Abstract
The insulin-like growth factor pathway plays a central role in the normal and abnormal growth of tissues; however, nutritional determinants of insulin-like growth factor I (IGF-I) and its binding proteins in normal individuals are not well-defined. Three test diets: high fat diet (HF; 40% energy as fat), low fat diet (LF; 20% energy as fat) and low fat, high omega-3 fatty acids diet (LFn3; 23% energy as fat) were tested in a randomized cross-over designed controlled feeding trial in healthy postmenopausal women. Plasma IGF-I, IGFBP-3, insulin, glucose and ratio of IGF-I:IGFBP-3 concentrations were measured in response to diets. Insulin sensitivity was calculated using the homeostatic model assessment of insulin resistance (HOMA-IR). We hypothesized that IGF-I, insulin and glucose concentrations would decrease and IGF binding protein-3 (IGFBP-3) concentration would increase in response to the low fat diets. 8 weeks of the LFn3 diet increased circulating IGF-I (P < 0.001) and IGFBP-3 (P = 0.01) and the LF diet increased IGFBP-3 (P = 0.04) resulting in trends towards an increased IGF-I:IGFBP-3 ratio with the LFn3 diet and a decreased IGF-I:IGFBP-3 ratio with the LF diet (P = 0.13 for both comparisons). No statistically significant differences were detected between treatments at baseline or 8 weeks for IGF-I, IGFBP-3 or IGFBP-3. Insulin, glucose and HOMA-IR were not altered by the interventions. Low fat diet with high n-3 fatty acids may increase circulating IGF-I concentrations without adversely affecting insulin sensitivity in healthy individuals.
Keywords: Diet, Dietary Fat, Omega-3 Fatty Acids, insulin-like growth factor I (IGF-I), IGF binding protein-3 (IGFBP-3), insulin, glucose
1. Introduction
Insulin-like growth factor I (IGF-I) is a peptide hormone predominantly secreted by the liver in response to pituitary-derived growth hormone (GH) [1]. IGF-I is generated to a lesser degree in peripheral tissues and acts in an autocrine and paracrine fashion in these tissues [2]. In the blood, approximately 90% of IGF-I is complexed with insulin-like growth factor binding protein-3 (IGFBP-3; one of six IGF binding proteins) and acid labile subunit in a 1:1:1 ratio which increases the half-life of IGF-I [3]. IGF-I is involved in both the normal and neoplastic growth of tissues via mediation of cell proliferation, cell cycle progression and programmed cell death [1, 2, 4] and inhibition of the IGF signaling pathway is a target for cancer therapy [5]. In contrast, aging is associated with decreased GH and IGF-I concentrations accompanied by decreased bone mineral density, decreased lean body tissue, and increased adiposity along with a higher risk vascular profile associated with increased cardiovascular mortality and morbidity [6].
IGF-I levels are markedly reduced with malnutrition [7, 8], protein and calorie restriction [9] and in cancer cachexia [10]; however the nutritional determinants of IGF-I and its binding proteins are less well-defined in normal, adequately-fed individuals. Cross-sectional studies have shown associations between concentrations of IGF-I and IGFBP-3 and dietary fat intake as assessed by food frequency questionnaire [11–13], although all studies did not report an association [14–16]. Vegans who reportedly consumed significantly more polyunsaturated fat than meat-eaters and vegetarians had reduced concentrations of IGF-I [17], while intake of omega-3 (n-3) fatty acids was associated with increased concentrations of IGFBP-3 [13].
Insulin modulates the bioavailability of IGF-1 by reducing levels of IGF binding proteins, modulating GH receptor density on liver cells, and stimulating hepatic IGF-1 synthesis [3, 18]. High total fat intake was positively associated with fasting insulin concentrations [19, 20] and negatively associated with insulin sensitivity [21] in cross-sectional studies in non-diabetic individuals. Low fat/high carbohydrate diets improved insulin sensitivity [22–24] and fasting insulin concentrations [22, 25, 26] in several intervention studies relative to a high fat [23, 24] or habitual [22, 25, 26] diets in healthy individuals.
We previously reported the effects of three test diets with varying amounts and types of dietary fat on circulating sex hormones [27] in healthy postmenopausal women. As an ancillary analysis to that study, we hypothesized that IGF-I, insulin and glucose concentrations would decrease and IGF binding protein-3 (IGFBP-3) concentration would increase in response to low fat diet interventions with or without omega-3 fatty acids. Insulin sensitivity was assessed using the homeostatic model assessment of insulin resistance (HOMA-IR) [28, 29].
2. Methods and Materials
2.1 Experimental protocol
This study is part of a trial that was designed to determine the effect of three test diets with varying amounts of dietary fat and n-3 fatty acids on plasma sex hormone profile and urinary eicosanoids in postmenopausal women [27]. Each of the 3 controlled diets, high fat (40% energy from fat; HF), low fat (20% energy from fat; LF) and low fat, high n-3 fatty acids (23% energy from fat; 3% of energy from n-3 fatty acids; LFn3) diets were provided to subjects in a randomized, cross-over design to all subjects. The diets were provided for 8 weeks with a washout period of 6–12 weeks between diets. During the washout periods, the subjects consumed their habitual diets. During the intervention periods, study participants picked up packaged study meals (breakfast, lunch, dinner, and a snack) that were prepared in the metabolic kitchen of the University of Minnesota General Clinical Research Center. Subjects recorded any foods that were consumed in addition to the study meals and any foods from the prepared study meals that were not consumed on a daily compliance questionnaire that was monitored by the study staff. At meal pick-up times subjects also recorded their weight.
The University of Minnesota Committee for the Use of Human Subjects in Research and the U.S. Army Medical Research and Materiel Command’s Human Subjects Research Review Board approved the protocol for the study. All participants gave written informed consent prior to enrollment in the study.
2.2 Subjects
Postmenopausal women were recruited from Minneapolis/St Paul, MN and the surrounding area. Details on study recruitment were detailed previously [27]. Participants were 45–70 years old and postmenopausal (= 1 year since last menstrual period plus follicle stimulating hormone concentration = 23 IU/L at screening or = 55 years old); had a body mass index of 19–32 kg/m2 with minimal weight fluctuation in the 6 months prior to study participation; were willing to refrain from taking non-steroidal anti-inflammatory drugs and aspirin during the course of the study; and had not taken hormone replacement therapy or fish oil supplements for 2 months prior to study enrollment. Potential participants were excluded if they were current smokers, had hormone-related cancer in the past, used non-steroidal anti-inflammatory drugs or prescription medication (excluding high blood pressure medication), had a bilateral oophrectomy (an exclusion criteria pertaining to the larger study on circulating sex hormones in the same study subjects) or had diagnosed chronic concurrent disease (e.g. diabetes mellitus, inflammatory disease). A medical history screening questionnaire was used to exclude participants with known disease and those taking medications prohibited by the study protocol.
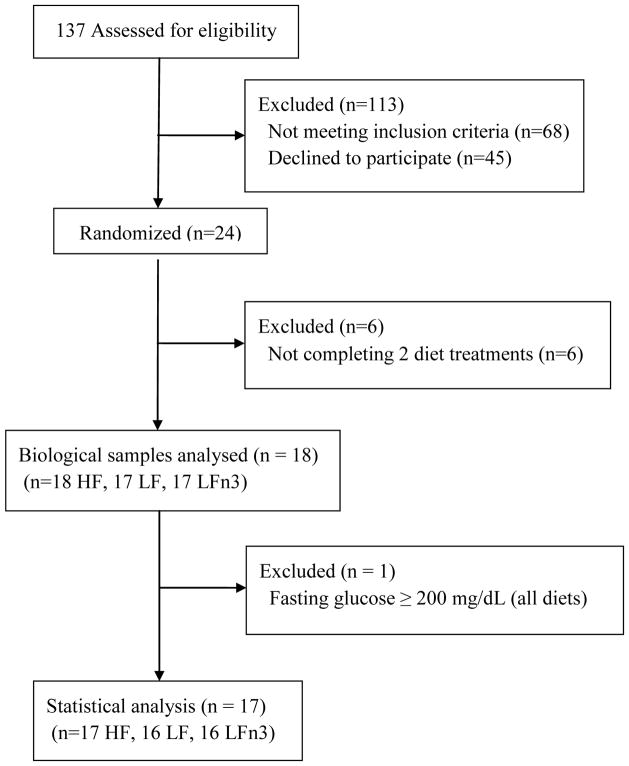
A total of 17 subjects completed all aspects of the study (Figure 1). An additional subject completed two diet treatments (missing LF diet period). One subject was excluded from the statistical analysis due to multiple fasting glucose measurements = 11.1 mmol/L (indicating the presence of type II diabetes). One subject was excluded from the analysis for the LFn3 diet (missing samples). One subject was excluded from the LFn3 diet statistical analysis for insulin and HOMA-IR due to an inexplicably high insulin value (> 5 standard deviations above the mean) at week 8.
Figure 1.
Flow diagram of participants from screening to data analysis
2.3 Dietary treatments
The three test diets have been described in detail previously [27]. Briefly, the three diets were isoenergenic high fat (HF; 40% energy from fat, 15% energy from protein, 45% energy from carbohydrate), low fat (LF; 20% energy from fat, 15% energy from protein, and 65% energy from carbohydrate) and low fat, high n-3 (LFn3; 23% energy from fat, 15% energy from protein, and 62% energy from carbohydrate) diets prepared from common, commercially available foods. The HF and LF diets contained minimal n-3 fatty acids and had similar proportions of saturated to monounsaturated to polyunsaturated fatty acids (1:1:1). The LFn3 diet included 3% of energy from n-3 fatty acids from foods naturally rich in these fats (salmon, flax seed oil and walnuts). Nutrients in the test diets were determined using nutrient analysis software (Nutritionist V).
The Harris-Benedict equation [655.1 + 9.56 x weight (kg) + 1.85 x height (cm) − 4.68 x age (y)] multiplied by an activity factor (1.4 – 1.7, mean 1.6) was used to predict an energy level appropriate for weight maintenance for each subject. The registered dietitian made a clinical assessment of activity level based on reported work and exercise habits [30]. Total energy of the diets was increased or decreased by 840 kJ (200 kcal) if a subject’s weigh fluctuated by 1.0 kg. Deviation from the study diets was calculated from the daily compliance questionnaires and was reported previously [27].
2.4 Plasma collection and analysis
Blood was drawn from fasting subjects between 7am and 10am at 0 and 8 weeks of each experimental diet period. Blood samples were centrifuged and plasma was flash frozen on dry ice and stored in 1mL aliquots at −80°C until analysis. Samples were assayed for IGF-I and IGFBP-3 using enzyme-linked immunosorbent assay kits (Diagnostics Systems Laboratories, Austin, TX) and samples were assayed for insulin by enzyme-linked immunosorbent assay kits (IBL America, Minneapolis, MN). Glucose was assayed using colorimetric assay kits (Cayman Chemical Company, Ann Arbor, MI). All samples from a given subject were assayed in the same batch. Intra-assay coefficients of variations were 5.9% for IGF-I, 3.3% for IGFBP-3, 4.3% for insulin, and 0.5% for glucose. Inter-batch variation was assessed using an internal control blood sample, and inter-batch coefficients of variation were 14.0% for IGF-I, 12.7% for IGFBP-3, 7.0% for insulin, and 7.4% for glucose. IGF-I:IGFBP-3 molar ratio was calculated as (0.130 x IGF-I concentration [ng/mL] )/ (0.036 x IGFBP-3 concentration [ng/mL]) [31]. HOMA-IR, calculated as (fasting plasma glucose [mmol/L] x fasting plasma insulin [mU/L]) / 22.5), was used as a measure of insulin resistance [28, 29].
2.5 Statistical analyses
A general linear mixed model (SAS Proc Mixed, SAS® 9.2, Cary, NC: SAS Institute Inc., 2002–2008) was fit to the data for each of the primary endpoints, with correlation between multiple measurements within each subject modeled by a random effect for subject. The mixed model accommodated missing values and was used to assess carryover and period effects. Randomization to diet sequences was unbalanced. Least squares means and their standard errors from the mixed model were the basis of pairwise comparisons between diets. Paired t-tests were used for within-diet comparisons. A P-value of < 0.05 denotes statistical significance.
3. Results
Baseline reported dietary intake and characteristics of the study subjects were reported previously [27]. At the study baseline, the subjects were 57 ± 6 years of age with a body mass index of 28 ± 4 kg/m2. The study diets were well-tolerated by the study subjects and reported deviation from the treatment diets was estimated from the daily compliance questionnaires to be less than 1% of total energy and less than 0.5% for n-3 intake [27]. Baseline body weight did not differ among the three diets (P = 0.83) although mean body weight decreased significantly from baseline within all diets (−1.1 kg with the HF, P < 0.001; −1.3 kg with the LF, P < 0.0001; and −0.7 kg with the LFn3, P = 0.02) although weights did not differ among the diets at 8 weeks (P = 0.46).
Consumption of the LFn3 resulted in increased IGF-I (P < 0.001, Table 1) and IGFBP-3 (P = 0.01) while consumption of the LF increased IGFBP-3 only (P = 0.04). Because of these changes, the ratio of IGF-I:IGFBP-3 increased with the LFn3 and decreased with the LF, although the changes in the ratio were not statistically significant (P = 0.13 for both comparisons). Baseline concentration of IGF-I was lower with the LFn3 (P = 0.24), which may have contributed to the magnitude of the increase observed. No statistically significant differences were detected between treatments at baseline or 8 weeks for IGF-I, IGFBP-3 or IGFBP-3. No changes within or among the diets were observed with insulin, glucose, or HOMA-IR. As previously published [27], the plasma phospholipid n-3 fatty acids in the participants were significantly increased following the LFn3 diet intervention.
Table 1.
Baseline and 8 weeks values for serum glucose, insulin, IGF-1, IGFPB-3, HOMA-IR, and IGF-I:IGFBP-3 ratio for the HF, LF, and LFn3.
| HF n = 17 |
LF n = 16 |
LFn31 n = 16 |
P-value2 | |
|---|---|---|---|---|
| Glucose (mmol/L) | ||||
| Baseline | 5.98 ± 0.3 | 5.96 ± 0.3 | 5.78 ± 0.3 | 0.43 |
| 8 weeks | 5.95 ± 0.2 | 5.67± 0.2 | 6.05 ± 0.2 | 0.47 |
| P-value3 | 0.92 | 0.20 | 0.36 | |
| Insulin (pmol/L) | ||||
| Baseline | 73.6 ± 6.9 | 76.4 ± 6.9 | 70.8 ± 6.9 | 0.56 |
| 8 weeks | 72.2 ± 7.6 | 73.6 ± 7.6 | 76.4 ± 7.6 | 0.60 |
| P-value3 | 0.83 | 0.66 | 0.31 | |
| IGF-I (nmol/L) | ||||
| Baseline | 19.6 ± 1.5 | 19.6 ± 1.5 | 17.8 ± 1.5 | 0.24 |
| 8 weeks | 19.3 ± 1.6 | 20.3 ± 1.6 | 21.4 ± 1.6* | 0.10 |
| P-value3 | 0.74 | 0.61 | < 0.001 | |
| IGFBP-3 (nmol/L) | ||||
| Baseline | 183.0 ± 14.4 | 167.1 ± 14.9 | 183.5 ± 14.9 | 0.60 |
| 8 weeks | 193.0 ± 15.4 | 179.6 ± 16.0* | 199.9 ± 16.0* | 0.60 |
| P-value3 | 0.10 | 0.04 | 0.01 | |
| HOMA-IR | ||||
| Baseline | 2.9 ± 0.4 | 3.1 ± 0.4 | 2.8 ± 0.4 | 0.42 |
| 8 weeks | 2.8 ± 0.3 | 2.7 ± 0.3 | 3.1 ± 0.3 | 0.26 |
| P-value3 | 0.66 | 0.19 | 0.31 | |
| IGF-I:IGFBP-3 ratio | ||||
| Baseline | 0.120 ± 0.016 | 0.129 ± 0.017 | 0.112 ± 0.017 | 0.42 |
| 8 weeks | 0.111 ± 0.016 | 0.119 ± 0.016 | 0.123 ± 0.016 | 0.74 |
| P-value3 | 0.20 | 0.13 | 0.13 | |
| Body Weight (kg) | ||||
| Baseline | 73.2 ± 2.8 | 73.6 ± 2.8 | 73.4 ± 2.8 | 0.83 |
| 8 weeks | 72.1 ± 2.74* | 72.3 ± 2.7* | 72.7 ± 2.7* | 0.46 |
| P-value3 | < 0.001 | < 0.0001 | 0.02 |
For the final analysis, 17 participants completed the high fat (HF) diet, 16 participants completed the low fat (LF) diet, and 16 participants completed the low fat + n-3 fatty acids (LFn3) diet. A general linear mixed model was used for data analysis. All values are means ± standard errors.
One subject was excluded from insulin and HOMA-IR analysis in the LFn3, thus n=15 for these endpoints.
P-value for effect of treatment across the three diets.
P-value for paired t-test comparing baseline and 8 weeks means within each diet.
8 weeks mean is significantly different from baseline mean.
4. Discussion
The results of this well-controlled dietary feeding study in healthy, non-diabetic postmenopausal women indicate that circulating IGF-I and IGFBP-3 concentrations were increased by a diet low in fat containing 3% of energy from n-3 fatty acids, resulting in a trend towards increased IGF-I:IGFBP-3 ratio. As such, we rejected our research hypothesis that the low fat diets would reduce IGF, insulin and glucose. IGFBP-3 was increased by the low fat diets as we hypothesized. As both IGF-I and IGFBP-3 are GH-dependent; these results may indicate a greater effect of the LFn3 diet compared to the LF diet on growth hormone levels. Few data are available regarding the effects of n-3 fatty acids on circulating IGF-I concentrations, however a cross-sectional study in men and women in Singapore reported an association between increased IGFBP-3 and high intake of n-3 fatty acids [13].
The effects of a low fat, high fiber diet on serum IGF-I, IGFBP-3, insulin, and glucose were the focus of an ancillary study involving 750 subjects who participated in the Polyp Prevention Trial (PPT) [32]. The investigators observed no significant differences in IGF-I, IGFBP-3, insulin or glucose concentrations at one year or four years follow-up in the intervention group compared to baseline, or the control group, although the intervention group achieved self-reported reductions in dietary fat from 35% of energy to 22.7% of energy and an increase in fiber intake from 18.9 g/day to 33.6 g/day at four years. The diets in the current study were controlled and not self-reported; therefore the results are not likely affected by reporting errors. There was also no change in IGF-I, IGFBP-1 and IGFBP-3 after a 12-month low fat/high fiber dietary intervention in premenopausal women conducted by Gann et al [33]. Neither the PPT authors nor Gann et al addressed n-3 fatty acid composition of the diets. Our low fat dietary intervention was much shorter in duration than both the PPT and Gann et al trial and it is unknown whether the increase in IGFBP-3 that we observed with the LF diet or the increases in IGF-I and IGFBP-3 that we observed with the LFn3 diet are transitory changes or whether concentrations could be altered long-term by a diet low in total fat and high in n-3 fatty acids. This point deserves further investigation in a longer-term trial. Others have also shown no association between low fat diet and omega-3 fatty acids and IGF-I [34, 35] although these studies were conducted in men with prostate cancer.
There was no indication that insulin sensitivity was altered by the test diets in this study, although the study subjects were healthy, non-diabetic individuals. However, insulin sensitivity-related endpoints did change following the multifactor intervention of the Diet and Androgens (DIANA) Randomized Trail [36]. The subjects in the DIANA trial intervention group were instructed to alter a number of dietary factors simultaneously and follow a Mediterranean/macrobiotic type diet, which among other aims, intended to reduce animal fat intake and increase n-3 fatty acids intake. Following 4 months of the intervention, the subjects reported decreased intake of animal protein, total fat (reduced from 37.1 to 30.8% of energy), animal fat, saturated fat, monounsaturated fat, cholesterol, and total energy and increased intake of vegetable protein, vegetable fat, polyunsaturated fat, and carbohydrates [37]. While IGF-I and IGFBP-3 concentrations were not altered in the intervention group, concentrations of IGFBP-1 and -2 were increased, and C-peptide and fasting glucose decreased. There was no change in fasting insulin with the intervention, although area under the curve of insulin during the oral glucose tolerance test was reduced in the intervention group [36]. Importantly, there was also a significant deficit in energy intake in the DIANA trial intervention group and significant changes attributed to the intervention were attenuated when adjustments for weight and waist circumference were applied. Therefore the effects observed with the intervention may be due more to weight loss and the accompanying body composition changes than to the change in dietary composition. Our subjects lost a small but statistically significant amount of weight with each dietary treatment; however the amount of weight lost and mean weights at 8 weeks of each treatment did not differ.
IGF-I concentrations decrease with protein and calorie restriction and with malnutrition [38]. Increasing protein intake in adequately-fed individuals may also affect IGF-I and IGF binding protein concentrations. Addition of a soy protein supplement to a low fat/high fiber dietary intervention or usual diet reduced IGFBP-3 concentrations and increased IGF-I:IGFBP-3 ratio in premenopausal women [33]. In post-menopausal women, addition of either a 40g/day milk protein or soy protein supplement to their usual diets increased circulating IGF-I concentrations, although the women concomitantly reduced their protein intake from other sources and ended the 3-month study with a similar total protein intake to baseline [39]. Similarly, IGF-I concentrations were significantly increased by consumption of soy protein supplements in healthy men [40] and prostate cancer patients [41]. In the present study, the diets were designed so that the percentage of energy from protein was equivalent (15% of energy) among the three test diets, therefore the significant changes that we observed in IGF-I (LFn3) and IGFBP-3 (LF and LFn3) were not due to differential protein intake.
IGF-I concentrations decrease with age, and the effects of aging (decreased bone mineral density and lean body tissue, increased fat mass, and a high-risk cardiovascular profile) mirror the symptoms of GH deficiency [6]. Increasing the concentration of IGF-I with pharmacological GH replacement is accompanied by a number of symptoms related to water retention [42], and therefore the possibility of raising IGF-I concentrations with a dietary intervention is desirable. Further studies employing the addition of n-3 fatty acids to the diet and assessment of IGF-I concentrations and effects on the symptoms of aging are warranted.
On the other hand, there have been reports of associations between IGF-I and breast cancer risk. This association appears to depend on menopausal status, with most studies showing no association between relatively increased IGF-I concentrations and breast cancer risk in postmenopausal women [43]. Current data do not suggest that the increase in IGF-I with the LFn3 diet in our postmenopausal female study participants is likely to increase risk of breast cancer.
Strengths of this study include the controlled nature of the study. Participants were provided with all food for each 8-week treatment; therefore diet was not self-reported. Also, participants crossed-over from one-treatment to another and this attributed increased statistical power for each participant.
This study is not without limitations. The results of this trial should be regarded with caution due to the small sample size. Even though our study was a cross-over trial, imparting stronger statistical power than if each subject had completed only one dietary treatment, the sample size is still regarded as being relatively small and differences in the endpoints measured may not have been detected because of the high variability and small sample size. Also, as previously mentioned, the length of each dietary intervention was 8-weeks, which may not be long enough to show long-term changes in the analytes.
The results of this controlled dietary intervention in postmenopausal women indicate that the addition of n-3 fatty acids to a low fat diet increases IGF-I and IGFBP-3 concentrations while low fat diet with no additional n-3 fatty acids increases IGFBP-3 only. The effects of increasing IGF-I concentrations on cancer risk is unknown, however, an increase in circulating IGF-I may be beneficial in preventing the reduced bone and lean mass associated with aging.
Acknowledgments
We would like to thank the subjects for their serious commitment to this study and the GCRC staff for their assistance with clinical work and preparation of the study diets. We thank Douglas Yee for his comments and review of the manuscript. Funding for this work was provided by grants from the Department of Defense (W81XWH-04-1-0448 and W81XWH-06-1-0778) and the National Center for Research Resources, National Institutes of Health (Mo1-RR00400). Salmon of the Americas, Inc. donated salmon fillets for the controlled diets.
Abbreviations
- GH
growth hormone
- IGF-I
insulin-like growth factor I
- IGFBP-3
IGF binding protein-3
- HOMA-IR
homeostatic model assessment-insulin resistance (fasting insulin (mU/L) x fasting glucose (mmol/L)/22.5)
Footnotes
The authors declare no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ibrahim YH, Yee D. Insulin-like growth factor-I and cancer risk. Growth Horm IGF Res. 2004;14:261–269. doi: 10.1016/j.ghir.2004.01.005. [DOI] [PubMed] [Google Scholar]
- 2.Martin MB, Stoica A. Insulin-like growth factor-I and estrogen interactions in breast cancer. J Nutr. 2002;132:3799S–3801S. doi: 10.1093/jn/132.12.3799S. [DOI] [PubMed] [Google Scholar]
- 3.Giovannucci E. Nutrition, insulin, insulin-like growth factors and cancer. Horm Metab Res. 2003;35:694–704. doi: 10.1055/s-2004-814147. [DOI] [PubMed] [Google Scholar]
- 4.Hadsell DL. The insulin-like growth factor system in normal mammary gland function. Breast Dis. 2003;17:3–14. doi: 10.3233/bd-2003-17102. [DOI] [PubMed] [Google Scholar]
- 5.Sachdev D, Yee D. Disrupting insulin-like growth factor signaling as a potential cancer therapy. Mol Cancer Ther. 2007;6:1–12. doi: 10.1158/1535-7163.MCT-06-0080. [DOI] [PubMed] [Google Scholar]
- 6.Toogood AA. The somatopause: An indication for growth hormone therapy? Treat Endocrinol. 2004;3:201–209. doi: 10.2165/00024677-200403040-00001. [DOI] [PubMed] [Google Scholar]
- 7.Hintz RL, Suskind R, Amatayakul K, Thanangkul O, Olson R. Plasma somatomedin and growth hormone values in children with protein-calorie malnutrition. J Pediatr. 1978;92:153–156. doi: 10.1016/s0022-3476(78)80099-7. [DOI] [PubMed] [Google Scholar]
- 8.Smith IF, Latham MC, Azubuike JA, Butler WR, Phillips LS, Pond WG, et al. Blood plasma levels of cortisol, insulin, growth hormone and somatomedin in children with marasmus, kwashiorkor, and intermediate forms of protein-energy malnutrition. Proc Soc Exp Biol Med. 1981;167:607–611. doi: 10.3181/00379727-167-41222. [DOI] [PubMed] [Google Scholar]
- 9.Smith WL, Underwood LE, Clemmons DR. Effects of caloric or protein restriction on insulin-like growth factor-I (IGF-I) and IGF-binding proteins in children and adults. J Clin Endocrinol Metab. 1995;80:443–9. doi: 10.1210/jcem.80.2.7531712. [DOI] [PubMed] [Google Scholar]
- 10.Pollak M. The question of a link between insulin-like growth factor physiology and neoplasia. Growth Horm IGF Res. 2000;10:S21–24. doi: 10.1016/s1096-6374(00)80005-2. [DOI] [PubMed] [Google Scholar]
- 11.Kaklamani VG, Linos Kaklamani AE, Markaki I, Koumantaki Y, Mantzoros CS. Dietary fat and carbohydrates are independently associated with circulating insulin-like growth factor 1 and insulin-like growth factor-binding protein 3 concentrations in healthy adults. J Clin Oncol. 1999;17:3291–3298. doi: 10.1200/JCO.1999.17.10.3291. [DOI] [PubMed] [Google Scholar]
- 12.Nagata C, Shimizu H, Takami R, Hayashi M, Takeda N, Yasuda K. Dietary soy and fats in relation to serum insulin-like growth factor-1 and insulin-like growth factor-binding protein-3 levels in premenopausal Japanese women. Nutr Cancer. 2003;45:185–189. doi: 10.1207/S15327914NC4502_07. [DOI] [PubMed] [Google Scholar]
- 13.Probst-Hensch NM, Wang H, Goh VH, Seow A, Lee HP, Yu MC. Determinants of circulating insulin-like growth factor I and insulin-like growth factor binding protein 3 concentrations in a cohort of Singapore men and women. Cancer Epidemiol Biomarkers Prev. 2003;12:739–746. [PubMed] [Google Scholar]
- 14.Holmes MD, Pollak MN, WC, Willett WC, Hankinson SE. Dietary correlates of plasma insulin-like growth factor I and insulin-like growth factor binding protein 3 concentrations. Cancer Epidemiol Biomarkers Prev. 2002;11:852–61. [PubMed] [Google Scholar]
- 15.Norat T, Dossus L, Rinaldi S, Overvad K, Grønbaek H, Tjønneland A, et al. Diet, serum insulin-like growth factor-I and IGF-binding protein-3 in European women. Eur J Clin Nutr. 2007;61:91–8. doi: 10.1038/sj.ejcn.1602494. [DOI] [PubMed] [Google Scholar]
- 16.Crowe FL, Key TJ, Allen NE, Appleby PN, Roddam A, Overvad K, et al. The association between diet and serum concentrations of IGF-I, IGFBP-1, IGFBP-2, and IGFBP-3 in the european prospective investigation into cancer and nutrition, Cancer Epidemiol. Biomarkers Prev. 2009;18:1333–1340. doi: 10.1158/1055-9965.EPI-08-0781. [DOI] [PubMed] [Google Scholar]
- 17.Allen NE, Appleby PN, Davey GK, Kaaks R, Rinaldi S, Key TJ. The associations of diet with serum insulin-like growth factor I and its main binding proteins in 292 women meat-eaters, vegetarians, and vegans. Cancer Epidemiol Biomarkers Prev. 2002;11:1441–1448. [PubMed] [Google Scholar]
- 18.Baxter RC, Turtle JR. Regulation of hepatic growth hormone receptors by insulin. Biochem Biophys Res Commun. 1978;84:350–357. doi: 10.1016/0006-291x(78)90177-8. [DOI] [PubMed] [Google Scholar]
- 19.Mayer EJ, Newman B, Quesenberry CP, Selby JV. Usual dietary fat intake and insulin concentrations in healthy women twins. Diabetes Care. 1993;16:1459–1469. doi: 10.2337/diacare.16.11.1459. [DOI] [PubMed] [Google Scholar]
- 20.Parker DR, Weiss ST, Troisi R, Cassano PA, Vokonas PS, Landsberg L. Relationship of dietary saturated fatty acids and body habitus to serum insulin concentrations: The normative aging study. Am J Clin Nutr. 1993;58:129–36. doi: 10.1093/ajcn/58.2.129. [DOI] [PubMed] [Google Scholar]
- 21.Lovejoy J, DiGirolamo M. Habitual dietary intake and insulin sensitivity in lean and obese adults. Am J Clin Nutr. 1992;55:1174–1179. doi: 10.1093/ajcn/55.6.1174. [DOI] [PubMed] [Google Scholar]
- 22.Fukagawa NK, Anderson JW, Hageman G, Young VR, Minaker KL. High-carbohydrate, high-fiber diets increase peripheral insulin sensitivity in healthy young and old adults. Am J Clin Nutr. 1990;52:524–528. doi: 10.1093/ajcn/52.3.524. [DOI] [PubMed] [Google Scholar]
- 23.Lovejoy JC, Windhauser MM, Rood JC, de la Bretonne JA. Effect of a controlled high-fat versus low-fat diet on insulin sensitivity and leptin levels in African-American and Caucasian women. Metabolism. 1998;47:1520–1524. doi: 10.1016/s0026-0495(98)90080-4. [DOI] [PubMed] [Google Scholar]
- 24.Straznicky NE, O’Callaghan CJ, Barrington VE, Louis WJ. Hypotensive effect of low-fat, high-carbohydrate diet can be independent of changes in plasma insulin concentrations. Hypertension. 1999;34:580–585. doi: 10.1161/01.hyp.34.4.580. [DOI] [PubMed] [Google Scholar]
- 25.Tymchuk CN, Tessler SB, Barnard RJ. Changes in sex hormone-binding globulin, insulin, and serum lipids in postmenopausal women on a low-fat, high-fiber diet combined with exercise. Nutr Cancer. 2000;38:158–162. doi: 10.1207/S15327914NC382_3. [DOI] [PubMed] [Google Scholar]
- 26.Hall WD, Feng Z, George VA, Lewis CE, Oberman A, Huber M, et al. Low-fat diet: Effect on anthropometrics, blood pressure, glucose, and insulin in older women. Ethn Dis. 230(13):337–343. [PubMed] [Google Scholar]
- 27.Young LR, Kurzer MS, Thomas W, Redmon JB, Raatz SK. Effects of dietary fat and omega-3 fatty acid intake on urinary eicosanoids and sex hormone levels in postmenopausal women: A randomized controlled feeding trial. Nutr Cancer. 2011;63:930–939. doi: 10.1080/01635581.2011.589957. [DOI] [PubMed] [Google Scholar]
- 28.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: Insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 29.Duncan MH, Singh BM, Wise PH, Carter G, Alaghband-Zadeh J. A simple measure of insulin resistance. Lancet. 1995;346:120–1. doi: 10.1016/s0140-6736(95)92143-5. [DOI] [PubMed] [Google Scholar]
- 30.World Health Organization. World Health Organization technical report series 724. Geneva, Switzerland: 1985. Energy and protein requirements. Report of a joint FAO/WHO/UNU expert consultation. [PubMed] [Google Scholar]
- 31.Lee HY, Chang YS, Han JY, Liu DD, Lee JJ, Lotan R, et al. Effects of 9-cis-retinoic acid on the insulin-like growth factor axis in former smokers. J Clin Oncol. 2005;23:4439–4449. doi: 10.1200/JCO.2005.04.572. [DOI] [PubMed] [Google Scholar]
- 32.Flood A, Mai V, Pfeiffer R, Kahle L, Remaley AT, Rosen CJ, et al. The effects of a high-fruit and -vegetable, high-fiber, low-fat dietary intervention on serum concentrations of insulin, glucose, IGF-I and IGFBP-3. Eur J Clin Nutr. 2008;62:186–196. doi: 10.1038/sj.ejcn.1602726. [DOI] [PubMed] [Google Scholar]
- 33.Gann PH, Kazer R, Chatterton R, Gapstur S, Thedford K, Helenowski I, et al. Sequential, randomized trial of a low-fat, high-fiber diet and soy supplementation: Effects on circulating IGF-I and its binding proteins in premenopausal women. Int J Cancer. 2005;116:297–303. doi: 10.1002/ijc.21042. [DOI] [PubMed] [Google Scholar]
- 34.Chan JM, Weinberg V, Magbanua MJ, Sosa E, Simko J, Shinohara K, Federman S, et al. Nutritional supplements, COX-2 and IGF-1 expression in men on active surveillance for prostate cancer. Cancer Causes Control. 2011;1:141–150. doi: 10.1007/s10552-010-9684-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Aronson WJ, Kobayashi N, Barnard RJ, Henning S, Huang M, Jardack PM, et al. Phase II porspective randomized trial of a low-fat diet with fish oil supplementation in men undergoing radical prostatectomy. Cancer Prev Res (Philla) 2011;12:2062–2071. doi: 10.1158/1940-6207.CAPR-11-0298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kaaks R, Bellati C, Venturelli E, Rinaldi S, Secreto G, Biessy C, et al. Effects of dietary intervention on IGF-I and IGF-binding proteins, and related alterations in sex steroid metabolism: The diet and androgens (DIANA) randomised trial. Eur J Clin Nutr. 2003;57:1079–1088. doi: 10.1038/sj.ejcn.1601647. [DOI] [PubMed] [Google Scholar]
- 37.Berrino F, Bellati C, Secreto G, Camerini E, Pala V, Panico S, et al. Reducing bioavailable sex hormones through a comprehensive change in diet: The diet and androgens (DIANA) randomized trial. Cancer Epidemiol Biomarkers Prev. 2002;10:25–33. [PubMed] [Google Scholar]
- 38.Thissen JP, Ketelslegers JM, Underwood LE. Nutritional regulation of the insulin-like growth factors. Endocr Rev. 1994;15:80–101. doi: 10.1210/edrv-15-1-80. [DOI] [PubMed] [Google Scholar]
- 39.Arjmandi BH, Khalil DA, Smith BJ, Lucas EA, Juma S, Payton ME, et al. Soy protein has a greater effect on bone in postmenopausal women not on hormone replacement therapy, as evidenced by reducing bone resorption and urinary calcium excretion. J Clin Endocrinol Metab. 2003;88:1048–1054. doi: 10.1210/jc.2002-020849. [DOI] [PubMed] [Google Scholar]
- 40.Khalil DA, Lucas EA, Juma S, Smith BJ, Payton ME, Arjmandi BH. Soy protein supplementation increases serum insulin-like growth factor-I in young and old men but does not affect markers of bone metabolism. J Nutr. 2002;132:2605–2608. doi: 10.1093/jn/132.9.2605. [DOI] [PubMed] [Google Scholar]
- 41.Spentzos D, Mantzoros C, Regan MM, Morrissey ME, Duggan S, Flickner-Garvey S, et al. Minimal effect of a low-fat/high soy diet for asymptomatic, hormonally naive prostate cancer patients. Clin Cancer Res. 2003;9:3282–3287. [PubMed] [Google Scholar]
- 42.Carroll PV, Christ ER, Bengtsson BA, Carlsson L, Christiansen JS, Clemmons D, et al. Growth hormone deficiency in adulthood and the effects of growth hormone replacement: A review. J Clin Endocrinol Metab. 1998;83:382–395. doi: 10.1210/jcem.83.2.4594. [DOI] [PubMed] [Google Scholar]
- 43.Renehan AG, Zwahlen M, Minder C, O’Dwyer ST, Shalet SM, Egger M. Insulin-like growth factor (IGF)-I, IGF binding protein-3, and cancer risk: Systematic review and meta-regression analysis. Lancet. 2004;363:1346–1353. doi: 10.1016/S0140-6736(04)16044-3. [DOI] [PubMed] [Google Scholar]