Abstract
The purpose of this study is to delineate the immediate sources and fractional turnover of high density lipoprotein (HDL) esterified cholesterol in man. Various labeled preparations were administered in 11 experiments to six subjects who had either a complete bile fistula (maximally stimulated cholesterol metabolism) or an intact enterohepatic circulation. The administered tracers included [3H]mevalonic acid; [14C]cholesterol bound to albumin; low density lipoprotein (LDL) free [3H] or [14C]cholesterol; HDL free [3H] or [14C]cholesterol; HDL esterified [3H]cholesterol; and LDL esterified [3H]cholesterol. Blood samples were obtained at frequent intervals for up to 5 d after the administration of tracers. The mass and radioactivity in individual plasma lipoprotein (very low density lipoprotein [VLDL], HDL, and LDL) free and esterified cholesterol were determined.
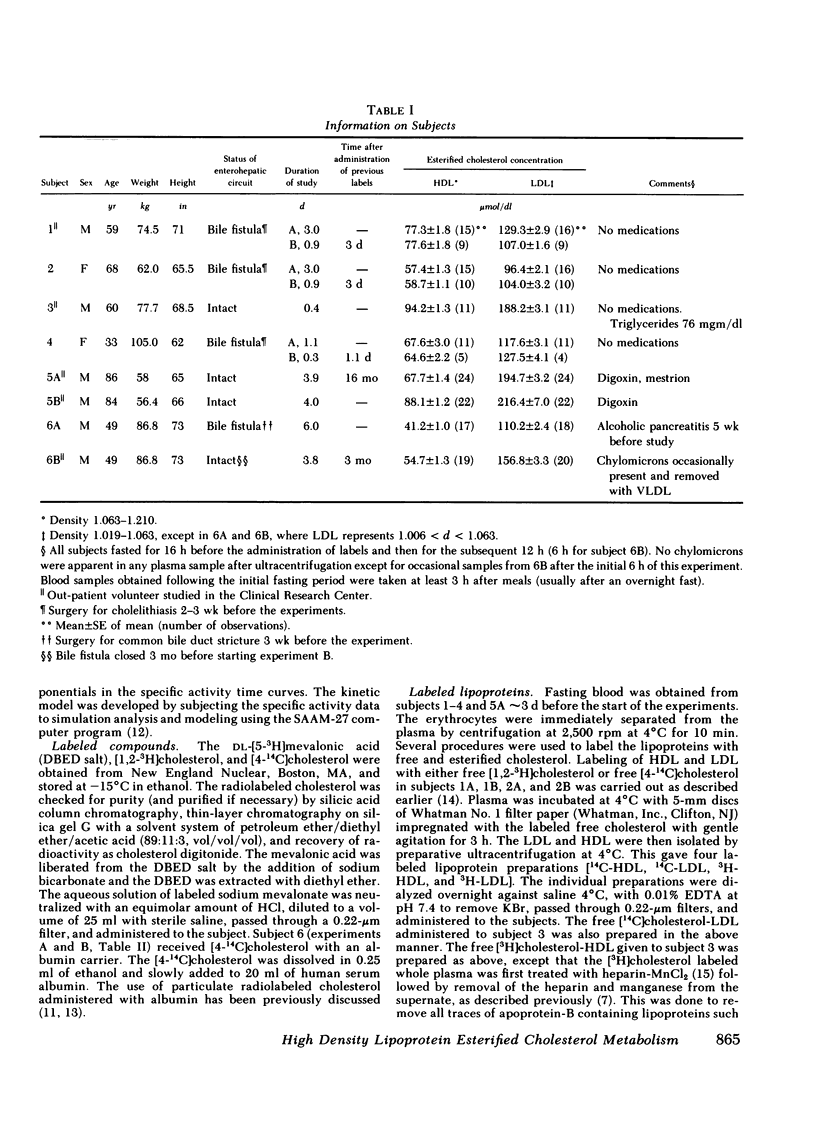
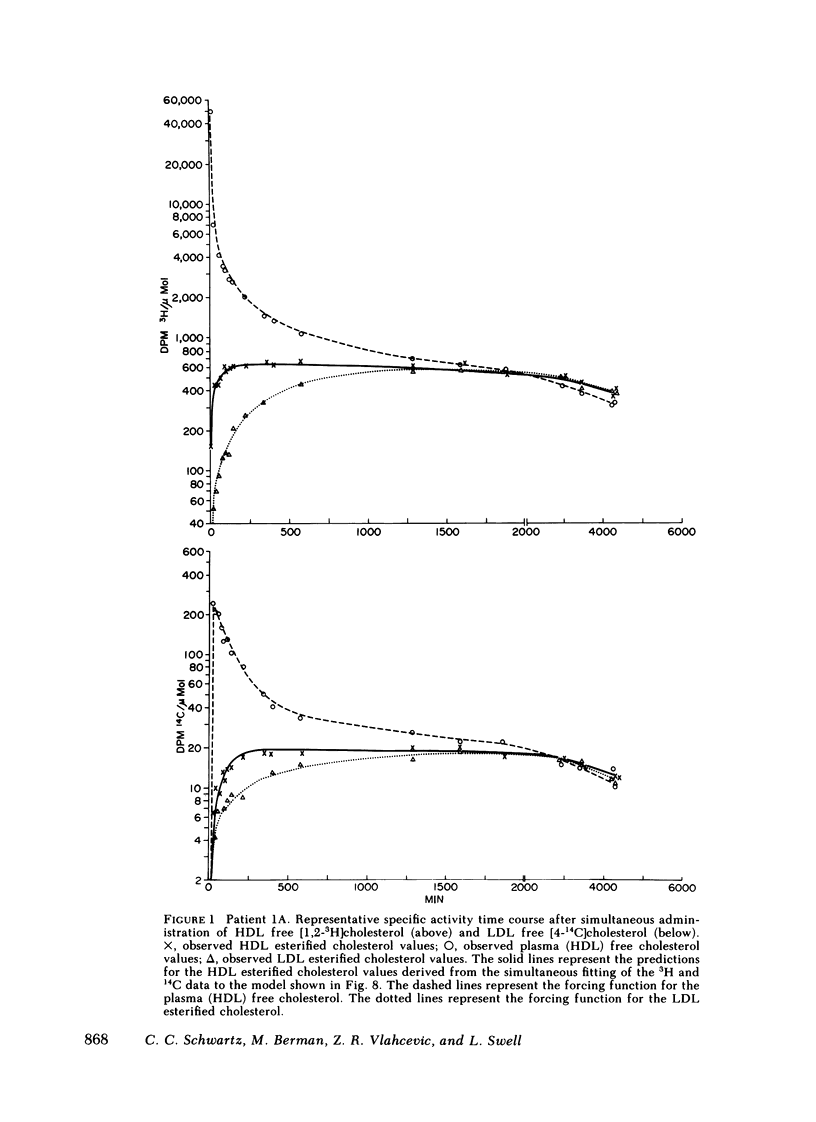
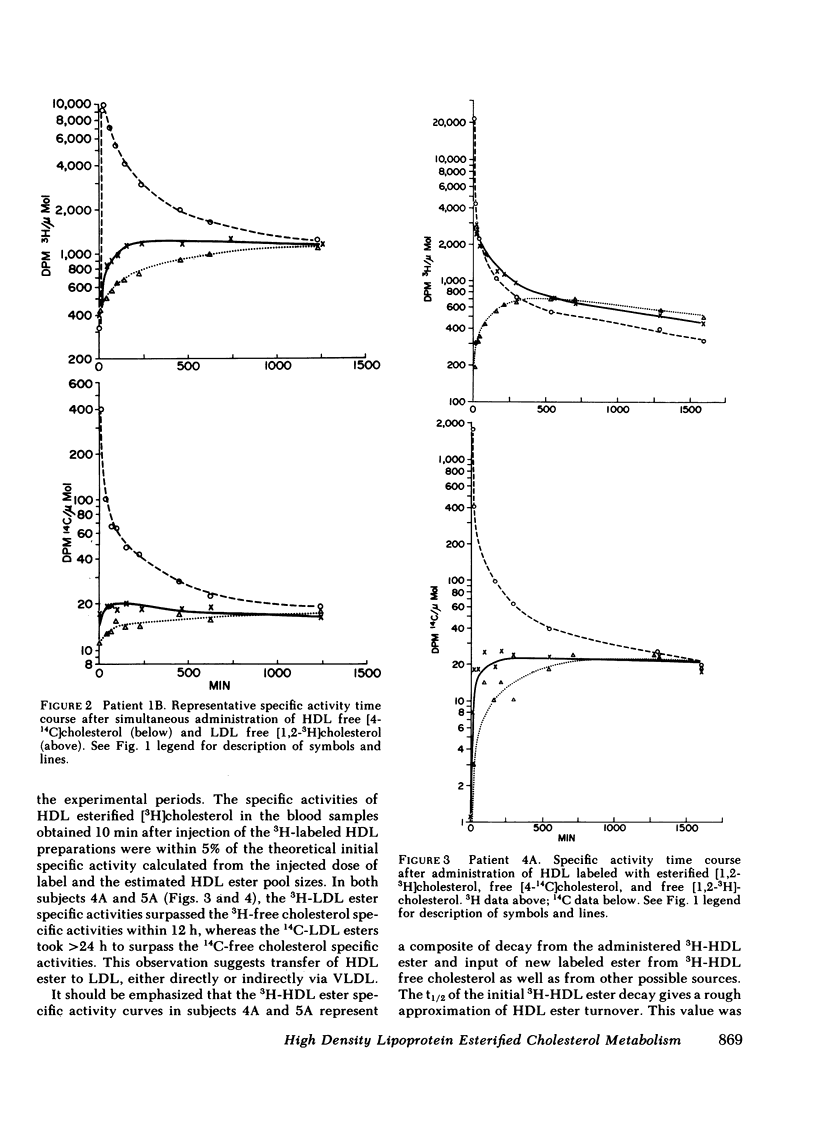
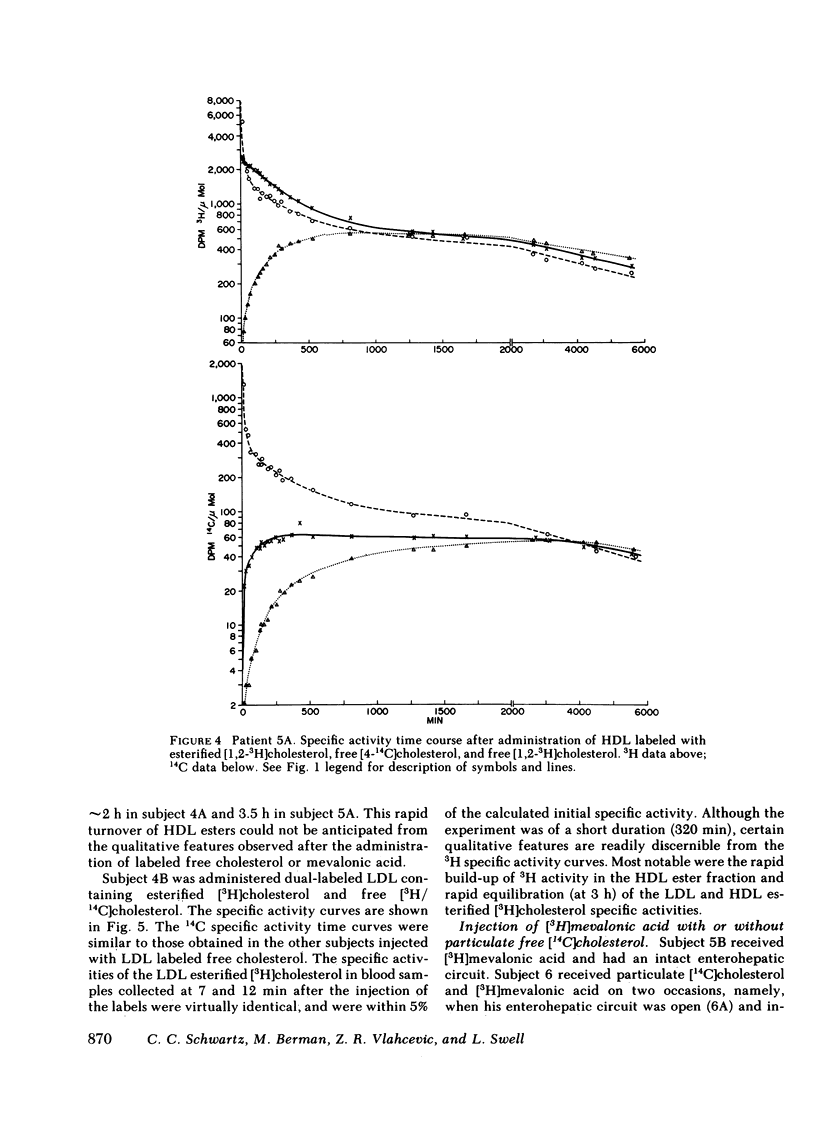
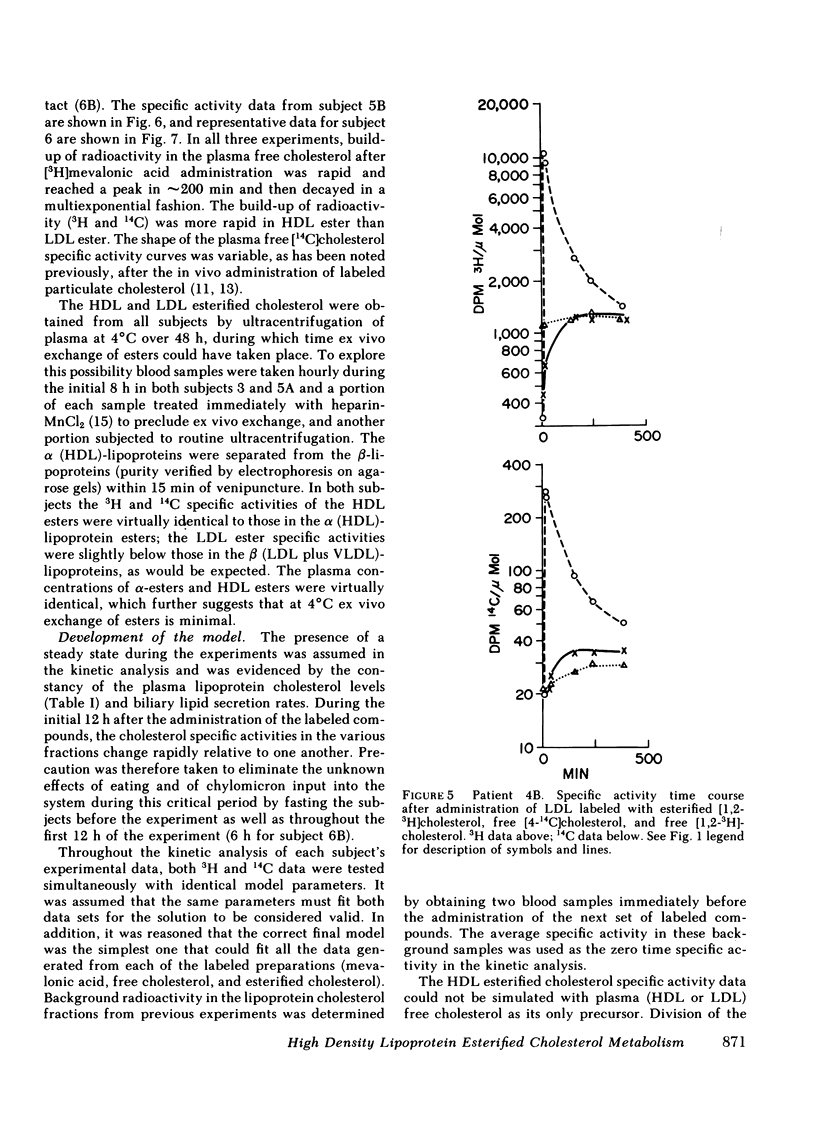
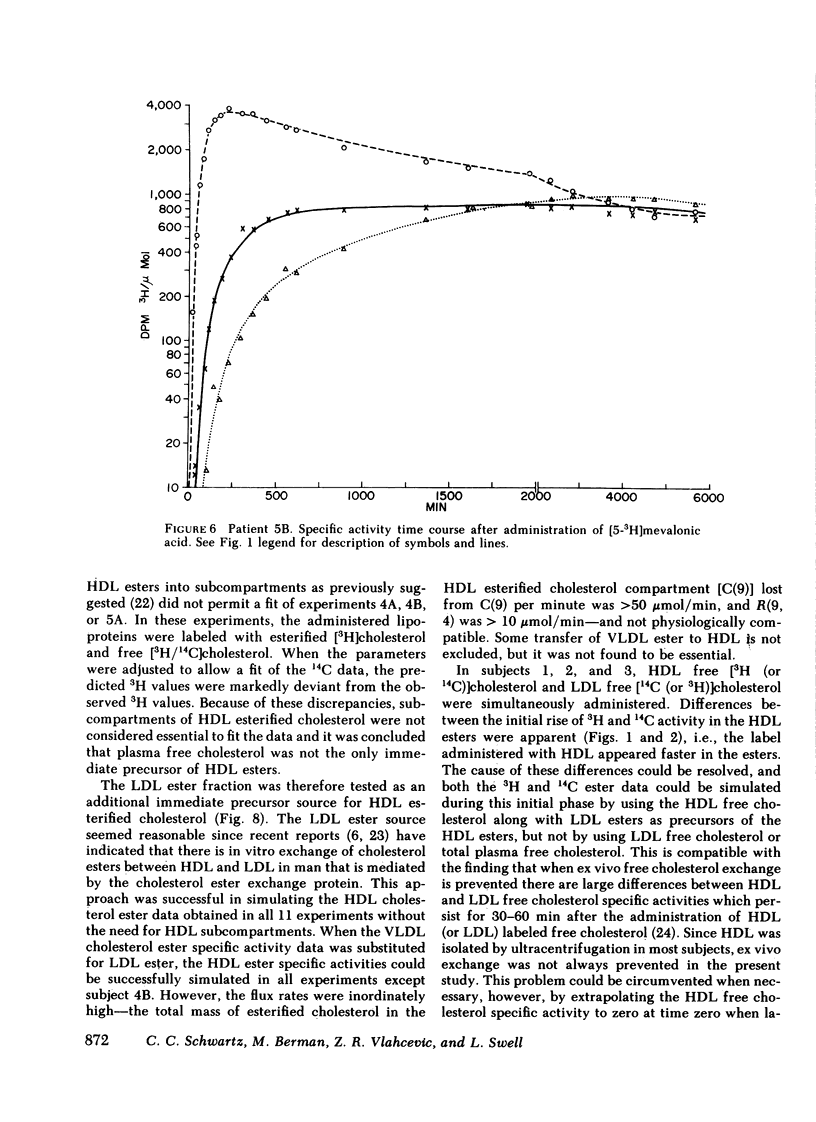
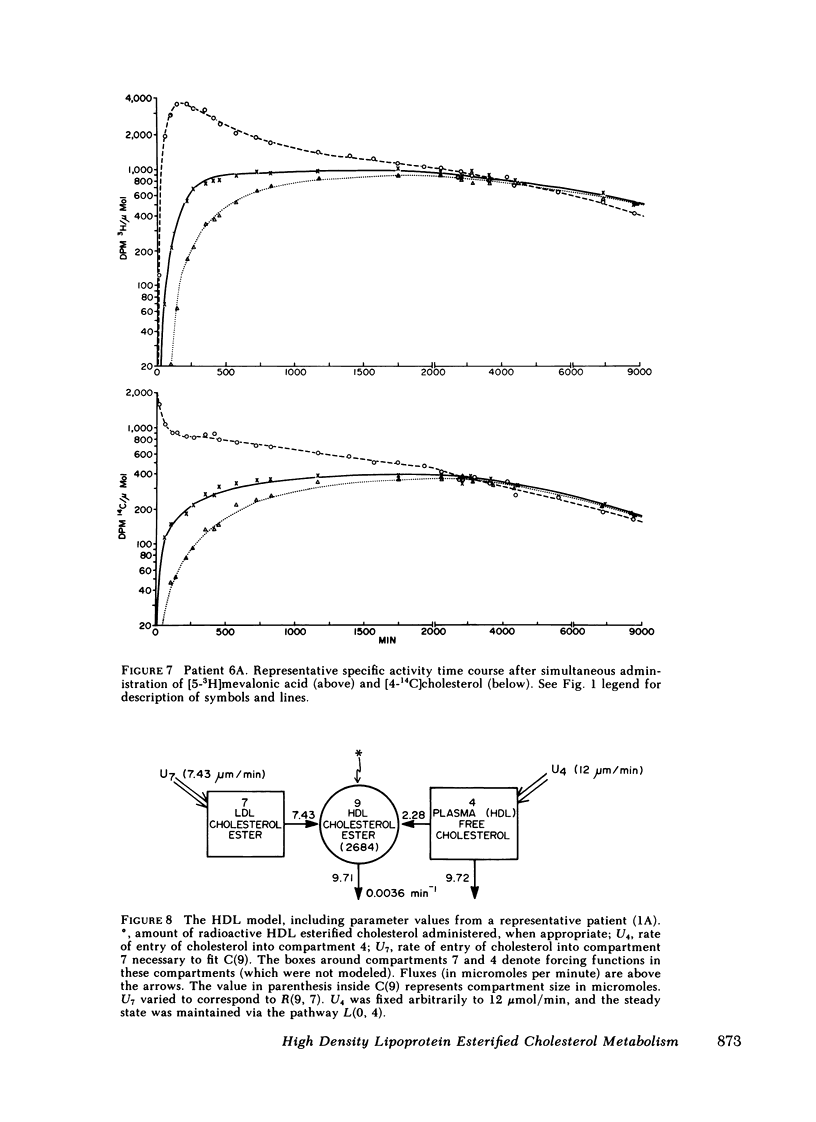
The data were subjected to multicompartmental analysis using the SAAM-27 computer program. The analysis revealed that plasma free cholesterol was not the only immediate source of either a single- or two-compartment HDL ester system. When LDL esters and plasma (HDL) free cholesterol were tested together as sources of one HDL ester compartment, data from all the experiments were readily fit.
The fluxes arrived at with the final model indicated that only ∼20% of the esterified cholesterol in HDL was newly synthesized from plasma (HDL) free cholesterol (2.36 μmol/min); the remaining 80% was from LDL ester (8.92 μmol/min). The presence of a bile fistula had no obvious effect on HDL esterified cholesterol metabolism. The rate of HDL cholesterol ester turnover was 3-12 times/d, indicating that the ester component of the HDL particle is in a very dynamic state.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barter P. J., Conner W. E. The transport of esterified cholesterol in plasma high density lipoproteins of human subjects: a mathematical model. J Lab Clin Med. 1976 Oct;88(4):627–639. [PubMed] [Google Scholar]
- Barter P. J., Jones M. E. Kinetic studies of the transfer of esterified cholesterol between human plasma low and high density lipoproteins. J Lipid Res. 1980 Feb;21(2):238–249. [PubMed] [Google Scholar]
- Barter P. J., Lally J. I. In vitro exchanges of esterified cholesterol between serum lipoprotein fractions: studies of humans and rabbits. Metabolism. 1979 Mar;28(3):230–236. doi: 10.1016/0026-0495(79)90068-4. [DOI] [PubMed] [Google Scholar]
- Barter P. J., Lally J. I. The metabolism of esterified cholesterol in rabbit plasma low density lipoproteins. Biochim Biophys Acta. 1979 Mar 29;572(3):510–518. doi: 10.1016/0005-2760(79)90158-9. [DOI] [PubMed] [Google Scholar]
- Blum C. B., Levy R. I., Eisenberg S., Hall M., 3rd, Goebel R. H., Berman M. High density lipoprotein metabolism in man. J Clin Invest. 1977 Oct;60(4):795–807. doi: 10.1172/JCI108833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown M. S., Kovanen P. T., Goldstein J. L. Regulation of plasma cholesterol by lipoprotein receptors. Science. 1981 May 8;212(4495):628–635. doi: 10.1126/science.6261329. [DOI] [PubMed] [Google Scholar]
- Burstein M., Scholnick H. R. Lipoprotein-polyanion-metal interactions. Adv Lipid Res. 1973;11(0):67–108. [PubMed] [Google Scholar]
- Chajek T., Fielding C. J. Isolation and characterization of a human serum cholesteryl ester transfer protein. Proc Natl Acad Sci U S A. 1978 Jul;75(7):3445–3449. doi: 10.1073/pnas.75.7.3445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FOLCH J., LEES M., SLOANE STANLEY G. H. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957 May;226(1):497–509. [PubMed] [Google Scholar]
- Fielding C. J., Fielding P. E. Purification and substrate specificity of lecithin-cholesterol acyl transferase from human plasma. FEBS Lett. 1971 Jul 8;15(5):355–358. doi: 10.1016/0014-5793(71)80333-2. [DOI] [PubMed] [Google Scholar]
- Fielding P. E., Fielding C. J. A cholesteryl ester transfer complex in human plasma. Proc Natl Acad Sci U S A. 1980 Jun;77(6):3327–3330. doi: 10.1073/pnas.77.6.3327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GOODMAN D. S. THE IN VIVO TURNOVER OF INDIVIDUAL CHOLESTEROL ESTERS IN HUMAN PLASMA LIPOPROTEINS. J Clin Invest. 1964 Nov;43:2026–2036. doi: 10.1172/JCI105077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glomset J. A., Norum K. R., King W. Plasma lipoproteins in familial lecithin: cholesterol acyltransferase deficiency: lipid composition and reactivity in vitro. J Clin Invest. 1970 Oct;49(10):1827–1837. doi: 10.1172/JCI106400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glomset J. A. The plasma lecithins:cholesterol acyltransferase reaction. J Lipid Res. 1968 Mar;9(2):155–167. [PubMed] [Google Scholar]
- Goodman D. S., Noble R. P. Cholesteryl ester turnover in human plasma lipoproteins during cholestyramine and clofibrate therapy. J Lipid Res. 1970 May;11(3):183–189. [PubMed] [Google Scholar]
- HAVEL R. J., EDER H. A., BRAGDON J. H. The distribution and chemical composition of ultracentrifugally separated lipoproteins in human serum. J Clin Invest. 1955 Sep;34(9):1345–1353. doi: 10.1172/JCI103182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HIRSCH J., AHRENS E. H., Jr The separation of complex lipide mixtures by the use of silicic acid chromatography. J Biol Chem. 1958 Aug;233(2):311–20. [PubMed] [Google Scholar]
- Ha Y. C., Calvert G. D., McIntosh G. H., Barter P. J. A physiologic role for the esterified cholesterol transfer protein: in vivo studies in rabbits and pigs. Metabolism. 1981 Apr;30(4):380–383. doi: 10.1016/0026-0495(81)90119-0. [DOI] [PubMed] [Google Scholar]
- Heinen R. J., Herbert P. N., Fredrickson D. S. Properties of the plasma very low and low density lipoproteins in Tangier disease. J Clin Invest. 1978 Jan;61(1):120–132. doi: 10.1172/JCI108910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heller F. R., Desager J. P., Harvengt C. Plasma lipid concentrations and lecithin:cholesterol acyltransferase activity in normolipidemic subjects given fenofibrate and colestipol. Metabolism. 1981 Jan;30(1):67–71. doi: 10.1016/0026-0495(81)90221-3. [DOI] [PubMed] [Google Scholar]
- Myant N. B., Balasubramaniam S., Moutafis C. D., Mancini M., Slack J. Turnover of cholesteryl esters in plasma low-density and high-density lipoproteins in familial hyperbetalipoproteinaemia. Clin Sci Mol Med. 1973 Oct;45(4):551–560. doi: 10.1042/cs0450551. [DOI] [PubMed] [Google Scholar]
- Nestel P. J., Reardon M., Billington T. In vivo transfer of cholesteryl esters from high density lipoproteins to very low density lipoproteins in man. Biochim Biophys Acta. 1979 May 25;573(2):403–407. doi: 10.1016/0005-2760(79)90073-0. [DOI] [PubMed] [Google Scholar]
- Nilsson-Ehle P., Garfinkel A. S., Schotz M. C. Lipolytic enzymes and plasma lipoprotein metabolism. Annu Rev Biochem. 1980;49:667–693. doi: 10.1146/annurev.bi.49.070180.003315. [DOI] [PubMed] [Google Scholar]
- Nilsson A., Zilversmit D. B. Fate of intravenously administered particulate and lipoprotein cholesterol in the rat. J Lipid Res. 1972 Jan;13(1):32–38. [PubMed] [Google Scholar]
- Noble R. P. Electrophoretic separation of plasma lipoproteins in agarose gel. J Lipid Res. 1968 Nov;9(6):693–700. [PubMed] [Google Scholar]
- Pattnaik N. M., Montes A., Hughes L. B., Zilversmit D. B. Cholesteryl ester exchange protein in human plasma isolation and characterization. Biochim Biophys Acta. 1978 Sep 28;530(3):428–438. doi: 10.1016/0005-2760(78)90163-7. [DOI] [PubMed] [Google Scholar]
- SPERRY W. M., WEBB M. A revision of the Schoenheimer-Sperry method for cholesterol determination. J Biol Chem. 1950 Nov;187(1):97–106. [PubMed] [Google Scholar]
- Schwartz C. C., Berman M., Vlahcevic Z. R., Halloran L. G., Gregory D. H., Swell L. Multicompartmental analysis of cholesterol metabolism in man. Characterization of the hepatic bile acid and biliary cholesterol precursor sites. J Clin Invest. 1978 Feb;61(2):408–423. doi: 10.1172/JCI108952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz C. C., Halloran L. G., Vlahcevic Z. R., Gregory D. H., Swell L. Preferential utilization of free cholesterol from high-density lipoproteins for biliary cholesterol secretion in man. Science. 1978 Apr 7;200(4337):62–64. doi: 10.1126/science.204996. [DOI] [PubMed] [Google Scholar]
- Schwartz C. C., Vlahcevic Z. R., Berman M., Meadows J. G., Nisman R. M., Swell L. Central role of high density lipoprotein in plasma free cholesterol metabolism. J Clin Invest. 1982 Jul;70(1):105–116. doi: 10.1172/JCI110582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz C. C., Vlahcevic Z. R., Halloran L. G., Swell L. An in vivo evaluation in man of the transfer of esterified cholesterol between lipoproteins and into the liver and bile. Biochim Biophys Acta. 1981 Jan 26;663(1):143–162. doi: 10.1016/0005-2760(81)90201-0. [DOI] [PubMed] [Google Scholar]
- Sniderman A., Teng B., Vezina C., Marcel Y. L. Cholesterol ester exchange between human plasma high and low density lipoproteins mediated by a plasma protein factor. Atherosclerosis. 1978 Nov;31(3):327–333. doi: 10.1016/0021-9150(78)90067-9. [DOI] [PubMed] [Google Scholar]



