Abstract
Rabbit transferrin in vitro is shown to load ferrous iron at random on its specific binding sites. The release of iron to reticulocytes is shown to be an all-or-none phenomenon. The two monoferric transferrins have similar in vivo plasma iron clearance rates and tissue distribution. Diferric transferrin, while giving a similar tissue distribution of radioiron, has a plasma iron clearance rate approximately twice that of the monoferric transferrins at low plasma iron concentrations. This difference diminishes as the plasma iron concentration increases. These results are consistent with a progressively greater in vivo conversion of mono- to diferric transferrin as transferrin saturation increases. The in vivo plasma iron turnover in the rabbit increases progressively as the plasma iron increases, from a mean value of ∼0.8 mg/dl whole blood per d at a plasma iron concentration of 50 μg/dl to 2.0 at a plasma iron concentration of 300.
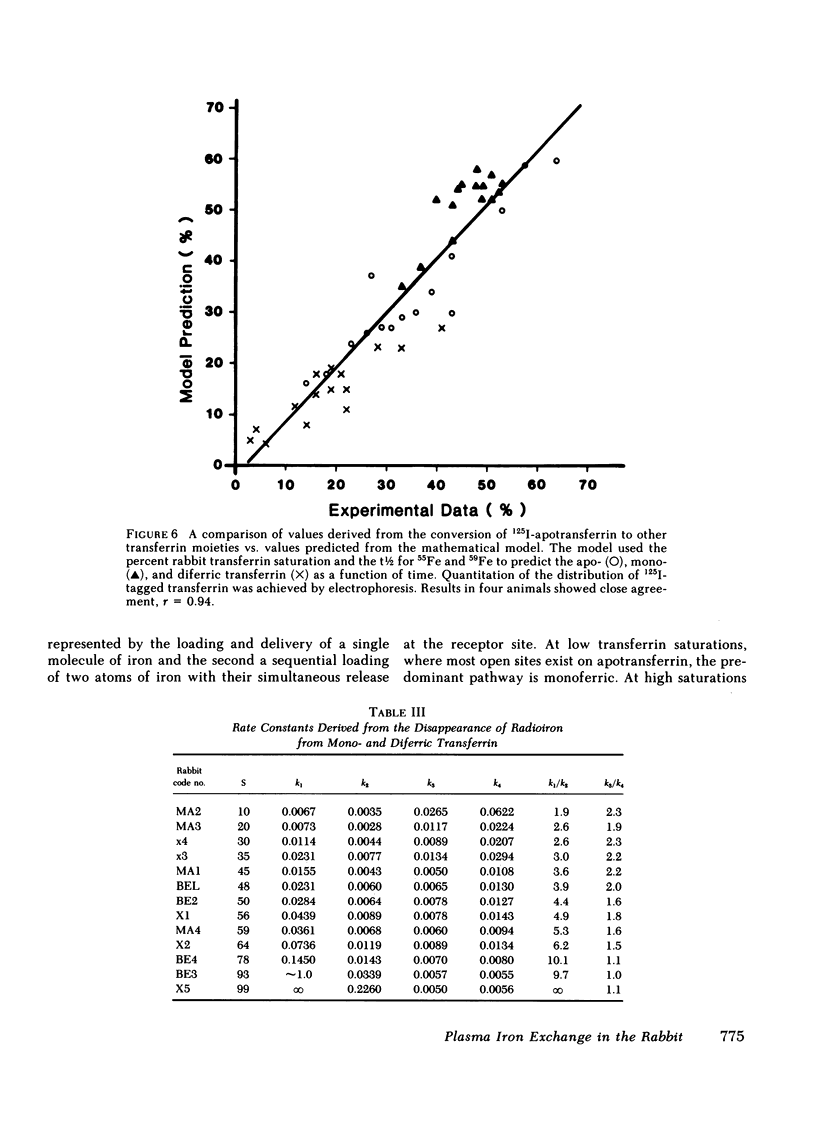
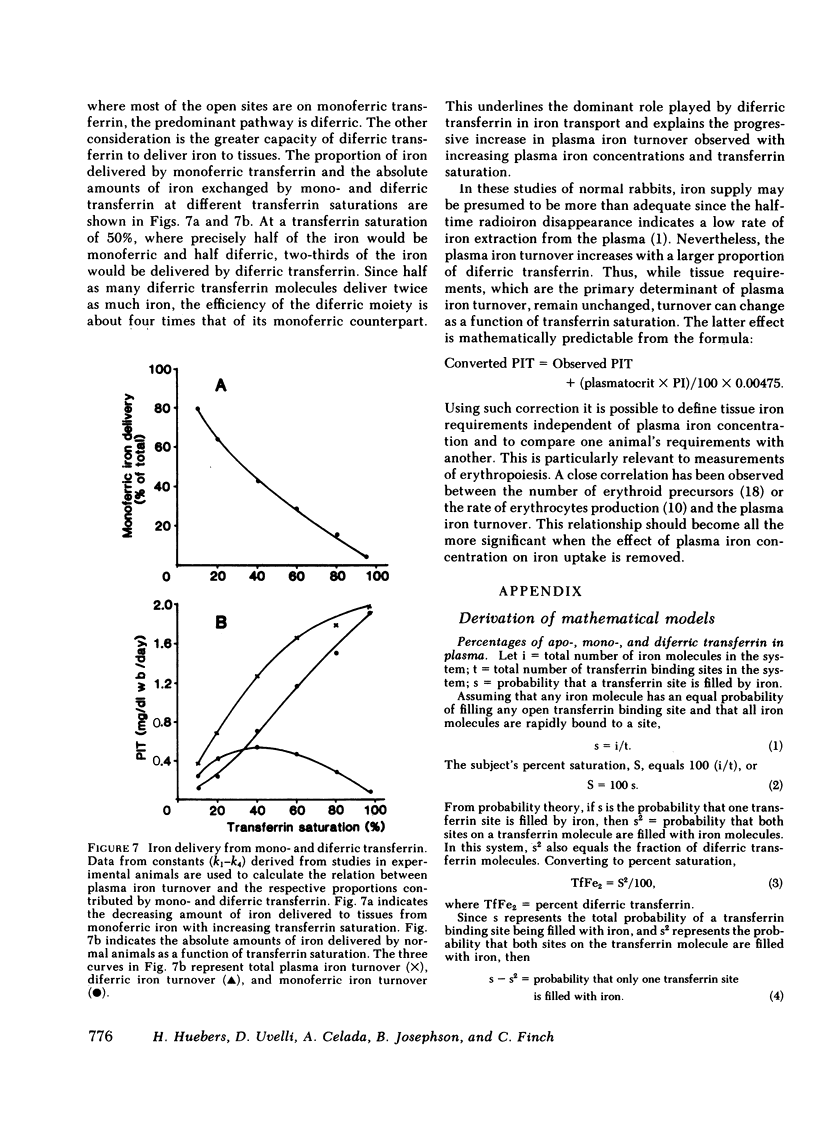
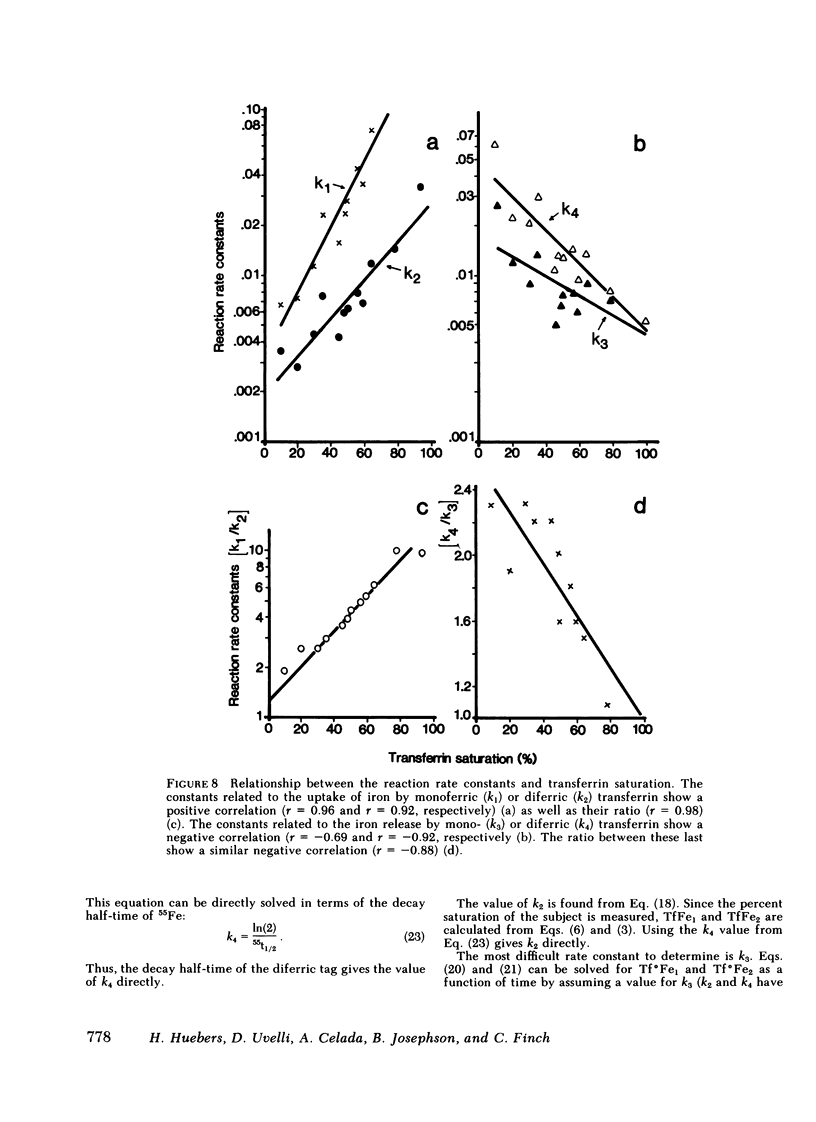
The molecular behavior of transferrin and its iron over this range was investigated using 125I-transferrin, [55Fe]monoferric transferrin, and [59Fe]diferric transferrin. The equilibrium distribution of transferrin between its apo-, mono-, and diferric moieties was similar to that predicted on the basis of the percent saturation and random distribution. Rate constants of iron loading and unloading calculated from the percent saturation and from the clearance rates of [55Fe]monoferric and [59Fe]diferric transferrin were similar to those derived from changes in injected 125I-apotransferrin. On the basis of these data, it is concluded that the plasma transferrin pool is nonhomogeneous and that the relative size of the mono- and diferric cycles depends on transferrin saturation. A formula is proposed for correcting the plasma iron turnover, thereby eliminating the effect of plasma iron concentration, so as to reflect directly the number of tissue transferrin receptors.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aisen P., Brown E. B. The iron-binding function of transferrin in iron metabolism. Semin Hematol. 1977 Jan;14(1):31–53. [PubMed] [Google Scholar]
- Bauer W., Stray S., Huebers H., Finch C. The relationship between plasma iron and plasma iron turnover in the rat. Blood. 1981 Feb;57(2):239–242. [PubMed] [Google Scholar]
- Cook J. D. An evaluation of adsorption methods for measurement of plasma iron-binding capacity. J Lab Clin Med. 1970 Sep;76(3):497–506. [PubMed] [Google Scholar]
- Eakins J. D., Brown D. A. An improved method for the simultaneous determination of iron-55 and iron-59 in blood by liquid scintillation counting. Int J Appl Radiat Isot. 1966 Jul;17(7):391–397. doi: 10.1016/0020-708x(66)90065-2. [DOI] [PubMed] [Google Scholar]
- Finch C. A., Deubelbeiss K., Cook J. D., Eschbach J. W., Harker L. A., Funk D. D., Marsaglia G., Hillman R. S., Slichter S., Adamson J. W. Ferrokinetics in man. Medicine (Baltimore) 1970 Jan;49(1):17–53. doi: 10.1097/00005792-197001000-00002. [DOI] [PubMed] [Google Scholar]
- Groen R., Hendricksen P., Young S. P., Leibman A., Aisen P. Molecular ferrokinetics in the rabbit. Br J Haematol. 1982 Jan;50(1):43–53. doi: 10.1111/j.1365-2141.1982.tb01889.x. [DOI] [PubMed] [Google Scholar]
- Hillman R. S., Henderson P. A. Control of marrow production by the level of iron supply. J Clin Invest. 1969 Mar;48(3):454–460. doi: 10.1172/JCI106002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huebers H., Bauer W., Huebers E., Csiba E., Finch C. The behavior of transferrin iron in the rat. Blood. 1981 Feb;57(2):218–228. [PubMed] [Google Scholar]
- Huebers H., Csiba E., Josephson B., Huebers E., Finch C. Interaction of human diferric transferrin with reticulocytes. Proc Natl Acad Sci U S A. 1981 Jan;78(1):621–625. doi: 10.1073/pnas.78.1.621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huebers H., Huebers E., Csiba E., Finch C. A. Iron uptake from rat plasma transferrin by rat reticulocytes. J Clin Invest. 1978 Nov;62(5):944–951. doi: 10.1172/JCI109223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huebers H., Josephson B., Huebers E., Csiba E., Finch C. Uptake and release of iron from human transferrin. Proc Natl Acad Sci U S A. 1981 Apr;78(4):2572–2576. doi: 10.1073/pnas.78.4.2572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makey D. G., Seal U. S. The detection of four molecular forms of human transferrin during the iron binding process. Biochim Biophys Acta. 1976 Nov 26;453(1):250–256. doi: 10.1016/0005-2795(76)90270-1. [DOI] [PubMed] [Google Scholar]
- Wenn R. V., Williams J. The isoelectric fractionation of hen's-egg ovotransferrin. Biochem J. 1968 Jun;108(1):69–74. doi: 10.1042/bj1080069. [DOI] [PMC free article] [PubMed] [Google Scholar]



