Abstract
Pulmonary surfactant deficiency caused by mutations in ABCA3 (ATP-binding cassette transporter of the A subfamily, member 3) gene results in diffuse parenchymal lung disease (DPLD) in children. So far, systemic steroids are the main treatment, with however limited efficacy. We report the case of a young boy showing a dramatic long-term improvement of respiratory disease by low-dose azithromycin (AZM) with no side effect after 6 years of treatment. Cellular and molecular studies are ongoing to progress in the understanding of the mechanisms involved. On behalf of the National Reference Center for rare lung diseases in France (Respirare, http://www.respirare.fr), clinical studies on AZM in various forms of DPLD in children have been initiated and should provide information on the types of paediatric DPLD that may benefit from this treatment.
Background
Mutations in ABCA3 (ATP-binding cassette transporter of the A subfamily, member 3) gene result in pulmonary surfactant deficiency and are associated with the development of diffuse parenchymal lung disease (DPLD) in children.1 So far, the treatment of this respiratory disorder has been limited to the use of systemic corticosteroids with, however, poor efficacy in most situations and ineluctable progression towards irreversible respiratory failure. Several novel therapeutical strategies aimed at restoring lung parenchyma homeostasis, are currently discussed. Potential candidates include macrolides.2 We report the first dramatic long-term improvement of respiratory disease by azithromycin (AZM) in a young boy with severe DPLD and documented mutations in ABCA3.
Case presentation
In 1999, a full-term born boy developed, a few hours after birth, severe DPLD with refractory hypoxaemia and pulmonary hypertension. He was admitted in the intensive care unit and needed a high-pressure mechanical ventilation for 2 weeks, with oxygen therapy up to 100% FiO2. Infectious search remained negative. Chest radiographs showed bilateral infiltrates consistent with DPLD, confirmed by CT. Because of the oxygen requirement and the respiratory distress persistence, an open lung biopsy was performed at the age of 4 months that revealed extensive alveolar septal thickening and hyperplasia of type 2 alveolar epithelial cells (AEC). Electron microscopic analysis showed the presence of abnormal lamellar bodies evocative of intracellular phospholipids accumulation. Supported by the familial history of unexplained chronic lung diseases in several members, of an older sister's death at the age of 7 months caused by respiratory failure, and of parental consanguinity, testing for genetic lung disorders was performed. No mutations in surfactant protein C and protein B genes were found. The search for ABCA3 mutational defects revealed a homozygous single nucleotide substitution leading to a histidine into aspartic acid in codon 253 (D253H). Familial genetic screening confirmed that the parents and the deceased sister carried, respectively, one and two ABCA3-D253H mutations. Monthly intravenous methylprednisolone pulses were initiated at the age of 4 months, with a dose of 30 mg/kg daily for three consecutive days, along with daily oral prednisone 2–0.5 mg/kg/J, continuous oxygen therapy and gastrostomy tube feeding. However, despite the high steroid regimen, the patient condition remained critical with resting tachypnoea, clubbing, diffuse crackles, severe hypoxaemia and poor growth curve. Chest CT performed at the age of 2 and 4 years showed similar diffuse bilateral infiltrates with ground glass pattern (figure 1).
Figure 1.
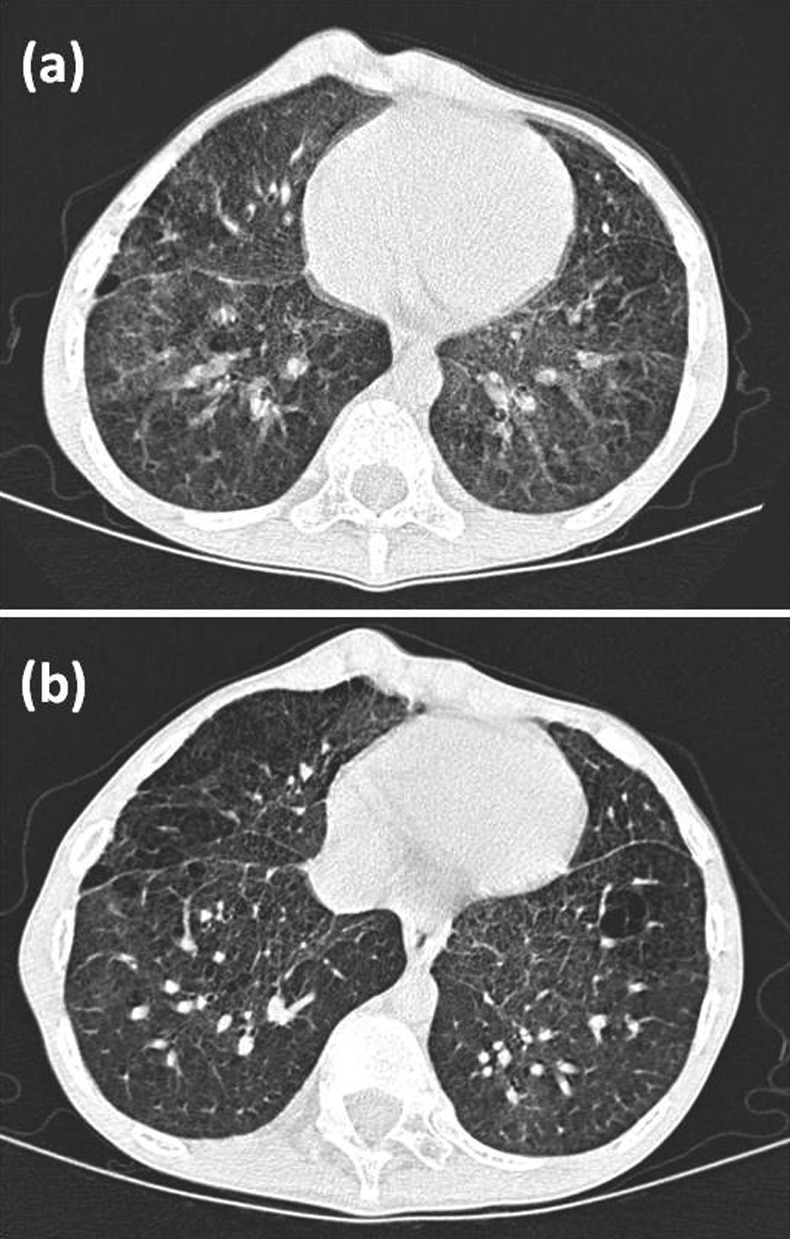
Chest CT of the patient at the age of 6 years showed bilateral ground glass opacities, interlobular septal thickening and some pulmonary cysts (A). CT at the age of 12 years (after 6 years of azithromycin) indicated partial clearing of ground glass attenuation without appearance of new cysts or alveolar condensation (B).
Treatment
At the age of 6 years, his severe respiratory insufficiency despite the high corticosteroid regimen led us to introduce oral AZM at a dose of 20 mg/kg thrice a week.
Outcome and follow-up
Surprisingly, after only few weeks of AZM, parents reported a dramatic improvement of the child respiratory condition, and a significant decrease in resting tachypnoea and oxygen requirement was documented. After 10 months of macrolides, he was breathing room air and no longer received steroids. Arterial PaO2 progressively increased from 62 to 75 mm Hg. Nutritional support could progressively be stopped.
At the age of 12 years, after 6 years of good adherence to AZM treatment, the growth curve, clinical condition and the quality-of-life perception were good. Scholar course was normal. Neither pulmonary exacerbations nor adverse events were reported. The thoracic CT indicated partial clearing of the lung infiltrates (figure 1). Lung function tests documented normal lung volume parameters, diffusing capacity for carbon monoxide and blood gases. Medium overnight room air oxygen saturation was 96% (min 74%, max 99%, time duration under 88%=44 s=0.1% of time). Exercise training programme was started.
Discussion
DPLD due to ABCA3 deficiency is rare and of poor prognosis. The largest study reporting seven cases with homozygous or compound heterozygous ABCA3 mutations highlighted the severity of the respiratory disease.3 Although in the present case we cannot exclude some spontaneous improvement of the patient condition, the impressive rapidity of the beneficial response to AZM observed after several years of sustained management with steroids provides some evidence of macrolide therapy efficacy in this pathological situation. This is further supported by a recent publication documenting benefit of AZM associated with hydroxychloroquine in a young patient with a severe respiratory insufficiency due to ABCA3 deficiency.4
The effectiveness of macrolides in the treatment of various forms of chronic lung diseases has been discussed in several reports.2 5 6 In addition to their antibacterial effect, there is growing evidence that these molecules also exert beneficial effects through anti-inflammatory actions. Several experimental and clinical reports have documented an anti-inflammatory action associated with a decreased synthesis of proinflammatory molecules such as interleukin (IL)-8 in a number of pathological conditions including surfactant deficiency. Recent studies also suggest that macrolides could interfere with cellular processes associated with lung epithelial function and alveolar parenchymal repair.7
In the present case of DPLD linked to ABCA3 mutations, the documented long-term efficacy of AZM is more likely to be due to its anti-inflammatory action rather than its antimicrobial property. Several molecular mechanisms can be discussed. ABCA3 plays an important role in alveolar epithelium homeostasis as a transporter of phospholipids, a step required for a proper surfactant assembly and secretion. In situation of abnormal ABCA3 proteins, AZM may exert its beneficial effect by targeting the altered surfactant metabolism. Indeed, the abnormal lamellar structures documented in the patient lung tissue biopsy are evocative of intracellular phospholipids accumulation, with, as a consequence, an increased AEC stress and injury. Through its capacity to modulate phospholipidosis, AZM may improve alveolar lipid metabolism and surfactant trafficking, contributing to stimulate the defence process against cellular stress.8 Anti-inflammatory actions of AZM may also be proposed, such as downregulation of IL-8 whose exaggerated production has been observed in cells harbouring ABCA3 mutations.3 Other possible explanations include epigenetic modulation, as epigenetic alterations can contribute to various forms of chronic inflammatory lung disorders including DPLD.9 Among the molecular targets is histone deacetylase (HDAC) 2, with a decrease in its level and activity documented in situations of lung parenchymal injury similar to those observed in surfactant disorders. Of interest, recent studies have documented a role of macrolides on restoring HDAC2 activity, thus providing the potential to regenerate lung tissue.10
The present observation is the first documenting a rapid, impressive and sustained clinical improvement of low-dose AZM in a young patient with DPLD associated with ABCA3 mutations. Of importance, the careful monitoring during the follow-up did not document any complications or side effects. This report is of tremendous interest for the lung paediatric community considering the limited efficacy of systemic steroids in the majority of young patients with DPLD. Clinical studies gathering patients with DPLD associated with various forms of surfactant deficiency have been started, under the coordination of the National Reference Center for rare lung diseases in France (Respirare, http://www.respirare.fr).11 They should provide information on the types of paediatric DPLD that may benefit from AZM. Cellular and molecular studies are also ongoing to progress in the understanding of the mechanisms involved.
Learning points.
Steroids have been so far the main treatment of diffuse parenchymal lung disease due to surfactant deficiency in children with, however, a limited efficacy.
Azithromycin represents a new therapeutic strategy in these pathological situations, most likely through its ability to decrease the inflammatory burden and to favour alveolar parenchymal repair.
A long-term beneficial effect of azithromycin can be observed without side effects.
Acknowledgments
We thank Professor Hubert Ducou le Pointe for lung imaging, Professor Aurore Coulomb for pathological analysis, Dr Loïc Guillot for ABCA3 and phospholipids studies, Dr Michèle Boulé for lung functional tests and the Association ‘Respirer c'est Grandir’ and ‘Comite Belleherbe’.
Footnotes
Contributors: GT, NN, RE and AC wrote the manuscript. AC is in charge of the patient and reviewed the manuscript.
Competing interests: None.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Clement A, Nathan N, Epaud R, et al. Interstitial lung diseases in children. Orphanet J Rare Dis 2010;2013:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kanoh S, Rubin BK. Mechanisms of action and clinical application of macrolides as immunomodulatory medications. Clin Microbiol Rev 2010;2013:590–615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Flamein F, Riffault L, Muselet-Charlier C, et al. Molecular and cellular characteristics of ABCA3 mutations associated with diffuse parenchymal lung diseases in children. Hum Mol Genet 2012;2013:765–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hayes D, Jr, Lloyd EA, Fitch JA, et al. ABCA3 transporter deficiency. Am J Respir Crit Care Med 2012;2013:807. [DOI] [PubMed] [Google Scholar]
- 5.Li H, Zhou Y, Fan F, et al. Effect of azithromycin on patients with diffuse panbronchiolitis: retrospective study of 51 cases. Intern Med 2011;2013:1663–9 [DOI] [PubMed] [Google Scholar]
- 6.Guillot L, Tabary O, Nathan N, et al. Macrolides: new therapeutic perspectives in lung diseases. Int J Biochem Cell Biol 2011;2013:1241–6 [DOI] [PubMed] [Google Scholar]
- 7.Ribeiro CM, Hurd H, Wu Y, et al. Azithromycin treatment alters gene expression in inflammatory, lipid metabolism, and cell cycle pathways in well-differentiated human airway epithelia. PLoS ONE 2009;2013:e5806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Anderson N, Borlak J. Drug-induced phospholipidosis. FEBS Lett 2006;2013:5533–40 [DOI] [PubMed] [Google Scholar]
- 9.Rabinovich EI, Kapetanaki MG, Steinfeld I, et al. Global methylation patterns in idiopathic pulmonary fibrosis. PLoS ONE 2012;2013:e33770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li M, Zhong X, He Z, et al. Effect of erythromycin on cigarette-induced histone deacetylase protein expression and nuclear factor-kappaB activity in human macrophages in vitro. Int Immunopharmacol 2012;2013:643–50 [DOI] [PubMed] [Google Scholar]
- 11.Nathan N, Abou Taam R, Epaud R, et al. A national internet-linked based database for pediatric interstitial lung diseases: the French network. Orphanet J Rare Dis 2012;2013:40. [DOI] [PMC free article] [PubMed] [Google Scholar]


