Abstract
Although phenol-extracted gram-negative bacterial lipopolysaccharides (LPS) have been used to study the properties of endotoxins for many years, nothing is known about the behavior of native (unextracted) LPS in vivo. Accordingly, we have compared extracted and native forms of LPS with regard to their biological activity, their ability to bind to plasma high density lipoproteins (HDL), and their fate after intravenous injection into rats. The LPS of Salmonella typhimurium G-30 were labeled with [3H]galactose, and whole bacteria, bacterial outer membranes, outer membrane fragments (harvested from the bacterial culture supernatant), and phenol extracts of the bacteria were prepared. After defining the LPS, phospholipid, and protein composition of these preparations, we compared the activity of the LPS in phenol extracts and membrane fragments in two assays. In both the limulus lysate assay and the rabbit pyrogen test, the LPS in phenol extracts were slightly more potent than the LPS in membrane fragments. We next studied the ability of the LPS in each preparation to bind to rat lipoproteins in vitro, and each preparation was then injected intravenously into rats for measurements of LPS-HDL binding and tissue uptake in vivo. Two patterns of lipoprotein binding were observed. Less than 25% of the LPS in both outer membranes and whole bacteria bound to HDL in vitro. When the outer membranes and whole bacteria were injected into rats, their LPS again bound poorly to HDL and they were rapidly removed from plasma into the liver and spleen. In contrast, >50% of the LPS in both culture supernatant membrane fragments and phenol-water extracts bound to HDL in vitro. When these preparations were injected into rats, ∼50% of the LPS in the membrane fragments and phenol-water extracts bound to HDL and remained in the plasma over the 10-min study period. Moreover, the LPS in these preparations accumulated in the ovary and the adrenal gland, two tissues that use HDL-cholesterol for hormone synthesis. Binding to HDL thus greatly influenced the plasma half-life and tissue uptake of both extracted and native LPS.
We conclude that extraction of S. typhimurium LPS with phenol does not significantly alter the biological activity or the lipoprotein binding behavior of the LPS and that the in vivo fates of phenol-extracted and membrane fragment LPS are essentially identical. The results thus provide important support for many previous studies that have used phenol-extracted LPS to mimic the activities of native LPS in vivo. However, the only native LPS that resembled the behavior of extracted LPS were the LPS that had been shed from the bacteria in fragments of membrane that had reduced amounts of protein and phospholipid. Removal of LPS from other outer membrane constituents, whether by chemical extraction or by a natural process of surface shedding, thus alters the behavior of the LPS; the most important feature of this alteration appears to be the ability of these LPS to bind readily to HDL.
Full text
PDF





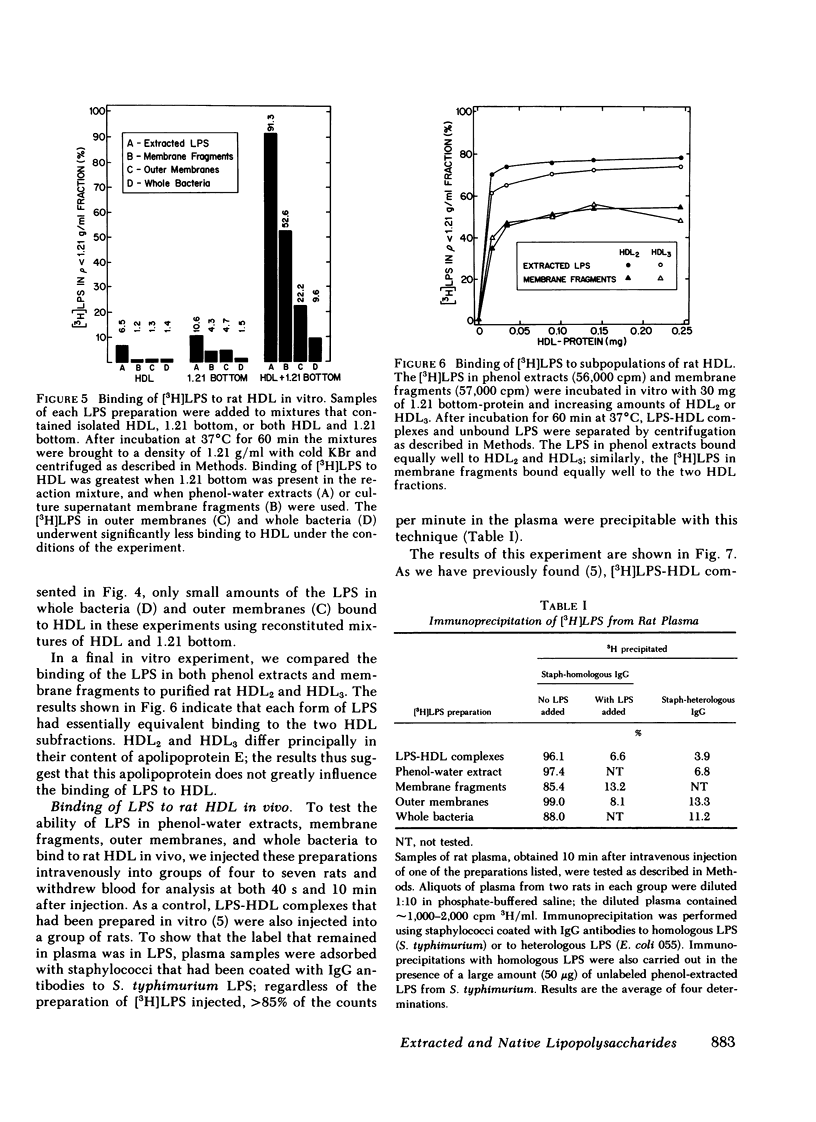

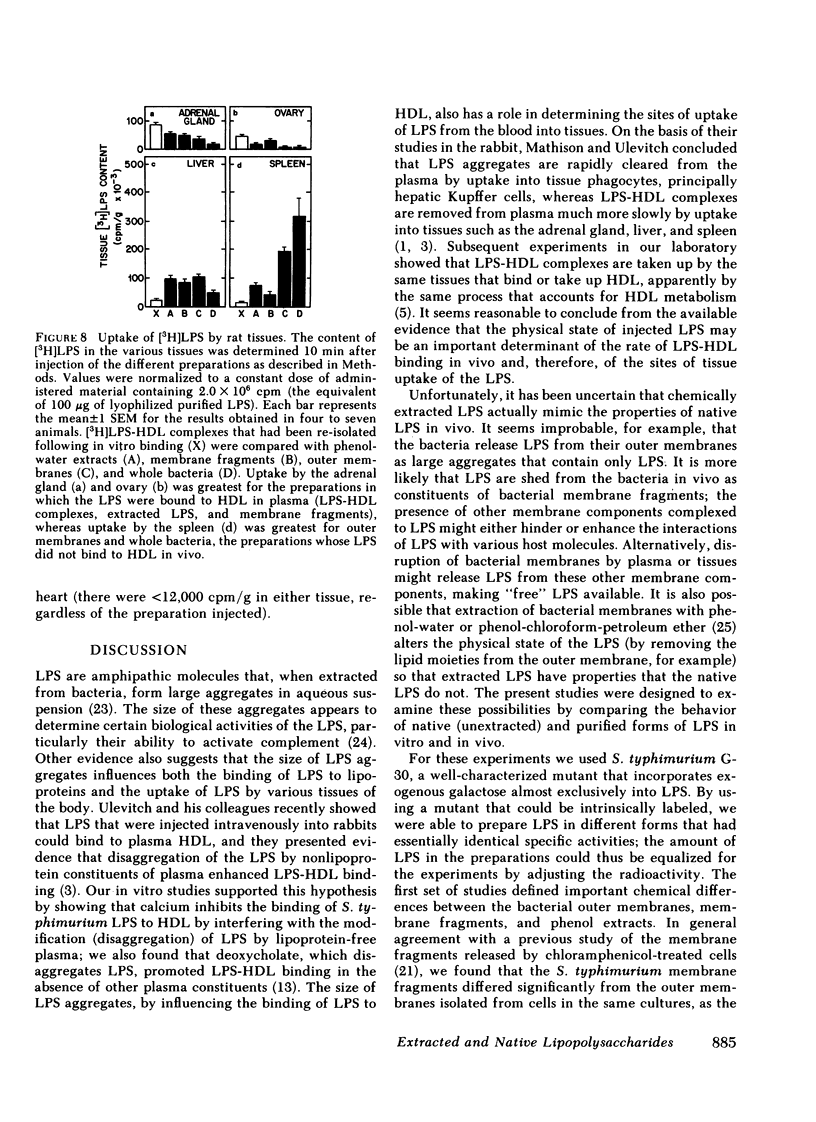



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ames G. F. Lipids of Salmonella typhimurium and Escherichia coli: structure and metabolism. J Bacteriol. 1968 Mar;95(3):833–843. doi: 10.1128/jb.95.3.833-843.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berlin R. D., Fera J. P., Pfeiffer J. R. Reversible phagocytosis in rabbit polymorphonuclear leukocytes. J Clin Invest. 1979 Jun;63(6):1137–1144. doi: 10.1172/JCI109407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berman N. S., Siegel S. E., Nachum R., Lipsey A., Leedom J. Cerebrospinal fluid endotoxin concentrations in gram-negative bacterial meningitis. J Pediatr. 1976 Apr;88(4 Pt 1):553–556. doi: 10.1016/s0022-3476(76)80004-2. [DOI] [PubMed] [Google Scholar]
- Cook J. A., Wise W. C., Halushka P. V. Elevated thromboxane levels in the rat during endotoxic shock: protective effects of imidazole, 13-azaprostanoic acid, or essential fatty acid deficiency. J Clin Invest. 1980 Jan;65(1):227–230. doi: 10.1172/JCI109655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crutchley M. J., Marsh D. G., Cameron J. Biological studies on free endotoxin and a non-toxic material from culture supernatant fluids of Escherichia coli 078K80. J Gen Microbiol. 1968 Mar;50(3):413–420. doi: 10.1099/00221287-50-3-413. [DOI] [PubMed] [Google Scholar]
- Crutchley M. J., Marsh D. G., Cameron J. Free Endotoxin. Nature. 1967 Jun 3;214(5092):1052–1052. doi: 10.1038/2141052a0. [DOI] [PubMed] [Google Scholar]
- Elin R. J., Sandberg A. L., Rosentreich D. L. Comparison of the pyrogenicity, Limulus activity mitogenicity and complement reactivity of several bacterial endotoxins and related compounds. J Immunol. 1976 Oct;117(4):1238–1242. [PubMed] [Google Scholar]
- Freudenberg M. A., Bøg-Hansen T. C., Back U., Galanos C. Interaction of lipopolysaccharides with plasma high-density lipoprotein in rats. Infect Immun. 1980 May;28(2):373–380. doi: 10.1128/iai.28.2.373-380.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galanos C., Lüderitz O., Westphal O. A new method for the extraction of R lipopolysaccharides. Eur J Biochem. 1969 Jun;9(2):245–249. doi: 10.1111/j.1432-1033.1969.tb00601.x. [DOI] [PubMed] [Google Scholar]
- Goto Y., Baez S., Orkin L. R. The effect of endotoxin on microcirculatory and lymphatic dynamics in the rat (Shwartzman phenomenon). Circ Shock. 1981;8(5):533–542. [PubMed] [Google Scholar]
- Jorgensen J. H., Lee J. C. Rapid diagnosis of gram-negative bacterial meningitis by the Limulus endotoxin assay. J Clin Microbiol. 1978 Jan;7(1):12–17. doi: 10.1128/jcm.7.1.12-17.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler S. W. Rapid isolation of antigens from cells with a staphylococcal protein A-antibody adsorbent: parameters of the interaction of antibody-antigen complexes with protein A. J Immunol. 1975 Dec;115(6):1617–1624. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Markwell M. A., Haas S. M., Bieber L. L., Tolbert N. E. A modification of the Lowry procedure to simplify protein determination in membrane and lipoprotein samples. Anal Biochem. 1978 Jun 15;87(1):206–210. doi: 10.1016/0003-2697(78)90586-9. [DOI] [PubMed] [Google Scholar]
- Mathison J. C., Ulevitch R. J. The clearance, tissue distribution, and cellular localization of intravenously injected lipopolysaccharide in rabbits. J Immunol. 1979 Nov;123(5):2133–2143. [PubMed] [Google Scholar]
- Milner K. C., Finkelstein R. A. Bioassay of endotoxin: correlation between pyrogenicity for rabbits and lethality for chick embryos. J Infect Dis. 1966 Dec;116(5):529–536. doi: 10.1093/infdis/116.5.529. [DOI] [PubMed] [Google Scholar]
- Munford R. S., Andersen J. M., Dietschy J. M. Sites of tissue binding and uptake in vivo of bacterial lipopolysaccharide-high density lipoprotein complexes: studies in the rat and squirrel monkey. J Clin Invest. 1981 Dec;68(6):1503–1513. doi: 10.1172/JCI110404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munford R. S., Hall C. L., Dietschy J. M. Binding of Salmonella typhimurium lipopolysaccharides to rat high-density lipoproteins. Infect Immun. 1981 Dec;34(3):835–843. doi: 10.1128/iai.34.3.835-843.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munford R. S., Hall C. L. Radioimmunoassay for Gram-negative bacterial lipopolysaccharide O antigens: influence of antigen solubility. Infect Immun. 1979 Oct;26(1):42–48. doi: 10.1128/iai.26.1.42-48.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munford R. S., Hall C. L., Rick P. D. Size heterogeneity of Salmonella typhimurium lipopolysaccharides in outer membranes and culture supernatant membrane fragments. J Bacteriol. 1980 Nov;144(2):630–640. doi: 10.1128/jb.144.2.630-640.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munford R. S. Quantitative Limulus lysate assay for endotoxin activity: aggregation of radioiodinated coagulogen monomers. Anal Biochem. 1978 Dec;91(2):509–515. doi: 10.1016/0003-2697(78)90537-7. [DOI] [PubMed] [Google Scholar]
- OSBORN M. J., ROSEN S. M., ROTHFIELD L., HORECKER B. L. Biosynthesis of bacterial lipopolysaccharide. I. Enzymatic incorporation of galactose in a mutant strain of Salmonella. Proc Natl Acad Sci U S A. 1962 Oct 15;48:1831–1838. doi: 10.1073/pnas.48.10.1831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osborn M. J., Gander J. E., Parisi E. Mechanism of assembly of the outer membrane of Salmonella typhimurium. Site of synthesis of lipopolysaccharide. J Biol Chem. 1972 Jun 25;247(12):3973–3986. [PubMed] [Google Scholar]
- Osborn M. J., Rick P. D., Rasmussen N. S. Mechanism of assembly of the outer membrane of Salmonella typhimurium. Translocation and integration of an incomplete mutant lipid A into the outer membrane. J Biol Chem. 1980 May 10;255(9):4246–4251. [PubMed] [Google Scholar]
- ROGERS D. E., MELLY M. A. Studies on bacteriemia. III. The blood stream clearance of Escherichia coli in rabbits. J Exp Med. 1957 Feb 1;105(2):113–124. doi: 10.1084/jem.105.2.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rickles F. R., Levin J., Rosenthal D. I., Atkins E. Functional interaction of concanavalin A and bacterial endotoxin (lipopolysaccharide): effects on the measurement of endogenous pyrogen release, human mononuclear cell tissue factor activation, lymphocyte DNA synthesis, and gelation of Limulus amebocyte lysate. J Lab Clin Med. 1979 Jan;93(1):128–145. [PubMed] [Google Scholar]
- Rothfield L., Pearlman-Kothencz M. Synthesis and assembly of bacterial membrane components. A lipopolysaccharide-phospholipid-protein complex excreted by living bacteria. J Mol Biol. 1969 Sep 28;44(3):477–492. doi: 10.1016/0022-2836(69)90374-x. [DOI] [PubMed] [Google Scholar]
- Tai J. Y., Seid R. C., Jr, Huhn R. D., Liu T. Y. Studies on Limulus amoebocyte lysate II. Purification of the coagulogen and the mechanism of clotting. J Biol Chem. 1977 Jul 25;252(14):4773–4776. [PubMed] [Google Scholar]
- Tomasulo P. A., Levin J., Murphy P. A., Winkelstein J. A. Biological activities of tritiated endotoxins: correlation of the Limulus lysate assay with rabbit pyrogen and complement-activation assays for endotoxin. J Lab Clin Med. 1977 Feb;89(2):308–315. [PubMed] [Google Scholar]
- Ulevitch R. J., Johnston A. R., Weinstein D. B. New function for high density lipoproteins. Isolation and characterization of a bacterial lipopolysaccharide-high density lipoprotein complex formed in rabbit plasma. J Clin Invest. 1981 Mar;67(3):827–837. doi: 10.1172/JCI110100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulevitch R. J., Johnston A. R., Weinstein D. B. New function for high density lipoproteins. Their participation in intravascular reactions of bacterial lipopolysaccharides. J Clin Invest. 1979 Nov;64(5):1516–1524. doi: 10.1172/JCI109610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiser M. M., Rothfield L. The reassociation of lipopolysaccharide, phospholipid, and transferase enzymes of the bacterial cell envelope. Isolation of binary and ternary complexes. J Biol Chem. 1968 Mar 25;243(6):1320–1328. [PubMed] [Google Scholar]
- Zimmer J. A., Lipton J. M. Central and peripheral injections of ACTH (1-24) reduce fever in adrenalectomized rabbits. Peptides. 1981 Winter;2(4):413–417. doi: 10.1016/s0196-9781(81)80097-6. [DOI] [PubMed] [Google Scholar]



