokyo, Japan) through a color video camera (VK-C150; Hitachi, Tokyo, Japan) or a charge-coupled device video camera (XC-77; Hamamatsu, Hamamatsu City, Japan), respectively. The images were recorded with a DVD recorder (SR-MV50; JVC, Wayne, NJ). A video timer (Time-Date Generator WJ-810; Panasonic, Osaka, Japan) was connected to the monitor to record time and date. The diameters of the selected cremaster muscle microvessels were measured by video analysis software (ImageJ software version 1.37; NIH, Bethesda, MD) on a personal computer (G4 Macintosh; Apple, Cupertino City, CA). Fluorescein isothiocyanate-dextran (5%) 10 mL/kg (150,000 molecular weight; Sigma Chemicals, St. Louis, MO) was infused via a cannulated jugular vein. Photoactivation of fluorescein isothiocyanate-dextran (excitation, 495 nm; emission, 519 nm) within the selected microvessels was achieved by epi-illumination with a 175-W xenon lamp (Lambda LS, Sutter, CA) and a fluorescein filter cube (HQ-FITC; Chroma Technology, Bellows Falls, VT). The excitation power density was measured daily (ILT 1700 Radiometer, SED033 detector; International Light Technologies, Peabody, MA) and maintained within 1% of 0.77 W/cm2, as previously described.27,28
Second- or third-order arterioles with a diameter of 30 to 50 μm, a minimum length of 100 μm, and a wall shear rate >500/s were randomly selected in the mouse cremaster muscle to study thrombus formation. Epi-illumination was continuously applied to the vessels, and thrombus formation was quantified by determining the time of onset of platelet deposition/aggregation (onset time) and the time required for complete cessation for >60 seconds (cessation time). Epi-illumination was discontinued once blood flow ceased in the vessel under study. The results from each arteriole were averaged from two to three thrombi produced in each mouse.
The light/dye method was used to monitor thrombus formation in the following experimental groups: i) control WT mice receiving an intrascrotal injection of 0.2 mL of saline at 5 hours before vessel photoactivation (n = 7); ii) control WT mice injected intrascrotally with recombinant mouse IL-6 (R&D Systems, Minneapolis, MN) at a concentration of either 10 (n = 5), 100 (n = 5), or 500 ng (n = 5) per mouse (dissolved in 0.2 mL of normal saline) at 5 hours before photoactivation; iii) WT mice on water (n = 10); iv) DSS-treated WT mice (WT-DSS) (n = 10); v) anti–IL-6 antibody (BD Biosciences) treated (100 μg/mouse administered 24 hours before photoactivation) WT-DSS mice (n = 8); and iv) DSS-treated IL-6−/− mice (n = 9).
Production of Bone Marrow Chimeras
In some mice, bone marrow from IL-6−/− donor mice was transplanted into WT recipients to produce IL-6−/−⇒WT chimeras, wherein only circulating blood cells are IL-6 deficient. IL-6–deficient BM cells (5 × 106) were transferred into the irradiated recipient mice, as previously described.29 Bone marrow chimerization was detected after 8 weeks, and only mice with >90% chimerization were used.
Statistics
Data were analyzed with standard statistical analysis, that is, one-way analysis of variance, Fisher’s post hoc test, Neuman-Keuls multicomparison test for >2 groups, or a Student’s t-test for only two groups of animals. All values are reported as means ± SEM with more than five mice per group.
Results
Colonic Inflammation Is Associated with IL-6–Dependent Thrombocytosis and Appearance of Immature Platelets
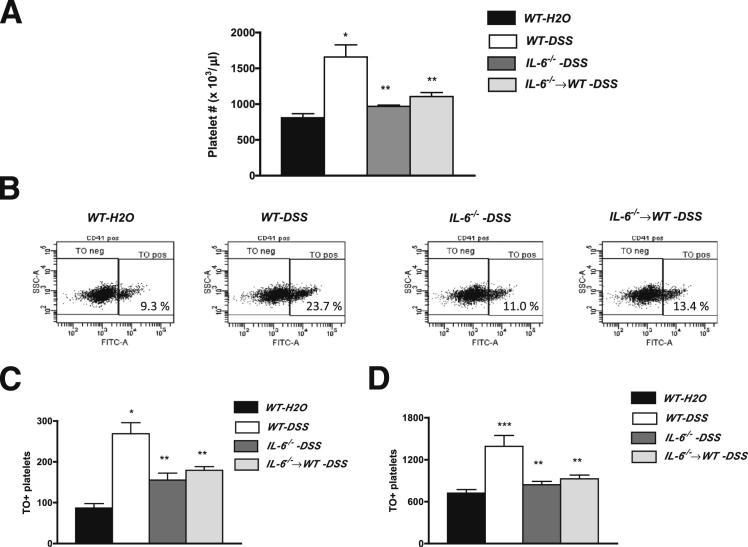
A well-characterized response to human IBD is thrombocytosis.4,8,30 Figure 1A shows that DSS-induced colonic inflammation in WT mice is also associated with a significant increase in total platelet count on day 6 of DSS treatment. This response was accompanied by increases in the numbers of immature (TO+) and mature (TO−) platelets (Figure 1, B–D). These increases in total, immature, and mature platelets were not detected in either IL-6−/− mice or in IL-6−/−⇒WT bone marrow chimeras, suggesting that IL-6 derived from cells of myeloid origin mediates the thrombocytosis associated with DSS-induced colitis. A role for IL-6 in mediating DSS-induced thrombocytosis is also supported by the observation (data not shown) that WT-DSS mice treated on day 5 with an IL-6rβ neutralizing antibody (936 ± 163 × 103 platelets/μL), but not an IL-6rα antibody (1368 ± 94 × 103 platelets/μL), resulted in a significant reduction in platelet count on day 6 of DSS, compared with untreated colitic (day 6) mice (1741 ± 215 × 103 platelets/μL).
Figure 1.
Platelet levels in control and DSS-fed mice. Changes in blood levels of total platelets (A), immature platelets (C), and mature platelets (D) in mice placed on regular drinking water (WT-H2O) or 3% DSS. Representative flow cytometric analyses of mature (TO−) and immature (TO+) platelets in the different experimental groups (WT-H2O, n = 5; WT-DSS, n = 7; IL-6−/−-DSS, n = 5; IL-6−/−⇒WT, n = 5) B: The platelet populations were gated on CD41+ cells. WT, IL-6−/−, and IL-6−/−⇒WT mice were studied. ∗P < 0.001 versus WT-H2O; ∗∗P < 0.01 versus WT-DSS; and ∗∗∗P < 0.01 versus WT-DSS.
IL-6–Dependent Colitis-Induced Thrombocytosis Does Not Result from a Prolongation of Platelet Life Span
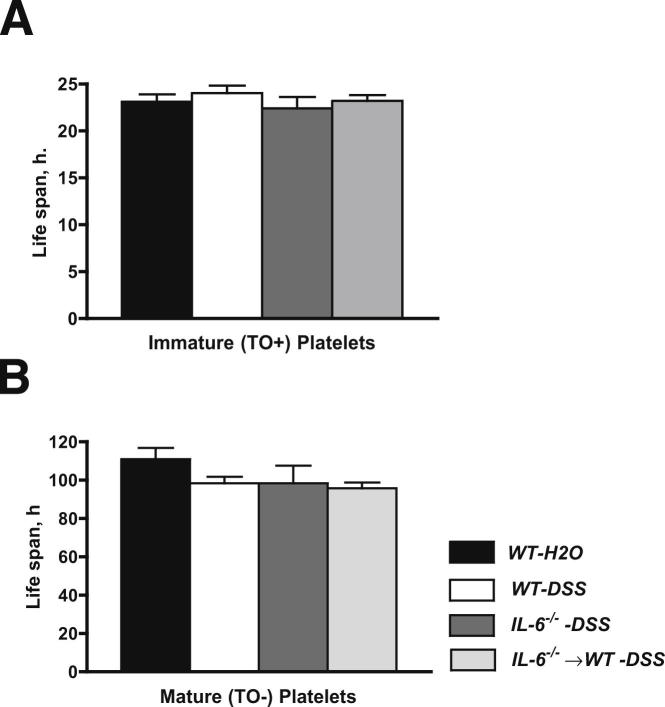
The IL-6–dependent increase in immature platelets in DSS colitic mice is consistent with the known ability of this cytokine to enhance platelet production.17 Because an increased platelet count can also result from an extended platelet life span, we also evaluated the influence of DSS-induced colonic inflammation on the life span of mature and immature platelets of both WT and IL-6−/− mice. As shown in Figure 2, A and B, the life span of immature (23.10 ± 0.8 hours) and mature (110 ± 5.7 hours) platelets in WT noncolitic (control) mice was not significantly altered by DSS treatment in WT, IL-6−/−, or IL-6−/−⇒WT mice.
Figure 2.
Platelet life span. Life span determinations for immature (TO+) (A) and mature (TO−) (B) biotinylated platelets in WT mice placed on regular drinking water (WT-H2O, n = 5) or 3% DSS in drinking water (n = 7). IL-6−/−-DSS (n = 5) and IL-6−/−⇒WT (n = 5) chimeras are also shown. No differences were noted between experimental groups.
Hyperreactivity of Platelets to Thrombin in DSS Colitic Mice Is Mediated by IL-6
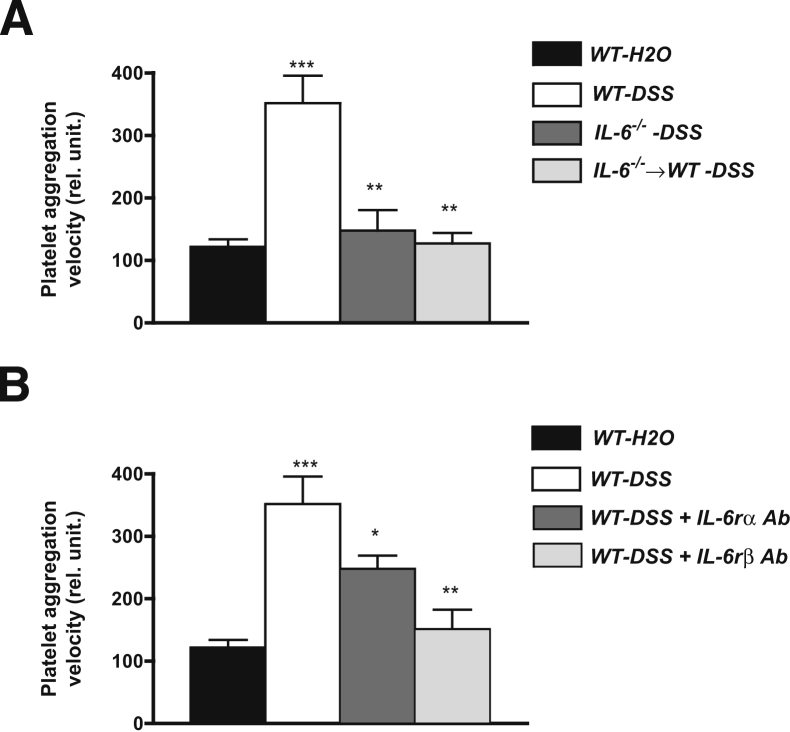
Platelets from patients with IBD are known to exhibit a hypersensitivity to aggregation in response to different agonists.31 This phenomenon is also evident in platelets derived from mice with DSS colitis.14 Figure 3A shows the large increase in platelet aggregation velocity observed in platelet-rich plasma from DSS colitic WT mice after exposure to an EC50 dose of thrombin. This 2.5-fold increase (compared with WT controls) in platelet aggregation velocity was not evident in platelet-rich plasma derived from either IL-6−/− or IL-6−/−⇒WT mice treated with DSS, suggesting that IL-6 (derived from myeloid cells) plays an important role in the altered platelet aggregation response that accompanies DSS colitis.
Figure 3.
Thrombin-induced platelet aggregation. A: Detection of platelet aggregation velocity (in relative units) after exposure to an EC50 dose (0.63 U/mL) of thrombin in WT mice on regular drinking water (WT-H2O, n = 7), WT-DSS (n = 7), IL-6−/−-DSS (n = 7), and IL-6−/−⇒WT-DSS (n = 6). ∗∗∗P < 0.001 versus WT-H2O; ∗∗P < 0.01 versus WT-DSS. B: Platelet aggregation velocity after thrombin exposure in WT-H2O (n = 7), untreated WT-DSS (n = 7), and WT-DSS mice receiving a blocking antibody directed against either the IL-6 receptor α (IL-6rα) (n = 5) or β (IL-6rβ) chain (n = 6). ∗∗∗P < 0.001 versus WT-H2O; ∗∗P < 0.01 versus WT-DSS; ∗P < 0.05 versus WT-DSS.
To further evaluate the contribution of IL-6 to colitis-induced platelet hyperaggregation, WT DSS colitic mice were treated with a blocking antibody directed against either the α or β (gp130) subunit of the IL-6 receptor (Figure 3B). The results of these experiments indicated that immunoblockade of the β-subunit of IL-6r completely prevented the exaggerated aggregation response of platelets from colitic mice to thrombin. Although blockade of the α-subunit (membrane-bound) of IL-6r also reduced the aggregation response to thrombin, this intervention was not as effective as β-subunit blockade. Measurements of platelet count in arterial blood of WT controls (992 ± 60 × 103/μL), WT-DSS mice (1741 ± 215 × 103/μL), and mice treated with the IL-6r antibodies indicated that the DSS-induced thrombocytosis was significantly blunted by treatment with the IL-6rβ monoclonal antibody (936 ± 163 × 103/μL; P < 0.01), but not with the IL-6rα monoclonal antibody (1368 ± 94 × 103/μL).
Administration of Murine IL-6 in WT Mice Promotes Microvascular Thrombus Formation
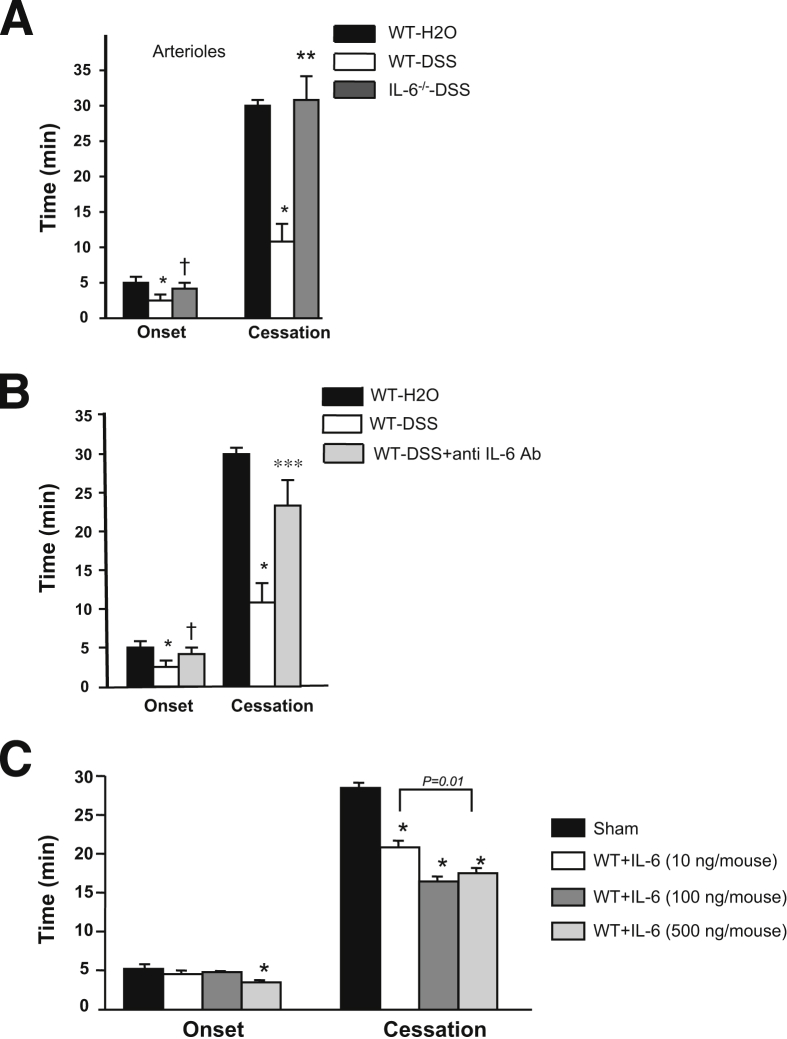
Although some pro-inflammatory cytokines (eg, TNF-α, IL-1β) have been shown to promote a microvascular thrombosis response similar to experimental colitis,14,15 the prothrombogenic effects of IL-6 have not been previously assessed. Figure 4C shows that intrascrotal administration of murine IL-6 induces a dose-dependent acceleration of light/dye-induced thrombosis, as reflected by a reduced time to flow cessation (which is evident at all doses) and a shorter time of onset of thrombus formation (evident only at the higher dose).
Figure 4.
Light/dye-induced thrombus formation in cremaster arterioles. Effects of IL-6−/− (n = 9) (A) and IL-6 immunoblocking (n = 8) (B) on light/dye-induced microvascular thrombosis in arterioles. ∗P < 0.05 versus WT-H2O (n = 10); ∗∗P < 0.01 versus WT-DSS (n = 10); †P < 0.05 versus WT-DSS. Effects of intrascrotal administration of IL-6 (10, 100, or 1000 ng/mouse; n = 5 for each one) dissolved in 0.2 mL of normal saline on thrombus formation (5 hours after injection) in cremaster muscle arterioles (C). ∗P < 0.05 versus sham-operated mice (n = 7).
IL-6 Mediates the Accelerated Thrombus Development Associated with DSS Colitis
The contribution of IL-6 to colitis-enhanced thrombus development was evaluated with both immunoblockade and genetic deletion strategies (Figure 4). A comparison of the thrombosis responses in WT-DSS and IL-6−/−-DSS mice indicated that IL-6 deficiency completely prevents the reductions in both time of onset and time to flow cessation induced by DSS after light/dye injury (Figure 4A). Similarly, immunoblockade of the IL-6 in colitic WT mice was nearly as effective as IL-6 deficiency in reversing the colitis-induced thrombosis response (Figure 4B).
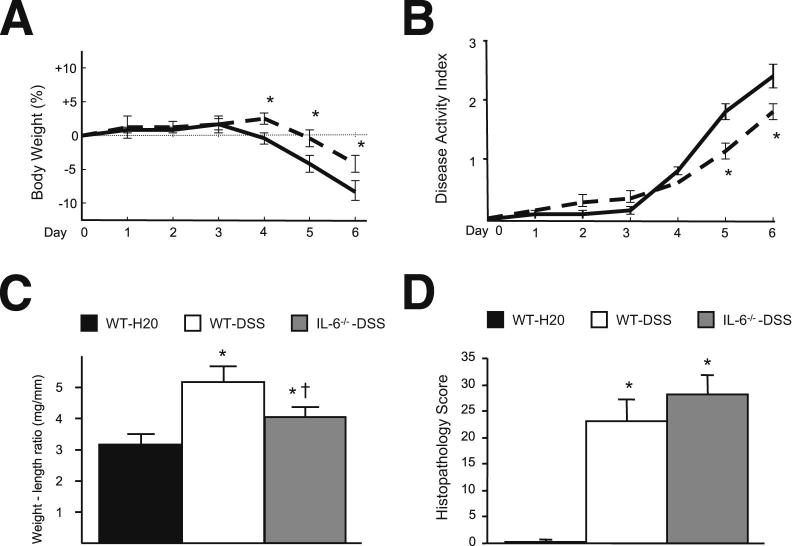
IL-6–deficient mice treated with DSS exhibited a small yet significant reduction in disease activity index and colon weight–length ratio (Figure 5, A–C). Disease activity index values for IL-6−/−⇒WT chimeras on DSS were higher than WT-DSS on days −4 and −5, but significantly lower on day 6. However, no significant differences were noted between WT-DSS and IL-6–deficient mice for total histology score (Figure 5D). Similarly, no differences were noted for the individual components of the total histological score, that is, severity of inflammation, depth of injury, and crypt damage.
Figure 5.
Effects of IL-6−/− on disease progression in DSS-treated mice. A and B represent changes in body weight and disease activity index of WT (solid line) and IL-6−/− (dotted line) mice treated with 3% DSS; WT-DSS (n = 9), IL-6−/−-DSS (n = 5) mice. C: Shown are the changes in colon weight-to-length ratio in WT-H2O control mice (n = 10), WT-DSS (n = 10), and IL-6−/−-DSS mice (n = 10). D: Shown are the histological scores (total) obtained in WT-H2O control (n = 6), WT-DSS (n = 6), and IL-6−/−-DSS (n = 6) mice. ∗P < 0.05 versus WT-H2O; †P < 0.05 versus WT-DSS.
Discussion
Although it is generally appreciated that abnormalities in both coagulation and platelet function may contribute to the enhanced thrombus formation that is associated with IBD, most attention has been focused on altered coagulation as the underlying molecular basis for the thrombogenic phenotype assumed by the vasculature in IBD.1,3 Less attention has been devoted to the potential contribution of platelets despite numerous reports that describe elevated blood platelet levels and platelet hyperreactivity in both human and experimental colitis.3,14 Previous studies from our laboratory have indicated that the platelet abnormalities and enhanced thrombus development described in human IBD are recapitulated in both acute (DSS) and chronic (T-cell transfer) models of experimental colitis. For example, we have reported that DSS- and T-cell transfer-induced colitis are accompanied by nearly identical changes in thrombus development.14 More recently, we have reported that the two distinct models of experimental colitis exhibit nearly identical responses for thrombocytosis, appearance of mature and immature platelets, increased number of activated circulating platelets, and increased number of platelet–leukocyte aggregates.32
Here, the DSS model was used to address the role of altered platelet production and reactivity in the enhanced thrombus development that accompanies colitis and specifically examined the role of IL-6, a cytokine that has been implicated in IBD pathogenesis,33 as a gut-derived factor that targets the production and reactivity of platelets, ultimately resulting in enhanced thrombus development. Our findings implicate IL-6 as a major factor underlying the accelerated microvascular thrombosis detected in extra-intestinal tissue during experimental IBD and lend support to the possibility that increased platelet production and enhanced platelet reactivity are important targets of IL-6 action that predispose the vasculature to thrombus development during colonic inflammation.
IBD is often accompanied by an increased blood platelet count (reactive thrombocytosis), with 50% to 100% increases in platelet count commonly reported for patients with active IBD, compared with control subjects.6,7,34 The association of thrombocytosis with active IBD has led to the proposal that platelet count may be a useful marker of disease activity.4,8 Our study has indicated that the colonic inflammation induced by DSS administration in mice results in an increased platelet count (50% to 100%) that is comparable with that noted in patients with IBD. The results of this study also provide two lines of evidence to suggest that the increased platelet count induced by DSS results from increased platelet production. First, we detected a significant increase in the number of circulating immature (TO staining) platelets. Second, no change in platelet life span was noted when the DSS-treated WT mice were compared with WT mice on regular drinking water. The latter observation is consistent with a clinical report that described a normal life span of platelets labeled with indium-111 in patients with active IBD.7
IL-6 has been shown to act on the maturation stages of megakaryocytosis to stimulate platelet production in mice17,35 and other species.35,36 The cytokine is also able to indirectly stimulate platelet production by enhancing hepatic output of TPO, a potent thrombopoeitic agent that also targets the megakaryocyte.37 Our finding that IL-6 deficiency largely prevented the thrombocytosis and appearance of immature platelets without affecting platelet life span in DSS-treated mice provides strong evidence that IL-6 is largely responsible for the increased platelet production that is elicited by experimental colitis. Furthermore, our observation that immunoblockade of the β-subunit (gp130), but not the membrane bound α-subunit, of the IL-6 receptor blunts the DSS-induced thrombocytosis, as previously described in IL-6–infused dogs treated with anti-gp130,38 suggests that IL-6 stimulates its receptors on the megakaryocyte by binding to the circulating soluble isoform of the IL-6 receptor, which then engages with gp130 to initiate a trans-signaling mechanism that results in increased platelet production.38–42
Another characteristic feature of the altered platelet function in IBD is hyperreactivity to agonist stimulation.7,32 Platelets from patients with IBD exhibit an exaggerated aggregation response to stimulation by agonists such ADP and collagen.7,32 Our focus on thrombin is based on the critical role of this prothrombotic agent in mediating the enhanced extra-intestinal thrombosis that accompanies colonic inflammation in the DSS model.43 However, the present study extends our previous work on colitis-induced alterations in platelet function by implicating IL-6 as a mediator of the platelet hyperreactivity that accompanies DSS-induced colonic inflammation.19 We show that the exaggerated platelet aggregation response to the EC50 dose of thrombin in DSS-treated mice is not observed in either IL-6−/− or IL-6−/−⇒WT mice. Similarly, we noted that immunoblockade of either the α- or β-subunit of IL-6r blunts the response, with gp130 blockade providing complete protection, whereas the α-subunit (that targets the membrane bound form) is only partially protective. The latter observation suggests that both membrane-associated and soluble IL-6r participate in the IL-6–dependent platelet hyperreactivity in DSS colitis. Although the contribution of TPO to the altered platelet reactivity remains unclear, previous work has shown that the infusion of TPO in dogs does not alter platelet responses to thrombin,44 which differs from the increased platelet reactivity to thrombin noted after IL-6 infusion.19,44
IL-6 may mediate its effects on platelet reactivity by either acting directly on platelets and via stimulation of thrombopoiesis.44 Reports describe IL-6–induced activation of human platelets when the cytokine is incubated with platelet-rich plasma.45–47 By contrast, although dogs treated with IL-6 exhibit platelet hyperreactivity,18,19,44 direct ex vivo incubation of dog platelets with IL-6 does not alter platelet activation,19,44 suggesting that the cytokine is indirectly enhancing platelet reactivity in vivo. The latter observation has led to the proposal that increased IL-6 levels are more likely to alter platelet reactivity via an action on hematopoietic tissue.18,48 Because young (immature) platelets are considered to be more reactive to agonist stimulation,10,18,44 it follows that stimulants of thrombopoiesis (such as IL-6) would increase the percentage of circulating platelets that are immature and hyperreactive,44 thereby resulting in a phenotypic change in the reactivity of the total platelet population.
Our study also provides several lines of evidence that implicates IL-6 as a mediator of the accelerated light/dye injury-induced thrombosis observed in cremaster muscle arterioles of DSS-treated mice. First, we present the novel observation that exogenous IL-6 elicits a dose-dependent acceleration of thrombus development in arterioles of WT mice (without colitis). Second, we show that immunoblockade of IL-6 affords significant protection against DSS-induced thrombogenesis. Finally, mice that are genetically deficient in IL-6 exhibit complete protection against colitis-enhanced thrombus formation. Although we do not provide definitive evidence on the mechanisms that underlie the ability of IL-6 to mediate the enhanced thrombosis associated with colitis, it is tempting to speculate that the accelerated IL-6–dependent thrombosis is a direct reflection of the proposed actions of the cytokine on platelet production and reactivity in colitic mice. Such a connection has been reported in a zebra fish model of arterial thrombosis, wherein it was shown that young, hyperreactive thrombocytes initiate thrombus formation by rapidly accumulating at the site of vessel damage, releasing agonists that recruit more young thrombocytes, and then recruiting more mature (less reactive) thrombocytes into the growing thrombus.49
Consideration should be given to the known effects of IL-6 on the coagulation system. For example, IL-6 stimulates coagulation in humans as reflected in increased thrombin-antithrombin III complexes and elevated levels of prothrombin activation of fragment F1 and F2.50 Elimination of IL-6 attenuates the activation of coagulation.38,51 IL-6 is also known to enhance the production of other cytokines, such as TNF-α and IL-1β, which can also influence the coagulation system and promote thrombus formation. TNF-α and IL-1β are also known to stimulate IL-6 production via an NF-κB–dependent mechanism. This tight interdependence of IL-6 with TNF-α and IL-1β likely explains why TNF-α and IL-1β have also been shown to contribute to DSS colitis-enhanced thrombus formation.41,52 Although the hierarchy of IL-6, TNF-α, and IL-1β as mediators of the abnormal platelet function and enhanced thrombus development in colitis remains unclear, the available evidence points to a dominant role for IL-6. For example, we have previously reported that individual immunoblockade of TNF-α and IL-1β affords only partial protection against colitis-accelerated thrombus formation, whereas simultaneous blockade of both cytokines affords complete protection similar to IL-6 immunoblockade alone.14,15 Similarly, although the exaggerated thrombin-induced platelet aggregation response to DSS is absent in IL-6–deficient mice (Figure 3), IL-1β deficiency affords no protection.14
The role of IL-6 in mediating the colonic inflammation that is elicited by DSS treatment is another important consideration. Previous studies in murine models of colitis have reported an attenuation of macroscopic (disease activity index, colon weight–length ratio) and microscopic (histopathology) indices of colonic inflammation and disease activity with IL-6 deficiency.53–55 However, the findings of our study indicate that IL-6 deficiency confers moderate protection against DSS-induced loss of body weight and colonic thickening, without improving the degree of colonic inflammation or damage (Figure 5). These observations lessen the likelihood that IL-6 deficiency blunts the platelet abnormalities and enhanced extra-intestinal thrombosis indirectly by limiting the intensity of the colonic inflammation. Further support for a more direct action of IL-6 on the thrombogenic responses is evidenced by our observation (Figure 3B) that immunoblockade of IL-6 on day 5 of DSS treatment, when inflammation was already well established, resulted in a significant attenuation of the exaggerated thrombin-mediated platelet aggregation response normally observed on day 6 of DSS treatment.
In conclusion, the findings of this study provide evidence to implicate IL-6 as a mediator of the thrombocytosis, platelet hyperreactivity, and enhanced thrombus development that are associated with experimental colitis. The IL-6–dependent increase in blood platelet count elicited during DSS-induced colonic inflammation appears to result from an enhancement of platelet production, rather than a prolongation of platelet life span. The appearance of immature platelets during colitis supports the view that enhanced thrombus development in extra-intestinal tissues results from an increased percentage of young, hemostatically active platelets that more readily aggregate in blood and accumulate at an accelerated rate at sites of vessel injury.
Acknowledgments
E.Y.S., S.K., and D.N.G. designed the research and wrote the paper; E.Y.S., S.K., L.D.A.-P., L.-S.Y., and S.Z. performed experiments and analyzed results; and E.Y.S., K.S., and J.R. prepared the figures.
Footnotes
Supported by the National Institute of Diabetes and Digestive and Kidney Diseases grant P01 DK43785-20.
E.Y.S. and S.K. contributed equally to this work.
References
- 1.Yoshida H., Granger D.N. Inflammatory bowel disease: a paradigm for the link between coagulation and inflammation. Inflamm Bowel Dis. 2009;15:1245–1255. doi: 10.1002/ibd.20896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Larsen S., Bendtzen K., Nielsen O.H. Extraintestinal manifestations of inflammatory bowel disease: epidemiology, diagnosis, and management. Ann Med. 2010;42:97–114. doi: 10.3109/07853890903559724. [DOI] [PubMed] [Google Scholar]
- 3.Danese S., Papa A., Saibeni S., Repici A., Malesci A., Vecchi M. Inflammation and coagulation in inflammatory bowel disease: the clot thickens. Am J Gastroenterol. 2007;102:174–186. doi: 10.1111/j.1572-0241.2006.00943.x. [DOI] [PubMed] [Google Scholar]
- 4.Harries A.D., Beeching N.J., Rogerson S.J., Nye F.J. The platelet count as a simple measure to distinguish inflammatory bowel disease from infective diarrhoea. J Infect. 1991;22:247–250. doi: 10.1016/s0163-4453(05)80006-4. [DOI] [PubMed] [Google Scholar]
- 5.Collins C.E., Rampton D.S., Rogers J., Williams N.S. Platelet aggregation and neutrophil sequestration in the mesenteric circulation in inflammatory bowel disease. Eur J Gastroenterol Hepatol. 1997;9:1213–1217. [PubMed] [Google Scholar]
- 6.Heits F., Stahl M., Ludwig D., Stange E.F., Jelkmann W. Elevated serum thrombopoietin and interleukin-6 concentrations in thrombocytosis associated with inflammatory bowel disease. J Interferon Cytokine Res. 1999;19:757–760. doi: 10.1089/107999099313604. [DOI] [PubMed] [Google Scholar]
- 7.Webberley M.J., Hart M.T., Melikian V. Thromboembolism in inflammatory bowel disease: role of platelets. Gut. 1993;34:247–251. doi: 10.1136/gut.34.2.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Harries A.D., Fitzsimons E., Fifield R., Dew M.J., Rhoades J. Platelet count: a simple measure of activity in Crohn’s disease. Br Med J (Clin Res Ed) 1983;286:1476. doi: 10.1136/bmj.286.6376.1476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ault K.A., Knowles C. In vivo biotinylation demonstrates that reticulated platelets are the youngest platelets in circulation. Exp Hematol. 1995;23:996–1001. [PubMed] [Google Scholar]
- 10.Thompson C.B., Jakubowski J.A., Quinn P.G., Deykin D., Valeri C.R. Platelet size and age determine platelet function independently. Blood. 1984;63:1372–1375. [PubMed] [Google Scholar]
- 11.Irving P.M., Pasi K.J., Rampton D.S. Thrombosis and inflammatory bowel disease. Clin Gastroenterol Hepatol. 2005;3:617–628. doi: 10.1016/s1542-3565(05)00154-0. [DOI] [PubMed] [Google Scholar]
- 12.Alex P., Zachos N.C., Nguyen T., Gonzales L., Chen T.E., Conklin L.S., Centola M., Li X. Distinct cytokine patterns identified from multiplex profiles of murine DSS and TNBS-induced colitis. Inflamm Bowel Dis. 2009;15:341–352. doi: 10.1002/ibd.20753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Reinecker H.C., Steffen M., Witthoeft T., Pflueger I., Schreiber S., MacDermott R.P., Raedler A. Enhanced secretion of tumour necrosis factor-alpha, IL-6, and IL-1 beta by isolated lamina propria mononuclear cells from patients with ulcerative colitis and Crohn’s disease. Clin Exp Immunol. 1993;94:174–181. doi: 10.1111/j.1365-2249.1993.tb05997.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yoshida H., Russell J., Senchenkova E.Y., Almeida Paula L.D., Granger D.N. Interleukin-1beta mediates the extra-intestinal thrombosis associated with experimental colitis. Am J Pathol. 2010;177:2774–2781. doi: 10.2353/ajpath.2010.100205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yoshida H., Yilmaz C.E., Granger D.N. Role of tumor necrosis factor-alpha in the extraintestinal thrombosis associated with colonic inflammation. Inflamm Bowel Dis. 2011;17:2217–2223. doi: 10.1002/ibd.21593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Levi M., van der Poll T. Two-way interactions between inflammation and coagulation. Trends Cardiovasc Med. 2005;15:254–259. doi: 10.1016/j.tcm.2005.07.004. [DOI] [PubMed] [Google Scholar]
- 17.Ishibashi T., Kimura H., Shikama Y., Uchida T., Kariyone S., Hirano T., Kishimoto T., Takatsuki F., Akiyama Y. Interleukin-6 is a potent thrombopoietic factor in vivo in mice. Blood. 1989;74:1241–1244. [PubMed] [Google Scholar]
- 18.Burstein S.A. Effects of interleukin 6 on megakaryocytes and on canine platelet function. Stem Cells. 1994;12:386–393. doi: 10.1002/stem.5530120405. [DOI] [PubMed] [Google Scholar]
- 19.Peng J., Friese P., George J.N., Dale G.L., Burstein S.A. Alteration of platelet function in dogs mediated by interleukin-6. Blood. 1994;83:398–403. [PubMed] [Google Scholar]
- 20.Mutlu G.M., Green D., Bellmeyer A., Baker C.M., Burgess Z., Rajamannan N., Christman J.W., Foiles N., Kamp D.W., Ghio A.J., Chandel N.S., Dean D.A., Sznajder J.I., Budinger G.R. Ambient particulate matter accelerates coagulation via an IL-6-dependent pathway. J Clin Invest. 2007;117:2952–2961. doi: 10.1172/JCI30639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yoshida H., Russell J., Stokes K.Y., Yilmaz C.E., Esmon C.T., Granger D.N. Role of the protein C pathway in the extraintestinal thrombosis associated with murine colitis. Gastroenterology. 2008;135:882–888. doi: 10.1053/j.gastro.2008.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dieleman L.A., Palmen M.J., Akol H., Bloemena E., Pena A.S., Meuwissen S.G., Van Rees E.P. Chronic experimental colitis induced by dextran sulphate sodium (DSS) is characterized by Th1 and Th2 cytokines. Clin Exp Immunol. 1998;114:385–391. doi: 10.1046/j.1365-2249.1998.00728.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Matic G.B., Chapman E.S., Zaiss M., Rothe G., Schmitz G. Whole blood analysis of reticulated platelets: improvements of detection and assay stability. Cytometry. 1998;34:229–234. doi: 10.1002/(sici)1097-0320(19981015)34:5<229::aid-cyto4>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 24.Robinson M., MacHin S., Mackie I., Harrison P. In vivo biotinylation studies: specificity of labelling of reticulated platelets by thiazole orange and mepacrine. Br J Haematol. 2000;108:859–864. doi: 10.1046/j.1365-2141.2000.01939.x. [DOI] [PubMed] [Google Scholar]
- 25.Mindukshev I., Gambaryan S., Kehrer L., Schuetz C., Kobsar A., Rukoyatkina N., Nikolaev V.O., Krivchenko A., Watson S.P., Walter U., Geiger J. Low angle light scattering analysis: a novel quantitative method for functional characterization of human and murine platelet receptors. Clin Chem Lab Med. 2012;50:1253–1263. doi: 10.1515/CCLM.2011.817. [DOI] [PubMed] [Google Scholar]
- 26.Gavins F.N., Russell J., Senchenkova E.L., De Almeida Paula L., Damazo A.S., Esmon C.T., Kirchhofer D., Hebbel R.P., Granger D.N. Mechanisms of enhanced thrombus formation in cerebral microvessels of mice expressing hemoglobin-S. Blood. 2011;117:4125–4133. doi: 10.1182/blood-2010-08-301366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rumbaut R.E., Randhawa J.K., Smith C.W., Burns A.R. Mouse cremaster venules are predisposed to light/dye-induced thrombosis independent of wall shear rate, CD18, ICAM-1, or P-selectin. Microcirculation. 2004;11:239–247. doi: 10.1080/10739680490425949. [DOI] [PubMed] [Google Scholar]
- 28.Rumbaut R.E., Slaff D.W., Burns A.R. Microvascular thrombosis models in venules and arterioles in vivo. Microcirculation. 2005;12:259–274. doi: 10.1080/10739680590925664. [DOI] [PubMed] [Google Scholar]
- 29.Stokes K.Y., Calahan L., Russell J.M., Gurwara S., Granger D.N. Role of platelets in hypercholesterolemia-induced leukocyte recruitment and arteriolar dysfunction. Microcirculation. 2006;13:377–388. doi: 10.1080/10739680600745877. [DOI] [PubMed] [Google Scholar]
- 30.Morowitz D.A., Allen L.W., Kirsner J.B. Thrombocytosis in chronic inflammatory bowel disease. Ann Intern Med. 1968;68:1013–1021. doi: 10.7326/0003-4819-68-5-1013. [DOI] [PubMed] [Google Scholar]
- 31.Andoh A., Yoshida T., Yagi Y., Bamba S., Hata K., Tsujikawa T., Kitoh K., Sasaki M., Fujiyama Y. Increased aggregation response of platelets in patients with inflammatory bowel disease. J Gastroenterol. 2006;41:47–54. doi: 10.1007/s00535-005-1721-x. [DOI] [PubMed] [Google Scholar]
- 32.Yan S.L., Russell J., Harris N.R., Senchenkova E.Y., Yildirim A., Granger D.N. Platelet abnormalities during colonic inflammation. Inflamm Bowel Dis. 2013;19:1245–1253. doi: 10.1097/MIB.0b013e318281f3df. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Atreya R., Neurath M.F. Involvement of IL-6 in the pathogenesis of inflammatory bowel disease and colon cancer. Clin Rev Allergy Immunol. 2005;28:187–196. doi: 10.1385/CRIAI:28:3:187. [DOI] [PubMed] [Google Scholar]
- 34.Collins C.E., Rampton D.S. Review article: platelets in inflammatory bowel disease–pathogenetic role and therapeutic implications. Aliment Pharmacol Ther. 1997;11:237–247. doi: 10.1046/j.1365-2036.1997.153328000.x. [DOI] [PubMed] [Google Scholar]
- 35.Ishibashi T., Kimura H., Uchida T., Kariyone S., Friese P., Burstein S.A. Human interleukin 6 is a direct promoter of maturation of megakaryocytes in vitro. Proc Natl Acad Sci U S A. 1989;86:5953–5957. doi: 10.1073/pnas.86.15.5953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Asano S., Okano A., Ozawa K., Nakahata T., Ishibashi T., Koike K., Kimura H., Tanioka Y., Shibuya A., Hirano T. In vivo effects of recombinant human interleukin-6 in primates: stimulated production of platelets. Blood. 1990;75:1602–1605. [PubMed] [Google Scholar]
- 37.Kaser A., Brandacher G., Steurer W., Kaser S., Offner F.A., Zoller H., Theurl I., Widder W., Molnar C., Ludwiczek O., Atkins M.B., Mier J.W., Tilg H. Interleukin-6 stimulates thrombopoiesis through thrombopoietin: role in inflammatory thrombocytosis. Blood. 2001;98:2720–2725. doi: 10.1182/blood.v98.9.2720. [DOI] [PubMed] [Google Scholar]
- 38.Harrison P., Downs T., Friese P., Wolf R., George J.N., Burstein S.A. Inhibition of the acute-phase response in vivo by anti-gp130 monoclonal antibodies. Br J Haematol. 1996;95:443–451. [PubMed] [Google Scholar]
- 39.Hill R.J., Warren M.K., Levin J. Stimulation of thrombopoiesis in mice by human recombinant interleukin 6. J Clin Invest. 1990;85:1242–1247. doi: 10.1172/JCI114559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kishimoto T., Akira S., Taga T. IL-6 receptor and mechanism of signal transduction. Int J Immunopharmacol. 1992;14:431–438. doi: 10.1016/0192-0561(92)90173-i. [DOI] [PubMed] [Google Scholar]
- 41.Mudter J., Neurath M.F. IL-6 signaling in inflammatory bowel disease: pathophysiological role and clinical relevance. Inflamm Bowel Dis. 2007;13:1016–1023. doi: 10.1002/ibd.20148. [DOI] [PubMed] [Google Scholar]
- 42.Peters M., Muller A.M., Rose-John S. Interleukin-6 and soluble interleukin-6 receptor: direct stimulation of gp130 and hematopoiesis. Blood. 1998;92:3495–3504. [PubMed] [Google Scholar]
- 43.Yoshida H., Russell J., Granger D.N. Thrombin mediates the extraintestinal thrombosis associated with experimental colitis. Am J Physiol Gastrointest Liver Physiol. 2008;295:G904–G908. doi: 10.1152/ajpgi.90400.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Peng J., Friese P., Wolf R.F., Harrison P., Downs T., Lok S., Dale G.L., Burstein S.A. Relative reactivity of platelets from thrombopoietin- and interleukin-6-treated dogs. Blood. 1996;87:4158–4163. [PubMed] [Google Scholar]
- 45.Oleksowicz L., Mrowiec Z., Zuckerman D., Isaacs R., Dutcher J., Puszkin E. Platelet activation induced by interleukin-6: evidence for a mechanism involving arachidonic acid metabolism. Thromb Haemost. 1994;72:302–308. [PubMed] [Google Scholar]
- 46.Oleksowicz L., Puszkin E., Mrowiec Z., Isaacs R., Dutcher J.P. Alterations in platelet function in patients receiving interleukin-6 as cytokine therapy. Cancer Invest. 1996;14:307–316. doi: 10.3109/07357909609012156. [DOI] [PubMed] [Google Scholar]
- 47.Oleksowicz L., Mrowiec Z., Isaacs R., Dutcher J.P., Puszkin E. Morphologic and ultrastructural evidence of interleukin-6 induced platelet activation. Am J Hematol. 1995;48:92–99. doi: 10.1002/ajh.2830480205. [DOI] [PubMed] [Google Scholar]
- 48.Burstein S.A., Peng J., Friese P., Wolf R.F., Harrison P., Downs T., Hamilton K., Comp P., Dale G.L. Cytokine-induced alteration of platelet and hemostatic function. Stem Cells. 1996;14(Suppl 1):154–162. doi: 10.1002/stem.5530140720. [DOI] [PubMed] [Google Scholar]
- 49.Thattaliyath B., Cykowski M., Jagadeeswaran P. Young thrombocytes initiate the formation of arterial thrombi in zebrafish. Blood. 2005;106:118–124. doi: 10.1182/blood-2004-10-4118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Stouthard J.M., Levi M., Hack C.E., Veenhof C.H., Romijn H.A., Sauerwein H.P., van der Poll T. Interleukin-6 stimulates coagulation, not fibrinolysis, in humans. Thromb Haemost. 1996;76:738–742. [PubMed] [Google Scholar]
- 51.van der Poll T., Levi M., Hack C.E., ten Cate H., van Deventer S.J., Eerenberg A.J., de Groot E.R., Jansen J., Gallati H., Buller H.R., ten Cate J.W., Aarden L.A. Elimination of interleukin 6 attenuates coagulation activation in experimental endotoxemia in chimpanzees. J Exp Med. 1994;179:1253–1259. doi: 10.1084/jem.179.4.1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mitsuyama K., Matsumoto S., Masuda J., Yamasakii H., Kuwaki K., Takedatsu H., Sata M. Therapeutic strategies for targeting the IL-6/STAT3 cytokine signaling pathway in inflammatory bowel disease. Anticancer Res. 2007;27:3749–3756. [PubMed] [Google Scholar]
- 53.Suzuki A., Hanada T., Mitsuyama K., Yoshida T., Kamizono S., Hoshino T., Kubo M., Yamashita A., Okabe M., Takeda K., Akira S., Matsumoto S., Toyonaga A., Sata M., Yoshimura A. CIS3/SOCS3/SSI3 plays a negative regulatory role in STAT3 activation and intestinal inflammation. J Exp Med. 2001;193:471–481. doi: 10.1084/jem.193.4.471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Naito Y., Takagi T., Uchiyama K., Kuroda M., Kokura S., Ichikawa H., Yanagisawa R., Inoue K., Takano H., Satoh M., Yoshida N., Okanoue T., Yoshikawa T. Reduced intestinal inflammation induced by dextran sodium sulfate in interleukin-6-deficient mice. Int J Mol Med. 2004;14:191–196. [PubMed] [Google Scholar]
- 55.Weigmann B., Lehr H.A., Yancopoulos G., Valenzuela D., Murphy A., Stevens S., Schmidt J., Galle P.R., Rose-John S., Neurath M.F. The transcription factor NFATc2 controls IL-6-dependent T cell activation in experimental colitis. J Exp Med. 2008;205:2099–2110. doi: 10.1084/jem.20072484. [DOI] [PMC free article] [PubMed] [Google Scholar]