Abstract
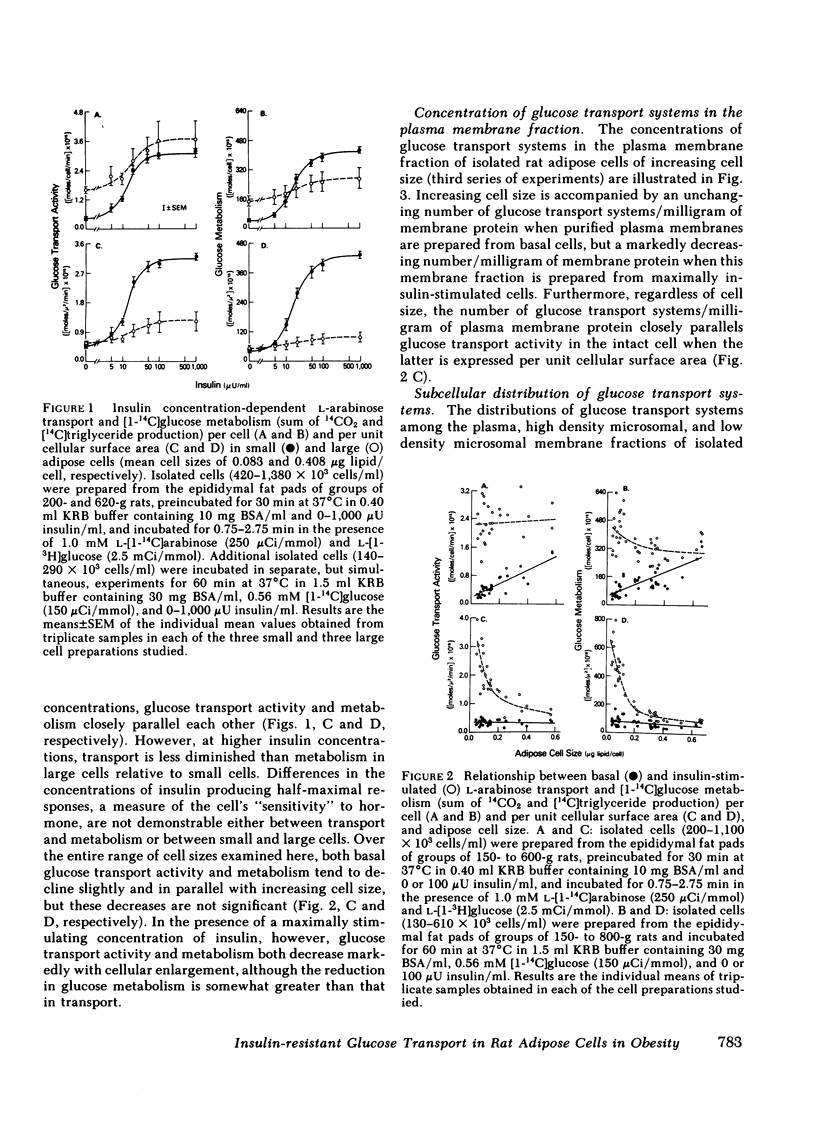
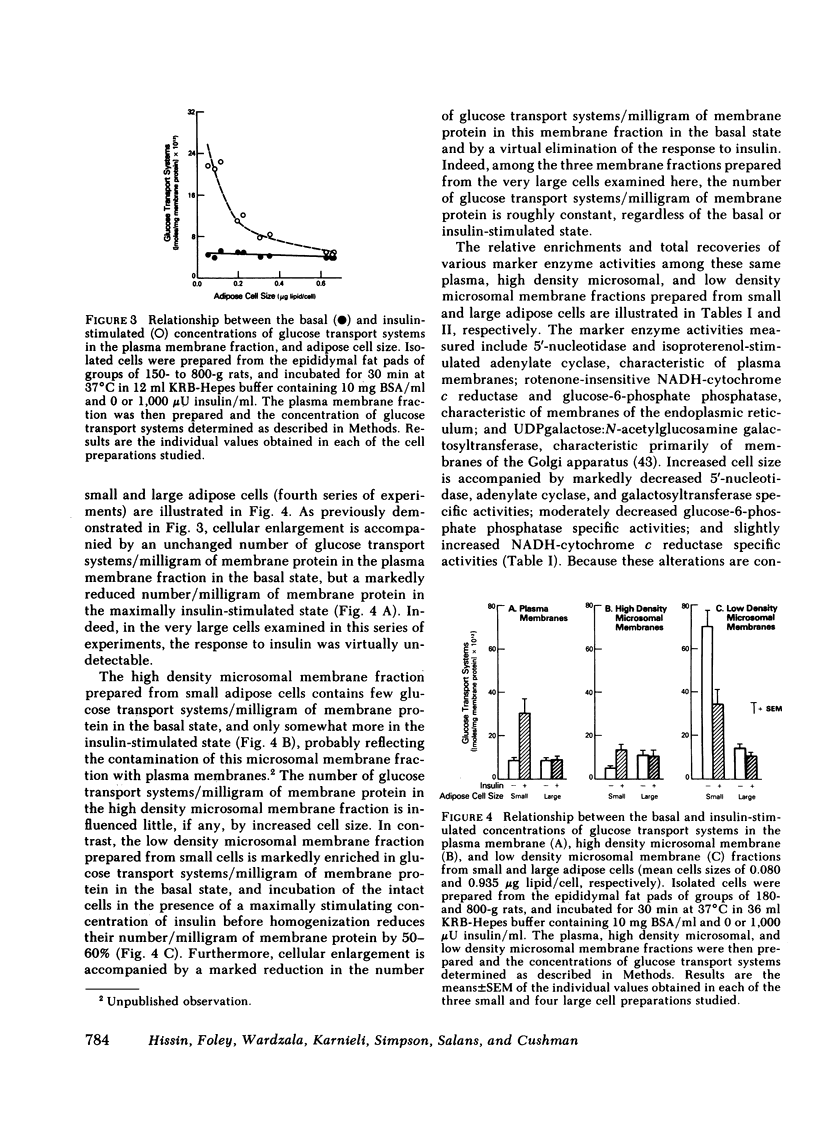
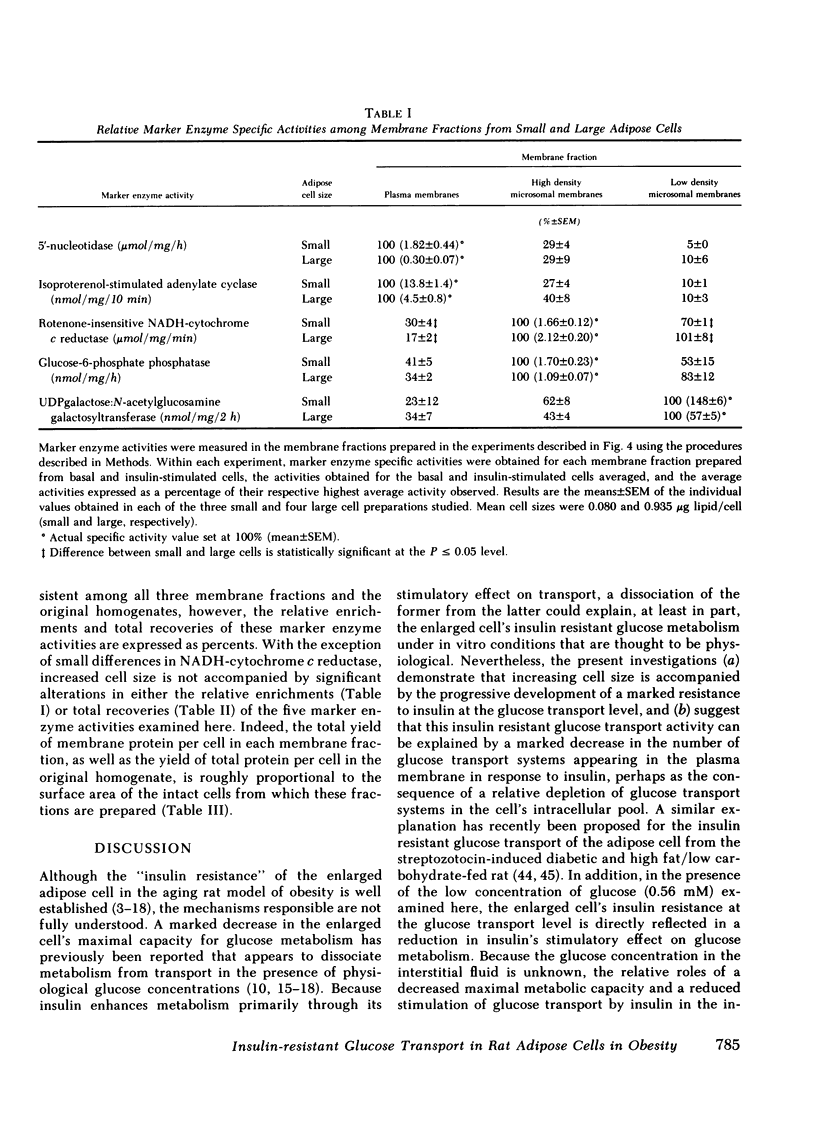
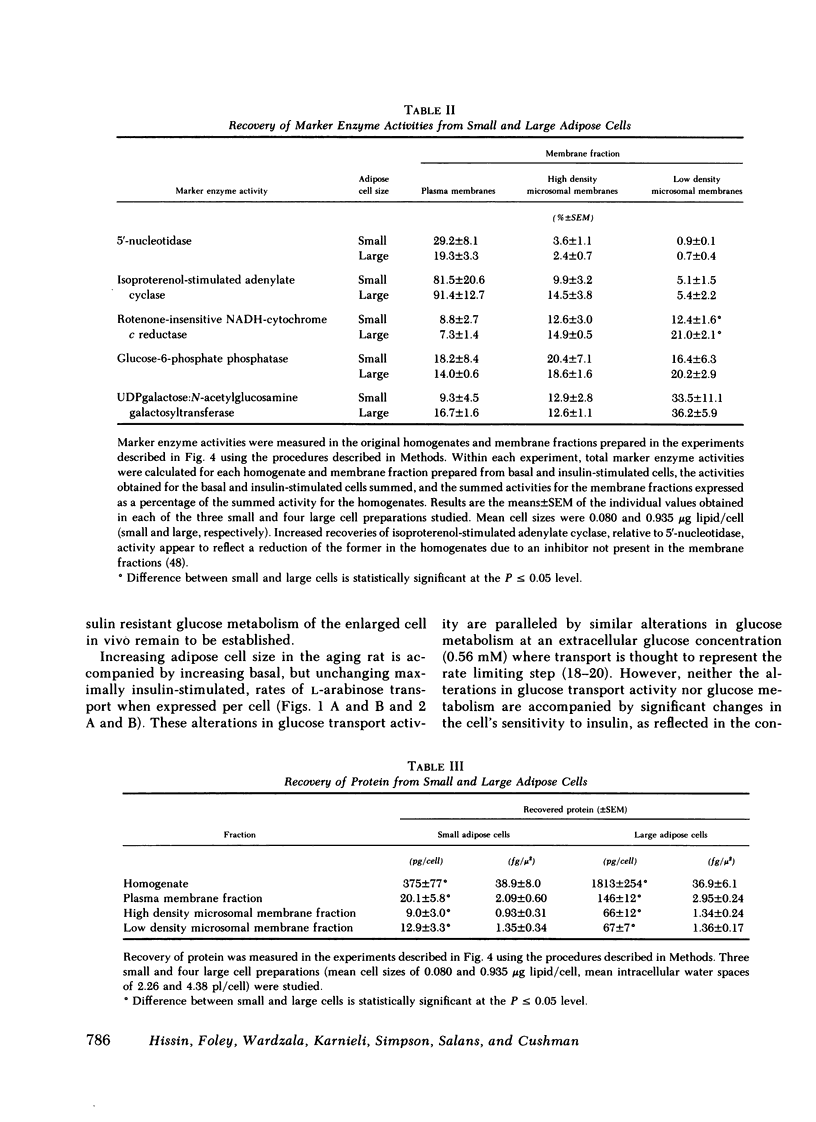
The effects of increasing cell size on glucose transport activity and metabolism and on the concentrations of glucose transport systems in both the plasma and low density microsomal membranes in isolated adipose cells from the aging rat model of obesity have been examined. Glucose transport activity was assessed by measuring l-arabinose transport and the concentration of glucose transport systems estimated by measuring specific d-glucose-inhibitable cytochalasin B-binding. Basal glucose transport activity increases from 0.3 to 1.4 fmol/cell/min with a 10-fold increase in cell size, but remains constant per unit cellular surface area and is accompanied by a constant 5 pmol of glucose transport systems/mg of membrane protein in the plasma membrane fraction. Maximally insulin-stimulated glucose transport activity, on the other hand, remains constant at 2.3 fmol/cell per min with increasing cell size, but markedly decreases per unit cellular surface area and is accompanied by a decrease from 30 pmol of glucose transport systems/mg of plasma membrane protein to the basal level. These diminished effects of insulin on glucose transport activity and the number of glucose transport systems in the plasma membrane fraction in enlarged cells are paralleled by an 80% decrease in the basal number of glucose transport systems/mg of membrane protein in the low density microsomal membrane fraction, the source of those glucose transport systems appearing in the plasma membrane in response to insulin. The effects of cell size on the metabolism of a low concentration of [1-14C]glucose (0.56 mM) directly parallel those on glucose transport activity and the concentration of glucose transport systems in the plasma membrane fraction, and are not associated with significant alterations in the cell's sensitivity to insulin. Thus, adipose cellular enlargement is accompanied by the development of a marked “insulin resistance” at the glucose transport level, which may be the consequence of a relative depletion of glucose transport systems in the intracellular pool.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Avruch J., Wallach D. F. Preparation and properties of plasma membrane and endoplasmic reticulum fragments from isolated rat fat cells. Biochim Biophys Acta. 1971 Apr 13;233(2):334–347. doi: 10.1016/0005-2736(71)90331-2. [DOI] [PubMed] [Google Scholar]
- CROFFORD O. B., RENOLD A. E. GLUCOSE UPTAKE BY INCUBATED RAT EPIDIDYMAL ADIPOSE TISSUE. CHARACTERISTICS OF THE GLUCOSE TRANSPORT SYSTEM AND ACTION OF INSULIN. J Biol Chem. 1965 Aug;240:3237–3244. [PubMed] [Google Scholar]
- CROFFORD O. B., RENOLD A. E. GLUCOSE UPTAKE BY INCUBATED RAT EPIDIDYMAL ADIPOSE TISSUE. RATE-LIMITING STEPS AND SITE OF INSULIN ACTION. J Biol Chem. 1965 Jan;240:14–21. [PubMed] [Google Scholar]
- Cushman S. W., Noda D., Salans L. B. Adipose cell size-function relationships: insulin binding and degradation. Am J Physiol. 1981 Feb;240(2):E166–E174. doi: 10.1152/ajpendo.1981.240.2.E166. [DOI] [PubMed] [Google Scholar]
- Cushman S. W., Rizack M. A. Structure-function relationships in the adipose cell. 3. Effects of bovine serum albumin on the metabolism of glucose and the release of nonesterified fatty acids and glycerol by the isolated adipose cell. J Cell Biol. 1970 Aug;46(2):354–361. doi: 10.1083/jcb.46.2.354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cushman S. W., Salans L. B. Determinations of adipose cell size and number in suspensions of isolated rat and human adipose cells. J Lipid Res. 1978 Feb;19(2):269–273. [PubMed] [Google Scholar]
- Cushman S. W. Structure-function relationships in the adipose cell. I. Ultrastructure of the isolated adipose cell. J Cell Biol. 1970 Aug;46(2):326–341. doi: 10.1083/jcb.46.2.326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cushman S. W., Wardzala L. J. Potential mechanism of insulin action on glucose transport in the isolated rat adipose cell. Apparent translocation of intracellular transport systems to the plasma membrane. J Biol Chem. 1980 May 25;255(10):4758–4762. [PubMed] [Google Scholar]
- Czech M. P. Cellular basis of insulin insensitivity in large rat adipocytes. J Clin Invest. 1976 Jun;57(6):1523–1532. doi: 10.1172/JCI108422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dallner G., Siekevitz P., Palade G. E. Biogenesis of endoplasmic reticulum membranes. II. Synthesis of constitutive microsomal enzymes in developing rat hepatocyte. J Cell Biol. 1966 Jul;30(1):97–117. doi: 10.1083/jcb.30.1.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Girolamo M., Rudman D. Variations in glucose metabolism and sensitivity to insulin of the rat's adipose tissue, in relation to age and body weight. Endocrinology. 1968 Jun;82(6):1133–1141. doi: 10.1210/endo-82-6-1133. [DOI] [PubMed] [Google Scholar]
- DiGirolamo M., Howe M. D., Esposito J., Thurman L., Owens J. L. Metabolic patterns and insulin responsiveness of enlarging fat cells. J Lipid Res. 1974 Jul;15(4):332–338. [PubMed] [Google Scholar]
- Faust I. M., Johnson P. R., Stern J. S., Hirsch J. Diet-induced adipocyte number increase in adult rats: a new model of obesity. Am J Physiol. 1978 Sep;235(3):E279–E286. doi: 10.1152/ajpendo.1978.235.3.E279. [DOI] [PubMed] [Google Scholar]
- Fleischer B. Isolation and characterization of Golgi apparatus and membranes from rat liver. Methods Enzymol. 1974;31:180–191. doi: 10.1016/0076-6879(74)31020-8. [DOI] [PubMed] [Google Scholar]
- Foley J. E., Cushman S. W., Salans L. B. Glucose transport in isolated rat adipocytes with measurements of L-arabinose uptake. Am J Physiol. 1978 Feb;234(2):E112–E119. doi: 10.1152/ajpendo.1978.234.2.E112. [DOI] [PubMed] [Google Scholar]
- Foley J. E., Cushman S. W., Salans L. B. Intracellular glucose concentration in small and large rat adipose cells. Am J Physiol. 1980 Feb;238(2):E180–E185. doi: 10.1152/ajpendo.1980.238.2.E180. [DOI] [PubMed] [Google Scholar]
- Foley J. E., Laursen A. L., Sonne O., Gliemann J. Insulin binding and hexose transport in rat adipocytes. Relation to cell size. Diabetologia. 1980 Sep;19(3):234–241. doi: 10.1007/BF00275275. [DOI] [PubMed] [Google Scholar]
- Gliemann J., Osterlind K., Vinten J., Gammeltoft S. A procedure for measurement of distribution spaces in isolated fat cells. Biochim Biophys Acta. 1972 Nov 24;286(1):1–9. doi: 10.1016/0304-4165(72)90082-7. [DOI] [PubMed] [Google Scholar]
- Hirsch J., Gallian E. Methods for the determination of adipose cell size in man and animals. J Lipid Res. 1968 Jan;9(1):110–119. [PubMed] [Google Scholar]
- Hissin P. J., Karnieli E., Simpson I. A., Salans L. B., Cushman S. W. A possible mechanism of insulin resistance in the rat adipose cell with high-fat/low-carbohydrate feeding. Depletion of intracellular glucose transport systems. Diabetes. 1982 Jul;31(7):589–592. doi: 10.2337/diab.31.7.589. [DOI] [PubMed] [Google Scholar]
- Ho R., Sutherland E. W. cAMP-mediated feedback regulation in target cells. Adv Cyclic Nucleotide Res. 1975;5:533–548. [PubMed] [Google Scholar]
- Karnieli E., Hissin P. J., Simpson I. A., Salans L. B., Cushman S. W. A possible mechanism of insulin resistance in the rat adipose cell in streptozotocin-induced diabetes mellitus. Depletion of intracellular glucose transport systems. J Clin Invest. 1981 Sep;68(3):811–814. doi: 10.1172/JCI110318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karnieli E., Zarnowski M. J., Hissin P. J., Simpson I. A., Salans L. B., Cushman S. W. Insulin-stimulated translocation of glucose transport systems in the isolated rat adipose cell. Time course, reversal, insulin concentration dependency, and relationship to glucose transport activity. J Biol Chem. 1981 May 25;256(10):4772–4777. [PubMed] [Google Scholar]
- Kono T., Suzuki K., Dansey L. E., Robinson F. W., Blevins T. L. Energy-dependent and protein synthesis-independent recycling of the insulin-sensitive glucose transport mechanism in fat cells. J Biol Chem. 1981 Jun 25;256(12):6400–6407. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Livingston J. N., Cuatrecasa P., Lockwood D. H. Insulin insensitivity of large fat cells. Science. 1972 Aug 18;177(4049):626–628. doi: 10.1126/science.177.4049.626. [DOI] [PubMed] [Google Scholar]
- Livingston J. N., Lockwood D. H. Direct measurements of sugar uptake in small and large adipocytes from young and adult rats. Biochem Biophys Res Commun. 1974 Dec 11;61(3):989–996. doi: 10.1016/0006-291x(74)90253-8. [DOI] [PubMed] [Google Scholar]
- Lockwood D. H., Livingston J. N., Amatruda J. M. Relation of insulin receptors to insulin resistance. Fed Proc. 1975 Jun;34(7):1564–1569. [PubMed] [Google Scholar]
- McKeel D. W., Jarett L. Preparation and characterization of a plasma membrane fraction from isolated fat cells. J Cell Biol. 1970 Feb;44(2):417–432. doi: 10.1083/jcb.44.2.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olefsky J. M. Insensitivity of large rat adipocytes to the antilipolytic effects of insulin. J Lipid Res. 1977 Jul;18(4):459–464. [PubMed] [Google Scholar]
- Olefsky J. M. Mechanisms of decreased insulin responsiveness of large adipocytes. Endocrinology. 1977 Apr;100(4):1169–1177. doi: 10.1210/endo-100-4-1169. [DOI] [PubMed] [Google Scholar]
- Olefsky J. M., Reaven G. M. Effects of age and obesity on insulin binding to isolated adipocytes. Endocrinology. 1975 Jun;96(6):1486–1498. doi: 10.1210/endo-96-6-1486. [DOI] [PubMed] [Google Scholar]
- Olefsky J. M. The effects of spontaneous obesity on insulin binding, glucose transport, and glucose oxidation of isolated rat adipocytes. J Clin Invest. 1976 Apr;57(4):842–851. doi: 10.1172/JCI108360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olefsky J. M. The insulin receptor: its role in insulin resistance of obesity and diabetes. Diabetes. 1976 Dec;25(12):1154–1162. doi: 10.2337/diab.25.12.1154. [DOI] [PubMed] [Google Scholar]
- Peterson G. L. A simplification of the protein assay method of Lowry et al. which is more generally applicable. Anal Biochem. 1977 Dec;83(2):346–356. doi: 10.1016/0003-2697(77)90043-4. [DOI] [PubMed] [Google Scholar]
- RODBELL M. METABOLISM OF ISOLATED FAT CELLS. I. EFFECTS OF HORMONES ON GLUCOSE METABOLISM AND LIPOLYSIS. J Biol Chem. 1964 Feb;239:375–380. [PubMed] [Google Scholar]
- Richardson D. K., Czech M. P. Primary role of decreased fatty acid synthesis in insulin resistance of large rat adipocytes. Am J Physiol. 1978 Feb;234(2):E182–E189. doi: 10.1152/ajpendo.1978.234.2.E182. [DOI] [PubMed] [Google Scholar]
- Salans L. B., Bray G. A., Cushman S. W., Danforth E., Jr, Glennon J. A., Horton E. S., Sims E. A. Glucose metabolism and the response to insulin by human adipose tissue in spontaneous and experimental obesity. Effects of dietary composition and adipose cell size. J Clin Invest. 1974 Mar;53(3):848–856. doi: 10.1172/JCI107625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salans L. B., Dougherty J. W. The effect of insulin upon glucose metabolism by adipose cells of different size. Influence of cell lipid and protein content, age, and nutritional state. J Clin Invest. 1971 Jul;50(7):1399–1410. doi: 10.1172/JCI106623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salans L. B., Knittle J. L., Hirsch J. The role of adipose cell size and adipose tissue insulin sensitivity in the carbohydrate intolerance of human obesity. J Clin Invest. 1968 Jan;47(1):153–165. doi: 10.1172/JCI105705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salomon Y., Londos C., Rodbell M. A highly sensitive adenylate cyclase assay. Anal Biochem. 1974 Apr;58(2):541–548. doi: 10.1016/0003-2697(74)90222-x. [DOI] [PubMed] [Google Scholar]
- Suzuki K., Kono T. Evidence that insulin causes translocation of glucose transport activity to the plasma membrane from an intracellular storage site. Proc Natl Acad Sci U S A. 1980 May;77(5):2542–2545. doi: 10.1073/pnas.77.5.2542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wardzala L. J., Cushman S. W., Salans L. B. Mechanism of insulin action on glucose transport in the isolated rat adipose cell. Enhancement of the number of functional transport systems. J Biol Chem. 1978 Nov 25;253(22):8002–8005. [PubMed] [Google Scholar]
- Zinder O., Arad R., Shapiro B. Effect of cell size on the metabolism of isolated fat cells. Isr J Med Sci. 1967 Nov-Dec;3(6):787–791. [PubMed] [Google Scholar]



