Abstract
Studies on the feedback inhibition of ACTH release by steroid hormones and on the binding of tritiated steroids by the pituitary have prompted the hypothesis that receptors in addition to or other than classical glucocorticoid receptors may mediate steroid hormone effects in this tissue. Accordingly, we have asked whether more than one glucocorticoid-binding species, distinct from corticosteroid binding globulin, can be found in rat anterior pituitary gland.
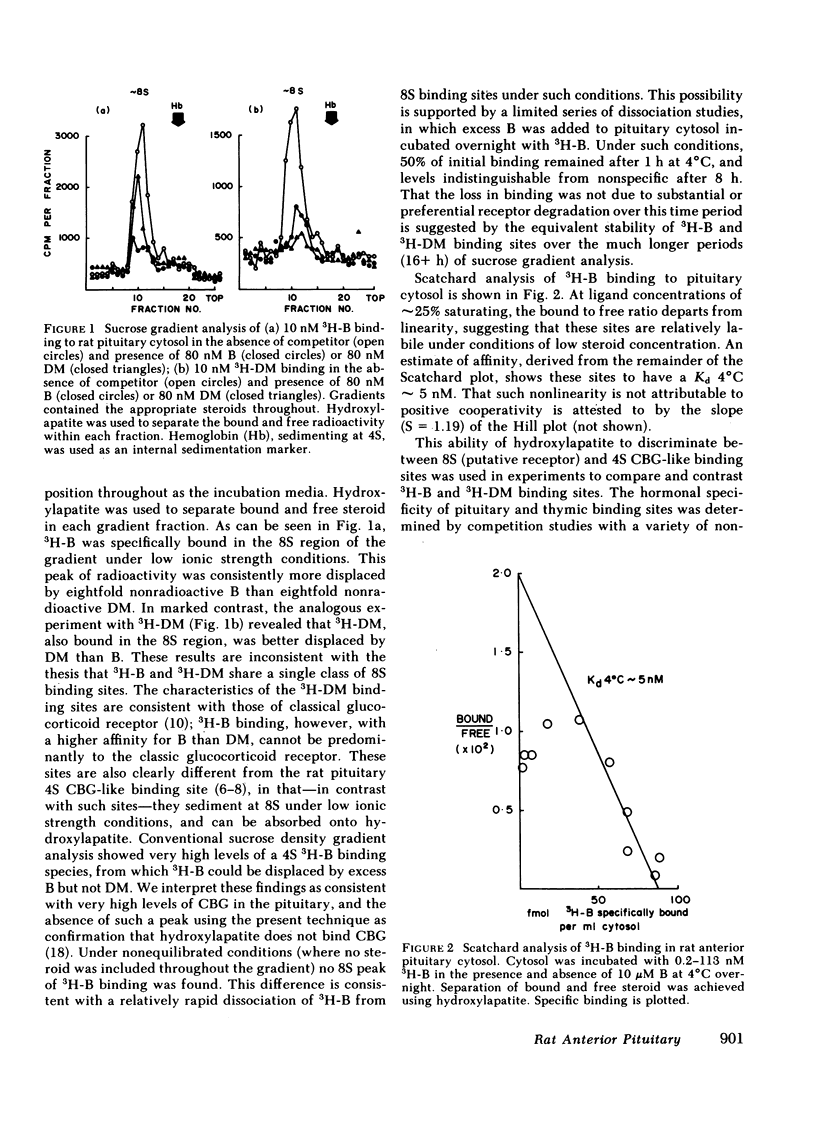
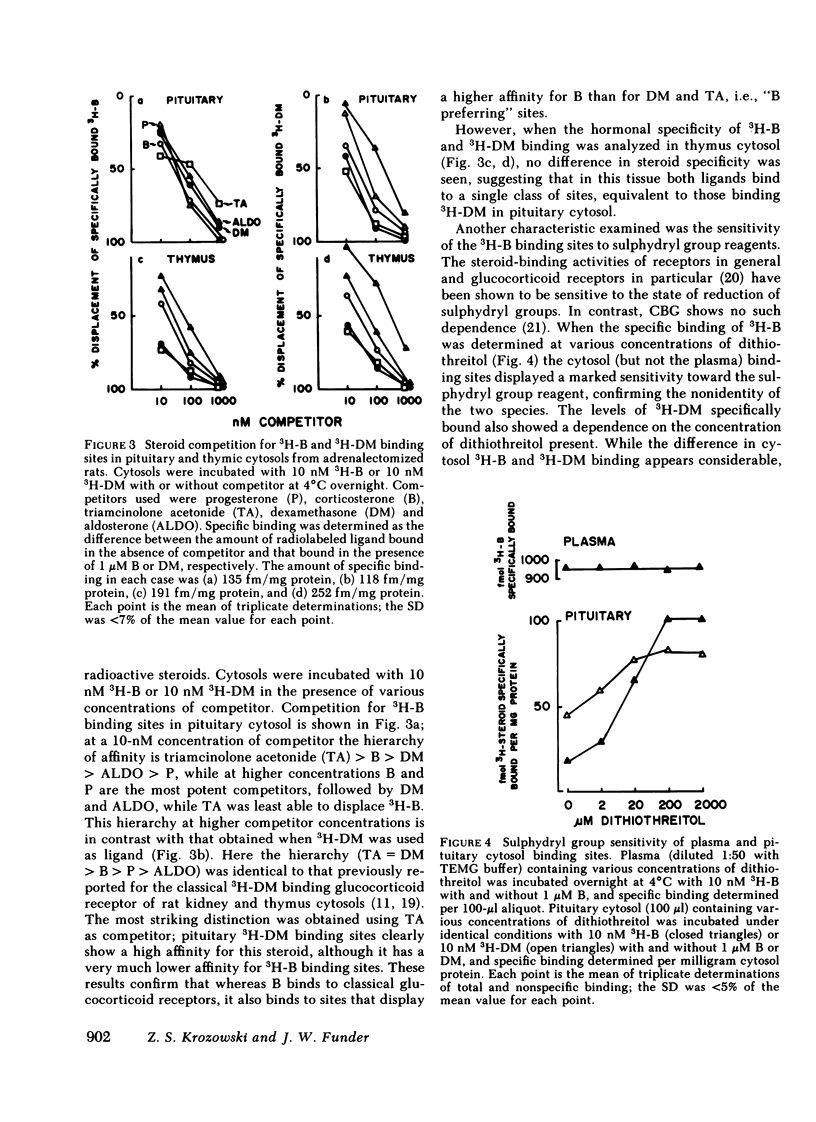
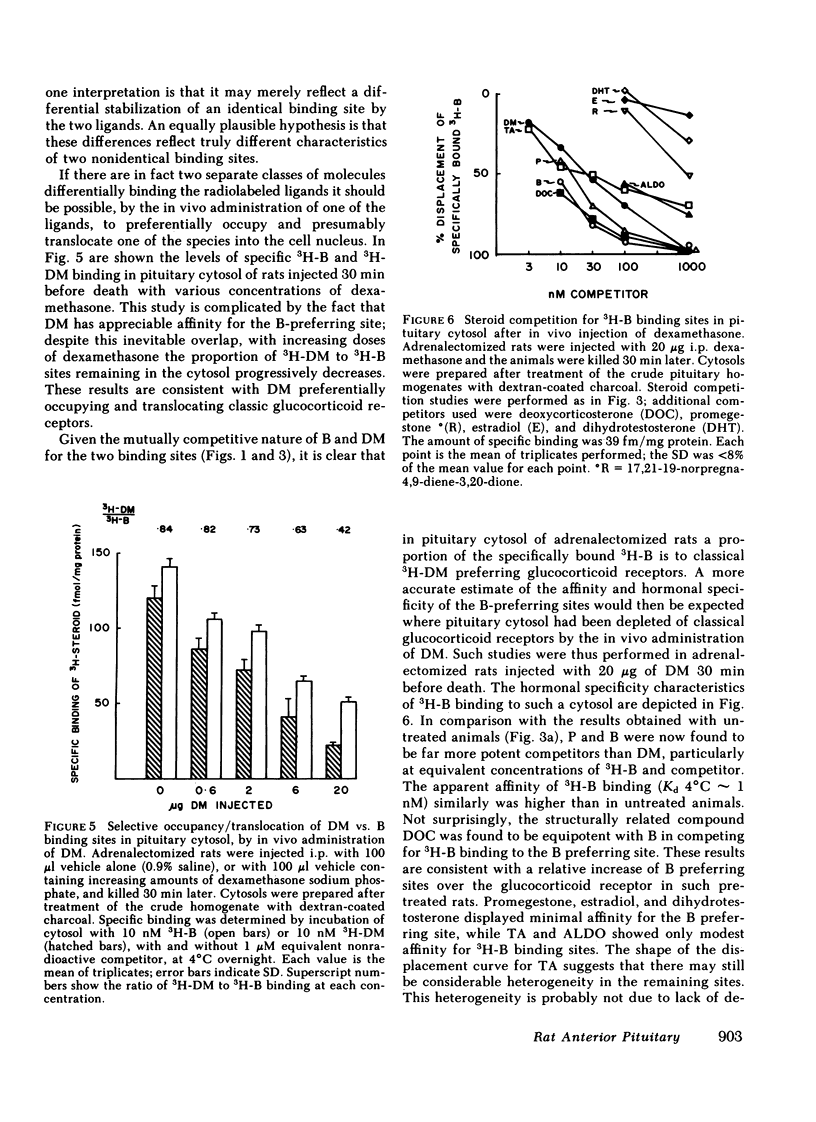
In our study we have demonstrated high affinity (Kd 4°C ∼ 1 nM) binding sites for tritiated corticosterone (3H-B) in rat pituitary cytosol, distinct from classical glucocorticoid receptors and transcortin-like sites. Unlike 3H-B-transcortin complexes, 3H-B bound to such sites is adsorbed onto hydroxylapatite and is stabilized by sulphydryl group reducing agents. Sucrose density gradient analysis in low ionic strength buffer under equilibrium conditions (3H-B±nonradioactive competitors throughout) showed 3H-B to sediment as a single, ∼8S peak, from which 3H-B was consistently better displaced by B than dexamethasone (DM); 3H-DM similarly bound to an ∼8S peak, from which it was better displaced by DM than B. The existence of two species of pituitary glucocorticoid receptors is further supported by clear differences in specificity for a range of steroids, and in the differential depletion of cytoplasmic sites after in vivo DM administration. Similar “B-preferring” sites were not found in thymus cytosols. These results demonstrate that there exist in the pituitary high affinity intracellular binding sites for naturally occurring glucocorticoids, distinct from classical glucocorticoid receptors and transcortin-like sites. Physiological roles as glucocorticoid receptors remain to be established for these B-preferring sites.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baxter J. D., Harris A. W., Tomkins G. M., Cohn M. Glucocorticoid receptors in lymphoma cells in culture: relationship to glucocorticoid killing activity. Science. 1971 Jan 15;171(3967):189–191. doi: 10.1126/science.171.3967.189. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- De Kloet E. R., McEwen B. S. A putative glucocorticoid receptor and a transcortin-like macromolecule in pituitary cytosol. Biochim Biophys Acta. 1976 Jan 14;421(1):115–123. doi: 10.1016/0304-4165(76)90175-6. [DOI] [PubMed] [Google Scholar]
- De Kloet R., Wallach G., McEwen B. S. Differences in corticosterone and dexamethasone binding to rat brain and pituitary. Endocrinology. 1975 Mar;96(3):598–609. doi: 10.1210/endo-96-3-598. [DOI] [PubMed] [Google Scholar]
- Duncan M. R., Duncan G. R. An in vivo study of the action of antiglucocorticoids on thymus weight ratio, antibody titre and the adrenal-pituitary-hypothalamus axis. J Steroid Biochem. 1979 Mar;10(3):245–259. doi: 10.1016/0022-4731(79)90250-4. [DOI] [PubMed] [Google Scholar]
- Fehm H. L., Voigt K. H., Kummer G., Lang R., Pfeiffer E. F. Differential and integral corticosteroid feedback effects on ACTH secretion in hypoadrenocorticism. J Clin Invest. 1979 Feb;63(2):247–253. doi: 10.1172/JCI109296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman D., Funder J. W., Edelman I. S. Evidence for a new class of corticosterone receptors in the rat kidney. Endocrinology. 1973 May;92(5):1429–1441. doi: 10.1210/endo-92-5-1429. [DOI] [PubMed] [Google Scholar]
- Funder J. W., Feldman D., Edelman I. S. Glucocorticoid receptors in rat kidney: the binding of tritiated-dexamethasone. Endocrinology. 1973 Apr;92(4):1005–1013. doi: 10.1210/endo-92-4-1005. [DOI] [PubMed] [Google Scholar]
- Funder J. W., Feldman D., Edelman I. S. The roles of plasma binding and receptor specificity in the mineralocorticoid action of aldosterone. Endocrinology. 1973 Apr;92(4):994–1004. doi: 10.1210/endo-92-4-994. [DOI] [PubMed] [Google Scholar]
- Granberg J. P., Ballard P. L. The role of sulfhydryl groups in the binding of glucocorticoids by cytoplasmic receptors of lung and other mammalian tissues. Endocrinology. 1977 Apr;100(4):1160–1168. doi: 10.1210/endo-100-4-1160. [DOI] [PubMed] [Google Scholar]
- Harrison R. W., Fairfield S., Orth D. N. Multiple glucocorticoid binding components of intact AtT-20/D-1 mouse pituitary tumor cells. Biochim Biophys Acta. 1976 Sep 24;444(2):487–496. doi: 10.1016/0304-4165(76)90392-5. [DOI] [PubMed] [Google Scholar]
- Jones M. T., Hillhouse E. W. Structure-activity relationship and the mode of action of corticosteroid feedback on the secretion of corticotrophin-releasing factor (corticoliberin). J Steroid Biochem. 1976 Nov-Dec;7(11-12):1189–1202. doi: 10.1016/0022-4731(76)90054-6. [DOI] [PubMed] [Google Scholar]
- Koch B., Lutz-Bucher B., Briaud B., Mialhe C. Specific interaction of corticosteroids with binding sites in the plasma membranes of the rat anterior pituitary gland. J Endocrinol. 1978 Nov;79(2):215–222. doi: 10.1677/joe.0.0790215. [DOI] [PubMed] [Google Scholar]
- Koch B., Lutz B., Briaud B., Mialhe C. Heterogeneity of pituitary glucocorticoid binding evidence for a transcortin-like compound. Biochim Biophys Acta. 1976 Sep 24;444(2):497–507. doi: 10.1016/0304-4165(76)90393-7. [DOI] [PubMed] [Google Scholar]
- Krozowski Z., Funder J. W. Mineralocorticoid receptors in rat anterior pituitary: toward a redefinition of "mineralocorticoid hormone". Endocrinology. 1981 Oct;109(4):1221–1224. doi: 10.1210/endo-109-4-1221. [DOI] [PubMed] [Google Scholar]
- MacLusky N. J., McEwen B. S. Oestrogen modulates progestin receptor concentrations in some rat brain regions but not in others. Nature. 1978 Jul 20;274(5668):276–278. doi: 10.1038/274276a0. [DOI] [PubMed] [Google Scholar]
- MacLusky N. J., Turner B. B., McEwen B. S. Corticosteroid binding in rat brain and pituitary cytosols: resolution of multiple binding components by polyacrylamide gel based isoelectric focusing. Brain Res. 1977 Jul 22;130(3):564–571. doi: 10.1016/0006-8993(77)90119-6. [DOI] [PubMed] [Google Scholar]
- McEwen B. S., de Kloet R., Wallach G. Interactions in vivo and in vitro of corticoids and progesterone with cell nuclei and soluble macromolecules from rat brain regions and pituitary. Brain Res. 1976 Mar 19;105(1):129–136. doi: 10.1016/0006-8993(76)90928-8. [DOI] [PubMed] [Google Scholar]
- Muldoon T. G., Westphal U. Steroid-protein interactions. XV. Isolation and characterization of corticosteroid-binding globulin from human plasma. J Biol Chem. 1967 Dec 10;242(23):5636–5643. [PubMed] [Google Scholar]
- Naylor P. H., Gilani S. S., Milholland R. J., Rosen F. Antiglucocorticoids: in vivo assay and evaluation of cortexolone, progesterone, and 6-beta-bromoprogesterone. Endocrinology. 1980 Jul;107(1):117–121. doi: 10.1210/endo-107-1-117. [DOI] [PubMed] [Google Scholar]
- Rousseau G. G., Baxter J. D., Tomkins G. M. Glucocorticoid receptors: relations between steroid binding and biological effects. J Mol Biol. 1972 Jun 14;67(1):99–115. doi: 10.1016/0022-2836(72)90389-0. [DOI] [PubMed] [Google Scholar]
- SEAL U. S., DOE R. P. Corticosteroid-binding globulin. I. Isolation from plasma of diethylstilbestrol-treated men. J Biol Chem. 1962 Oct;237:3136–3140. [PubMed] [Google Scholar]
- de Kloet E. R., Burbach P., Mulder G. H. Localization and role of transcortin-like molecules in the anterior pituitary. Mol Cell Endocrinol. 1977 May;7(3):261–273. doi: 10.1016/0303-7207(77)90058-2. [DOI] [PubMed] [Google Scholar]



