Abstract
Recently it has been observed that Ca excretion in laboratory rats does not follow a Gaussian distribution, with ∼10% of them excreting Ca at a rate of 2 SD above the group mean. This phenomenon has been described as spontaneous hypercalciuria (SH). Our studies were designed to define its mechanism. 48 Wistar rats were subjected to metabolic studies to identify SH, prospectively defined as Ca excretion 2 SD above the group mean during 7 d of dietary Ca deprivation (≤0.03% by analysis), in the absence of hypercalcemia, PO4 depletion, or exaggerated natriuresis.
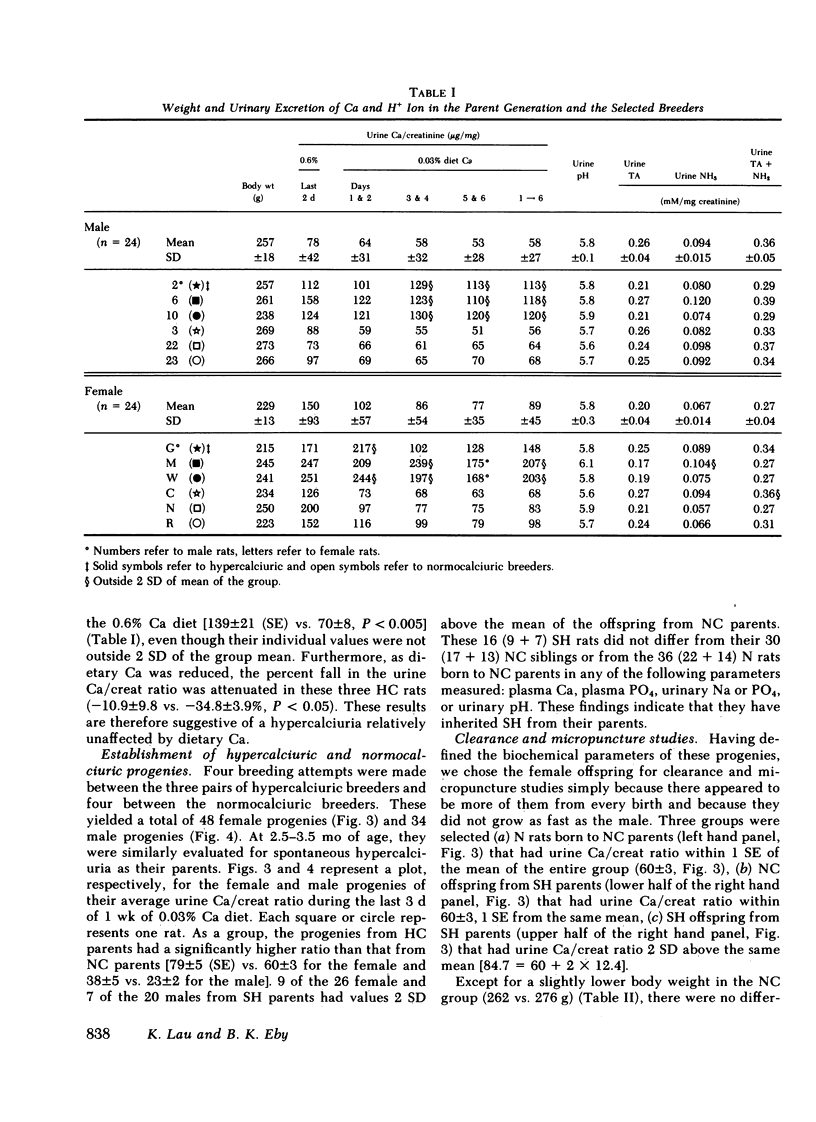
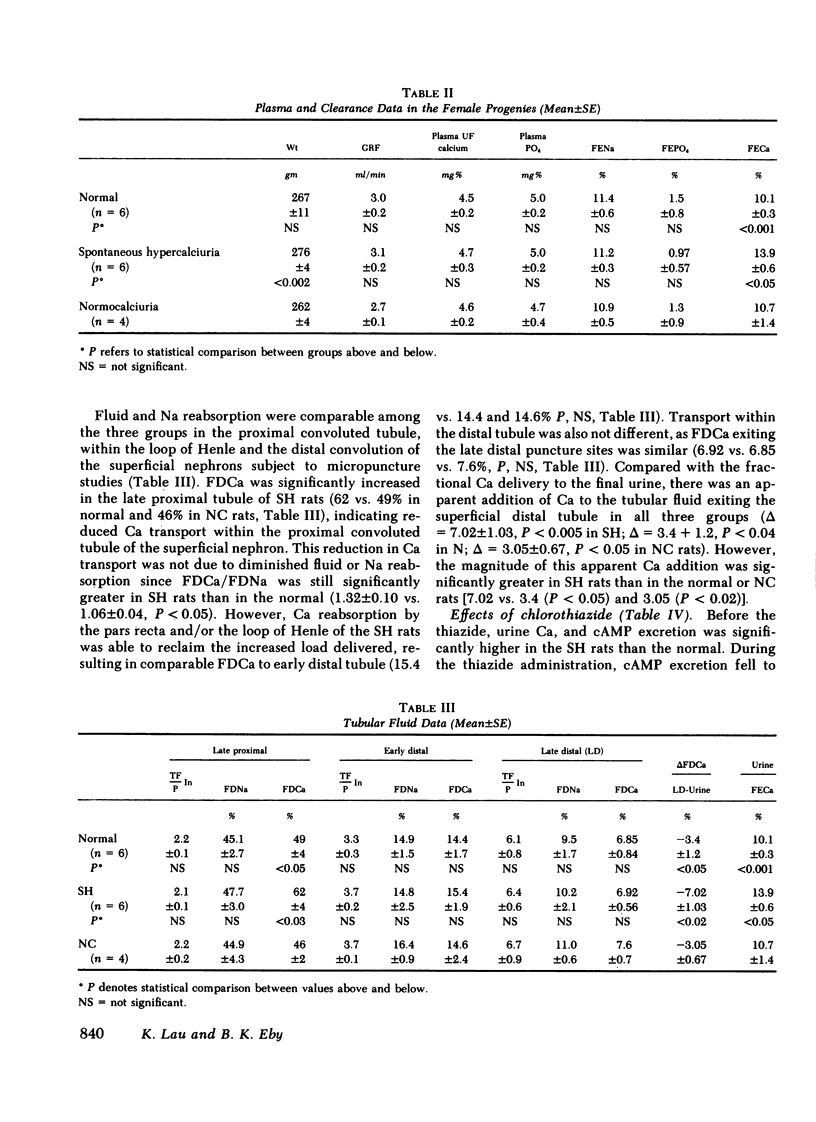
Progenies from SH rats were found to have significantly higher urine Ca/creatinine (micrograms per milligram) (male = 38 vs. 23, P < 0.05; female = 79 vs. 60, P < 0.005) with 7/20 males and 9/26 females having values 2 SD above the means of normal. After a 12-h fast and during 10% volume expansion with saline, clearance and micropuncture studies were performed on three groups of acutely parathyroidectomized female rats; (a) normocalciuric (N) progenies from the normal, (b) normocalciuric (NC) progenies from SH, and (c) hypercalciuric (HC) progenies from SH rats. Among these groups, there was no significant difference in body weights, glomerular filtration rate, plasma ultrafiltrable Ca (4.5, 4.6 vs. 4.7 mg/100 g), PO4, and the fractional excretion (FE) of Na or FEPO4. FE Ca was significantly higher in HC rats (13.9%) than N (10.1%) and NC (10.7%). Segmental reabsorption of fluid and Na was comparable among the three groups. Fractional delivery (FD) of Ca was, however, significantly increased in the late proximal tubule of HC rats (62 vs. 49 and 46%, P < 0.05). The increased FDCa was no longer apparent in early or late distal tubule (6.9 vs. 6.9 and 7.6%, P = NS). Although FECa exceeded late distal FDCa in all three groups, the increment was significantly greater in HC rats (7.02%) than both N (3.4, P < 0.05) and NC rats (3.05, P < 0.02).
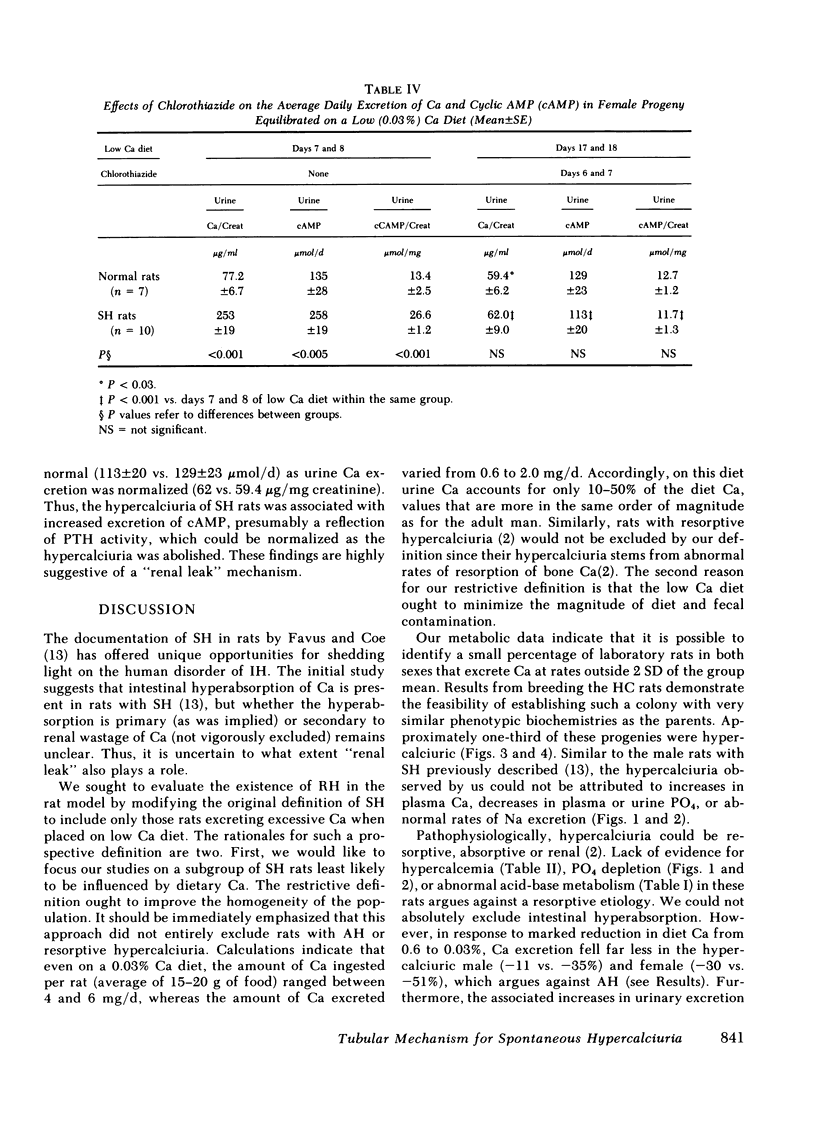
The effects of chlorothiazide (27.5 mg/kg/d, i.p. × 7 d) were evaluated in the female offsprings of the SH rats. Before chlorothiazide, average urine Ca/creatinine (253 vs. 77.2) and cyclic AMP (26.6 vs. 13.4 μmol/mg creatinine, P < 0.001) on days 7 and 8 of the Ca-deprived diet were higher than the normal. On days 6 and 7 of chlorothiazide, average cyclic AMP (cAMP) excretion fell to normal range (11.7 vs. 12.7 μmol/mg creatinine) as Ca excretion was reduced to normal (62 vs. 59.4 μg Ca/mg creatinine).
We conclude: (a) SH, as defined in this study, is an inheritable biochemical marker and renal in origin. (b) The hypercalciuria is independent of parathyroid hormone, changes in plasma Ca and tubular handling of Na. (c) As studied in the PTX and volume expanded conditions of our experiments, decreased Ca reabsorption in superficial proximal convoluted tubule is demonstrable, but the hypercalciuria is probably mediated by diminished Ca transport by the deep nephron. The unlikely possibility of increased secretion by the terminal nephron, however, remains to be excluded. (d) In normal rats, there is internephron heterogeneity in regard to Ca transport during saline loading.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agus Z. S., Chiu P. J., Goldberg M. Regulation of urinary calcium excretion in the rat. Am J Physiol. 1977 Jun;232(6):F545–F549. doi: 10.1152/ajprenal.1977.232.6.F545. [DOI] [PubMed] [Google Scholar]
- Barilla D. E., Tolentino R., Kaplan R. A., Pak C. Y. Selective effects of thiazide on intestinal absorption of calcium and adsorptive and renal hypercalciurias. Metabolism. 1978 Feb;27(2):125–131. doi: 10.1016/0026-0495(78)90158-0. [DOI] [PubMed] [Google Scholar]
- Battilana C. A., Dobyan D. C., Lacy F. B., Bhattacharya J., Johnston P. A., Jamison R. L. Effect of chronic potassium loading on potassium secretion by the pars recta or descending limb of the juxtamedullary nephron in the rat. J Clin Invest. 1978 Nov;62(5):1093–1103. doi: 10.1172/JCI109215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bengele H. H., Alexander E. A., Lechene C. P. Calcium and magnesium transport along the inner medullary collecting duct of the rat. Am J Physiol. 1980 Jul;239(1):F24–F29. doi: 10.1152/ajprenal.1980.239.1.F24. [DOI] [PubMed] [Google Scholar]
- Bordier P., Ryckewart A., Gueris J., Rasmussen H. On the pathogenesis of so-called idiopathic hypercalciuria. Am J Med. 1977 Sep;63(3):398–409. doi: 10.1016/0002-9343(77)90278-9. [DOI] [PubMed] [Google Scholar]
- Bourdeau J. E., Burg M. B. Effect of PTH on calcium transport across the cortical thick ascending limb of Henle's loop. Am J Physiol. 1980 Aug;239(2):F121–F126. doi: 10.1152/ajprenal.1980.239.2.F121. [DOI] [PubMed] [Google Scholar]
- Brickman A. S., Massry S. G., Coburn J. W. changes in serum and urinary calcium during treatment with hydrochlorothiazide: studies on mechanisms. J Clin Invest. 1972 Apr;51(4):945–954. doi: 10.1172/JCI106889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broadus A. E., Dominguez M., Bartter F. C. Pathophysiological studies in idiopathic hypercalciuria: use of an oral calcium tolerance test to characterize distinctive hypercalciuric subgroups. J Clin Endocrinol Metab. 1978 Oct;47(4):751–760. doi: 10.1210/jcem-47-4-751. [DOI] [PubMed] [Google Scholar]
- CHANEY A. L., MARBACH E. P. Modified reagents for determination of urea and ammonia. Clin Chem. 1962 Apr;8:130–132. [PubMed] [Google Scholar]
- Coe F. L., Canterbury J. M., Firpo J. J., Reiss E. Evidence for secondary hyperparathyroidism in idiopathic hypercalciuria. J Clin Invest. 1973 Jan;52(1):134–142. doi: 10.1172/JCI107156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coe F. L., Parks J. H., Moore E. S. Familial idiopathic hypercalciuria. N Engl J Med. 1979 Feb 15;300(7):337–340. doi: 10.1056/NEJM197902153000703. [DOI] [PubMed] [Google Scholar]
- Higashihara E., Stokes J. B., Kokko J. P., Campbell W. B., DuBose T. D., Jr Cortical and papillary micropuncture examination of chloride transport in segments of the rat kidney during inhibition of prostaglandin production. Possible role for prostaglandins in the chloruresis of acute volume expansion. J Clin Invest. 1979 Nov;64(5):1277–1287. doi: 10.1172/JCI109583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holt W. F., Lechene C. ADH-PGE2 interactions in cortical collecting tubule. II. inhibition of Ca and P reabsorption. Am J Physiol. 1981 Oct;241(4):F461–F467. doi: 10.1152/ajprenal.1981.241.4.F461. [DOI] [PubMed] [Google Scholar]
- Imai M. Calcium transport across the rabbit thick ascending limb of Henle's loop perfused in vitro. Pflugers Arch. 1978 May 31;374(3):255–263. doi: 10.1007/BF00585603. [DOI] [PubMed] [Google Scholar]
- Jamison R. L., Frey N. R., Lacy F. B. Calcium reabsorption in the thin loop of Henle. Am J Physiol. 1974 Sep;227(3):745–751. doi: 10.1152/ajplegacy.1974.227.3.745. [DOI] [PubMed] [Google Scholar]
- Knox F. G., Haas J. A., Berndt T., Marchand G. R., Youngberg S. P. Phosphate transport in superficial and deep nephrons in phosphate-loaded rats. Am J Physiol. 1977 Aug;233(2):F150–F153. doi: 10.1152/ajprenal.1977.233.2.F150. [DOI] [PubMed] [Google Scholar]
- Lau K., Agus Z. S., Goldberg M., Goldfarb S. Renal tubular sites of altered calcium transport in phosphate-depleted rats. J Clin Invest. 1979 Dec;64(6):1681–1687. doi: 10.1172/JCI109630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau Y. K., Wasserstein A., Westby G. R., Bosanac P., Grabie M., Mitnick P., Slatopolsky E., Goldfarb S., Agus Z. S. Proximal tubular defects in idiopathic hypercalciuria: resistance to phosphate administration. Miner Electrolyte Metab. 1982;7(5):237–249. [PubMed] [Google Scholar]
- Pak C. Y., Kaplan R., Bone H., Townsend J., Waters O. A simple test for the diagnosis of absorptive, resorptive and renal hypercalciurias. N Engl J Med. 1975 Mar 6;292(10):497–500. doi: 10.1056/NEJM197503062921002. [DOI] [PubMed] [Google Scholar]
- Pak C. Y., Oata M., Lawrence E. C., Snyder W. The hypercalciurias. Causes, parathyroid functions, and diagnostic criteria. J Clin Invest. 1974 Aug;54(2):387–400. doi: 10.1172/JCI107774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peraino R. A., Suki W. N. Urine HCO3- augments renal Ca2+ absorption independent of systemic acid-base changes. Am J Physiol. 1980 May;238(5):F394–F398. doi: 10.1152/ajprenal.1980.238.5.F394. [DOI] [PubMed] [Google Scholar]
- Rouse D., Ng R. C., Suki W. N. Calcium transport in the pars recta and thin descending limb of Henle of the rabbit, perfused in vitro. J Clin Invest. 1980 Jan;65(1):37–42. doi: 10.1172/JCI109657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shareghi G. R., Stoner L. C. Calcium transport across segments of the rabbit distal nephron in vitro. Am J Physiol. 1978 Oct;235(4):F367–F375. doi: 10.1152/ajprenal.1978.235.4.F367. [DOI] [PubMed] [Google Scholar]
- Stein J. H., Osgood R. W., Kunau R. T., Jr Direct measurement of papillary collecting duct sodium transport in the rat. Evidence for heterogeneity of nephron function during Ringer loading. J Clin Invest. 1976 Oct;58(4):767–773. doi: 10.1172/JCI108527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiner A. L., Parker C. W., Kipnis D. M. Radioimmunoassay for cyclic nucleotides. I. Preparation of antibodies and iodinated cyclic nucleotides. J Biol Chem. 1972 Feb 25;247(4):1106–1113. [PubMed] [Google Scholar]
- Suki W. N. Calcium transport in the nephron. Am J Physiol. 1979 Jul;237(1):F1–F6. doi: 10.1152/ajprenal.1979.237.1.F1. [DOI] [PubMed] [Google Scholar]
- Suki W. N., Rouse D., Ng R. C., Kokko J. P. Calcium transport in the thick ascending limb of Henle. Heterogeneity of function in the medullary and cortical segments. J Clin Invest. 1980 Nov;66(5):1004–1009. doi: 10.1172/JCI109928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutton R. A., Walker V. R. Responses to hydrochlorothiazide and acetazolamide in patients with calcium stones. Evidence suggesting a defect in renal tubular function. N Engl J Med. 1980 Mar 27;302(13):709–713. doi: 10.1056/NEJM198003273021302. [DOI] [PubMed] [Google Scholar]
- Zerwekh J. E., Pak C. Y. Selective effects of thiazide therapy on serum 1 alpha,25-dihydroxyvitamin D and intestinal calcium absorption in renal and absorptive hypercalciurias. Metabolism. 1980 Jan;29(1):13–17. doi: 10.1016/0026-0495(80)90091-8. [DOI] [PubMed] [Google Scholar]
- de Rouffignac C., Morel F., Moss N., Roinel N. Micropuncture study of water and electrolyte movements along the loop of Henle in psammomys with special reference to magnesium, calcium and phosphorus. Pflugers Arch. 1973 Nov 30;344(4):309–326. doi: 10.1007/BF00592784. [DOI] [PubMed] [Google Scholar]



