Abstract
The bisphosphonates (3-amino-1-hydroxypropylidene)-1,1-bisphosphonate (APD) and disodium dichloromethylidene bisphosphonate (Cl2MDP) effectively inhibit the accelerated bone resorption associated with some skeletal disorders, e.g., Paget's disease. However, it has not been established whether these compounds exert their inhibitory effect by rendering the bone mineral more resistant to degradation, by diminishing the activity of resorbing cells, or through some combination of both activities. In this study, we have tested these possibilities using an in vitro resorption assay system consisting of elicited rat peritoneal macrophages co-cultured with particles of 45Ca-labeled, devitalized rat bone. This assay system permits the quantitative assessment of the action of APD and Cl2MDP on the two major phases of bone resorption (cell-substrate attachment and osteolysis) under circumstances where the drugs are present continuously or, most importantly for the issues in question, after the separate pretreatment of the particles or the resorbing cells.
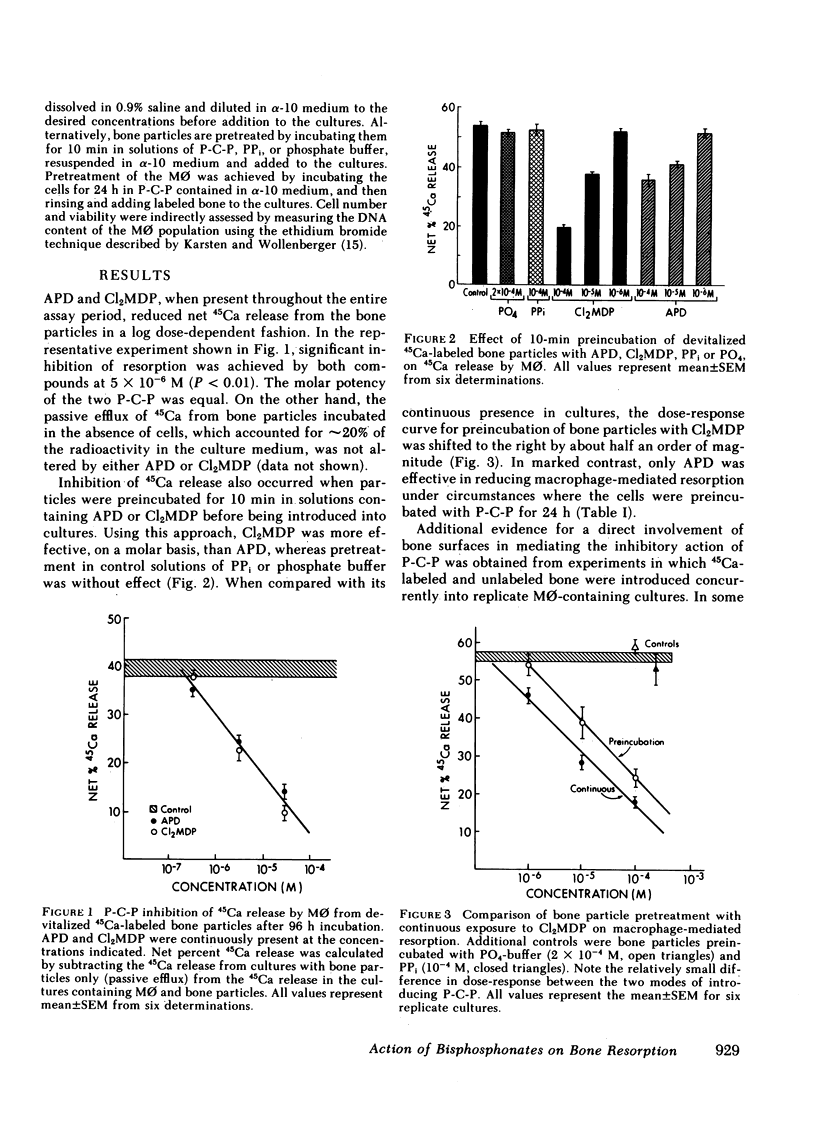
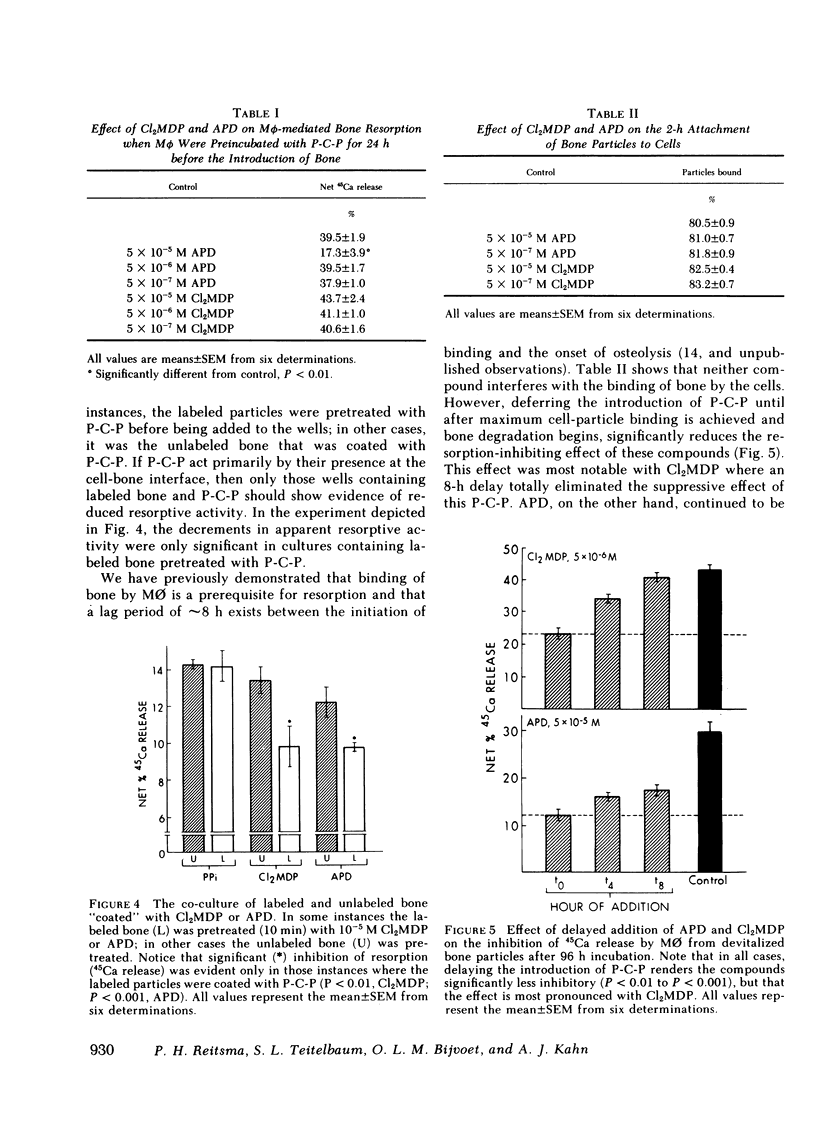
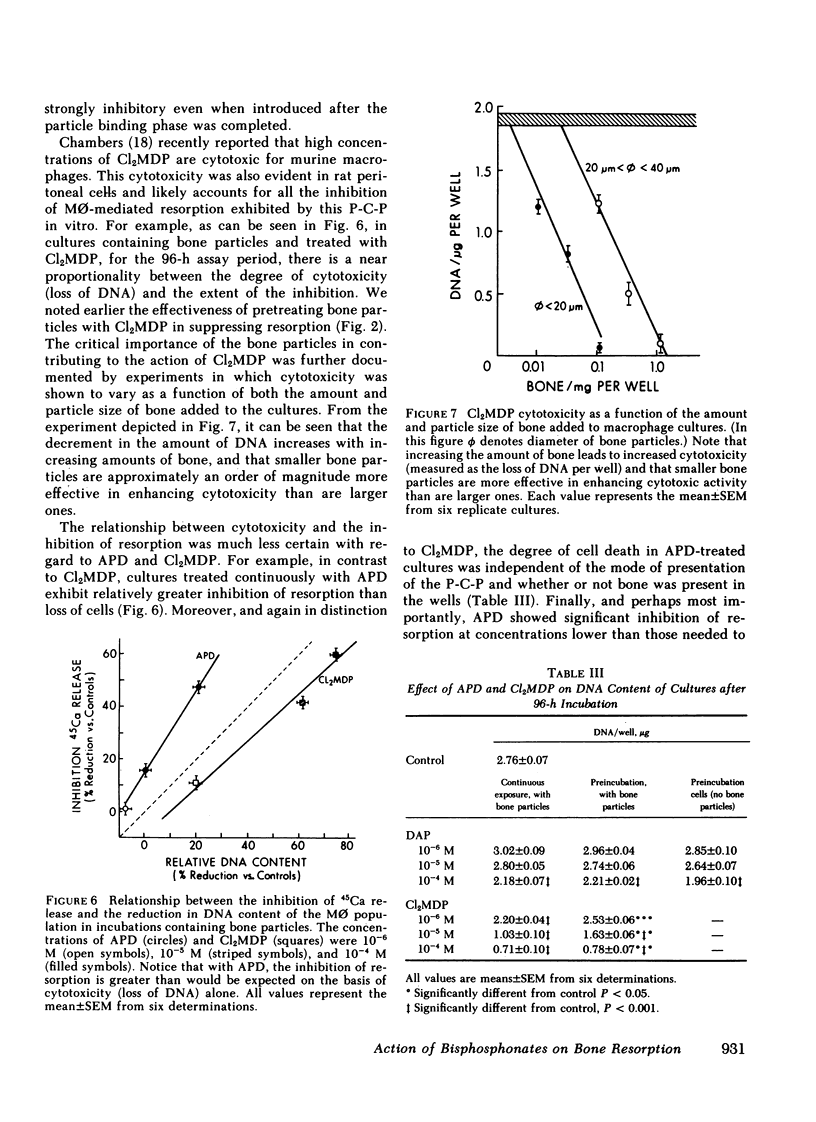
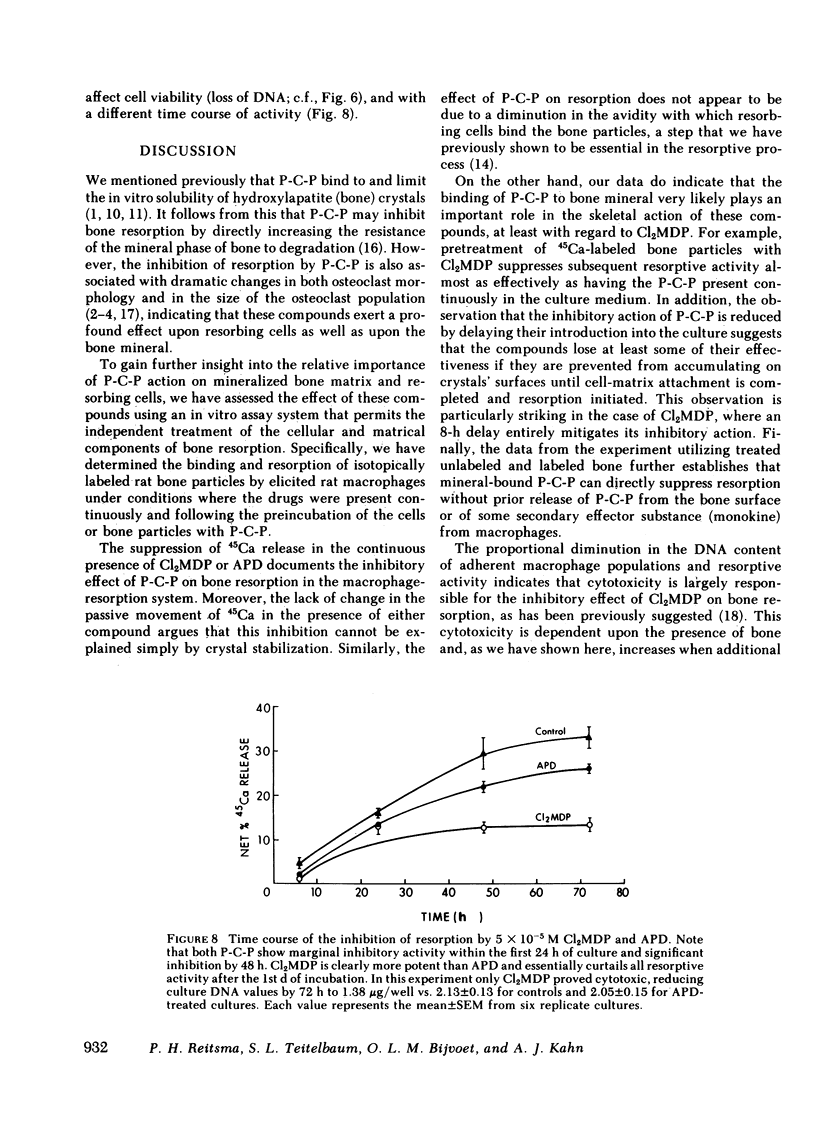
Our data indicate that (a) Both APD and Cl2MDP at concentrations ≥5 × 10-6 M diminish macrophage-mediated 45Ca release (i.e., bone resorption) in a log dose-dependent fashion. (b) A 10-min pretreatment of bone particles with either bisphosphonate (P-C-P) similarly inhibits resorptive activity, but is most pronounced with Cl2MDP. However, only APD is effective in reducing resorption when cells are preincubated (for 24 h) with P-C-P. (c) In cultures containing both labeled and unlabeled bone, significant inhibition occurs only when the labeled particles are coated with P-C-P (indicating that the action of P-C-P-treated bone is highly localized). (d) P-C-P does not diminish cell-bone particle attachment, an essential step in the resorptive process. On the other hand, delaying the addition of P-C-P until after cell-bone attachment is completed significantly reduces the resorption-inhibiting effect of these compounds. (e) Cl2MDP reduces culture DNA content in proportion to its inhibitory effect on resorption, and both the inhibitory and cytotoxic actions of this P-C-P are dependent upon the presence of bone. On the other hand, APD is cytotoxic only at very high concentrations (10-4 M), acts independently of the presence of bone, and inhibits resorption without killing cells.
We conclude that the mechanisms of action of APD and Cl2MDP are markedly different. Cl2MDP is a potent cytotoxin in the presence of bone and apparently exerts its inhibitory effect in this manner. APD is noncytotoxic at levels adequate to suppress resorption and, therefore, must inhibit macrophage activity by some other mechanism. Neither P-C-P appears to limit resorption by decreasing the solubility of mineralized bone matrix.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chambers T. J. Diphosphonates inhibit bone resorption by macrophages in vitro. J Pathol. 1980 Nov;132(3):255–262. doi: 10.1002/path.1711320307. [DOI] [PubMed] [Google Scholar]
- Fast D. K., Felix R., Dowse C., Neuman W. F., Fleisch H. The effects of diphosphonates on the growth and glycolysis of connective-tissue cells in culture. Biochem J. 1978 Apr 15;172(1):97–107. doi: 10.1042/bj1720097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleisch H., Russell R. G., Francis M. D. Diphosphonates inhibit hydroxyapatite dissolution in vitro and bone resorption in tissue culture and in vivo. Science. 1969 Sep 19;165(3899):1262–1264. doi: 10.1126/science.165.3899.1262. [DOI] [PubMed] [Google Scholar]
- Francis M. D. The inhibition of calcium hydroxypatite crystal growth by polyphosphonates and polyphosphates. Calcif Tissue Res. 1969;3(2):151–162. doi: 10.1007/BF02058658. [DOI] [PubMed] [Google Scholar]
- Frijlink W. B., Bijvoet O. L., te Velde J., Heynen G. Treatment of Paget's disease with (3-amino-1-hydroxypropylidene)-1, 1-bisphosphonate (A.P.D.). Lancet. 1979 Apr 14;1(8120):799–803. doi: 10.1016/s0140-6736(79)91318-7. [DOI] [PubMed] [Google Scholar]
- Jung A., Bisaz S., Fleisch H. The binding of pyrophosphate and two diphosphonates by hydroxyapatite crystals. Calcif Tissue Res. 1973 Mar 30;11(4):269–280. doi: 10.1007/BF02547227. [DOI] [PubMed] [Google Scholar]
- Karsten U., Wollenberger A. Improvements in the ethidium bromide method for direct fluorometric estimation of DNA and RNA in cell and tissue homogenates. Anal Biochem. 1977 Feb;77(2):464–470. doi: 10.1016/0003-2697(77)90259-7. [DOI] [PubMed] [Google Scholar]
- Meunier P. J., Chapuy M. C., Alexandre C., Bressot C., Edouard C., Vignon C., Mathieu L., Trechsel U. Effects of disodium dichloromethylene diphosphonate on Paget's disease of bone. Lancet. 1979 Sep 8;2(8141):489–492. doi: 10.1016/s0140-6736(79)91551-4. [DOI] [PubMed] [Google Scholar]
- Miller S. C., Jee W. S. The comparative effects of dichloromethylene diphosphonate (C12MDP) and ethane-1-hydroxy-1,1-diphosphonate (EHDP) on growth and modeling of the rat tibia. Calcif Tissue Res. 1977 Oct 20;23(3):207–214. doi: 10.1007/BF02012787. [DOI] [PubMed] [Google Scholar]
- Reitsma P. H., Bijvoet O. L., Verlinden-Ooms H., van der Wee-Pals L. J. Kinetic studies of bone and mineral metabolism during treatment with (3-amino-1-hydroxypropylidene)-1,1-bisphosphonate (APD) in rats. Calcif Tissue Int. 1980;32(2):145–157. doi: 10.1007/BF02408534. [DOI] [PubMed] [Google Scholar]
- Rowe E. J., Hausmann E. The alteration of osteoclast morphology by diphosphonates in bone organ culture. Calcif Tissue Res. 1976 Apr 13;20(1):53–60. doi: 10.1007/BF02546397. [DOI] [PubMed] [Google Scholar]
- Russell R. G., Fleisch H. Pyrophosphate and diphosphonates in skeletal metabolism. Physiological, clinical and therapeutic aspects. Clin Orthop Relat Res. 1975 May;(108):241–263. doi: 10.1097/00003086-197505000-00038. [DOI] [PubMed] [Google Scholar]
- Schenk R., Merz W. A., Mühlbauer R., Russell R. G., Fleisch H. Effect of ethane-1-hydroxy-1,1-diphosphonate (EHDP) and dichloromethylene diphosphonate (Cl 2 MDP) on the calcification and resorption of cartilage and bone in the tibial epiphysis and metaphysis of rats. Calcif Tissue Res. 1973 Mar 12;11(3):196–214. doi: 10.1007/BF02547219. [DOI] [PubMed] [Google Scholar]
- Siris E. S., Sherman W. H., Baquiran D. C., Schlatterer J. P., Osserman E. F., Canfield R. E. Effects of dichloromethylene diphosphonate on skeletal mobilization of calcium in multiple myeloma. N Engl J Med. 1980 Feb 7;302(6):310–315. doi: 10.1056/NEJM198002073020602. [DOI] [PubMed] [Google Scholar]
- Teitelbaum S. L., Stewart C. C., Kahn A. J. Rodent peritoneal macrophages as bone resorbing cells. Calcif Tissue Int. 1979 Jul 3;27(3):255–261. doi: 10.1007/BF02441194. [DOI] [PubMed] [Google Scholar]
- van Breukelen F. J., Bijvoet O. L., van Oosterom A. T. Inhibition of osteolytic bone lesions by (3-amino-1-hydroxypropylidene)-1, 1-bisphosphonate (A.P.D.). Lancet. 1979 Apr 14;1(8120):803–805. doi: 10.1016/s0140-6736(79)91319-9. [DOI] [PubMed] [Google Scholar]



