Abstract
Background:
Vomiting is one of the most prevalent side effects of chemotherapy in cancer patients. The aim of this study was to evaluate the effect of ginger plant on chemotherapy-induced vomiting, since the previous studies were somehow imperfect and have provided controversial results.
Materials and Methods:
This randomized double-blind placebo-controlled clinical trial was conducted on 80 women with breast cancer undergoing chemotherapy and suffering from vomiting in Imam Khomeini Hospital, Tehran, Iran, between July and December 2009. During a convenience sampling the participants were randomly allocated into treatment and placebo groups after taking a written informed consent. Two groups were matched based on the age and emetic risk of chemotherapy drugs. The treatment group received 250 mg ginger powder capsules (Zintoma) and placebo group 250 mg starch capsules 4 times a day (1 g/day) for 6 days since 3 days before chemotherapy session. A two-part self-made questionnaire was used to assess the effect of ginger. Patients completed the instrument every day. Then by STATA software version 8, the gathered data were analyzed using Fisher’s exact, Kruskal-Wallis, and Chi-square tests.
Results:
The 2 groups had no significant age differences and were matched (ginger: 41.8±8.4 vs placebo: 45.1±10, P = 0.1). Vomiting cases were significantly lower in ginger group at anticipatory (P = 0.04), acute (P = 0.04), and delayed (P = 0.003) phases. Also, heartburn was the only and venial reported side effect (P > 0.05).
Conclusions:
Taking ginger capsules (for 6 days since 3 days before chemotherapy) accompanied by the routine antiemetic treatment could relieve chemotherapy-induced vomiting in all phases.
Keywords: Cancer, chemotherapy, complementary and alternative therapy, ginger, vomiting
INTRODUCTION
Anticipatory vomiting occurs during the 72 h before chemotherapy session, and acute and delayed vomiting happen ≤24 h and 24-72 h post chemotherapy, respectively.[1–3] Chemotherapy-induced vomiting (CIV) is a common complaint in patients with cancer that in spite of receiving numerous chemical antiemetic drugs, such as serotonin-receptor antagonists (5-HT3), almost 70% of patients suffer from this problem and bear various occupational and personal difficulties.[4–7]
Heavy use of synthetic antiemetic drugs cause problems, such as extrapyramidal complications, hypotension, headache, and so on.[8] Thus, recently the intention to use complementary and alternative therapy (CAT), especially herbal medicine as a safer and cheaper method has dramatically increased, because chemical drugs are severely more toxic and expensive compared with herbal plants.[2] Ginger rhizome powder (Zingiber officinale) contains substances like 6-gingerol and 6-shagaol that have antiemetic effects.[7] Ginger has been used since ancient times for the treatment of gastrointestinal disorders, such as nausea, vomiting, and dyspepsia,[9,10] and for alleviation of pregnancy gravidarum[11,12] and motion sickness nausea recently.[13]
Health care practitioners, especially nurses have the role, duty, and ability to assess, diagnose, control, cure, and prevent the emesis as the most common chemotherapy-induced complication. Therefore, nurses can use CAT, especially herbal medicine as an effective and applicable method to relieve CIV and increase cancer patients’ welfare and comfort.[2]
Previous researches in this field have been contradicted to the results. As Sontakke et al,[4] and Ryan et al,[14] reported in their studies, in patients receiving chemotherapy, taking ginger powder leads to decrease of CIV. In contrast, Manusirivithaya et al,[15] and Zick et al,[7] rejected its effectiveness and stated that consumption of ginger has no palliative effect on CIV in cancer patients. Also, only 2 recent studies examined the effect of ginger on the delayed CIV and results were negative, too.[7,15] On the other hand, although 20%-30% of patients expected to suffer from anticipatory CIV[8] but so far no research has been conducted on that. Therefore, the present study covered the all anticipatory, acute, and delayed phases. In addition, it seems that another defect of previous studies was no inclusion of patients with a single type of cancer. So, this study was only performed on the women with breast cancer as the most prevalent cancer among women, because any type of cancer has its own particular chemotherapy protocol with different emetogenicity.[16,17]
Four objectives were followed: (1) to compare the vomiting in anticipatory phase between the ginger and placebo groups, (2) to evaluate the vomiting in acute phase between the 2 groups, (3) to compare vomiting in delayed phase, and (4) to appraise the safety of ginger capsules through the discovery, evaluation, and comparison of resulting side effects.
In the present study, the antiemetic effect of ginger capsules was investigated on various phases of CIV in women with breast cancer. Therefore, nurses could reduce this most prevalent complication of chemotherapy by providing appropriate facilities and training programs about the method of consumption of ginger capsules.
MATERIAL AND METHODS
This study was a randomized, double-blind, placebo-controlled clinical trial that was conducted on 80 women with breast cancer undergoing chemotherapy in the Department of Chemotherapy at the Cancer Institute Center “2” in Imam Khomeini Hospital affiliated to Tehran University of Medical Sciences, Tehran, Iran, between July and December 2009. Inclusion criteria were age ≥ 18 years; histologic diagnosis of breast cancer, history of receiving at least one chemotherapy injection, receiving single-day cycles of chemotherapy (each cycle separated from next by ≥ 2 weeks); experiencing vomiting in previous sessions, and having normal values of hematologic and biomedical laboratory parameters. Patients were excluded if they were receiving multiple-day chemotherapy; receiving concurrent radiotherapy with high risk of causing emesis (ie, total body, hemi body, upper abdomen, and craniospinal radiation); taking therapeutic doses of warfarin, aspirin, or heparin; had a history of bleeding disorder(s) like severe thrombocytopenia; had an allergy to ginger or had taken it in the last week; had gastrointestinal disorders and cancers; and had other emesis-inducing diseases, such as hypertension, liver, and renal failure. Also, patients who met the following criteria were excluded: forgotten to take capsules ≥ 3 consecutive times; used other antiemetic drugs or therapeutic methods except the routine antiemetic; had severe gastrointestinal problems during the study; and refusal to continue participating in trial. The study began after obtaining approval from the ethics committee of Tehran University of Medical Sciences and registering in Iranian Registry of Clinical Trials (IRCT) with code of IRCT138811203319N1.
After selecting breast cancer women among total patients, through convenience sampling 80 concerned patients were approached and enrolled by providing written informed consent.
Based on the results of previous studies we supposed that we could reduce the emesis from 50% to 20%. So, using the following formula and considering confidence interval 95% (α = ./05) and test power 80% (β = ./20) and p1= 50 and p2= 20%, the sample size was calculated for both groups.
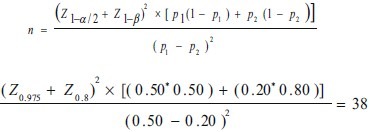
The patients were randomly allocated to treatment (ginger) group and control (placebo) group using the 20 block random tables. The treatment group received red capsules of ginger (Zintoma) manufactured by Goldaru Company, Isfahan, Iran, and the control group received placebo capsules containing starch with exactly the same size, shape, color, taste, and dosage as Zintoma capsules. Each Zintoma capsule contained 250 mg dry powdered ginger root that was prepared with 10:1 ratio of ethanol 50% and ginger root and included 5.38 mg (2.15%) 6-gingerol, 1.8 mg (0.72%) 8-gingerol, 4.19 mg (1.78%) 10-gingerol, and 0.92 mg (0.37%) 6-shagaol.
Coding and blinding of the 2 groups were performed privately by the pharmacologist consultant, and all of the samples, data analyzers, and all participants too, were unaware of the real content of the capsules.
Intervention lasted for 6 days starting 3 days before the chemotherapy session. At this time all participants received four 250 mg capsules (Zintoma) with 6 h intervals (1 g/day) accompanied by the routine antiemetic regimen, including Kytril or Granisetron hydrochloride tablets (1 mg/day) and dexamethasone ampoules (8 mg/day). The vomiting times were recorded in the instruments each night just before sleep. The dosage of capsules was selected based on the results of clinical trials conducted by Sontakke et al and Ryan et al.[4,14]
A self-made, two-part self-reporting instrument was used to measure the number of vomiting cases in both the groups, after checking the content validity. This instrument was a reliable standard table that has been used in various studies as well.[4,7,8,11,15] First part contained a table to record the number of CIV, and the second section included 3 questions about the: (1) probable use of other antiemetics; (2) missed cases of capsule taking; (3) probable side effects due to capsules intake. The patients were asked to fill the questionnaires out every night during the study and in any cases of intolerable complications stop the consumption and contact the researchers for more information.
Professional blind interviewers collected and recorded the information and then data entry and analysis were performed by a professional blind statistician. Data were analyzed using STATA software version 8 and the application of descriptive and inferential statistics. The t test and Fisher’s exact test were applied to compare the 2 groups regarding demographic characteristics, such as age (continuous variable) and education (ranked qualitative variance). To examine differences in the profile of diseases, such as duration of disease (discrete variance) and emetic risk of chemotherapy drugs (ranked qualitative variance), Kruskal-Wallis and Chi-square test were applied. Finally the Kruskal-Wallis and Chi-square tests were applied to compare the number of CIV (discrete variance) between the 2 groups in different phases. After completing the data analysis the consultant pharmacologist broke the codes and introduced the groups.
RESULTS
In the present study 18 patients (9 in each group) interrupted their participation (5 for general weakness and canceling of the chemotherapy; 6 because of unwilling to continue the study; 6 for not filling the tools; and 1 due to die). Sampling time was extended to maintain the sample size (80 persons).
There were no significant differences between the ginger and placebo groups regarding age variable (mean ± SD: 41.8±8.4 vs 45.1±10, P = 0.1, respectively). Most participants of both groups had high school education and were homogenous (P = 0.46) according to Table 1.
Table 1.
Baseline characteristics of the randomization groups
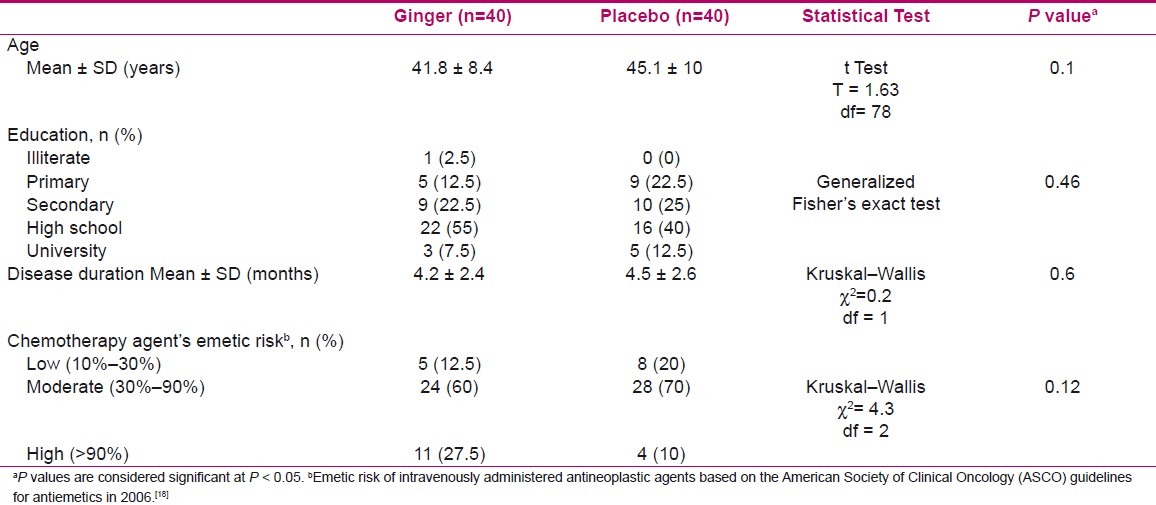
Clinical characteristics of groups, including duration of disease and emetic risk of chemotherapy agents are presented in Table 1. The findings indicated no significant differences between the two groups in terms of stated profiles.
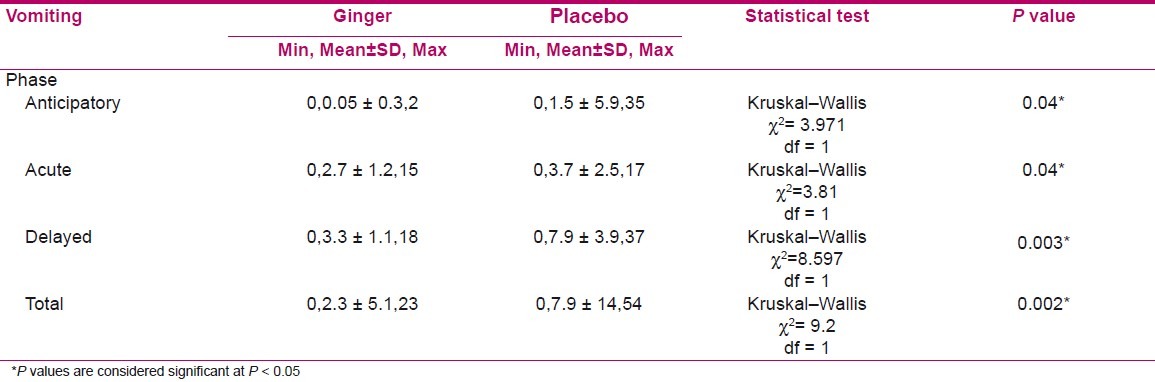
Vomiting cases were significantly lower in ginger group at anticipatory (P = 0.04), acute (P = 0.04), and delayed (P = 0.003) phases and total sum of 6 days (P = 0.002) according to Table 2.
Table 2.
Distribution and comparison of Chemotherapy-induced vomiting in various phases between ginger and placebo groups
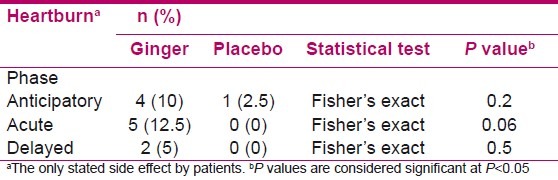
The only reported side effect during the study was the heartburn. But there were no significant differences between 2 groups at anticipatory (P = 0.2), acute (P = 0.06), and delayed (P = 0.5) phases [Table 3].
Table 3.
Prevalence of side effects in treatment groups
DISCUSSION
In addition to controversial reports, there are few studies about the effect of ginger extract on the CIV. It seems that characteristics of those studies could affect their results. For example, they have targeted wide range and kind of cancers.[7,15] Meanwhile, each of them has special therapeutic protocol and each protocol has also its own special emetogenicity in comparison to the others. Hence, we cannot easily approve or refuse the effectiveness of ginger plant on CIV and also we cannot generalize the results to other types of cancer.
On the other hand, no studies have examined the anticipatory vomiting that is very bothersome for many patients. Therefore, this study was carried out to address those issues that have been less studied.
Only women with breast cancer were attended to increase the accuracy and generalizability of the results because breast cancer is the most common type of cancer among women in Iran and in the world[16,17] and almost the same chemotherapy protocol is used to treat it.
The number of vomiting cases in the ginger group were significantly lower than the placebo group at anticipatory, acute, delayed, and total phases (P = 0.04, P = 0.04, P = 0.003, and P = 0.002, respectively) according to Table 2. There is no published study about the effect of ginger on the anticipatory vomiting except Ryan et al,[14] who performed their intervention from 3 days before to three days after chemotherapy session, but presented the results of the acute phase. Similar to our findings, Sontakke et al,[4] indicated that in cancer patients undergoing chemotherapy the antiemetic effect of metoclopramide was equivalent to ginger (1 g/day) in acute phase (58% vs 62%, respectively).
In contrast, Zick et al,[7] reported that in cancer patients undergoing chemotherapy, ginger capsules could not relieve the CIV in acute (P=0.88) and delayed (P=0.35) phases in comparison to the control group[7] that could be probably due to the use of cisplatin as a high cytotoxic agent. Moreover, Manusirivithaya et al,[15] expressed that there was no significant differences between ginger and placebo groups related to the complete control of acute and delayed vomiting that could be concerned using cisplatin in chemotherapy protocol.
The results of other studies about the effect of ginger extract on pregnancy-induced vomiting (PIV) are the same as ours. As Ozgoli et al,[11] stated, the use of ginger (1 g/day) significantly (P < 0.05) reduced the cases of PIV. Also, Jenabi et al (2009) revealed that taking ginger powder (1 g/day) significantly (P < 0.05) relieved PIV.[12]
The heartburn was the only complaint due to taking the ginger capsules in the present study, but with no significant differences with placebo group at anticipatory, acute, and delayed phases (P = 0.2, P = 0.06, and P = 0.5, respectively). This finding is consistent with the results of Zick et al and Manusirivithaya et al studies.[7,15] Thus, it could be concluded that ginger capsules are safe and danger free.
This study was only performed on women with breast cancer undergoing single-day courses of chemotherapy. This matter has its own privileges but also limitations. The most important limitation of this study was that our results were only devoted and applicable for patients with breast cancer, and thus we cannot generalize the results to other types of cancers. So, it should be suggested that to conduct more widespread studies about the effect of ginger on the different types of cancers and also to assess the effect of ginger on patients undergoing multiple-day chemotherapy to achieve more accurate information about the efficacy of this herb.
According to the findings of this study, it should be declared that taking ginger capsules (1 g/day) may relieve the CIV safely. Nurses are dealing directly with cancer patients and are responsible for providing educational programs for patients and their families about how to deal with their drug regimens and its side effects. Therefore, nurses should introduce and teach those patients to take these capsules, as the effectiveness and safety of ginger are confirmed.
ACKNOWLEDGMENTS
This study was carried out by the financial support of research deputy of Tehran University of Medical Sciences and with the favor of Cancer Research Centre of Imam Khomeini Hospital. Hereby we would like to express our appreciation to all the dear participants and personnel of chemotherapy department of Cancer Institute Centre “2”.
Footnotes
Source of Support: Tehran University of Medical Sciences and with the favor of Cancer Research Centre of Imam Khomeini Hospital
Conflict of Interest: Nil.
REFERENCES
- 1.Grunberg SM, Deuson RR, Mavros P, Geling O, Hansen M, Cruciani G, et al. Incidence of chemotherapy-induced nausea and emesis after modern antiemetics. Cancer. 2004;100:2261–8. doi: 10.1002/cncr.20230. [DOI] [PubMed] [Google Scholar]
- 2.Black JM, Hawks JH. Medical surgical nursing: Clinical management for positive outcomes. 8th ed. Vol 1. Saunders: Elsevier; 2009. [Google Scholar]
- 3.Levine ME, Gillis MG, Koch SY, Voss AC, Stern RM, Koch KL. Protein and ginger for the treatment of chemotherapy-induced delayed nausea. J Altern Complement Med. 2008;14:545–51. doi: 10.1089/acm.2007.0817. [DOI] [PubMed] [Google Scholar]
- 4.Sontakke S, Thawani V, Naik MS. Ginger as an antiemetic in nausea and vomiting induced by chemotherapy: A randomized, cross-over, double blind study. Indian J Pharmacol. 2003;35:32–6. [Google Scholar]
- 5.Henry DH, Viswanathan HN, Elkin EP, Traina S, Wade S, Cella D. Symptoms and treatment burden associated with cancer treatment: Results from a cross-sectional national survey in the U.S. Support Care Cancer. 2008;16:791–801. doi: 10.1007/s00520-007-0380-2. [DOI] [PubMed] [Google Scholar]
- 6.Bloechl-Daum B, Deuson RR, Mavros P, Hansen M, Herrstedt J. Delayed nausea and vomiting continue to reduce patients’ quality of life after highly and moderately emetogenic chemotherapy despite antiemetic treatment. J Clin Oncol. 2006;24:4472–8. doi: 10.1200/JCO.2006.05.6382. [DOI] [PubMed] [Google Scholar]
- 7.Zick SM, Ruffin MT, Lee J, Normolle DP, Siden R, Alrawi S, et al. Phase II trial of encapsulated ginger as a treatment for chemotherapy-induced nausea and vomiting. Support Care Cancer. 2009;17:563–72. doi: 10.1007/s00520-008-0528-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hickok JT, Roscoe JA, Morrow GR, Ryan JL. A phase II/III randomized, placebo-controlled, double-blind clinical trial of ginger (zingiber officinale) for nausea caused by chemotherapy for cancer: A currently accruing URCC CCOP cancer control study. Support Cancer Ther. 2007;4:247–50. doi: 10.3816/SCT.2007.n.022. [DOI] [PubMed] [Google Scholar]
- 9.Mills S, Bone K. Principles and practice of phytotherapy. Oxford: Churchill LivingStone; 2000. [Google Scholar]
- 10.Hoffman D. The new holistic herbal. Shaftesburry, Dorset: Element Books Limited; 1983. [Google Scholar]
- 11.Ozgoli G, Goli M, Simbar M. Effects of ginger capsules on pregnancy nausea and vomiting. J Altern Complement Med. 2009;15:243–6. doi: 10.1089/acm.2008.0406. [DOI] [PubMed] [Google Scholar]
- 12.Jenabi E, Mohammad-Alizadeh S. Comparing ginger and vitamin B6 for the treatment of nausea and vomiting in pregnancy: A randomized controlled trial. Midwifery. 2008;5:35–7. doi: 10.1016/j.midw.2007.10.013. [DOI] [PubMed] [Google Scholar]
- 13.Lien HC, Sun WM, Chen YH, Kim H, Hasler W, Owyang C. Effect of ginger on motion sickness and gastric slow-wave dysrhythmias induced by circular vection. Am J Physiol Gastrointest Liver Physiol. 2003;284:481–9. doi: 10.1152/ajpgi.00164.2002. [DOI] [PubMed] [Google Scholar]
- 14.Ryan JL, Hickok JT, Roscoe JA, Morrow GR. Ginger for chemotherapy-related nausea in cancer patients: A URCC CCOP randomized, double-blind, placebo-controlled clinical trial of 644 cancer patients. J Clin Oncol. 2009;27:15. [Google Scholar]
- 15.Manusirivithaya S, Sripramote M, Tangjitgamol S, Sheanakul C, Leelahakorn S, Thavaramara T, et al. Antiemetic effect of ginger in gynecologic oncology patients receiving cisplatin. Int J Gynecol Cancer. 2004;14:1063–9. doi: 10.1111/j.1048-891X.2004.14603.x. [DOI] [PubMed] [Google Scholar]
- 16.Dundar EP, Ozmen D, Ozturk B, Haspolat G, Akyildiz F, Coban S. The knowledge and attitudes of breast self-examination and mamography in a group of women in a rural area in western Turkey. BMC Cancer. 2006;6:1–9. doi: 10.1186/1471-2407-6-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Edwards QT, Palomares MR. Assessment of risk for breast cancer utilizing history and Quantitative models in primary care. J Nurse Pract. 2008;10:361–9. [Google Scholar]
- 18.Kris MG, Hesketh PS, Somerfiels MR. American society of clinical oncology guideline for antiemetics in oncology: Update 2006. J Clin Oncol. 2006;24:2932–42. doi: 10.1200/JCO.2006.06.9591. [DOI] [PubMed] [Google Scholar]


