Abstract
Familial hemiplegic migraine is a rare subtype of migraine with aura which includes motor weakness. A 32-year-old woman with known familial hemiplegic migraine (point mutation in Exon 22 of the ATP1A2 gene) presented with an acute confusional state, after an initially typical migraine. On examination, she had fever (38°C), agitated, with a right hemiparesis and dysphasia. Electroencephalography showed slowing of α rhythm and continuous rhythmical δ activity in the left hemisphere. She recovered 48 h after the onset of encephalopathic episode. Electroencephalography after recovery showed resolution of the abnormal slowing of the α waveforms.
Background
Familial hemiplegic migraine (FHM) is a rare subtype of migraine with aura which includes motor weakness. It is classified in the International Headache Classification, and is described when there is one or more affected first-degree or second-degree relative.1 FHM has a prevalence of 0.01%,2 and is inherited as an autosomal dominant condition with variable penetrance. There are four genetic variants: FHM1, FHM2, FHM3 and FHM4. FHM1 and FHM2 are the most frequently occurring subtypes and are due to mutations in the CACNA1A gene and the ATP1A2 gene, respectively. In 2005, Dichgans et al3 reported SCN1A gene mutation in three FHM families. This subtype is termed as FHM3. FHM4 is described in one German/Native American family and the disease locus is found on chromosome 1q31,4 although the putative gene mutation is unknown.
This patient presented with an acute confusional episode and was initially suspected to have an infectious encephalitis. This case illustrates a rare but treatable cause of acute confusional state with depressed level of consciousness.
Case presentation
A 32-year-old woman with known FHM presented to the emergency room in an acute confusional state. Earlier during the day, she suffered a hemiplegic migraine attack. She told her husband that she saw zig-zag lines and black spots, after which her speech became slurred and she could not verbally express herself. This was followed by right-sided weakness and headache. As the symptoms were typical, she took some mefenamic acid and went to bed. 4 h later, her husband heard a noise from her room, and found her to be confused, disorientated and agitated. She was unable to recognise her husband. When admitted to hospital, she had fever at 38°C and had a Glasgow Coma Scale of 9/15 (eye open to voice, incomprehensible speech and withdrawal to pain). Neurological examination revealed right hemiparesis and dysphasia, but no other focal neurological deficits. There were no signs of meningeal irritation. This patient was empirically started on intravenous acyclovir, ceftriaxone and benzylpenicillin.
Investigations
Investigations included full blood count, renal, liver, toxicology and thyroid profile, serum ammonia, thrombophilia screen, vasculitic blood screen, blood cultures, PCR for meningococcus, pregnancy test and cerebrospinal fluid (CSF) examination, which were all normal or negative. CSF showed 0 white cell count, 102 red cell count, 4.7 mmol/L glucose (>2/3 of serum glucose), 0.35 g/L protein, negative Gram stain and culture for bacteria and mycobacterium. CSF viral PCR was negative for herpes simplex virus (HSV1 and HSV2), varicella zoster virus, Epstein-Barr virus, cytomegalovirus and human herpes virus 6. CT scan, MRI and MRA (MR angiography) of the brain were all normal.
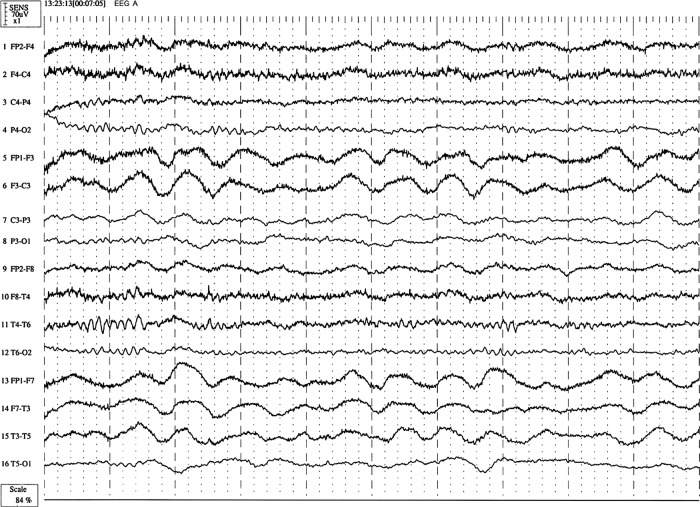
EEG performed during her acute confusional state showed slowing of α rhythm (8–10 Hz) and continuous rhythmical δ activity (1–5 Hz) in the left hemisphere (figure 1). There was no epileptiform activity. Based on the above investigations, the confusional state was attributed to channelopathy-induced encephalopathy. This patient had previously been shown to have a point mutation (2995 G→C substitution) in exon 22 of the ATP1A2 gene.5
Figure 1.
Patient's EEG during the acute confusional state. It shows left hemisphere low amplitude delta activity (0.5–1 Hz) with less prevalent α rhythm (8–10 Hz) on the left side. Continuous rhythmical δ activity (1–5 Hz) was observed over left hemisphere.
Differential diagnosis
Acute confusional states with encephalopathy may occur due to infectious, toxic, metabolic or immune-mediated disorder. Viral encephalitis is important to treat as early as possible if suspected. Antibody-associated encephalopathies such as anti-N-methyl-D-aspartate (NMDA) receptor, antivoltage-gated potassium channel (VGKC) antibody-associated encephalopathy or paraneoplastic disorders are important treatable encephalopathies.
Treatment
The patient was empirically started on intravenous acyclovir, ceftriaxone and benzylpenicillin and received supportive care in the acute phase of her illness.
Prior to discharge, she was started on topiramate as a migraine-preventative therapy.
Outcome and follow-up
The patient recovered 48 h after the onset of acute migraine attack.
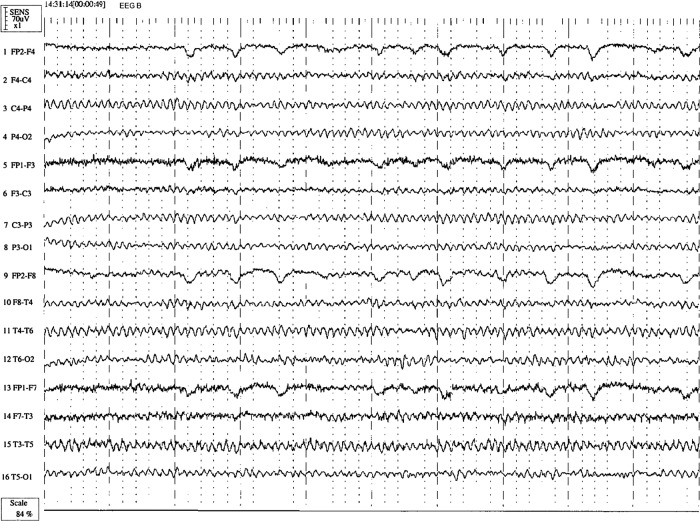
A repeat EEG was carried out after recovery showed resolution of the abnormal slowing of the α waveforms (figure 2).
Figure 2.
Patient's EEG after recovery, which shows normalisation of the focal slow wave activity with background dominated by well formed α rhythm (9 Hz) in a visually responsive symmetrically distribution.
Discussion
The patient belongs to a large FHM pedigree that was found to have the D999H mutation of the ATP1A2 gene.5 Seven of her 11 siblings and her father are affected with hemiplegic migraine, and all were found to have the D999H mutation. ATP1A2 encodes the α2 subunit of the A1A2 glial sodium–potassium ATPase exchange pump.5 There were no phenocopies within this family. This patient suffered from hemiplegic migraine since the age of 8 years and experienced 3–4 hemiplegic migraine attacks annually, although she never had a confusional episode. None of her relatives described acute confusion during their migraine attacks. Although epilepsy is described in patients with the D999H mutation, our patient does not have a history of seizures. Her EEG during the acute confusional episode also did not demonstrate any epileptiform activity.
A number of hypothesis for the pathophysiology of migraine with aura have been proposed including cortical spreading depression.1 The exact mechanism of topiramate is unclear, but it may bind to membrane channel complexes at phosphorylation sites and, thereby, allosterically modulate ionic conductance through the channels and reduce cortical spreading depression. Topiramate may act through blockage of voltage-dependent sodium channels, and augmentation of γ-aminobutyric acid activity at some subtypes of the GABA-A receptors.
Although confusion commonly occurs during migraine attacks and have been reported in FHM1, FHM2 and FHM4 patients,4 6–12 overt encephalopathy in FHM occurs less often. Recurrent encephalopathy occurring as part of hemiplegic migraine attacks has been described in a Native American kindred with an unknown mutation.13 Coma as a manifestation of encephalopathy has been reported to occur in 37% of FHM1, the most susceptible being those who have the T666M mutation.6 Coma has also been described in FHM2 families,7 10 11 14–16 and recurrent coma occurring with hemiplegic migraine attacks has been described in a patient with FHM4.4 Many cases of migraine associated coma were found to have cerebral oedema and head trauma appears to be a migraine trigger. EEG changes have been previously reported in hemiplegic migraine, but their diagnostic utility has not been clear.17 In the setting of a patient presenting with an acute confusional state or encephalopathy, we suggest that EEG may have a role in differentiating a channel-mediated encephalopathy from other causes of acute confusion, and may demonstrate dynamic changes over time. This case report demonstrates that encephalopathy with EEG changes can occur in the D999H mutation of the ATP1A2 gene.
Learning points.
Familial hemiplegic migraine can present with an acute confusional syndrome.
EEG can be a useful tool in the assessment of patients with acute confusional episodes as it provides dynamic data about cerebral activity.
In patients presenting with acute confusional states and normal brain imaging genetic disorders and metabolic conditions should be kept in mind.
Acknowledgments
The assistance of Mr Diarmaid Cowhie in preparation of the figures is gratefully acknowledged.
Footnotes
Contributors: AM, DF, BM and HH drafted the manuscript, provided the data and made critical revisions.
Competing interests: None.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.International Headache Society Classification Subcommittee International classification of headache disorders, 2nd edition. Cephalalgia 2004;2013(Suppl 1):1–160 [DOI] [PubMed] [Google Scholar]
- 2.Lykke Thomsen L, Kirchmann Eriksen M, Faerch Romer S, et al. An epidemiological survey of hemiplegic migraine. Cephalalgia 2002;2013:361–75 [DOI] [PubMed] [Google Scholar]
- 3.Dichgans M, Freilinger T, Eckstein G, et al. Mutation in the neuronal voltage-gated sodium channel SCN1A in familial hemiplegic migraine. Lancet 2005;2013:371–7 [DOI] [PubMed] [Google Scholar]
- 4.Gardner K, Barmada MM, Ptacek LJ, et al. A new locus for hemiplegic migraine maps to chromosome 1q31. Neurology 1997;2013:1231–8 [DOI] [PubMed] [Google Scholar]
- 5.Fernandez DM, Hand CK, Sweeney BJ, et al. A novel ATP1A2 gene mutation in an Irish familial hemiplegic migraine kindred. Headache 2008;2013:101–8 [DOI] [PubMed] [Google Scholar]
- 6.Ducros A, Denier C, Joutel A, et al. The clinical spectrum of familial hemiplegic migraine associated with mutations in a neuronal calcium channel. N Engl J Med 2001;2013:17–24 [DOI] [PubMed] [Google Scholar]
- 7.Ducros A, Joutel A, Vahedi K, et al. Mapping of a second locus for familial hemiplegic migraine to 1q21-q23 and evidence of further heterogeneity. Ann Neurol 1997;2013:885–90 [DOI] [PubMed] [Google Scholar]
- 8.Marconi R, De Fusco M, Aridon P, et al. Familial hemiplegic migraine type 2 is linked to 0.9Mb region on chromosome 1q23. Ann Neurol 2003;2013:376–81 [DOI] [PubMed] [Google Scholar]
- 9.Vanmolkot KR, Kors EE, Hottenga JJ, et al. Novel mutations in the Na+, K+-ATPase pump gene ATP1A2 associated with familial hemiplegic migraine and benign familial infantile convulsions. Ann Neurol 2003;2013:360–6 [DOI] [PubMed] [Google Scholar]
- 10.Jurkat-Rott K, Freilinger T, Dreier JP, et al. Variability of familial hemiplegic migraine with novel A1A2 Na+/K+-ATPase variants. Neurology 2004;2013:1857–61 [DOI] [PubMed] [Google Scholar]
- 11.Spadaro M, Ursu S, Lehmann-Horn F, et al. A G301R Na+/K+ -ATPase mutation causes familial hemiplegic migraine type 2 with cerebellar signs. Neurogenetics 2004;2013:177–85 [DOI] [PubMed] [Google Scholar]
- 12.Vanmolkot KR, Stroink H, Koenderink JB, et al. Severe episodic neurological deficits and permanent mental retardation in a child with a novel FHM2 ATP1A2 mutation. Ann Neurol 2006;2013:310–14 [DOI] [PubMed] [Google Scholar]
- 13.Spacey SD, Vanmolkot KR, Murphy C, et al. Familial hemiplegic migraine presenting as recurrent encephalopathy in a Native Indian family. Headache 2005;2013:1244–9 [DOI] [PubMed] [Google Scholar]
- 14.Kaunisto MA, Harno H, Vanmolkot KR, et al. A novel missense ATP1A2 mutation in a Finnish family with familial hemiplegic migraine type 2. Neurogenetics 2004;2013:141–6 [DOI] [PubMed] [Google Scholar]
- 15.Pierelli F, Grieco GS, Pauri F, et al. A novel ATP1A2 mutation in a family with FHM type II. Cephalalgia 2006;2013:324–8 [DOI] [PubMed] [Google Scholar]
- 16.de Vries B, Stam AH, Kirkpatrick M, et al. Familial hemiplegic migraine is associated with febrile seizures in an FHM2 family with a novel de novo ATP1A2 mutation. Epilepsia 2009;2013:2503–4 [DOI] [PubMed] [Google Scholar]
- 17.Campbell JK, Zagami A. Hemiplegic migraine. In: Olesen J, Tfelt-Hansen P, Welch KMA, eds. The headaches. Raven Press, 1993:409–11 [Google Scholar]