Abstract
We report a case of acute myelitis associated with hepatitis C virus (HCV) infection. A Japanese woman developed left calf pain and weakness, but this quickly generalised to paraplegia. We diagnosed acute myelitis based on the results of clinical manifestations, an MRI examination and a cerebrospinal fluid (CSF) examination. The clinical condition and spinal cord lesions improved following intravenous administration of methylprednisolone. The patient had been diagnosed with HCV infection 11 years before the onset. We detected HCV RNA in the CSF, supporting the strong association of our patient's myelitis. However, it is difficult to conclude whether the neurological condition was caused directly by the viral load or indirectly by the immune response. We suggest that testing for HCV infection is important in patients with myelitis. In particular, anti-HCV antibody and HCV RNA should be measured in the patients’ serum as well as CSF.
Background
Acute myelitis is a focal inflammatory disease of the spinal cord characterised by muscle weakness and sensory abnormalities in the lower extremities as well as autonomic dysfunctions.1 This clinical condition may be associated with several disorders, such as infectious, neoplastic, demyelinating and compressive diseases as well as autoimmune connective tissue disorders. It may also be associated with exposure to radiation or it may be idiopathic.2 3 However, acute myelitis associated with hepatitis C virus (HCV) infection has been considered to be a rare condition.4 In the present study, we report the clinical and neuroradiological findings of a patient with acute myelitis and HCV infection. This is the rare case in which both anti-HCV antibody and HCV RNA have been detected in the cerebrospinal fluid (CSF).
Case presentation
A 63-year-old woman developed pain and muscle weakness in the left calf during the morning. She was admitted to our hospital by ambulance by the end of the same day because her symptoms had progressed. She noticed muscle weakness in both legs and gait disturbance in the evening. On admission, blood pressure was 132/80 and other vital signs were stable, and physical examination showed no abnormal findings. Neurological examination showed severe weakness of the lower extremities. She could not hold her left leg against gravity or walk even with assistance. There was hypoesthesia below the level of the navel that affected the left side more than the right side. The cranial nerve regions and upper extremities were normal. Tendon reflex were normal at the upper limbs, but decreasing at the lower extremities. On the second day, her neurological symptoms worsened. She also developed urinary retention and severe constipation.
She had a history of HCV infection 11 years before. According to her information, HCV infection had never been medically treated because her liver function was normal and the viral genotype was 1b. No history of liver biopsy was present.
We diagnosed the patient as having acute myelitis based on the results of the MRI examination and the clinical manifestations, although we could not determine the definitive cause at this time. As her clinical condition was acutely progressive, we started intravenous methylprednisolone (1 g/day) for 3 days (steroid-pulse therapy). After the treatment, the muscle weakness gradually improved, but bladder and rectal functions remained impaired. Therefore, we carried out a second course of steroid-pulse therapy beginning on the 10th hospital day. After this treatment, her clinical condition gradually improved. During the hospital stay, we received the results of immunological and viral examinations performed at the time of admission. As she was able to walk with a walker, the patient was discharged from the hospital to her home on the 45th hospital day. One year after the onset, she was able to walk without any assistance, although she had numbness in the lower extremities. The concentration of MBP in the CSF improved to the normal range (table 1). There was no evidence of recurrence of acute myelitis.
Table 1.
Results of laboratory examinations
| Exam | At admission | After 1 year |
|---|---|---|
| AST (13–33 IU/L) | 34 | 23 |
| ALT (6–27 IU/L) | 27 | 16 |
| Total bilirubin (0.2–1.2 mg/dL) | 1.01 | 0.69 |
| Glucose (70–109 mg/dL) | 88 | 92 |
| Albumin (3.8–5.3 g/dL) | 4.1 | 4.2 |
| Prothrombin time (70–140%) | 89 | 98 |
| Anti-HCV (CLEIA) | + | |
| HCV RNA Taq (<1.2 Log IU/mL) | 5.7 | 5.4 |
| Antiaquaporin 4 antibody | − | |
| Antinuclear antibody (<40) | 40 | |
| Homogeneous pattern | + | |
| Anti-SSA (<10) | 35.0 | |
| Anti-SSB (<15) | <5.0 | |
| C-ANCA (<3.5 U/mL) | <3.5 | |
| P-ANCA (<3.5 U/mL) | <1.3 | |
| CSF WBC (/μL) | 1 | |
| CSF protein (mg/dL) | 34 | 2 |
| CSF glucose (mg/dL) | 66 | 57 |
| CSF oligoclonal bands | − | |
| CSF IgG index (<0.73) | 0.49 | |
| CSF MBP (pg/mL) | 40.7 | 1.2 |
| CSF anti-HCV (CLEIA) | + | |
| CSF HCV RNA Taq (<1.2 Log IU/mL) | 2.0 | 1.1 |
+, positive; −, negative; ALT, alanine aminotransferase; Anti -ANCA, antineutrophil cytoplasmic antibody; AST, aspartate aminotransferase; CSF, cerebrospinal fluid; HCV, hepatitis C virus; WBC, white blood cell.
Investigations
At the time of admission, ECG was normal. Laboratory examinations were summarised in table 1. The results of peripheral blood counts and biochemical analysis were normal, including liver enzymes. The CSF showed normal initial pressure and a clear appearance. The serum was positive for antinuclear antibody at a titre of 1:40 (speckled pattern), anti-HCV antibody and anti-SSA/Ro antibody but negative for antiaquaporin-4 antibody. Cryoglobulin level was not measured.
The concentration of mononuclear cells in the CSF was 1/μL, the total protein concentration was 34 mg/dL, the glucose concentration was 66 mg/dL and the MBP concentration was 40.7 pg/mL. The IgG index was normal, and oligoclonal bands were absent. However, the CSF was positive for anti-HCV antibody, and HCV RNA was elevated at 2.0 log IU/mL.
Brain MRI showed no abnormal lesions. On the patient's second day in the hospital, MRI of the spinal cord revealed high-intensity lesions at the levels of Th10 and Th12 to L2 on T2-weighted images (figure 1). There was gadolinium enhanced lesion at the level of Th12 of the spinal cord.
Figure 1.
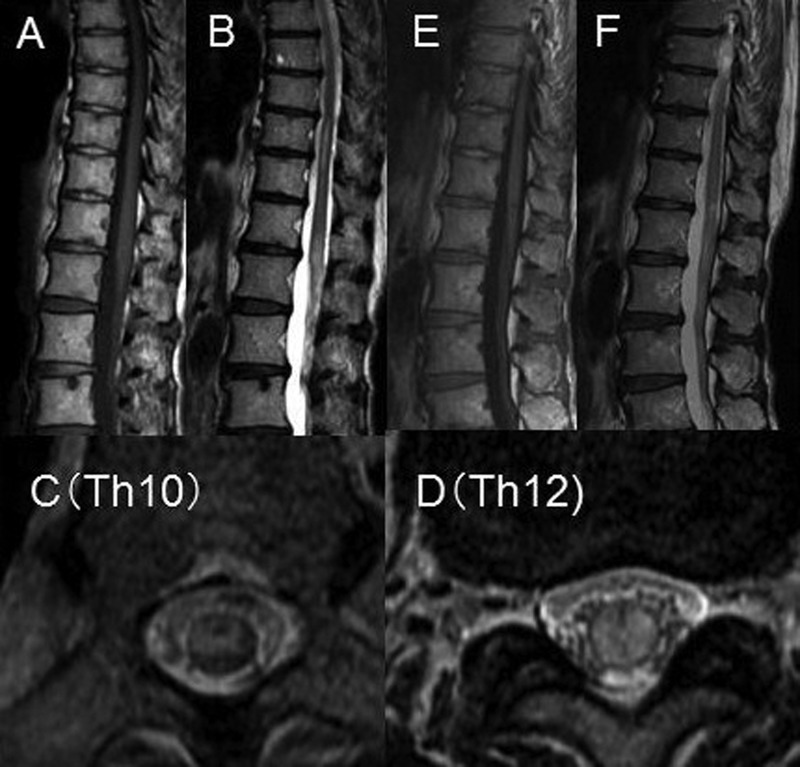
Spinal cord MRI of the patient was obtained at the time of onset (A–D) and 1 year later (E, F). (A) There was a mild swelling of the spinal cord at the level of L2. The lesions are of iso-intensity on a T1-weighted image. (B) T2-weighted image of the spinal cord shows highly intense lesions at the levels of Th10 and Th12 to L2 of the vertebral bodies. (C, D) On axial T2-weighted images, the lesions can be seen to be located in the grey and white matter at the level of Th10 and Th12. (E, F) 1 year later, there was an improvement of the hyperintense lesions and swelling of the spinal cord on T1-weighted (E) and T2-weighted (F) images.
Differential diagnosis
We diagnosed the patient as having acute myelitis based on the results of the MRI examination and the clinical manifestations. Acute myelitis may be associated with infectious, collagen, neoplastic, demyelinating and compressive diseases as well as exposure to radiation. In some instances, idiopathic form of acute myelitis is also reported.
On the basis of the clinical and laboratory examination, we believe that the neurological condition of our patient is not associated with multiple sclerosis or neuromyelitis optica. We could also rule out other infectious diseases.
Owing to the positivity of the serum level for anti-SSA/Ro antibody, we need to consider that her acute myelitis might be associated with Sjögren's syndrome. However, she did not show any clinical condition of Sjögren's syndrome. At this point, the clinical presentation of the patient does not strongly support myelitis associated with Sjögren's syndrome.
Treatment
Steroid-pulse therapy
Rehabilitation
Outcome and follow-up
She was able to walk with a walker at the 45th hospital day. She is now an outpatient of our hospital for 16 months. There was no evidence of a relapse of myelitis based on clinical and neuroradiological analyses.
Discussion
The present case is characterised by acute onset and progressive myelopathy that responded well to steroid therapy and by a positive test for HCV RNA in the patient's CSF that was obtained at the time of onset. Acute inflammatory neurological disorders with HCV infection are rare clinical condition including encephalitis,5 encephalomyelitis6 7 and polyradiculitis.8 To our knowledge, there are more than 10 reports of transverse myelitis associated with HCV infection3 9–16 (table 2). Zandman-Goddard et al3 reported a patient who had a history of HCV infection for 10 years. He developed acute transverse myelitis with paraplegia within a few hours. MRI showed hyperintense lesions at the level of Th4-5 of the spinal cord. Prednisolone was effective in treating the patient's symptoms. Most patients showed recurrent episodes of myelopathy over 6–16 months.9 11–14 In addition to the myelitis, one patient showed a brainstem lesion with impairment of consciousness.15
Table 2.
Cases of transverse acute myelitis associated with hepatitis C
| Author (year) | Age/sex | Onset form | Anti-HCV blood/CSF | HCV RNA blood/CSF | Treatment | Recurrence |
|---|---|---|---|---|---|---|
| Probst et al (1997) | 43/M | Acute | +/+ | +/+ | Steroid | Yes |
| Nolte et al (2002) | 64/M | Subacute | +/ND | +/+ | Steroid | None |
| Zandman-Goddard et al (2003) | 34/M | Acute | +/ND | +/ND | Steroid | None |
| Grewal et al (2004) | 46/M | Subacute | +/+ | +/− | Steroid | Yes |
| Annunziata et al (2005) | 60/F | Subacute | +/+ | +/− | Steroid, antibiotic | Yes |
| Aktipi et al (2007) | 58/F | Acute | +/ND | +/+ | Steroid, IVIg | Yes |
| 68/F | Acute | +/ND | +/ND | Steroid, IVIg | Yes | |
| 69/F | Acute | +/ND | +/ND | Steroid, IVIg | Yes | |
| 73/F | Acute | +/ND | +/ND | Steroid, IVIg | Yes | |
| 70/M | Acute | +/ND | +/ND | Steroid | Yes | |
| 34/M | Acute | +/ND | +/ND | Steroid | Yes | |
| 64/F | Acute | +/ND | +/ND | Steroid | Yes | |
| Takahashi et al (2009) | 63/F | Acute | +/ND | +/ND | Steroid | Yes |
| De Carli et al (2009) | 65/F | Subacute | +/+ | +/− | Antibiotic | None (death) |
| Mouelhi et al (2010) | 55/M | Acute | +/ND | +/ND | Steroid | None |
| Present case | 63/F | Acute | +/+ | +/+ | Steroid | None |
+, positive; −, negative; HCV, hepatitis C virus; IVIg, intravenous immunoglobulin.
ND, not determined;
Among these previous cases, there was no specific time course from the time of HCV infection to the onset of myelitis. In one previously reported case, HCV infection was estimated to have happened 10 years before myelitis.
The pathomechanism of myelitis associated with HCV infection has not been clarified. In some instances, anti-HCV antibody was detected in the serum and CSF. However, there was no evidence of HCV RNA in the CSF. In the present case, we successfully detected HCV RNA in the CSF. In a few previously reported cases, HCV RNA was also detected in the CSF.9 10 13 Those results suggest the robust association between the myelitis and HCV infection of the central nervous system. As chronic HCV infection can lead to several autoimmune disorders of the central and peripheral nervous systems, the previous cases, in fact, have been considered to be associated with continuous viral infection.3 9–16 However, it is still difficult to conclude whether the neurological condition of the spinal cord was caused directly by the viral load or indirectly by the immune response. In general, more than 50% of HCV-infected patients are positive for HCV RNA in their CSF.17–19
In the present case, the patient had been infected with HCV for more than 10 years. We did not give a specific treatment for HCV infection. As previously reported cases, steroid therapy improved her myelitis. Therefore, we suggested that myelitis with HCV may be associated with some immune response to HCV infection although we could not obtain the direct evidence of immunological abnormalities. Based on the literature reviews, a pathological analysis was reported of an individual with the recurrent type of myelitis with HCV infection.20 The biopsy specimen of the spinal cord showed severe infiltration of foamy macrophages and lymphocytes in the parenchyma with moderate gliosis. There was neither vasculitis nor evidence of HCV virus. The authors suggested that the spinal cord lesion was consistent with the acute demyelinating process of immune-mediated conditions. Since MBP was increased in the CSF, the present case may have similar pathological alterations.
Based on the patient's HCV genotype and normal level of aspartate aminotransferase and alanine aminotransferase, we did not start interferon treatment. Although the level of HCV RNA also decreased after the steroid administration, we need to continue to examine viral levels in CSF and serum.
In conclusion, we provide the clinical, laboratory and neuroradiological results of a patient with acute transverse myelitis associated with HCV infection. We speculated that the spinal cord lesion is associated with an immune-mediated condition instead of direct invasion of HCV. We also suggest that the analysis of HCV infection is important in individuals with acute myelitis. In particular, anti-HCV antibody and HCV RNA must be measured in the patients’ serum as well as CSF. Further analysis is important to determine the pathomechanism of myelitis associated with HCV infection.
Learning points.
Hepatitis C virus (HCV) infection must be considered in individuals with acute myelitis.
The serum and cerebrospinal fluid level of anti-HCV antibody and HCV RNA must be analysed.
Intravenous administration of methylprednisolone may be effective in acute phase.
Acknowledgments
This study was in part supported by Research on Measures for Intractable Diseases (Nanchi-ippan-063) and Comprehensive Brain Science Network.
Footnotes
Contributors: Dr Takao, Dr Katayama and Dr Mihara assisted my medical treatment of the patients.
Competing interests: None.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Transverse Myelitis Consortium Working Group Proposed diagnostic criteria and nosology of acute transverse myelitis. Neurology 2002;2013:499–505 [DOI] [PubMed] [Google Scholar]
- 2.Brito JC, Da Nobrega PV. Myelopathy: clinical considerations and etiological aspects. Arq Neuropsiquiatr 2003;2013(3B):816–21 [DOI] [PubMed] [Google Scholar]
- 3.Zandman-Goddard G, Levy Y, Weiss P, et al. Transverse myelitis associated with chronic hepatitis C. Clin Exp Rheumatol 2003;2013:111–13 [PubMed] [Google Scholar]
- 4.Stübgen JP. Immune-mediated myelitis associated with hepatitis virus infections. J Neuroimmunol 2011;2013:21–7 [DOI] [PubMed] [Google Scholar]
- 5.Seifert F, Struffert T, Hildebrandt M, et al. In vivo detection of hepatitis C virus (HCV) RNA in the brain in a case of encephalitis: evidence for HCV neuroinvasion. Eur J Neurol 2008;2013:214–18 [DOI] [PubMed] [Google Scholar]
- 6.Fujita H, Chuganji Y, Yaginuma M, et al. Case report: acute encephalitis immediately prior to acute onset of hepatitis C virus infection. J Gastroenterol Hepatol 1999;2013:1129–31 [DOI] [PubMed] [Google Scholar]
- 7.Bolay H, Soylemezoglu F, Nurlu G, et al. PCR detected hepatitis C virus genome in the brain of a case with progressive encephalomyelitis with rigidity. Clin Neurol Neurosurg 1996;2013:305–8 [DOI] [PubMed] [Google Scholar]
- 8.Gazzola P, Mavilio D, Costa P, et al. Possible hepatitis C virus involvement in acute meningoradiculitis/polyradiculitis of HIV-1-co-infected patients. AIDS 2001;2013:539–41 [DOI] [PubMed] [Google Scholar]
- 9.Probst T, Probst A, Nachbauer K, et al. Papillitis and vasculitis of the arteria spinalis anterior as complications of hepatitis C reinfection after liver transplantation. Transpl Int 1997;2013:234–7 [DOI] [PubMed] [Google Scholar]
- 10.Nolte CH, Endres AS, Meisel H. Sensory ataxia in myelopathy with chronic hepatitis C virus infection. Neurology 2002;2013:958. [DOI] [PubMed] [Google Scholar]
- 11.Grewal AK, Lopes MB, Berg CL, et al. Recurrent demyelinating myelitis associated with hepatitis C viral infection. J Neurol Sci 2004;2013:101–6 [DOI] [PubMed] [Google Scholar]
- 12.Annunziata P, Marroni M, Francisci D, et al. Acute transverse myelitis and hepatitis C virus. Infez Med 2005;2013:45–7 [PubMed] [Google Scholar]
- 13.Aktipi KM, Ravaglia S, Ceroni M, et al. Severe recurrent myelitis in patients with hepatitis C virus infection. Neurology 2007;2013:468–9 [DOI] [PubMed] [Google Scholar]
- 14.Takahashi-Fujigasaki J, Takagi S, Sakamoto T, et al. Spinal cord biopsy findings of anti-aquaporin-4 antibody-negative recurrent longitudinal myelitis in a patient with sicca and hepatitis C viral infection. Neuropathology 2009;2013:472–9 [DOI] [PubMed] [Google Scholar]
- 15.De Carli DM, Pannebeker J, Pedro FL, et al. Transverse myelitis associated to HCV infection. Braz J Infect Dis 2009;2013:147–52 [DOI] [PubMed] [Google Scholar]
- 16.Mouelhi L, Mekki H, Houissa F, et al. Transverse myelitis associated with chronic viral hepatitis C. Tunis Med 2010;2013:116–18 [PubMed] [Google Scholar]
- 17.Laskus T, Radkowski M, Bednarska A, et al. Detection and analysis of hepatitis C virus sequences in cerebrospinal fluid. J Virol 2002;2013:10064–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Morsica G, Bernardi MT, Novati R, et al. Detection of hepatitis C virus genomic sequences in the cerebrospinal fluid of HIV-infected patients. J Med Virol 1997;2013:252–4 [DOI] [PubMed] [Google Scholar]
- 19.Maggi F, Giorgi M, Fornai C, et al. Detection and quasispecies analysis of hepatitis C virus in the cerebrospinal fluid of infected patients. J Neurovirol 1999;2013:319–23 [DOI] [PubMed] [Google Scholar]
- 20.Seifert T, Enzinger C, Ropele S, et al. Relapsing acute transverse myelitis: a specific entity. Eur J Neurol 2005;2013:681–4 [DOI] [PubMed] [Google Scholar]


