Abstract
Prostate cancer (CaP) progresses to a castration-resistant state assisted by multifold molecular changes, most of which involve activation of the androgen receptor (AR). Having previously demonstrated the importance of the Lin28/let-7/Myc axis in CaP, we tested the hypothesis that Lin28 is overexpressed in CaP and that it activates AR and promotes growth of CaP cells. We analyzed human clinical CaP samples for the expression of Lin28 by quantitative real-time RT-PCR, Western blot analysis, and IHC. Growth characteristics of CaP cell lines transiently and stably expressing Lin28 were examined. The clonogenic ability of CaP cells expressing Lin28 was determined by colony formation and soft agar assays. Increase in expression of AR and subsequent increase in transcription of AR-target genes were analyzed by quantitative real-time RT-PCR, luciferase assays, and ELISA. LNCaP cells stably expressing Lin28 were injected into nude mice, and tumorigenesis was monitored. We found that Lin28 is overexpressed in clinical CaP compared to benign prostates. Overexpression of Lin28 enhanced, while down-regulation reduced, growth of CaP cells. Lin28 enhanced the ability of CaP cells to form colonies in anchorage-dependent and anchorage-independent conditions. LNCaP cells stably expressing Lin28 exhibited significantly higher tumorigenic ability in vivo. Lin28 induced expression of the AR and its target genes such as PSA and NKX3.1. Collectively, our findings demonstrate a novel role for Lin28 in CaP development and activation of the AR axis.
Prostate cancer (CaP) remains one of the cancers with high incidence and mortality rates among men in the United States. Progression of CaP to castration resistance is the major challenge facing efforts to develop effective therapies against CaP. Castration-resistant prostate cancer evades androgen ablation by activating androgen receptor (AR)–dependent signaling through alternative mechanisms. Previous reports have shown that castration-resistant prostate cancer may evolve by suppression of tumor-suppressor genes and miRNAs, activation of potential oncogenes, overexpression of the AR, and activation of other signaling cascades.1 miRNAs are small RNAs that regulate gene expression by binding to the untranslated regions of target mRNAs and inhibiting their translation. The let-7 family of miRNAs is regulated by Lin28 and Lin28B, two homologues of the heterochronic gene lin-28 in Caenorhabditis elegans.2 The let-7 family miRNAs are tumor suppressors and are implicated as prognostic factors in a multitude of cancers.3–5 Hence, Lin28/Lin28B, which inhibit maturation of let-7 miRNAs, may be potential oncogenes in several malignancies. Our previous studies indicated that the Lin28/let-7 double-negative feedback loop regulates AR-dependent signaling in human CaP and that let-7 miRNA expression is suppressed in most cases of primary and castration-resistant CaP.6,7
Lin28, a highly conserved RNA-binding protein and a master regulator of let-7 miRNA processing, is overexpressed in primary human tumors8,9 and is postulated to be one of the embryonic stem cell factors that promote oncogenesis and proliferation of cancer cells.10 Lin28 binds to the terminal loops of the precursors of let-7 family miRNAs and blocks their processing into mature miRNAs.11,12 Lin28 also derepresses c-Myc by repressing let-7, and c-Myc activates transcription of Lin28.13,14 This Lin28/let-7/c-Myc loop may play an important role in the deregulated miRNA expression signature observed in many cancers.15
In this study, we hypothesized that Lin28 may function as a prosurvival factor in CaP. To test this hypothesis, we analyzed expression levels of Lin28 in human CaP samples and found that Lin28 levels are up-regulated in CaP. We expressed Lin28 transiently and constitutively in LNCaP human CaP cells and found that Lin28 enhances growth, invasion, and soft agar colony formation. Expression of Lin28 also led to up-regulation of expression of AR and its target genes. Constitutive expression of Lin28 also promoted tumorigenicity of LNCaP cells in nude mice. Knockdown of endogenous Lin28 inhibited expression of the AR and led to reduced levels of cell growth. Taken together, these data demonstrate the functional importance of Lin28 in human CaP.
Materials and Methods
Cell Lines, Antibodies, and Other Reagents
LNCaP, C4-2B, and DU145 prostate cancer cell lines and the PZ-HPV7 normal prostate epithelial cell line were purchased from ATCC (Manassas, VA). LNCaP-S17 and LNCaP-IL6 cell lines were described previously.16 LNCaP cells stably expressing Lin28 (LN-Lin28) were generated by transfection of pLKO.1-Lin28 (Open Biosystems, Pittsburgh, PA) into LNCaP cells. Expression of Lin28 in the stable transfectants was confirmed by Western blot analysis. LNCaP cells expressing Tet-inducible Lin28 (LN/TR/Lin28) were generated according to the manufacturer’s instructions using the ViraPower Lentiviral Transduction System (Invitrogen, Grand Island, NY). Inducible expression of Lin28 was confirmed by Western blot analysis after induction with 0.5 μg/mL doxycycline (Sigma, St. Louis, MO). Antibodies against AR and tubulin (AR-441 and T5168) were from Santa Cruz Biotechnologies (Santa Cruz, CA), and Lin28 antibodies (Ab-71415) were from Abcam (San Francisco, CA).
Western Blot Analysis
Cells were lysed in high salt buffer containing 50 mmol/L HEPES (pH 7.9), 250 mmol/L NaCl, 1 mmol/L EDTA, 1% NP-40, 1 mmol/L phenylmethylsulfonyl fluoride, 1 mmol/L Na vanadate, 1 mmol/L NaF, and protease inhibitors (Roche, Indianapolis, IN), as previously described.6 Total protein was estimated using the Coomassie Protein Assay Reagent (Pierce, Rockford, IL). Equal amounts of protein were loaded onto 10% SDS-PAGE and transferred to nitrocellulose membranes. The membranes were blocked with 5% nonfat milk in PBST (1× PBS + 0.1% Tween-20) and probed with primary antibodies in 1% bovine serum albumin. The signal was detected by enhanced chemiluminescence (GE Healthcare, Waukesha, WI) after incubation with the appropriate horseradish peroxidase–conjugated secondary antibodies.
Real-Time RT-qPCR
Total RNAs were extracted using TRIzol (Invitrogen) and were subjected to digestion with RNase-free RQ1 DNase (Promega, Madison, WI) before reverse transcription. The resultant cDNAs were subjected to real-time quantitative RT-PCR (RT-qPCR) using SsoFast Eva Green supermix (Bio-Rad, Hercules, CA), as described previously.7 Each reaction was normalized by coamplification of actin. Triplicates of samples were run on default settings of a Bio-Rad CFX-96 real-time cycler. Sequences of primers used have been published previously.6,7,17
Clonogenic Assays
Anchorage-dependent clonogenic ability assays were performed as described previously.18 At the end of the experiment, colonies were fixed with methanol, stained with crystal violet, and counted.
Soft-Agar Colony Formation Assays
Anchorage-independent colony formation assays were performed as described previously.7 At the end of the experiment, colonies were stained with 0.005% crystal violet and counted.
Cell Growth Assays
PZ-HPV7, LNCaP, C4-2B, DU145, LNCaP-S17, LN-Lin28, and LN/TR/Lin28 cells were plated in 12-well plates in triplicate, and viable cell numbers were determined at 0, 24, and 48 hours using a Coulter cell counter (Beckman Coulter, Brea, CA).
Invasion Assays
Boyden chamber invasion assays were performed as described previously.19 Briefly, 1 × 105 cells were plated in defined medium on Matrigel-coated cell culture inserts (BD Biosciences, San Jose, CA), with complete medium in the lower chamber. After incubation at 37°C for 48 hours, uninvaded cells were scraped off, the membrane inserts were washed and stained, and invaded cells on the lower surface were counted.
Animals
Male nude mice (aged 6 to 8 weeks) were maintained in the Animal Facility at University of California (UC) Davis Medical Center (Sacramento). All experimental procedures using animals were approved by the Institutional Animal Care and Use Committee of UC Davis. Cells (2 × 106 per flank) were injected s.c. into both flanks, and tumors were allowed to grow. Tumors were measured twice weekly. At the end of the experiments, tumors were excised and sera were collected for measurement of prostate-specific antigen (PSA).
Measurement of PSA
PSA levels were measured in the culture supernatants and mouse sera using enzyme-linked immunosorbent assay (ELISA; United Biotech Inc., Mountain View, CA), according to the manufacturer’s instructions.
Human CaP Specimens
The paired benign and tumor prostate tissues used for RT-qPCR of Lin28 were previously described.20 The protein extracts from human prostate specimens used for Western blot analysis of Lin28 were previously described.21,22 Immunohistochemical (IHC) staining of Lin28 was performed using a TMA PROS-006 obtained from UC Davis Comprehensive Cancer Center Biorepository. Staining was performed by the pathology core facility, and staining intensity was scored on a scale of 0 to 3 (0, negative; 1, weak; 2, strong; and 3, very strong). Brown color was considered positive staining for Lin28, and nuclear staining was denoted by blue color.
Statistical Analysis
Data are shown as means ± SD. Multiple-group comparison was performed by one-way analysis of variance. P ≤ 0.05 was considered significant.
Results
Lin28 Is Overexpressed in Clinical Prostate Cancer Specimens
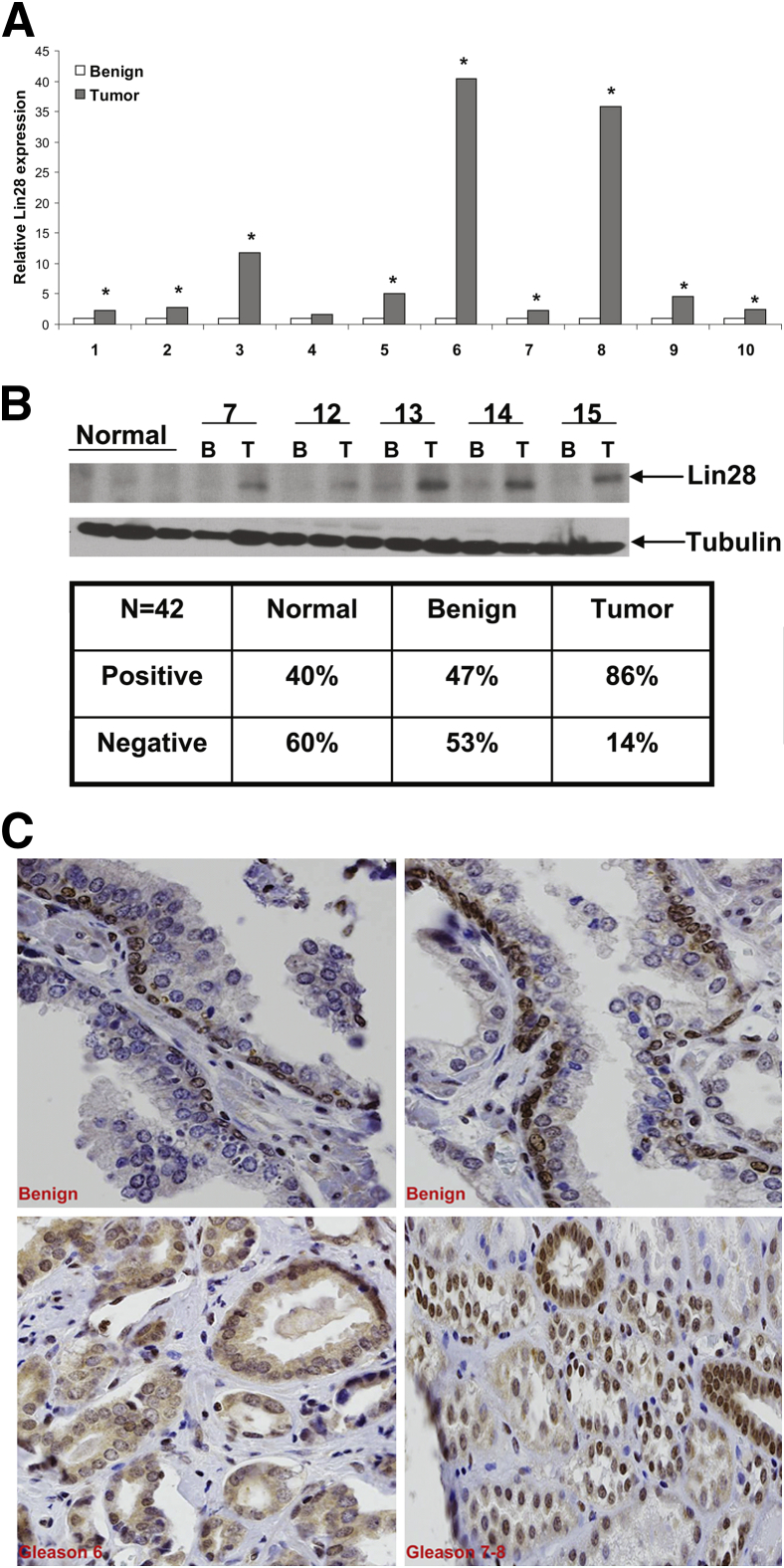
To determine the relative levels of expression of Lin28 in human CaP compared to benign prostates, we examined RNAs from 10 paired benign and tumor human CaP samples by RT-qPCR. Expression levels of Lin28 were found to be significantly elevated in 9 of 10 pairs of matched benign and tumor prostate specimens (Figure 1A). Extracts from archived human clinical prostatectomy specimens were also examined for expression of Lin28 by Western blot analysis. The data set contains 42 matched benign and cancer specimens, and expression levels of Lin28 were higher in cancer tissues (86% positive and 14% negative) compared to matched benign prostate tissues (47% positive and 53% negative) (Figure 1B). IHC was performed in formalin-fixed, paraffin-embedded prostate clinical specimens in a TMA PROS-006 (UC Davis Cancer Center Biorepository) with Lin28 antibody (Ab-7141523), and staining intensity was scored over a scale of 0 to 3 (0, negative; 1, weak; 2, strong; and 3, very strong). Lin28 was expressed in both nuclear and cytoplasmic compartments, with no significant differences in the pattern of expression with increasing Gleason grade (Figure 1C). We observed strong nuclear staining of Lin28 in benign prostate tissues almost exclusively in the basal cell layer, with no staining in the luminal epithelial compartment. In contrast, an apparent shift from mostly nuclear localization in benign prostate to a nuclear + cytoplasmic or mostly cytoplasmic localization appeared to occur in CaP (Table 1). Documentation of staining specificity for IHC is presented in Supplemental Figure S1.
Figure 1.
Lin28 is overexpressed in human prostate cancer. A: RT-qPCR analysis of Lin28 expression in 10 paired benign and tumor CaP tissues. Data are presented as means ± SD of two experiments performed in triplicate. ∗P ≤ 0.05. Lin28 mRNA expression levels were higher in cancer tissues compared to matched benign tissues. B: Western blot analysis of Lin28 expression in 42 paired benign (B) and tumor (T) samples. Representative Western blot analysis is shown. The table summarizes the results of Lin28 protein expression levels in the data set. C: IHC analysis of Lin28 expression in benign and cancer CaP tissues. Brown staining represents positive staining for expression of Lin28. Original magnification, ×200.
Table 1.
Summary of IHC Results for Lin28 in TMA PROS-006
| No. of cores | Benign(n = 19) | Gleason 6(n = 29) | Gleason 7-8(n = 26) |
|---|---|---|---|
| Nuclear alone | 12 (63) | 1 (3) | 0 (0) |
| Nuclear + cytoplasmic | 4 (21) | 17 (58) | 11 (42) |
| Cytoplasmic alone | 3 (15) | 11 (38) | 15 (57) |
Data are given as number (percentage).
Lin28 Enhances Growth of Prostate Cancer Cells
To test whether Lin28 activates a prosurvival mechanism in CaP cells, we transfected Lin28 into a panel of CaP cell lines (LNCaP, C4-2B, DU145, LNCaP-S17, and LNCaP-IL6) and a nontumorigenic prostate epithelial cell line (PZ-HPV7). Lin28 enhanced the growth rate of all CaP cell lines tested (Figure 2A). To confirm these results, LNCaP and C4-2B cells stably expressing Lin28 (LN-Lin28 and C4-2B-Lin28) were generated, and growth characteristics were examined. Compared to control LNCaP cells expressing the empty vector (LN-neo and C4-2B-neo), LN-Lin28 and C4-2B-Lin28 cells exhibited faster growth rates (Figure 2, B and C), suggesting that Lin28 promotes growth of prostate cancer cells in vitro.
Figure 2.
Lin28 promotes growth of CaP cells. A: LNCaP, PZ-HPV7, C4-2B, DU145, LNCaP-S17, and LNCaP-IL6 cells were transfected with empty vector or pLKO.1-Lin28, and growth was monitored at 0, 24, and 48 hours. Lin28-enhanced growth rates of all cell lines tested. B: LNCaP cells stably expressing Lin28 (LN-Lin28), and control (Con) LN-neo cells were plated in media containing complete FBS and growth was monitored at 0, 24, 48, and 72 hours. Left panel: Lin28 enhanced the growth of LNCaP cells. Right panel: The expression levels of Lin28 in LN-Lin28 and LN-neo cells. C: Left panel: C4-2B cells stably expressing Lin28 (C4-2B-Lin28), and control C4-2B-neo cells were plated in media containing complete FBS and growth was monitored at 0, 24, 48, and 72 hours. Right panel: The expression levels of Lin28 in C4-2B-Lin28 and C4-2B-neo cells. Data are presented as means ± SD of three experiments performed in triplicate.
To examine the effects of down-regulation of Lin28 on CaP growth, lentiviral vector-driven shRNA against Lin28 (Open Biosystems) was transfected into C4-2B cells, and cell growth was monitored. C4-2B cells transfected with Lin28 shRNA exhibited lower rates of growth, compared to cells transfected with shRNA against enhanced green fluorescent protein (EGFP) (Figure 3). Down-regulation of Lin28 was confirmed by Western blot analysis (Figure 3). These data demonstrated that down-regulation of Lin28 reduces proliferation of CaP cells.
Figure 3.
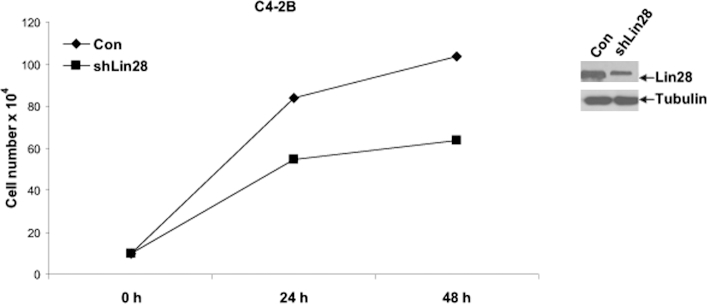
Down-regulation of endogenous Lin28 suppresses CaP cell growth. C4-2B cells were transfected with shRNA against Lin28, and growth was measured in media containing complete FBS. Left panel: Growth of C4-2B cells was abrogated with down-regulation of Lin28. Right panel: Reduced expression of Lin28 protein after transfection with shRNA. Data are presented as means ± SD of three experiments performed in triplicate.
Lin28 Increases Clonogenic Ability of LNCaP Cells
To test whether Lin28 influences the ability of CaP cells to form colonies in anchorage-dependent and anchorage-independent conditions, we performed clonogenic assays by transiently transfecting Lin28 into C4-2B and LNCaP-S17 cells. The results showed that the number of colonies formed by C4-2B cells expressing Lin28 was 356 ± 14, whereas the number of colonies formed by control C4-2B cells was 182 ± 10 (Figure 4A). Similarly, the number of colonies formed by LNCaP-S17 cells expressing Lin28 was 392 ± 19, whereas the number of colonies formed by control LNCaP-S17 cells was 212 ± 15 (Figure 4A). These experiments were confirmed using LN-Lin28 cells (LNCaP cells stably expressing Lin28), which exhibited a 3.4-fold increase in colony-forming ability compared to LN-neo cells (228 ± 21 versus 67 ± 13 colonies) (Figure 4B). These results were also confirmed using LN/TR/Lin28 cells (LNCaP cells expressing tet-inducible Lin28), which exhibited higher clonogenic ability compared to control LN/TR/Con cells upon doxycycline induction (Figure 4B). Collectively, these findings suggest that Lin28 enhances the ability of prostate cancer cells to form colonies in anchorage-dependent conditions.
Figure 4.
Lin28 promotes clonogenic ability of CaP cells. A: C4-2B and LNCaP-S17 cells were transfected with empty vector or pLKO.1-Lin28, and anchorage-dependent clonogenic assays were performed. Lin28 enhanced the colony-forming ability of both C4-2B and LNCaP-S17 cells. B: LN-Lin28 and control (Con) LN-neo cells were subjected to clonogenic assays. Lin28-expressing LNCaP cells exhibited higher clonogenic ability compared to control cells. LN/TR/Lin28 (LNCaP cells expressing Lin28 under a Tet-inducible promoter) and LN/TR/Con cells were subjected to clonogenic assays in media containing either complete FBS or CS-FBS. Induction of Lin28 expression by doxycycline (DOX) enhanced colony formation of LN/TR/Lin28 cells compared to control cells. C: C4-2B and LNCaP-S17 cells were transfected with empty vector or pLKO.1-Lin28 and subjected to anchorage-independent soft agar colony formation assays. Lin28 expression increased the number of colonies formed in soft agar by both cell lines. D: Left panel: LN-Lin28 and control LN-neo cells were subjected to soft agar assays. LN-Lin28 cells were able to form more colonies in soft agar compared to LN-neo cells. Right panel: Lin28 increases invasiveness of CaP cells. LN-Lin28 and LN-neo cells were subjected to Boyden chamber invasion assays. Inset: Representative images of invading cells. Data are presented as means ± SD of three experiments performed in triplicate. ∗P ≤ 0.05.
To further test whether Lin28 regulates anchorage-independent growth of CaP cells, we performed soft agar colony formation assays with C4-2B and LNCaP-S17 cells transfected with Lin28, as described in Materials and Methods. The results showed that Lin28 promoted the growth of both C4-2B (Figure 3C) and LNCaP-S17 (Figure 4C) cells in soft agar, compared to control C4-2B or LNCaP-S17 cells transfected with the empty vector. Similarly, LN-Lin28 cells exhibited significantly better ability to grow in soft agar, whereas control LN-neo cells failed to grow in soft agar (Figure 4D), demonstrating that Lin28 confers soft agar colony-forming ability on CaP cells.
Lin28 Increases Invasiveness of LNCaP Cells
To test whether Lin28 regulates the ability of CaP cells to invade through Matrigel in vitro, we performed Boyden chamber invasion assays using LN-Lin28 and control LN-neo cells. Cells were plated on Matrigel in the upper compartment of the Boyden chamber and allowed to invade toward the lower compartment filled with complete medium containing complete fetal bovine serum (FBS) or charcoal dextran-stripped FBS (CS-FBS). The number of LN-Lin28 cells invading through Matrigel in FBS-containing medium was 118 ± 10, whereas the number of control cells invading through Matrigel was 60 ± 5 (Figure 4D). Similarly, the number of LN-Lin28 cells invading through Matrigel in CS-FBS–containing medium was 54 ± 6, whereas the number of control cells was 4 ± 2 (Figure 4D), indicating that Lin28 induces invasion of CaP cells through basement membrane in vitro.
Lin28 Promotes Tumorigenicity of CaP Cells in Vivo
To test whether the growth-promoting effect of Lin28 can be recapitulated in vivo, we injected 2 × 106 LN-Lin28 cells or control LN-neo cells s.c. into each flank of male nude mice and monitored their tumorigenic ability. Tumors were measured twice weekly, and serum samples were collected at the end of the experiment to confirm that the tumor cells secreted PSA. We found that mice injected with LN-Lin28 cells exhibited significantly higher rates of incidence and growth of tumors compared to control LN-neo cells, which formed slow-growing tumors (Figure 5A). Higher levels of secretion of PSA were observed in mice bearing tumors expressing Lin28 compared to mice bearing control tumors (Figure 5B). Total RNA and protein extracts from the tumors were examined for expression levels of Lin28 and AR. Lin28 was highly expressed in the tumors, which was correlated positively with expression levels of AR (Figure 5C), indicating that higher expression of Lin28 enhances expression of AR and promotes tumor growth of LNCaP human CaP cells in vivo.
Figure 5.
Lin28 promotes tumor growth of CaP xenografts. A: Cells (2 × 106 per flank of LN-Lin28 or LN-neo) were injected s.c. into both flanks of male nude mice, and tumor growth was monitored. B: Secretion of PSA by the CaP xenografts was measured in the mouse serum samples. Tumors expressing Lin28 secreted more PSA compared to the control tumors. C: Higher expression levels of Lin28 (left panel) and AR (right panel) were confirmed by RT-qPCR in total RNAs from xenograft tissues. ∗P ≤ 0.05.
Lin28 Activates Androgen Receptor Signaling Axis
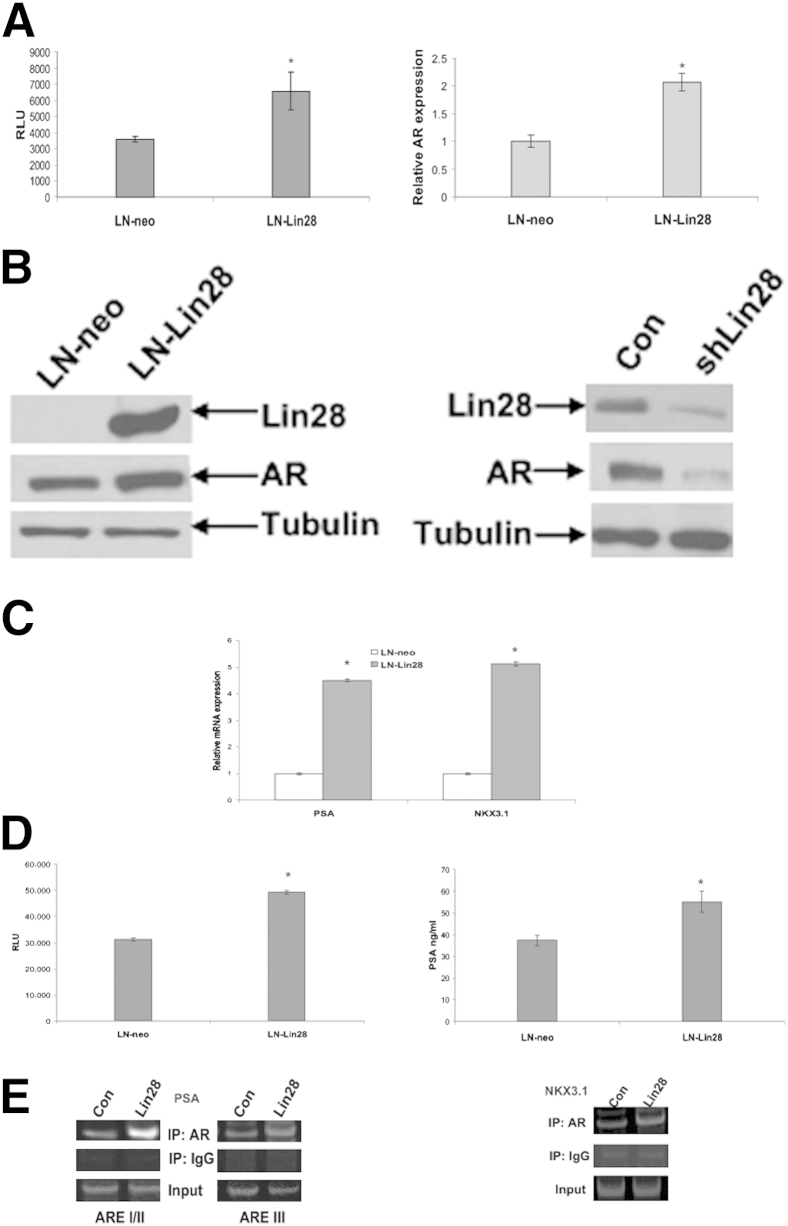
As we previously reported that hsa-let-7c, an miRNA regulated by Lin28, suppressed expression of the AR,6 we examined whether Lin28 regulates expression of the AR. We transfected a luciferase reporter vector driven by the full-length promoter of AR (pGL4-AR-Prom-Luc) into LN-neo and LN-Lin28 cells and performed luciferase assays. LN-Lin28 cells exhibited higher levels of activation of AR promoter compared to control cells (Figure 6A), indicating that Lin28 may activate transcription of the AR gene. Next, we analyzed the mRNA levels of AR in LN-neo and LN-Lin28 cells and found that LN-Lin28 cells exhibited significantly higher levels of AR mRNA (Figure 6A). These results were confirmed by Western blot analysis (Figure 6B). To determine whether down-regulation of Lin28 affects the expression of AR, we transfected shRNA against Lin28 into C4-2B cells and analyzed the protein levels of AR by Western blot analysis. The results showed that down-regulation of Lin28 led to a decrease in protein levels of AR (Figure 6B), suggesting that Lin28 expression is necessary for maintenance of AR expression in CaP cells.
Figure 6.
Lin28 enhances expression and activation of the AR. A: LN-Lin28 and LN-neo cells were transfected with pGL4-AR-Prom-Luc, and luciferase activities were measured. Activity of the full-length promoter of AR was increased in Lin28-expressing cells. RT-qPCR assays showing the increase in AR mRNA expression in Lin28-expressing LNCaP cells. B: Western blot analysis showing the increase in AR protein expression in LN-Lin28 cells compared to LN-neo cells. C4-2B cells were transfected with shRNA against Lin28, and protein expression of AR was analyzed. Down-regulation of Lin28 by shRNA reduced the expression of AR. C: Expression levels of AR target genes, PSA and NKX3.1, were measured by RT-qPCR in LN-neo and LN-Lin28 cells. mRNA levels of both genes were increased in Lin28-expressing cells compared to control (Con) cells. D: LN-Lin28 and LN-neo cells were transfected with PSA-E/P-Luc, and luciferase activities were measured. Left panel: Activation of PSA promoter was enhanced in Lin28-expressing cells compared to control cells. Right panel: Secretion of PSA by LN-Lin28 and LN-neo cells with measured by ELISA. Levels of PSA secreted by Lin28-expressing cells were higher than control cells. Data are presented as means ± SD of three experiments performed in triplicate. ∗P ≤ 0.05. E: Recruitment of AR to the ARE I/II and ARE III regions of PSA promoter and ARE in NKX3.1 promoter was analyzed by chromatin immunoprecipitation assays. Expression of Lin28 enhanced recruitment of AR to AREs in target gene promoters. IP, immunoprecipitation; RLU, relative light units.
To determine whether Lin28 regulates AR-dependent signaling, we analyzed the expression levels of PSA and NKX3.1, two typical AR target genes, in LN-neo and LN-Lin28 cells by RT-qPCR. The results showed that expression levels of PSA and NKX3.1 were enhanced in LN-Lin28 cells (Figure 6C). We analyzed the effect of Lin28 on the transactivating ability of AR using luciferase assays. A luciferase reporter vector driven by the full-length promoter of PSA (PSA-E/P-Luc) was transfected into LN-neo and LN-Lin28 cells, and luciferase assays were performed. The results showed that Lin28 induced the activity of PSA promoter (Figure 6D), indicating that Lin28 may contribute to increased transcription of AR-dependent genes by activating the AR. To confirm these findings, we analyzed levels of PSA in supernatants of LN-neo and LN-Lin28 cells by ELISA and found that secretion of PSA by LN-Lin28 cells was higher compared to LN-neo cells (Figure 6D). We also examined the effect of Lin28 on recruitment of AR to the promoters of PSA and NKX3.1 genes by chromatin immunoprecipitation assays. The results showed that recruitment of AR to AR-responsive element (ARE) I/II and ARE III regions in PSA (Figure 6E), and ARE in NKX3.1 (Figure 6E) promoters, was enhanced in Lin28-expressing cells compared to controls. Taken together, these results demonstrate that Lin28 activates the AR signaling axis.
Discussion
Lin28 is an RNA-binding protein postulated to be overexpressed in several malignancies.8,23–26 Lin28 controls the biogenesis of let-7 miRNAs27,28 and is one of the pluripotency factors that are responsible for the reprogramming of differentiated cells to stem cell like.29 In this study, we showed that Lin28 is overexpressed in human CaP compared to benign tissues, promotes growth of human CaP cells in vitro, and enhances growth of CaP xenografts. To our knowledge, this is the first report demonstrating the role of Lin28 in CaP cell proliferation and the ability of Lin28 to promote prostate tumor growth.
Lin28 was originally identified as a key regulator of developmental timing in C. elegans.30 It is a conserved cytoplasmic protein, but on export to the nucleus, regulates the translation or stability of mRNAs.31 In mammals, Lin28 is widely expressed in early development and in stem cells, but its expression is down-regulated with differentiation and is absent in most adult tissues.2,32 On the other hand, Lin28 is up-regulated in human tumors and promotes transformation and tumor progression.8 Depletion of Lin28 and expression of let-7 suppress bone metastasis.14 Lin28B, a homologue of Lin28, is overexpressed in hepatocellular carcinoma, and exogenous Lin28B promotes cancer cell proliferation.33 Lin28B plays an important role in Myc-dependent cellular proliferation.34 Several recent reports demonstrated that Lin28 expression correlates with survival of patients with malignant diseases.33 In ovarian cancer, patients with high Lin28B expression had shorter progression-free and overall survival times than those with low Lin28B expression.35 High Lin28B staining intensity in stage I/II colon cancers correlated with reduced survival and increased probability of tumor recurrence.25 A correlation between high expression of Lin28 and Lin28B and poor prognosis of patients with esophageal cancers was reported.23 Collectively, these reports emphasize the importance of Lin28 in development and cellular transformation and as a potential oncogene. Our current findings that Lin28 promotes CaP growth in vitro and in vivo are in accordance with the studies described herein and provide novel evidence for the role of Lin28 in prostate cancer.
We observed strong nuclear staining of Lin28 in benign prostate tissues almost exclusively in the basal cell layer, with no staining in the luminal epithelial compartment. This can be explained by the following: Lin28 is highly expressed in progenitor cells, and the basal cell compartment is generally considered to harbor putative prostate stem cells. Thus, it is conceivable that the benign prostate gland exhibits high expression of Lin28 in the basal cell layer. An apparent shift from mostly nuclear localization in benign prostate to a nuclear + cytoplasmic or mostly cytoplasmic localization appears to occur in CaP, which would have to be confirmed by further studies with a larger sample size.
We have demonstrated increased levels of Lin28 in human prostate tumors, which may likely result from the activation of c-Myc.36 c-Myc is a known target of let-7 miRNAs and also a transcriptional target of STAT3 and NF-κB2/p52, which we have already shown to be constitutively activated in prostate tumors.22,37 Alternatively, up-regulation of Lin28 in human CaP may occur due to stabilization of its mRNA owing to suppression of let-7 miRNAs. Our earlier studies showed that the double-negative feedback loop between let-7 and Lin28, encompassing c-Myc, likely plays a major role in prostate carcinogenesis.6,7 Recent evidence suggests that Lin28 does not rely solely on its regulation of let-7 miRNA biogenesis, but also modulates gene expression by altering translation38,39 and by modulating levels of splicing factors involved in alternative splicing of mature transcripts.40 Lin28 may regulate expression of prosurvival genes in CaP via one of these mechanisms. As Lin28 is being increasingly implicated in critical cellular processes, including development, transformation, and maintenance of stem cell signatures, the need to fully elucidate its multiple mechanisms of action becomes urgent to exploit its potential as a therapeutic target in human CaP.
In summary, we have shown that Lin28 is overexpressed in prostate carcinomas and promotes prostate tumor growth. Lin28 also activates AR-dependent signaling and enhances growth of human CaP cells. These findings underline the multifactorial nature of Lin28 and present it as an attractive target for therapeutic intervention in CaP.
Acknowledgments
We thank Drs. Leland W.K. Chung and Wen-Chin Huang (Cedars-Sinai Medical Center, Los Angeles, CA) and Dr. Donald Tindall (Mayo Clinic, Rochester, MN) for the pGL4-AR-Prom-Luc plasmid.
Footnotes
Supported, in part, by NIH/National Cancer Institute grants CA140468, CA118887, and Department of Defense grant PCRP PC080538, the US Department of Veterans Affairs, the Office of Research and Development (Veteran Affairs Merits I01 BX000526), and resources from the Veteran Affairs Northern California Health Care System (Sacramento) (to A.C.G.), and Department of Defense grant PCRP PC100502 (N.N.).
Contributor Information
Nagalakshmi Nadiminty, Email: nnadiminty@ucdavis.edu.
Allen C. Gao, Email: acgao@ucdavis.edu.
Supplemental Data
Sections of benign prostate were stained with secondary antibody alone for control. No brown staining was observed, which indicates that positive staining observed in the TMA is specific for the primary antibody Lin28.
References
- 1.Nadiminty N., Gao A.C. Mechanisms of persistent activation of the androgen receptor in CRPC: recent advances and future perspectives. World J Urol. 2012;30:287–295. doi: 10.1007/s00345-011-0771-3. [DOI] [PubMed] [Google Scholar]
- 2.Moss E.G., Tang L. Conservation of the heterochronic regulator Lin-28, its developmental expression and microRNA complementary sites. Dev Biol. 2003;258:432–442. doi: 10.1016/s0012-1606(03)00126-x. [DOI] [PubMed] [Google Scholar]
- 3.Takamizawa J., Konishi H., Yanagisawa K., Tomida S., Osada H., Endoh H., Harano T., Yatabe Y., Nagino M., Nimura Y., Mitsudomi T., Takahashi T. Reduced expression of the let-7 microRNAs in human lung cancers in association with shortened postoperative survival. Cancer Res. 2004;64:3753–3756. doi: 10.1158/0008-5472.CAN-04-0637. [DOI] [PubMed] [Google Scholar]
- 4.Shell S., Park S.M., Radjabi A.R., Schickel R., Kistner E.O., Jewell D.A., Feig C., Lengyel E., Peter M.E. Let-7 expression defines two differentiation stages of cancer. Proc Natl Acad Sci U S A. 2007;104:11400–11405. doi: 10.1073/pnas.0704372104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Akao Y., Nakagawa Y., Naoe T. let-7 microRNA functions as a potential growth suppressor in human colon cancer cells. Biol Pharm Bull. 2006;29:903–906. doi: 10.1248/bpb.29.903. [DOI] [PubMed] [Google Scholar]
- 6.Nadiminty N., Tummala R., Lou W., Zhu Y., Zhang J., Chen X., DeVere White R.W., Kung H.-J., Evans C.P., Gao A.C. MicroRNA let-7c suppresses androgen receptor expression and activity via regulation of Myc expression in prostate cancer cells. J Biol Chem. 2012;287:1527–1537. doi: 10.1074/jbc.M111.278705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nadiminty N., Tummala R., Lou W., Zhu Y., Shi X.-B., Zou J.X., Chen H., Zhang J., Chen X., Luo J., DeVere White R.W., Kung H.-J., Evans C.P., Gao A.C. MicroRNA let-7c is downregulated in prostate cancer and suppresses prostate cancer growth. PLoS One. 2012;7:e32832. doi: 10.1371/journal.pone.0032832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Viswanathan S.R., Powers J.T., Einhorn W., Hoshida Y., Ng T.L., Toffanin S., O’Sullivan M., Lu J., Phillips L.A., Lockhart V.L., Shah S.P., Tanwar P.S., Mermel C.H., Beroukhim R., Azam M., Teixeira J., Meyerson M., Hughes T.P., Llovet J.M., Radich J., Mullighan C.G., Golub T.R., Sorensen P.H., Daley G.Q. Lin28 promotes transformation and is associated with advanced human malignancies. Nat Genet. 2009;41:843–848. doi: 10.1038/ng.392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Iliopoulos D., Hirsch H., Struhl K. An epigenetic switch involving NF-kappaB, Lin28, Let-7 microRNA and IL6 links inflammation to cell transformation. Cell. 2009;139:693–706. doi: 10.1016/j.cell.2009.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Peng S., Maihle N., Huang Y. Pluripotency factors Lin28 and Oct4 identify a sub-population of stem cell-like cells in ovarian cancer. Oncogene. 2010;29:2153–2159. doi: 10.1038/onc.2009.500. [DOI] [PubMed] [Google Scholar]
- 11.Viswanathan S.R., Daley G.Q., Gregory R.I. Selective blockade of microRNA processing by Lin28. Science. 2008;320:97–100. doi: 10.1126/science.1154040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Viswanathan S.R., Daley G.Q. Lin28: a microRNA regulator with a macro role. Cell. 2010;140:445–449. doi: 10.1016/j.cell.2010.02.007. [DOI] [PubMed] [Google Scholar]
- 13.Chang T.-C., Zeitels L.R., Hwang H.-W., Chivukula R.R., Wentzel E.A., Dews M., Jung J., Gao P., Dang C.V., Beer M.A., Thomas-Tikhonenko A., Mendell J.T. Lin-28B transactivation is necessary for Myc-mediated let-7 repression and proliferation. Proc Natl Acad Sci U S A. 2009;106:3384–3389. doi: 10.1073/pnas.0808300106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dangi-Garimella S., Yun J., Eves E.M., Newman M., Erkeland S.J., Hammond S.M., Minn A.J., Rosner M.R. Raf kinase inhibitory protein suppresses a metastasis signalling cascade involving LIN28 and let-7. EMBO J. 2009;28:347–358. doi: 10.1038/emboj.2008.294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lu J., Getz G., Miska E.A., Alvarez-Saavedra E., Lamb J., Peck D., Sweet-Cordero A., Ebert B.L., Mak R.H., Ferrando A.A., Downing J.R., Jacks T., Horvitz H.R., Golub T.R. MicroRNA expression profiles classify human cancers. Nature. 2005;435:834–838. doi: 10.1038/nature03702. [DOI] [PubMed] [Google Scholar]
- 16.Chun J.Y., Nadiminty N., Dutt S., Lou W., Yang J.C., Kung H.-J., Evans C.P., Gao A.C. Interleukin-6 regulates androgen synthesis in prostate cancer cells. Clin Cancer Res. 2009;15:4815–4822. doi: 10.1158/1078-0432.CCR-09-0640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nadiminty N., Lou W., Sun M., Chen J., Yue J., Kung H.-J., Evans C.P., Zhou Q., Gao A.C. Aberrant activation of the androgen receptor by NF-κB2/p52 in prostate cancer cells. Cancer Res. 2010;70:3309–3319. doi: 10.1158/0008-5472.CAN-09-3703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nadiminty N., Chun J.Y., Lou W., Lin X., Gao A.C. NF-κB2/p52 enhances androgen-independent growth of human LNCaP cells via protection from apoptotic cell death and cell cycle arrest induced by androgen-deprivation. Prostate. 2008;68:1725–1733. doi: 10.1002/pros.20839. [DOI] [PubMed] [Google Scholar]
- 19.Yang J.C., Ok J.H., Busby J.E., Borowsky A.D., Kung H.J., Evans C.P. Aberrant activation of androgen receptor in a new neuropeptide-autocrine model of androgen-insensitive prostate cancer. Cancer Res. 2009;69:151–160. doi: 10.1158/0008-5472.CAN-08-0442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dunn T.A., Chen S., Faith D.A., Hicks J.L., Platz E.A., Chen Y., Ewing C.M., Sauvageot J., Isaacs W.B., De Marzo A.M., Luo J. A novel role of myosin VI in human prostate cancer. Am J Pathol. 2006;169:1843–1854. doi: 10.2353/ajpath.2006.060316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ni Z., Lou W., Lee S.O.K., Dhir R., De Miguel F., Grandis J.R., Gao A.C. Selective activation of members of the signal transducers and activators of transcription family in prostate carcinoma. J Urol. 2002;167:1859–1862. [PubMed] [Google Scholar]
- 22.Dhir R., Ni Z., Lou W., DeMiguel F., Grandis J.R., Gao A.C. Stat3 activation in prostatic carcinomas. Prostate. 2002;51:241–246. doi: 10.1002/pros.10079. [DOI] [PubMed] [Google Scholar]
- 23.Hamano R., Miyata H., Yamasaki M., Sugimura K., Tanaka K., Kurokawa Y., Nakajima K., Takiguchi S., Fujiwara Y., Mori M., Doki Y. High expression of Lin28 is associated with tumour aggressiveness and poor prognosis of patients in oesophagus cancer. Br J Cancer. 2012;106:1415–1423. doi: 10.1038/bjc.2012.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Helland Ã., Anglesio M.S., George J., Cowin P.A., Johnstone C.N., House C.M., Sheppard K.E., Etemadmoghadam D., Melnyk N., Rustgi A.K., Phillips W.A., Johnsen H., Holm R., Kristensen G.B., Birrer M.J., Pearson R.B., Børresen-Dale A.L., Huntsman D.G., deFazio A., Creighton C.J., Smyth G.K., Bowtell D.D., Australian Ovarian Cancer Study Group Deregulation of MYCN, LIN28B and LET7 in a molecular subtype of aggressive high-grade serous ovarian cancers. PLoS One. 2011;6:e18064. doi: 10.1371/journal.pone.0018064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.King C.E., Cuatrecasas M., Castells A., Sepulveda A.R., Lee J.-S., Rustgi A.K. LIN28B promotes colon cancer progression and metastasis. Cancer Res. 2011;71:4260–4268. doi: 10.1158/0008-5472.CAN-10-4637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.King C.E., Wang L., Winograd R., Madison B.B., Mongroo P.S., Johnstone C.N., Rustgi A.K. LIN28B fosters colon cancer migration, invasion and transformation through let-7-dependent and -independent mechanisms. Oncogene. 2011;30:4185–4193. doi: 10.1038/onc.2011.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Heo I., Joo C., Kim Y.-K., Ha M., Yoon M.-J., Cho J., Yeom K.-H., Han J., Kim V.N. TUT4 in concert with Lin28 suppresses microRNA biogenesis through pre-microRNA uridylation. Cell. 2009;138:696–708. doi: 10.1016/j.cell.2009.08.002. [DOI] [PubMed] [Google Scholar]
- 28.Lehrbach N.J., Miska E.A. Regulation of pre-miRNA processing. Adv Exp Med Biol. 2010;700:67–75. [PubMed] [Google Scholar]
- 29.Vêncio E.F., Nelson A.M., Cavanaugh C., Ware C.B., Miller D.G., Garcia J.C., Vêncio R.Z., Loprieno M.A., Liu A.Y. Reprogramming of prostate cancer-associated stromal cells to embryonic stem-like. Prostate. 2012;72:1453–1463. doi: 10.1002/pros.22497. [DOI] [PubMed] [Google Scholar]
- 30.Moss E.G., Lee R.C., Ambros V. The cold shock domain protein LIN-28 controls developmental timing in C. elegans and is regulated by the lin-4 RNA. Cell. 1997;88:637–646. doi: 10.1016/s0092-8674(00)81906-6. [DOI] [PubMed] [Google Scholar]
- 31.Balzer E., Moss E.G. Localization of the developmental timing regulator Lin28 to mRNP complexes, P-bodies and stress granules. RNA Biol. 2007;4:16–25. doi: 10.4161/rna.4.1.4364. [DOI] [PubMed] [Google Scholar]
- 32.Yang D.-H., Moss E.G. Temporally regulated expression of Lin-28 in diverse tissues of the developing mouse. Gene Expression Patterns. 2003;3:719–726. doi: 10.1016/s1567-133x(03)00140-6. [DOI] [PubMed] [Google Scholar]
- 33.Guo Y., Chen Y., Ito H., Watanabe A., Ge X., Kodama T., Aburatani H. Identification and characterization of lin-28 homolog B (LIN28B) in human hepatocellular carcinoma. Gene. 2006;384:51–61. doi: 10.1016/j.gene.2006.07.011. [DOI] [PubMed] [Google Scholar]
- 34.Shyh-Chang N., Daley G.Q. Lin28: primal regulator of growth and metabolism in stem cells. Cell Stem Cell. 2013;12:395–406. doi: 10.1016/j.stem.2013.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lu L., Katsaros D., Shaverdashvili K., Qian B., Wu Y., de la Longrais I.A.R., Preti M., Menato G., Yu H. Pluripotent factor lin-28 and its homologue lin-28b in epithelial ovarian cancer and their associations with disease outcomes and expression of let-7a and IGF-II. Eur J Cancer. 2009;45:2212–2218. doi: 10.1016/j.ejca.2009.05.003. [DOI] [PubMed] [Google Scholar]
- 36.Iliopoulos D., Hirsch H.A., Struhl K. An epigenetic switch involving NF-κB, Lin28, Let-7 microRNA, and IL6 links inflammation to cell transformation. Cell. 2009;139:693–706. doi: 10.1016/j.cell.2009.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nadiminty N., Dutt S., Tepper C., Gao A.C. Microarray analysis reveals potential target genes of NF-κB2/p52 in LNCaP prostate cancer cells. Prostate. 2010;70:276–287. doi: 10.1002/pros.21062. [DOI] [PubMed] [Google Scholar]
- 38.Huang Y. A mirror of two faces: Lin28 as a master regulator of both miRNA and mRNA. Wiley Interdiscip Rev RNA. 2012;3:483–494. doi: 10.1002/wrna.1112. [DOI] [PubMed] [Google Scholar]
- 39.Lei X.X., Xu J., Ma W., Qiao C., Newman M.A., Hammond S.M., Huang Y. Determinants of mRNA recognition and translation regulation by Lin28. Nucleic Acids Res. 2012;40:3574–3584. doi: 10.1093/nar/gkr1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wilbert M.L., Huelga S.C., Kapeli K., Stark T.J., Liang T.Y., Chen S.X., Yan B.Y., Nathanson J.L., Hutt K.R., Lovci M.T., Kazan H., Vu A.Q., Massirer K.B., Morris Q., Hoon S., Yeo G.W. LIN28 binds messenger RNAs at GGAGA motifs and regulates splicing factor abundance. Mol Cell. 2012;48:195–206. doi: 10.1016/j.molcel.2012.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Sections of benign prostate were stained with secondary antibody alone for control. No brown staining was observed, which indicates that positive staining observed in the TMA is specific for the primary antibody Lin28.