Abstract
Despite the widening use of combination anti-retroviral therapy (ART), neurocognitive impairment remains common among HIV-infected (HIV+) individuals. Associations between HIV-related neuromedical variables and magnetic resonance imaging indices of brain structural integrity may provide insight into the neural bases for these symptoms. A diverse HIV+ sample (n=251) was studied through the CNS HIV Antiretroviral Therapy Effects Research initiative. Multi-channel image analysis produced volumes of ventricular and sulcal cerebrospinal fluid (CSF), cortical and subcortical gray matter, total cerebral white matter, and abnormal white matter. Cross-sectional analyses employed a series of multiple linear regressions to model each structural volume as a function of severity of prior immunosuppression (CD4 nadir), current CD4 count, presence of detectable CSF HIV RNA, and presence of HCV antibodies; secondary analyses examined plasma HIV RNA, estimated duration of HIV infection, and cumulative exposure to ART. Lower CD4 nadir was related to most measures of the structural brain damage. Higher current CD4, unexpectedly, correlated with lower white and subcortical gray and increased CSF. Detectable CSF HIV RNA was related to less total white matter. HCV coinfection was associated with more abnormal white matter. Longer exposure to ART was associated with lower white matter and higher sulcal CSF. HIV neuromedical factors, including lower nadir, higher current CD4 levels, and detectable HIV RNA, were associated with white matter damage and variability in subcortical volumes. Brain structural integrity in HIV likely reflects dynamic effects of current immune status and HIV replication, superimposed on residual effects associated with severe prior immunosuppression.
Keywords: HIV, MRI, Neuroimaging, Immunospupression
Introduction
The widening use of combination antiretroviral therapies (ART) has decreased the incidence of HIV-associated dementia; however, neurocognitive impairment remains common among HIV-infected individuals (Antinori et al. 2007; Cysique and Brew 2009; Ellis et al. 2007; Giancola et al. 2006; Heaton et al. 2010; Heaton et al. 2011; Sacktor et al. 2002; Sevigny et al. 2007; Tozzi et al. 2008a, b). Although the neural bases for these symptoms remain uncertain, toxic HIV viral proteins secreted by infected macrophages and brain inflammatory pathways have been implicated (Crews et al. 2009).
Neuroimaging studies in HIV have linked cognitive deficits to global, fronto-parietal, or striatal atrophy (Cohen et al. 2010; Hestad et al. 1993; Kieburtz et al. 1996; Levin et al. 1990; Patel et al. 2002; Paul et al. 2002, 2008; Thompson et al. 2005). Cerebral white matter damage, including volume loss and signal abnormality, is commonly reported (Archibald et al. 2004; Aylward et al. 1995; Cardenas et al. 2009; Cohen et al. 2010; Heindel et al. 1994; Jernigan et al. 1993; Stout et al. 1998); and white matter signal abnormality has been associated with reductions in cortical dendritic arborization (Archibald et al. 2004). Using MR spectroscopy, increases in choline and myo-inositol have been observed, suggesting glial activation; and these have been associated with neurocognitive impairment (Chang et al. 2001, 2002, 2004, 2005; Cloak et al. 2004; Ernst et al. 2002, 2003; Sacktor et al. 2005; Tarasow et al. 2003; Yiannoutsos et al. 2004).
The CNS HIV Antiretroviral Therapy Effects Research (CHARTER) study is examining a diverse group of HIV+ individuals in the United States. CHARTER was designed with broad inclusion criteria, comprehensive neuromedical assessment, and a large sample size to afford reliable ascertainment of the frequency and severity of HIV-associated neurocognitive disorders (HAND), and the specific contributions of HIV and comorbidities to impairment. The recent work supports continued prevalence of HAND even within individuals with minimal comorbidities (Heaton et al. 2010).
Here, we describe the relationships between HIV-related neuromedical factors and measures of brain structural integrity for a subset of CHARTER participants with minimal comorbidities. The neuroimaging correlates of the severity of prior immunosuppression (CD4 nadir), current CD4 count, detectable HIV RNA in CSF, and coinfection with hepatitis C virus (HCV) were of primary interest. Additionally, we examined the effect of HIV RNA in plasma, the estimated duration of HIV infection, and the cumulative months of ART exposure. We hypothesized that lower CD4 nadir, lower current CD4, detectable HIV RNA in CSF, and HCV coinfection would be associated with brain structural damage as reflected in lower brain tissue volumes, increased ventricular and sulcal size, and increased abnormal white matter.
Materials and methods
All data reported here are from the first (baseline) CHARTER imaging visits; by study design, most visits occurred at participants’ 6-month follow-up after enrolling in the longitudinal study. All participants are seropositive for HIV. Neurobehavioral and neuromedical assessments and highresolution multi-channel structural MRI were acquired from 301 participants from which 50 were excluded as described below, leaving 251 for reported analyses. Gross morphological abnormalities rendered MRI data for 12 participants inappropriate for morphometric analysis; abnormalities included large focal or extensive lesions, cysts, or masses (not consistent with HIV-related neuropathology) or congenital brain malformations. To focus our study, most directly on the effects of HIV in this diverse CHARTER sample, we excluded 38 additional cases due to severely confounding comorbid conditions. As described in Heaton et al. (2010), these confounding cases were identified by thorough review of case histories for non-HIV-related neuromedical and neuro-psychiatric risks for brain dysfunction and neurocognitive impairment based on published criteria (Antinori et al. 2007). Confounded cases revealed comorbid conditions that could fully explain impairment and observed problems with everyday functioning; therefore, HIV effects could not be inferred reliably in these cases.
Study cohort
The participants were studied at five participating sites: Johns Hopkins University (Baltimore, MD, n=39), Mt. Sinai School of Medicine (New York, NY, n=52), University of California at San Diego (San Diego, CA, n=73), University of Texas Medical Branch (Galveston, TX, n=57), and University of Washington (Seattle, WA, n=30). Demographic, HIV disease, and treatment characteristics of the total sample (n=251) are summarized in Table 1; the sample for primary analyses was smaller based on the availability of CSF samples as described below.
Table 1.
Cohort characterization (n=251)
| Mean (SD) | Range | Proportion of Cohort (%) | |
|---|---|---|---|
| Age (years) | 44.1 (7.9) | 23–67 | |
| Education (years) | 13.0 (2.3) | 7–18 | |
| Global Deficit Score (GDSa) | 0.39 (0.5) | 0–3.5 | |
| CD4 nadir (per mm3) | 190.2 (183.3) | 0–1104 | |
| Current CD4 (per mm3) | 479.0 (262.0) | 7–1716 | |
| Estimated Months Seropositive | 132 (74) | 7–285 | |
| Estimated Cumulative Months on ART | 67 (55) | 0–215 | |
| Ethnicity | Caucasian | 42 | |
| African American | 45 | ||
| Hispanic | 10 | ||
| Other | 3 | ||
| Sex | 82 (male) | ||
| Neurocognitively Impaired | 37 | ||
| Detectable HIV RNA in CSF | 29 | ||
| Detectable HIV RNA in Plasma | 49 | ||
| ART History | Currently Using | 76 | |
| Past Use | 14 | ||
| Never Used | 10 | ||
| CDC Stage | |||
| CDC-A | 34 | ||
| CDC-B | 28 | ||
| CDC-C | 38 | ||
| AIDS | 66 | ||
| HCV Coinfection | 25 |
Criterion for Neurocognitive Impairment is a Global Deficit Score of greater than 0.5
Standard protocol approvals, registrations, and patient consents
These procedures were approved by the Human Subjects Protection Committees of each participating institution. Written informed consent was obtained from all study participants.
Neuromedical assessments
The assessments included medical history, structured medical and neurological examinations, and the collection of blood and urine samples. For those who consented, CSF was collected by lumbar puncture. Of the 251 participants, 226 (90%) had CSF samples collected at visits near the time to imaging (median elapsed time <3 days). The following clinical parameters were evaluated using structured interviews and laboratory assessments where appropriate: prescription of ART, including current and past exposure to specific drug classes, HIV disease markers (detectable HIV RNA in CSF and plasma and CD4 nadir), current CD4, and detectable hepatitis C virus. Plasma HIV viral loads were quantified by RT-PCR ultrasensitive assay (nominal lower quantitation limit 50 c/mL; Amplicor(r), Roche Diagnostic Systems, Indianapolis, IN). Current CD4 was measured by flow cytometry.
Structural MRI protocol
All imaging was performed on GE 1.5T magnets to include four series for morphometric analysis; scanners were assessed for quality annually, and the scanner was included as a variable in statistical analyses to control for scanner-related effects (Fennema-Notestine et al. 2007). Series 1 and 2 were coronal acquisitions with section thickness=2.0 mm, FOV 24 cm, matrix size 256× 256: 2D T2-weighted fast spin echo (FSE) with TR=5,700 ms, TE=90 ms, ETL=16; and 2D proton density (PD)-weighted FSE with TR=3,700 ms, TE=17 ms, ETL= 4. Series 3 and 4 were sagittal acquisitions with section thickness=1.3 mm, FOV 24 cm, matrix size 256×256× 124: 3D T1-weighted SPGR with TR=20 ms, TE=6 ms, flip angle=30; and 3D PD-weighted SPGR sequence with TR=20 ms, TE=6 ms, flip angle=5.
CHARTER morphometry
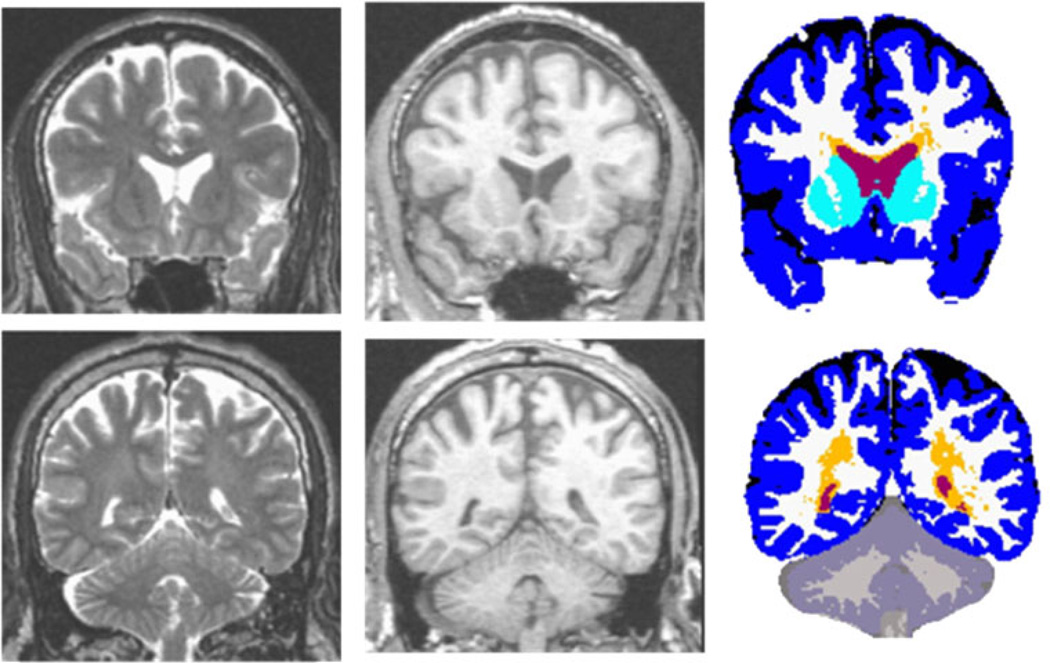
The standard morphometric analysis path employed in CHARTER, based on Jernigan et al. (2001), includes image inspection for motion and other artifacts, bias correction (with N3; Sled et al. 1998), coregistration of MRI volumes with a mutual information registration (Maes et al. 1997), reslicing in a standard space, anatomist selection of tissue samples (in gray matter, white matter, and CSF), removal of non-brain voxels (skull stripping), tissue segmentation, abnormal white matter designation, and anatomical segmentation (as illustrated below). To identify signal abnormalities in the white matter, neuroanatomists perform a semi-automated tissue sampling procedure to obtain the signal characteristics of regions of relatively normal gray matter, white matter, and CSF (Jernigan et al. 2001). The segmentation procedures use information from these sample regions from all four structural MRI volumes. Successive linear regressions on the tissue samples to separate CSF from brain and then to separate gray from white matter form the basis of tissue segmentation. With this analysis, abnormal white matter regions are defined as voxels within white matter that have signal values that fall in (or beyond) the distribution estimated from the gray matter sample (i.e., outside the range of normal white matter) and these voxels are segmented as “gray.” Trained anatomists processed the tissue-segmented images to separate manually the cerebellum from the cerebrum, the ventricles from sulcal fluid in the subarachnoid space, and the cortical from subcortical gray matter within the cerebrum. White matter abnormalities were also separated from gray matter structures using anatomical criteria. The outcome of the morphometric analysis is illustrated in Fig. 1. As reported previously (Jernigan et al. 2001), both interoperator and scan–rescan reliability for tissue segmentation with these methods have been estimated for each tissue class and these estimates range from .92 to .99.
Fig. 1.
Multi-channel morphometry. Coronal T2 (left), T1 (middle), and segmented (right) images for anterior (top) and posterior (bottom) sections. Volumes include: ventricular (red) and subarachnoid (black) CSF, abnormal (orange) and total (white + orange) white matter, cortical (blue) and subcortical (turquoise) gray matter
Statistical analysis
The analyses were designed to estimate the degree of association between HIV-related neuromedical variables and MRI indices of brain structural integrity. Structural integrity was assessed with the volumetric measures of subcortical and cortical gray matter, total and abnormal white matter, and ventricular and sulcal (subarachnoid) CSF. All volumetric measures were log-transformed, and CD4 count variables were square root transformed to symmetrize the distributions and stabilize the variances.
A series of six multiple linear regression models (one for each MRI measure) was used to address the main study question. In every regression model, six control covariates were included to account for some of the variance in MRI measures, although they were not of primary interest in the present study. These control variables included: supratentorial cranial volume (to control for differences in head size), age, gender, race/ethnicity, education, and scanner (including local scanner hardware changes; Fennema-Notestine et al. 2007).
Each model was used to predict one MRI measure with the control variables along with four neuromedical variables: (1) reported CD4 nadir, (2) measured current CD4 count, (3) presence of detectable HIV RNA in CSF, and (4) whether the participant was coinfected with HCV. This regression approach simultaneously examines the independent contributions of each variable to the structural volume, while controlling for other influences. For example, a significant effect of lower CD4 nadir in predicting lower total white matter volume is CD4 nadir’s independent contribution, accounting for other influences on white matter volume such as age, scanner, or detectable HIV RNA in CSF. For these analyses, 226 participants for whom CSF data was available were included. Thus, the primary effects reported here are those that persist in the presence of the six control covariates, as well as with the other four primary neuromedical variables in the model.
A treatment variable coding for whether the participant was on ART, had discontinued ART, or was ART naïve also was examined; however, its effect did not approach significance in any model. Therefore, this variable was not included as a covariate in the final regression models.
Two secondary analyses subsequently were performed with similar models: First, we examined the influence of detectable plasma HIV RNA by replacing the CSF variable with the plasma variable (n=251). Second, we examined the contributions of estimated duration of HIV infection and cumulative exposure to ART by adding these two additional variables to the primary regression model (n=209).
Results
All reported findings result from multiple regression models that simultaneously examine the independent contributions of each variable to the structural volume, while controlling for other influences (see the “Statistical analysis” subsection); that is, a significant effect of CD4 nadir on a volume measure reflects an independent effect controlling for other factors such as age, current CD4, and so on.
CD4 nadir, current CD4, HIV RNA in CSF, and HCV coinfection
The effects of the neuromedical variables as estimated in the primary regression model for each structural measure are summarized in Table 2. Accounting for control variables (e.g., scanner and age) and controlling for other neuromedical factors (e.g., current CD4), there was a consistent association between lower CD4 nadir and measures of brain damage (Fig. 2). Specifically, lower CD4 nadir was associated with lower volumes of total white and subcortical gray matter alongside more ventricular and sulcal CSF and more abnormal white matter. Detectable HIV RNA in CSF was associated with smaller white matter volumes and tended to predict more sulcal CSF.
Table 2.
Effects of neuromedical variables on morphometric volumes
| Abnormal white | Total white | Subcortical gray | Cortical gray | Ventricular CSF | Sulcal CSF | |
|---|---|---|---|---|---|---|
| CD4 Nadir | −2.12* | 3.87**** | 3.27**** | 0.05ns | −3.00*** | −3.85**** |
| Detectable HIV RNA in CSF | 0.58ns | −1.99* | 0.25ns | 0.20ns | 0.41ns | 1.79ns |
| Current CD4 | 0.59ns | −2.57* | −2.96*** | −0.02ns | 2.47* | 2.45* |
| HCV Coinfection | 2.33* | −0.13ns | 1.00ns | −0.63ns | 1.43ns | 0.47ns |
Reported t values are associated with the parameter estimates from each simultaneous regression analyses to demonstrate the strength of each neuromedical variable’s independent relationship with each neuroimaging measure. Scatterplots for some of these relationships are presented in Fig. 2 and Fig. 3. Sample size, n=226
ns not significant
p< .001
p < .005
p< .01
p< .05
Fig. 2.
Nadir CD4 and morphometric volumes. Partial correlation scatterplots (plots of partial residuals) show the relationship between square root transformed CD4 nadir and log-transformed morphometric volumes, after adjusting for all other variables in the regression models
Current CD4 was independently associated with volumes of ventricular and sulcal CSF, cerebral white matter, and subcortical gray matter, although the direction was unexpected. Controlling for CD4 nadir and other variables, a higher current CD4 was associated with higher CSF volumes and less white and subcortical gray matter (Fig. 3). There was no significant relationship between current CD4 and abnormal white matter. In other words, individuals with lower CD4 nadir but higher current CD4 had the most evidence for brain damage in terms of volume loss on MRI. Finally, HCV coinfection, after controlling for HIV-related factors and demographic variables, was associated with higher volumes of the abnormal white matter.
Fig. 3.
Current CD4 and morphometric volumes. Partial correlation scatterplots show the relationship between square root transformed current CD4 and log-transformed morphometric volumes, after adjusting for all other variables in the regression models
HIV RNA in plasma
More participants (n=251) had the measurements of HIV RNA in plasma than in CSF (n=226); secondary analyses replaced CSF with plasma detectable HIV RNA in the primary model. Detectable HIV RNA in plasma predicted larger ventricles (t=2.1, p=.03) and tended to predict smaller white matter volume (t=−1.8, p=.07). Other effects in the models were unchanged.
Duration of HIV infection and cumulative ART exposure
Furthermore, using the primary model which included CSF HIV RNA, we examined the influence of cumulative exposure to ART and estimated duration of HIV infection (n=209).
Longer ART exposure was associated with lower white matter (t=−2.2, p<.05) and higher sulcal (t=2.99, p<.005) volumes, after controlling for other variables. The association with higher ventricular volumes approached significance (t=3.2, p=0.075). The effect of estimated duration of HIV infection did not approach significance in any model. In no case did the roles of other variables in these models, such as CD4 nadir, differ from their roles in the primary models tested with the larger sample size (Table 2).
Discussion
Given minimal exclusion criteria, the diverse sample of HIV+ individuals in CHARTER provides a challenging context within which to interpret MRI evidence for brain tissue alterations. Individuals are at various stages of the infection and have widely differing histories of the comorbidity and ART exposure which could influence brain abnormalities. Since this population includes participants with substance use disorders and HCV as well as developmental and psychiatric conditions, we limited our analyses to individuals who were free of opportunistic infections, central nervous system malignancies, or present/ ongoing substance dependence disorders, as well as participants in whom severe comorbidities (confounding conditions) were present that could fully explain their neuropsychological deficits (Heaton et al. 2010). Our findings provide an initial description of the variability observed on MRI measures of the brain structure that may be associated with specific neuromedical factors in HIV, within the most informative participants of the CHARTER cohort at baseline.
The strongest and most consistent association with MRI measures was with CD4 nadir, an estimate of the patients’ most severe HIV-related immunosuppression. Low CD4 nadir may reflect prior high levels of the viral replication, and the associations we observed may reflect the persisting effects of these prior events. Low CD4 nadir was associated with virtually all the measures of brain tissue damage: larger ventricular and sulcal CSF volumes (an indication of brain volume loss), the lower volumes of cerebral white matter and subcortical gray matter, and increased abnormal white matter. These associations are consistent with previous evidence for prominent HIV effects on white matter and striatal structures, and with the findings of a recent study of demented and neurologically asymptomatic patients in which CD4 nadir was also related to brain volume loss (Cohen et al. 2010).
Interestingly, current CD4 level also was significantly associated with structural changes, but the associations were in an unexpected direction. Controlling for CD4 nadir and CSF viral load, higher current CD4 levels were associated with more MRI abnormality rather than less. Post hoc analyses revealed that although the effects of CD4 nadir were observable whether or not current CD4 was included in the model, the reverse was not the case. The associations with current CD4 could only be observed when CD4 nadir was in the model. The results suggest that the magnitude of the difference between current CD4 and CD4 nadir is somewhat more strongly associated with the severity of brain abnormality than is CD4 nadir alone, and therefore, the addition of current CD4 to the model improves the fit.
It is not clear, however, why lower current CD4 is associated with apparently less brain volume loss in the CHARTER cohort. This may be related to current treatment practices. The individuals currently on treatment are more likely to have had low CD4 nadirs that prompted the initiation of treatment and to have experienced ART-related rebound of CD4 counts. In contrast, untreated seropositive individuals are likely to be individuals with shorter duration of infection who may have lower current CD4 counts. Other factors may also contribute to counterintuitive associations between CD4 T cells and MRI measures. For example, there is some evidence that neuroinflammatory mechanisms may appear, in some cases, as increased tissue volumes, perhaps because of glial activation or even edema. Immune activation in individuals with current low CD4 values may have led to elevated volume estimates for gray matter, and commensurate decreases in CSF, essentially because of mild swelling of tissues. A similar explanation has been suggested for increases in cortical and subcortical gray matter volumes associated with recent stimulant dependence (Jacobsen et al. 2001; Jernigan et al. 2005). On the other hand, more neurodegeneration could accompany inflammatory processes associated with immune reconstitution in some participants, producing a correlation between relatively higher CD4 (in previously immunosuppressed individuals) and brain volume loss. Further studies are needed to characterize the influence of current CD4 on brain volumes. The analyses of the longitudinal results from CHARTER may provide insight, as these within-subjects effects will be less influenced by differences in treatment history.
While detectable HIV RNA in CSF was associated with smaller white matter volume (and perhaps more sulcal CSF), no significant association was found between the current use of ART and MRI measures. This may in part be because plasma viral levels were closely monitored in most participants and few remained untreated (Table 1). Given that most participants received ART, the associations detected in our models are likely to primarily represent relationships present in treated patients; when we computed the models in the currently treated participants, the relationships were essentially unchanged. Unfortunately, the sample size of untreated participants, including both medication naïve and recently treated individuals, did not allow sufficient power to test the models.
The estimates of cumulative duration of ART were associated with brain tissue damage, again in the form of smaller white matter volumes and larger CSF volumes. This association of longer treatment duration with volume loss did not appear to be mediated by self-reported longer duration of the infection. Cerebral white matter is among the earliest and most heavily affected tissue compartments in HIV (Archibald et al. 2004; Cardenas et al. 2009; Jernigan et al. 1993; Stout et al. 1998). Longer treatment duration may be an indicator of HIV disease severity or of damage from direct (toxic) or indirect (vascular) damage from antiretroviral agents.
Finally, HCV coinfection, a common comorbid illness with HIV, has been associated with worse neurocognitive impairment, higher HIV RNA levels, and higher levels in CSF of MCP-1, a proinflammatory chemokine (Chang et al. 2002; Taylor et al. 2007). To our knowledge, no previous studies have linked HCV coinfection to MRI-assessed brain tissue damage in HIV+ individuals. HCV-coinfected individuals had more abnormal white matter, even after controlling for demographic and HIV-associated factors, possibly reflecting HCV-mediated inflammation in the white matter. This inflammatory effect of HCV is consistent with a report of increased CCL2 (MCP-1), a proinflammatory chemokine, in the CSF of HCV-coinfected individuals (Letendre et al. 2005).
In summary, we observed that brain morphological variability in the CHARTER cohort is related to several indicators of HIV disease severity. Low CD4 nadir, possibly indicating prior exposure to HIV in the brain or other immunological abnormalities, was the most consistent predictor of less gray and white matter, more ventricular and sulcal CSF, and abnormalities in the remaining white matter. After controlling for CD4 nadir, higher CD4 was associated with lower subcortical volumes and larger CSF spaces, perhaps because of the transient inflammatory alterations of the tissues or because immune reconstitution in some individuals gives rise to increased neurodegeneration. Finally, HCV coinfection was associated with more abnormal white matter, consistent with proinflammatory effects. Future studies examining the relationship between neuroimaging markers and neurocognitive performance, particularly over time, will explore potentially dynamic episodes of injury and repair related to HIV and HAND.
Acknowledgments
Funding/Support The CNS HIV Anti-Retroviral Therapy Effects Research (CHARTER) is supported by award N01 MH22005 from the National Institutes of Health.
Footnotes
The CNS HIV Anti-Retroviral Therapy Effects Research (CHARTER) group is affiliated with the Johns Hopkins University, Mount Sinai School of Medicine, University of California, San Diego, University of Texas, Galveston, University of Washington, Seattle, Washington University, St. Louis and is headquartered at the University of California, San Diego and includes: Director: Igor Grant, M.D.; Co-Directors: J. Allen McCutchan, M.D., Ronald J. Ellis, M.D., Ph.D., Thomas D. Marcotte, Ph.D.; Center Manager: Donald Franklin, Jr.; Neuromedical Component: Ronald J. Ellis, M.D., Ph.D. (P.I.), J. Allen McCutchan, M.D., Terry Alexander, R.N.; Laboratory, Pharmacology and Immunology Component: Scott Letendre, M.D. (P.I.), Edmund Capparelli, Pharm.D.; Neurobehavioral Component: Robert K. Heaton, Ph.D. (P.I.), J. Hampton Atkinson, M.D., Steven Paul Woods, Psy.D., Matthew Dawson; Virology Component: Joseph K. Wong, M.D. (P.I.); Imaging Component: Christine Fennema-Notestine, Ph.D. (P.I.), Terry L., Jernigan, Ph.D., Michael J. Taylor, Ph.D., Rebecca J. Theilmann, Ph.D., John Hesselink, M.D.; Data Management Unit: Anthony C. Gamst, Ph.D. (P.I.), Clint Cushman; Statistics Unit: Ian Abramson, Ph.D. (P.I.), Florin Vaida, Ph.D.; Protocol Coordinating Component: Thomas D. Marcotte, Ph.D. (P.I.), Rodney von Jaeger, M.P.H.; Johns Hopkins University Site: Justin McArthur (P.I.), Mary Smith; Mount Sinai School of Medicine Site: Susan Morgello, M.D. (Co-P.I.) and David Simpson, M.D. (Co-P.I.), Letty Mintz, N.P., Cheuk Tang, Ph.D., and Thomas Naidich, M,D.; University of California, San Diego Site: J. Allen McCutchan, M.D. (P.I.), Will Toperoff, N.P..; University of Washington, Seattle Site: Ann Collier, M.D. (Co-P.I.) and Christina Marra, M.D. (Co-P.I.), Kenneth Maravilla, MD, KC Stegbauer, Ph.D.; Trudy Jones, M.N., A.R.N.P.; University of Texas, Galveston Site: Benjamin Gelman, M.D., Ph.D. (P.I.), Eleanor Head, R.N., B.S.N., Gregory Chaljub, M.D.; and Washington University, St. Louis Site: David Clifford, M.D. (P.I.), Muhammad Al-Lozi, M.D., Mengesha Teshome, M.D.
Disclaimer The views expressed in this article are those of the authors and do not reflect the official policy or position of the United States Government.
Contributor Information
Terry L. Jernigan, Department of Psychiatry, University of California, San Diego, 9500 Gilman Drive M/C 0115, La Jolla, CA, USA, tjernigan@ucsd.edu Department of Radiology, University of California, San Diego, 92093-0115, La Jolla, CA, USA.
Sarah L. Archibald, Department of Psychiatry, University of California, San Diego, 9500 Gilman Drive M/C 0115, La Jolla, CA, USA
Christine Fennema-Notestine, Department of Psychiatry, University of California, San Diego, 9500 Gilman Drive M/C 0115, La Jolla, CA, USA; Department of Radiology, University of California, San Diego, 92093-0115, La Jolla, CA, USA.
Michael J. Taylor, Department of Psychiatry, University of California, San Diego, 9500 Gilman Drive M/C 0115, La Jolla, CA, USA
Rebecca J. Theilmann, Department of Radiology, University of California, San Diego, 92093-0115, La Jolla, CA, USA
Michelle D. Julaton, Department of Psychiatry, University of California, San Diego, 9500 Gilman Drive M/C 0115, La Jolla, CA, USA
Randy J. Notestine, Department of Psychiatry, University of California, San Diego, 9500 Gilman Drive M/C 0115, La Jolla, CA, USA
Tanya Wolfson, Computational and Applied Statistics Laboratory (CASL), San Diego Supercomputer Center (SDSC), La Jolla, CA, USA.
Scott L. Letendre, Department of Medicine, University of California, San Diego, La Jolla, CA, USA
Ronald J. Ellis, Department of Neurosciences, University of California, San Diego, La Jolla, CA, USA
Robert K. Heaton, Department of Psychiatry, University of California, San Diego, 9500 Gilman Drive M/C 0115, La Jolla, CA, USA
Anthony C. Gamst, Department of Biostatistics and Bioinformatics, University of California, San Diego, La Jolla, CA, USA
Donald R. Franklin, Jr., Department of Psychiatry, University of California, San Diego, 9500 Gilman Drive M/C 0115, La Jolla, CA, USA
David B. Clifford, Department of Neurology, Washington University in St. Louis, St. Louis, MO, USA
Ann C. Collier, Department of Medicine, University of Washington, Seattle, WA, USA
Benjamin B. Gelman, Department of Pathology, University of Texas Medical Branch, Galveston, TX, USA
Christina Marra, Department of Medicine, University of Washington, Seattle, WA, USA; Department of Neurology, University of Washington, Seattle, WA, USA.
Justin C. McArthur, Department of Neurology, Johns Hopkins University, Baltimore, MD, USA
J. Allen McCutchan, Department of Medicine, University of California, San Diego, La Jolla, CA, USA.
Susan Morgello, Department of Pathology, Mount Sinai School of Medicine, New York, NY, USA.
David M. Simpson, Department of Neurology, Mount Sinai School of Medicine, New York, NY, USA
Igor Grant, Department of Psychiatry, University of California, San Diego, 9500 Gilman Drive M/C 0115, La Jolla, CA, USA.
References
- Antinori A, Arendt G, Becker JT, Brew BJ, Byrd DA, Cherner M, Clifford DB, Cinque P, Epstein LG, Goodkin K, Gisslen M, Grant I, Heaton RK, Joseph J, Marder K, Marra CM, McArthur JC, Nunn M, Price RW, Pulliam L, Robertson KR, Sacktor N, Valcour V, Wojna VE. Updated research nosology for HIV-associated neurocognitive disorders. Neurology. 2007;69:1789–1799. doi: 10.1212/01.WNL.0000287431.88658.8b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Archibald SL, Masliah E, Fennema-Notestine C, Marcotte TD, Ellis RJ, McCutchan JA, Heaton RK, Grant I, Mallory M, Miller A, Jernigan TL. Correlation of in vivo neuroimaging abnormalities with postmortem human immunodeficiency virus encephalitis and dendritic loss. Arch Neurol. 2004;61:369–376. doi: 10.1001/archneur.61.3.369. [DOI] [PubMed] [Google Scholar]
- Aylward EH, Brettschneider PD, McArthur JC, Harris GJ, Schlaepfer TE, Henderer JD, Barta PE, Tien AY, Pearlson GD. Magnetic resonance imaging measurement of gray matter volume reductions in HIV dementia. Am J Psychiatry. 1995;152:987–994. doi: 10.1176/ajp.152.7.987. [DOI] [PubMed] [Google Scholar]
- Cardenas V, Meyerhoff D, Studholme C, Kornak J, Rothlind J, Lampiris H, Neuhaus J, Grant R, Chao L, Truran D, Weiner M. Evidence for ongoing brain injury in human immunodeficiency virus-positive patients treated with antiretroviral therapy. J Neurovirol. 2009;15(4):324–333. doi: 10.1080/13550280902973960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang L, Speck O, Miller EN, Braun J, Jovicich J, Koch C, Itti L, Ernst T. Neural correlates of attention and working memory deficits in HIV patients. Neurology. 2001;57:1001–1007. doi: 10.1212/wnl.57.6.1001. [DOI] [PubMed] [Google Scholar]
- Chang L, Ernst T, Witt MD, Ames N, Gaiefsky M, Miller E. Relationships among brain metabolites, cognitive function, and viral loads in antiretroviral-naive HIV patients. Neuroimage. 2002;17:1638–1648. doi: 10.1006/nimg.2002.1254. [DOI] [PubMed] [Google Scholar]
- Chang L, Lee PL, Yiannoutsos CT, Ernst T, Marra CM, Richards T, Kolson D, Schifitto G, Jarvik JG, Miller EN, Lenkinski R, Gonzalez G, Navia BA. A multicenter in vivo proton-MRS study of HIV-associated dementia and its relationship to age. Neuroimage. 2004;23:1336–1347. doi: 10.1016/j.neuroimage.2004.07.067. [DOI] [PubMed] [Google Scholar]
- Chang L, Ernst T, Speck O, Grob CS. Additive effects of HIV and chronic methamphetamine use on brain metabolite abnormalities. Am J Psychiatry. 2005;162:361–369. doi: 10.1176/appi.ajp.162.2.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cloak CC, Chang L, Ernst T. Increased frontal white matter diffusion is associated with glial metabolites and psychomotor slowing in HIV. J Neuroimmunol. 2004;157:147–152. doi: 10.1016/j.jneuroim.2004.08.043. [DOI] [PubMed] [Google Scholar]
- Cohen RA, Harezlak J, Schifitto G, Hana G, Clark U, Gongvatana A, Paul R, Taylor M, Thompson P, Alger J, Brown M, Zhong J, Campbell T, Singer E, Daar E, McMahon D, Tso Y, Yiannoutsos CT, Navia B. Effects of nadir CD4 count and duration of human immunodeficiency virus infection on brain volumes in the highly active antiretroviral therapy era. J Neurovirol. 2010;16:25–32. doi: 10.3109/13550280903552420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crews L, Patrick C, Achim CL, Everall IP, Masliah E. Molecular pathology of neuro-AIDS (CNS-HIV) Int J Mol Sci. 2009;10:1045–1063. doi: 10.3390/ijms10031045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cysique LA, Brew BJ. Neuropsychological functioning and antiretroviral treatment in HIV/AIDS: a review. Neuropsychol Rev. 2009;19:169–185. doi: 10.1007/s11065-009-9092-3. [DOI] [PubMed] [Google Scholar]
- Ellis R, Langford D, Masliah E. HIV and antiretroviral therapy in the brain: neuronal injury and repair. Nat Rev Neurosci. 2007;8:33–4. doi: 10.1038/nrn2040. [DOI] [PubMed] [Google Scholar]
- Ernst T, Chang L, Jovicich J, Ames N, Arnold S. Abnormal brain activation on functional MRI in cognitively asymptomatic HIV patients. Neurology. 2002;59:1343–1349. doi: 10.1212/01.wnl.0000031811.45569.b0. [DOI] [PubMed] [Google Scholar]
- Ernst T, Chang L, Arnold S. Increased glial metabolites predict increased working memory network activation in HIV brain injury. Neuroimage. 2003;19:1686–1693. doi: 10.1016/s1053-8119(03)00232-5. [DOI] [PubMed] [Google Scholar]
- Fennema-Notestine C, Gamst AC, Quinn BT, Pacheco J, Jernigan TL, Thal L, Buckner R, Killiany R, Blacker D, Dale AM, Fischl B, Dickerson B, Gollub RL. Feasibility of multi-site clinical structural neuroimaging studies of aging using legacy data. Neuroinformatics. 2007;5:235–245. doi: 10.1007/s12021-007-9003-9. [DOI] [PubMed] [Google Scholar]
- Giancola ML, Lorenzini P, Balestra P, Larussa D, Baldini F, Corpolongo A, Narciso P, Bellagamba R, Tozzi V, Antinori A. Neuroactive antiretroviral drugs do not influence neurocognitive performance in less advanced HIV-infected patients responding to highly active antiretroviral therapy. J Acquir Immune Defic Syndr. 2006;41:332–337. doi: 10.1097/01.qai.0000197077.64021.07. [DOI] [PubMed] [Google Scholar]
- Heaton RK, Clifford DB, Franklin DR, Woods SP, Ake C, Vaida F, Ellis RJ, Letendre SL, Marcotte TD, Atkinson JH, Rivera-Mindt M, Vigil OR, Taylor MJ, Collier AC, Marra CM, Gelman BB, McArthur JC, Morgello S, Simpson DM, McCutchan JA, Abramson I, Gamst A, Fennema-Notestine C, Jernigan TL, Wong J, Grant I CHARTER Group. HIV-associated neurocognitive disorders persist in the era of potent antiretroviral therapy: CHARTER Study. Neurology. 2010;75:2087–2096. doi: 10.1212/WNL.0b013e318200d727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heaton RK, Franklin DR, Ellis RJ, McCutchan JA, Letendre SL, Leblanc S, Corkran SH, Duarte NA, Clifford DB, Woods SP, Collier AC, Marra CM, Morgello S, Mindt MR, Taylor MJ, Marcotte TD, Atkinson JH, Wolfson T, Gelman BB, McArthur JC, Simpson DM, Abramson I, Gamst A, Fennema-Notestine C, Jernigan TL, Wong J, Grant I CHARTER Group HNRC Group. HIV-associated neurocognitive disorders before and during the era of combination antiretroviral therapy: differences in rates, nature, and predictors. J Neurovirol. 2011;17:3–16. doi: 10.1007/s13365-010-0006-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heindel WC, Jernigan TL, Archibald SL, Achim CL, Masliah E, Wiley CA. The relationship of quantitative brain magnetic resonance imaging measures to neuropathologic indexes of human immunodeficiency virus infection. Arch Neurol. 1994;51:1129–1135. doi: 10.1001/archneur.1994.00540230067015. [DOI] [PubMed] [Google Scholar]
- Hestad K, McArthur JH, Dal Pan GJ, Selnes OA, Nance-Sproson TE, Aylward E, Mathews VP, McArthur JC. Regional brain atrophy in HIV-1 infection: association with specific neuropsychological test performance. Acta Neurol Scand. 1993;88:112–118. doi: 10.1111/j.1600-0404.1993.tb04201.x. [DOI] [PubMed] [Google Scholar]
- Jacobsen LK, Giedd JN, Gottschalk C, Kosten TR, Krystal JH. Quantitative morphology of the caudate and putamen in patients with cocaine dependence. Am J Psychiatry. 2001;158:486–489. doi: 10.1176/appi.ajp.158.3.486. [DOI] [PubMed] [Google Scholar]
- Jernigan TL, Archibald S, Hesselink JR, Atkinson JH, Velin RA, McCutchan JA, Chandler J, Grant I. Magnetic resonance imaging morphometric analysis of cerebral volume loss in human immunodeficiency virus infection. The HNRC Group. Arch Neurol. 1993;50:250–255. doi: 10.1001/archneur.1993.00540030016007. [DOI] [PubMed] [Google Scholar]
- Jernigan TL, Archibald SL, Fennema-Notestine C, Gamst AC, Stout JC, Bonner J, Hesselink JR. Effects of age on tissues and regions of the cerebrum and cerebellum. Neurobiol Aging. 2001;22:581–594. doi: 10.1016/s0197-4580(01)00217-2. [DOI] [PubMed] [Google Scholar]
- Jernigan TL, Gamst AC, Archibald SL, Fennema-Notestine C, Mindt MR, Marcotte TD, Heaton RK, Ellis RJ, Grant I. Effects of methamphetamine dependence and HIV infection on cerebral morphology. Am J Psychiatry. 2005;162:1461–1472. doi: 10.1176/appi.ajp.162.8.1461. [DOI] [PubMed] [Google Scholar]
- Kieburtz K, Ketonen L, Cox C, Grossman H, Holloway R, Booth H, Hickey C, Feigin A, Caine ED. Cognitive performance and regional brain volume in human immunodeficiency virus type 1 infection. Arch Neurol. 1996;53:155–158. doi: 10.1001/archneur.1996.00550020059016. [DOI] [PubMed] [Google Scholar]
- Letendre SL, Cherner M, Ellis RJ, Marquie-Beck J, Gragg B, Marcotte T, Heaton RK, McCutchan JA, Grant I. The effects of hepatitis C, HIV, and methamphetamine dependence on neuropsychological performance: biological correlates of disease. Aids. 2005;19(Suppl 3):S72–S78. doi: 10.1097/01.aids.0000192073.18691.ff. [DOI] [PubMed] [Google Scholar]
- Levin HS, Williams DH, Borucki MJ, Hillman GR, Williams JB, Guinto FC, Jr., Amparo EG, Crow WN, Pollard RB. Magnetic resonance imaging and neuropsychological findings in human immunodeficiency virus infection. J Acquir Immune Defic Syndr. 1990;3:757–762. [PubMed] [Google Scholar]
- Maes F, Collignon A, Vandermeulen D, Marchal G, Suetens P. Multimodality image registration by maximization of mutual information. IEEE Trans Med Imaging. 1997;16:187–198. doi: 10.1109/42.563664. [DOI] [PubMed] [Google Scholar]
- Patel SH, Kolson DL, Glosser G, Matozzo I, Ge Y, Babb JS, Mannon LJ, Grossman RI. Correlation between percentage of brain parenchymal volume and neurocognitive performance in HIV-infected patients. AJNR Am J Neuroradiol. 2002;23:543–549. [PMC free article] [PubMed] [Google Scholar]
- Paul R, Cohen R, Navia B, Tashima K. Relationships between cognition and structural neuroimaging findings in adults with human immunodeficiency virus type-1. Neurosci Biobehav Rev. 2002;26:353–359. doi: 10.1016/s0149-7634(02)00006-4. [DOI] [PubMed] [Google Scholar]
- Paul RH, Ernst T, Brickman AM, Yiannoutsos CT, Tate DF, Cohen RA, Navia BA. Relative sensitivity of magnetic resonance spectroscopy and quantitative magnetic resonance imaging to cognitive function among nondemented individuals infected with HIV. J Int Neuropsychol Soc. 2008;14:725–733. doi: 10.1017/S1355617708080910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacktor N, McDermott MP, Marder K, Schifitto G, Selnes OA, McArthur JC, Stern Y, Albert S, Palumbo D, Kieburtz K, De Marcaida JA, Cohen B, Epstein L. HIV-associated cognitive impairment before and after the advent of combination therapy. J Neurovirol. 2002;8:136–142. doi: 10.1080/13550280290049615. [DOI] [PubMed] [Google Scholar]
- Sacktor N, Skolasky RL, Ernst T, Mao X, Selnes O, Pomper MG, Chang L, Zhong K, Shungu DC, Marder K, Shibata D, Schifitto G, Bobo L, Barker PB. A multicenter study of two magnetic resonance spectroscopy techniques in individuals with HIV dementia. J Magn Reson Imaging. 2005;21:325–333. doi: 10.1002/jmri.20272. [DOI] [PubMed] [Google Scholar]
- Sevigny JJ, Albert SM, McDermott MP, Schifitto G, McArthur JC, Sacktor N, Conant K, Selnes OA, Stern Y, McClernon DR, Palumbo D, Kieburtz K, Riggs G, Cohen B, Marder K, Epstein LG. An evaluation of neurocognitive status and markers of immune activation as predictors of time to death in advanced HIV infection. Arch Neurol. 2007;64:97–102. doi: 10.1001/archneur.64.1.97. [DOI] [PubMed] [Google Scholar]
- Sled JG, Zijdenbos AP, Evans AC. A nonparametric method for automatic correction of intensity nonuniformity in MRI data. IEEE Trans Med Imaging. 1998;17:87–97. doi: 10.1109/42.668698. [DOI] [PubMed] [Google Scholar]
- Stout JC, Ellis RJ, Jernigan TL, Archibald SL, Abramson I, Wolfson T, McCutchan JA, Wallace MR, Atkinson JH, Grant I. Progressive cerebral volume loss in human immunodeficiency virus infection: a longitudinal volumetric magnetic resonance imaging study. HIV Neurobehavioral Research Center Group. Arch Neurol. 1998;55:161–168. doi: 10.1001/archneur.55.2.161. [DOI] [PubMed] [Google Scholar]
- Tarasow E, Wiercinska-Drapalo A, Kubas B, Dzienis W, Orzechowska-Bobkiewicz A, Prokopowicz D, Walecki J. Cerebral MR spectroscopy in neurologically asymptomatic HIV-infected patients. Acta Radiol. 2003;44:206–212. doi: 10.1080/j.1600-0455.2003.00028.x. [DOI] [PubMed] [Google Scholar]
- Taylor MJ, Schweinsburg BC, Alhassoon OM, Gongvatana A, Brown GG, Young-Casey C, Letendre SL, Grant I. Effects of human immunodeficiency virus and methamphetamine on cerebral metabolites measured with magnetic resonance spectroscopy. J Neurovirol. 2007;13:150–159. doi: 10.1080/13550280701194230. [DOI] [PubMed] [Google Scholar]
- Thompson PM, Dutton RA, Hayashi KM, Toga AW, Lopez OL, Aizenstein HJ, Becker JT. Thinning of the cerebral cortex visualized in HIV/AIDS reflects CD4+ T lymphocyte decline. Proc Natl Acad Sci U S A. 2005;102:15647–15652. doi: 10.1073/pnas.0502548102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tozzi V, Bellagamba R, Castiglione F, Amendola A, Ivanovic J, Nicastri E, Libertone R, D’Offizi G, Liuzzi G, Gori C, Forbici F, D’Arrigo R, Bertoli A, Salvatori MF, Capobianchi MR, Antinori A, Perno CF, Narciso P. Plasma HIV RNA decline and emergence of drug resistance mutations among patients with multiple virologic failures receiving resistance testing-guided HAART. AIDS Res Hum Retroviruses. 2008a;24:787–796. doi: 10.1089/aid.2007.0236. [DOI] [PubMed] [Google Scholar]
- Tozzi V, Libertone R, Liuzzi G. HIV pharmacogenetics in clinical practice: recent achievements and future challenges. Curr HIV Res. 2008b;6:544–554. doi: 10.2174/157016208786501535. [DOI] [PubMed] [Google Scholar]
- Yiannoutsos CT, Ernst T, Chang L, Lee PL, Richards T, Marra CM, Meyerhoff DJ, Jarvik JG, Kolson D, Schifitto G, Ellis RJ, Swindells S, Simpson DM, Miller EN, Gonzalez RG, Navia BA. Regional patterns of brain metabolites in AIDS dementia complex. Neuroimage. 2004;23:928–935. doi: 10.1016/j.neuroimage.2004.07.033. [DOI] [PubMed] [Google Scholar]