SYNOPSIS
The inflammatory pustular dermatoses constitute a spectrum of non-infectious conditions ranging from localized involvement to generalized disease with associated acute systemic inflammation and multi-organ involvement. Despite the variability in extent and severity of cutaneous presentation, each of these diseases is characterized by non-infectious neutrophilic intra-epidermal microabscesses. Many share systemic findings including fever, elevated inflammatory markers, inflammatory bowel disease and/or osteoarticular involvement, suggesting potential common pathogenic links (Figure 1). The recent discoveries of several genes responsible for heritable pustular diseases have revealed a distinct link between pustular skin disease and regulation of innate immunity. These genetic advances have led to a deeper exploration of common pathways in pustular skin disease and offer the potential for a new era of biologic therapy which targets these shared pathways.
This chapter provides a new categorization of inflammatory pustular dermatoses in the context of recent genetic and biologic insights. We will discuss recently-described monogenic diseases with pustular phenotypes, including deficiency of IL-1 receptor antagonist (DIRA), deficiency of the IL-36 receptor antagonist (DITRA), CARD14-associated pustular psoriasis (CAMPS), and pyogenic arthritis, pyoderma gangrenosum, acne (PAPA). We will then discuss how these new genetic advancements may inform how we view previously described pustular diseases, including pustular psoriasis and its clinical variants, with a focus on historical classification by clinical phenotype.
Keywords: Pustular psoriasis, Palmoplantar pustulosis, Subcorneal pustular dermatosis, Deficiency of IL-1 receptor antagonist (DIRA), PAPA syndrome, SAPHO syndrome
DIRA
In 2009, Goldbach-Mansky and colleagues described an autosomal recessive autoinflammatory disorder known as deficiency of the interleukin-1 (IL-1) receptor antagonist (DIRA)1–6. DIRA is caused by homozygous loss of function mutations in IL1RN, the gene encoding the IL-1 receptor antagonist. Mutations lead to unopposed IL-1 signaling and resultant uncontrolled life-threatening systemic inflammation. Heterozygous carriers of loss of function mutations in IL1RN appear to be asymptomatic1–6. To date, fewer than 20 cases from the United States, Canada, the Netherlands, Brazil and Puerto Rico have been described. First generation mutations in these distinct geographic populations are believed to be founder mutations. The allele frequencies of the founder mutations in Newfoundland and Puerto Rico are estimated at 0.2% and 1.3%, respectively.
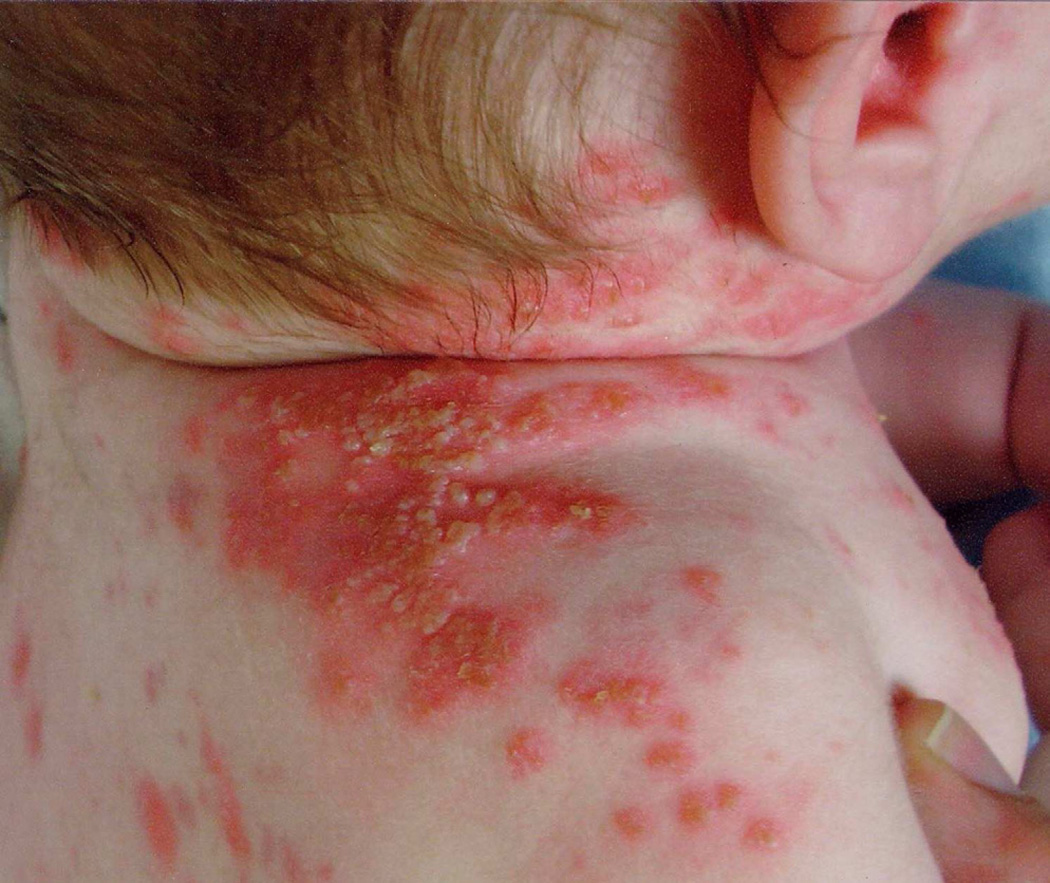
DIRA is characterized by perinatal onset pustular dermatitis resembling pustular psoriasis, multifocal aseptic osteomyelitis, periostitis, leukocytosis and elevated acute phase reactants. Affected individuals present between birth and 2.5 weeks of age with fetal distress, pustular rash, joint swelling, oral lesions and pain with movement. Premature birth is sometimes noted. Fever is typically absent. Cutaneous eruptions range from discrete crops of pustules to generalized pustulosis (Figure 2). Ichthyosiform changes can be seen. Nail changes include pitting and onychomadesis. Respiratory insufficiency and thrombotic events have also been reported. Bony changes include epiphyseal ballooning of long bones, anterior rib-end widening, periosteal elevation of long bones, and multifocal osteolytic lesions. Less commonly, widening of the clavicles, metaphyseal erosions and osteolytic skull lesions can be seen2. Mortality secondary to multi-organ failure in the setting of severe inflammatory response and pulmonary hemosiderosis with progressive interstitial fibrosis has been reported. Laboratory abnormalities include leukocytosis and marked elevation of serum erythrocyte sedimentation rate and C-reactive protein levels. Cutaneous histopathology is characterized by epidermal acanthosis and hyperkeratosis, extensive neutrophilic infiltration of epidermis and dermis by neutrophils and pustule formation1.
Figure 2. Deficiency of IL-2 receptor antagonist (DIRA).
Bright red plaques studded with crops of pustules in an infant. Photo courtesy of Raphaela Goldbach-Mansky.
The differential diagnosis of DIRA includes bacterial osteomyelitis, infantile cortical hyperostosis, infantile pustular psoriasis and chronic recurrent multifocal osteomyelitis (CRMO). Genetic sequencing is required for definitive diagnosis. DIRA can be effectively treated with the IL-1 receptor antagonist anakinra1, 5, 7, suggesting a potentially important role for IL-1 antagonism in the management of diseases with pustular phenotype. Individuals with less deleterious mutations have also been managed with corticosteroids and acitretin2. Given the severity of disease and availability of effective therapy, the key to successful management and reduced morbidity and mortality is early recognition of this condition and early implementation of anti-IL-1 therapy prior to the development of irreversible bony lesions, respiratory disease or other life-threatening events.
DITRA, a monogenic form of pustular psoriasis
Although the majority of pustular psoriasis patients lack a family history of similar disease, a number of familial cases have been reported, leading to a potential insight into common pathways of pustular skin disease8–15. In 2011, Marrakchi and colleagues identified inactivating mutations in the IL-36 receptor antagonist (IL-36RN) gene in 9 Tunisian families with an autosomal recessive form of generalized pustular psoriasis (GPP)13. This disease, known as deficiency of the IL-36 receptor antagonist (DITRA), was also reported in a second group of 5 individuals from the United Kingdom who did not have a family history of GPP14, as well as in one Japanese adult male16. IL-36 is an IL-1 family cytokine that binds to the IL-36 receptor, enabling the recruitment of the IL-1 receptor accessory protein and subsequent signal transduction involving nuclear factor kappa B (NFkB) and mitogen activated protein (MAP) kinases. The IL-36 receptor antagonist (IL-36RA) is encoded on chromosome 2 and competitively binds the IL-36 receptor, thereby providing negative feedback to IL-36 signaling13, 14. Deficiency of IL-36RA leads to an exaggerated inflammatory response, analogous to that resulting from IL-1RA deficiency in patients with DIRA, and further implicates the relevance of these pathways in pustular disease pathogenesis.
DITRA is characterized by sudden-onset, recurrent and severe GPP, high-grade fever, asthenia, neutrophilia and elevated inflammatory markers. While disease onset occurs during childhood in the majority of cases, adult-onset has also been described. Death due to septicemia has been reported in 5 cases13. Of note, concomitant psoriasis vulgaris, palmoplantar pustulosis, or psoriatic arthritis have not been reported, supporting the assertion that IL36RN mutations are responsible for a specific subtype of GPP. Histopathological studies have shown spongiform pustules, acanthosis with elongation of rete ridges and parakeratosis in the stratum corneum, consistent with histopathology of pustular psoriasis13. The differential diagnosis of DITRA includes generalized pustular psoriasis and acute generalized exanthematous pustulosis (AGEP). Acitretin, topical and oral steroids, adalimumab and phototherapy have been used with varying degrees of success in patients with DITRA13.
CAMPS
De novo mutations in caspase recruitment domain family member 14 (CARD14) have also been described in a monogenic form of childhood GPP called CARD14-mediated pustular psoriasis (CAMPS)9, 10. CARD14 mutations have also been previously implicated in plaque-type psoriasis and pityriasis rubra pilaris (PRP)9, 10, 17. CARD14 activates NFkB. Activating missense mutations in CARD14 upregulate NFkB, subsequently increasing the transcription of psoriasis-associated chemokines and cytokines, including IL-8, CCL20 and IL-3610.
CAMPS is characterized by childhood-onset generalized pustulosis, fevers, palmoplantar keratoderma and nail pitting. Psoriatic arthritis has not been described in CAMPS, although it has been reported in CARD14-associated plaque psoriasis and PRP9, 10. Poor response to methotrexate, cyclosporine, infliximab and anakinra have been reported, however, one case of CAMPS has been successfully managed with IL-12/23 antagonist ustekinumab18.
PAPA SYNDROME
The syndrome of pyogenic arthritis, pyoderma gangrenosum and acne (PAPA) was coined by Lindor and colleagues in 199719, although it was first described in 1975 in a patient with “streaking leukocyte factor”20. This rare autosomal dominant autoinflammatory syndrome is caused by mutations in the gene encoding CD2-binding protein 1, also known as proline-serine-threonine-phophatase-interacting protein1 (PSTPIP1). Mutations in PSTPIP1 lead to hyperphosphorylation of the protein and subsequent increased binding affinity to pyrin, thereby causing dysregulation of IL-1β production21–24. Genotype analyses of families have demonstrated variable penetrance, including genetic carriers without symptoms and others with typical PAPA syndrome features within the same family25.
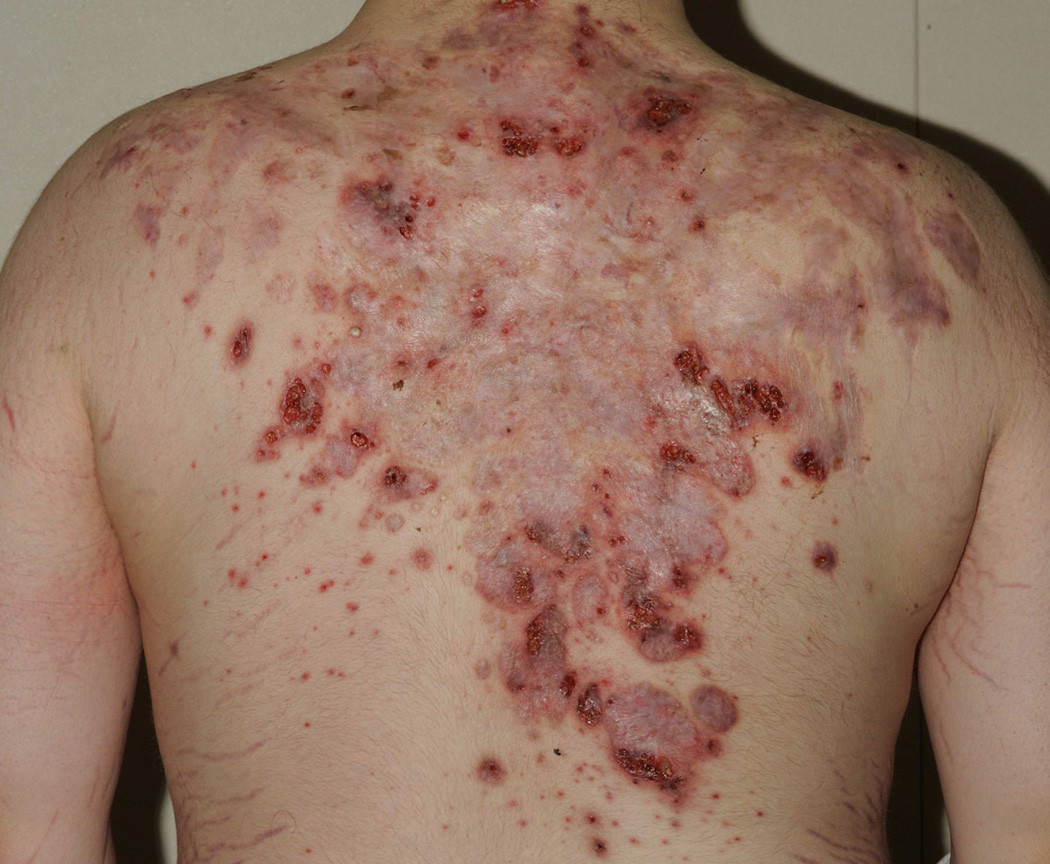
Clinically, PAPA syndrome is characterized by aseptic inflammation of the skin and joints. Painful, recurrent, sterile monoarticular arthritis with prominent neutrophilic infiltrate usually occurs in the first decade of life and may be the presenting sign of disease. Elbows, knees and ankles are most often involved, and the natural history of persistent disease leads to significant joint destruction. Skin involvement is variable. Pathergy is a commonly observed phenomenon and pustule formation followed by ulceration may be induced early in life upon vaccination or minimal trauma. (Figure 3) Severe nodulocystic acne and pyoderma gangrenosum tend to develop around puberty and may persist into adulthood19,20,26–32. Cystic acne persisting until the 7th decade of life has been reported19. Hidradenitis suppurativa has also been reported in a few cases, but is not a consistent clinical feature19. Other dermatologic manifestations described in the setting of PAPA include rosacea and psoriasis30. Sulfonamide-induced pancytopenia has also been reported in 23–40% of PAPA patients, although the significance of these observations is not well understood19,32.
Figure 3. PAPA syndrome.
Cribiform pyoderma gangrenosum ulcers in the setting of severe scarring from acne conglobata.
Laboratory findings in PAPA syndrome reflect a systemic inflammatory state with leukocytosis and elevated acute phase reactants, but are otherwise non-diagnostic. Elevations in IL-1β and TNFα production in peripheral blood leukocytes have been observed. Infectious processes, vasculopathy, coagulopathy and autoimmune disease should be ruled out in the evaluation of patients. Histopathology of cystic acne lesions reveals distended follicles with cystic spaces and follicular openings filled with keratinaceous debris and numerous bacteria. Ruptured cystic contents induce a brisk neutrophilic inflammatory infiltrate surrounding expanded follicles. Histopathology of pyoderma gangrenosum is similar to that seen with pyoderma gangrenosum in other settings. Early lesions demonstrate a neutrophilic vascular infiltrate. Actively expanding lesions demonstrate neutrophilic infiltrates with leukocytoclasia. Marked tissue necrosis with surrounding mononuclear cell infiltrates are seen in ulcers. Synovial fluid aspirations demonstrate sterile neutrophil-predominant infiltrate30.
The differential diagnosis for articular manifestations of PAPA syndrome includes monoarticular septic arthritis and, in children, chronic multifocal osteomyelitis (CRMO). The presence of both severe acne and PG, or the presence of PAPA findings in family members, should prompt consideration of PAPA syndrome, SAPHO syndrome and pyoderma gangrenosum secondary to underlying inflammatory bowel disease. Pyoderma, acne and suppurative hidradenitis (PASH) syndrome is a recently described autoinflammatory disorder that may also be considered in the differential diagnosis of PAPA syndrome33. Although mutations in PSTPIP1 were not found in the 2 reported patients with PASH, heterozygous repetition of the CCTG microsatellite motif in the PSTPIP1 promoter were detected. The significance of these findings is unknown. In contrast to PAPA syndrome, joint involvement was not reported in the 2 reported patients with PASH.
Medications targeting IL-1 and TNFα have been most successful in managing the manifestations of PAPA syndrome. The most consistent responses have been observed with the anti-TNFα antagonists etanercept, adalimumab and infliximab27,34–36. Response to anti-IL-1 agent, anakinra, has been more variable26, 28, 34, but appears to be more effective in the management of joint manifestations rather than cutaneous disease28. Joint disease is also responsive to corticosteroids, however acne may be exacerbated with systemic therapy. Joint effusions may also be surgically managed with drainage and/or intra-articular steroid injection. Combination therapy with anakinra and anti-TNF agents is associated with increased risk of infection, and should be undertaken with caution. Topical and systemic retinoids have been effective in combination with biologic agents for the management of cystic acne. Patients should be counseled regarding the pathergic component of disease, and advised to avoid trauma to the integument.
SAPHO SYNDROME
Since the 1960s, associations between pustular dermatologic manifestations and osteoarticular manifestations have been reported under various designations including pustulotic arthro-osteitis, sternocostoclavicular hyperostosis, acne-associated spondyloarthropathy, acquired hyperostosis syndrome, and chronic recurrent multifocal osteomyelitis. In 1987, Chamot and colleagues proposed a unifying syndrome known as synovitis, acne, pustulosis, hyperostosis, osteitis (SAPHO) syndrome37. The prevalence of this rare syndrome is estimated to be less than 4 in 10,000, with a female predilection. SAPHO can present at any age, however onset is most common in children and young adults. A similar condition first described in 1972, known as chronic recurrent multifocal osteomyelitis (CRMO), is now largely thought to represent a subset of SAPHO which predominates in the pediatric population38. CRMO, congenital dyserythropoietic anemia, and Sweets syndrome are features of Majeed syndrome, a very rare autosomal recessive disorder reported in 3 Middle Eastern families39–41. Other associated findings include fevers and growth failure. Mutations in the LPIN2 gene, which encodes lipin-2, are implicated in Majeed syndrome42. Lipin-2 is a protein which plays a role in lipid metabolism, however lipid abnormalities have not been reported in patients with Majeed syndrome. The genetic basis of SAPHO/CRMO is still unknown.
Osteoarticular manifestations are the hallmark of SAPHO syndrome, and occur regardless of the presence of dermatologic manifestations37. The most commonly involved areas include the anterior chest wall (65–90%) followed by the spine (30%), particular the thoracic spine. Sacroileitis may also occur, and mandibular lesions can be seen in up to 10% of adults43, 44. Long bone involvement is rare in adults, but involvement of the tibia and femur is common in children45, 46. Synovitis distant from sites of bony involvement is seen in up to 30% of adults, but rarely noted in children46. Surrounding soft tissue swelling and erythema can also develop, and morning stiffness is common. The essential features of SAPHO syndrome, including osteitis, hyperostosis and palmoplantar pustulosis (PPP), can also be seen in CRMO; however, bony involvement in CRMO frequently affects the metaphyses of tubular bones, a feature not typically seen in SAPHO47.
Dermatologic manifestations of SAPHO may occur concurrently, before or after osteoarticular disease, and are more frequent in adults than in children48–50. Seventy percent of children and 15% of adults never exhibit cutaneous disease. Cutaneous lesions consist of neutrophilic pustular dermatoses. Palmoplantar pustulosis is most common, affecting up to 60% of patients who develop dermatologic manifestations48. Acne conglobata and acne fulminans occur in approximately 25% of patients, with a notable male predominance48. Similarly, hidradenitis suppurativa may also be seen and predominates in men51, 52. Rarely, pyoderma gangrenosum and Sweet syndrome may occur53–55. Although systemic manifestations are uncommon in SAPHO syndrome, fever and moderate elevations in acute phase reactants may occur, and up to 10% of patients with SAPHO syndrome develop inflammatory bowel disease, most commonly after the onset of SAPHO symptoms46, 48, 53, 56. Histopathology of SAPHO-associated PPP is identical to that of PPP.
The differential diagnosis for the osteoarticular manifestations of SAPHO syndrome includes bacterial osteomyelitis, primary bone tumors, metastatic disease and eosinophilic granuloma. SAPHO syndrome should be considered in patients with palmoplantar pustulosis and/or nodulocystic acne in conjunction with a history of bony pain, particularly anterior chest wall pain. Dermatologic and osteoarticular findings should also be evaluated in patients with inflammatory bowel disease. Many individual signs and symptoms of SAPHO syndrome are nonspecific, and therefore it is a diagnosis of exclusion. Although validated diagnostic criteria do not exist, inclusion criteria for diagnosis have been proposed57. (Table 1) The presence of 1 of the 4 listed inclusion criteria is sufficient for diagnosis of SAPHO syndrome. Diagnostic criteria have also been established for CRMO and nonbacterial osteitis. (Table 2) The diagnosis of Majeed syndrome can be established by genetic evaluation performed in the setting of CRMO, anemia and inflammatory neutrophilic dermatosis.
Table 1.
Inclusion and exclusion criteria for SAPHO syndrome57
| Inclusion criteria | Osteo-articular manifestations of acne conglobata, acne fulminans, or hidradenitis suppurativa (i.e., inflammatory synovitis, anterior chest wall hyperostosis or osteitis, hyperostosis or osteitis at another site,or spondylitis or spondylodiscitis) |
| Osteo-articular manifestations of palmoplantar pustulosis (i.e., inflammatory synovitis, hyperostosis +/− osteitis, or spondylitis or spondylodiscitis ) | |
| Hyperostosis (of the anterior chest wall, limbs or spine) with or without dermatosis | |
| CRMO involving the axial or peripheral skeleton with or without dermatosis | |
| Exclusion criteria | Septic osteomyelitis |
| Infectious chest wall arthritis | |
| Infectious palmoplantar pustulosis | |
| Palmoplantar keratoderma | |
| Diffuse idiopathic skeletal hyperostosis (DISH), except for fortuitous association | |
| Osteoarticular manifestations of retinoid therapy | |
| Other reported features | Possible association with psoriasis vulgaris |
| Possible association with an inflammatory enterocolopathy | |
| Features of ankylosing spondylitis | |
| Presence of low-virulence bacterial infections | |
The presence of 1 of the 4 inclusion criteria is sufficient for diagnosis of SAPHO syndrome.
Table 2.
Proposed criteria for CRMO and non bacterial osteitis56
| Major diagnostic criteria | Minor diagnostic criteria |
|---|---|
|
|
Two major criteria or one major and three minor criteria is sufficient for diagnosis of CRMO.
Although the disease course is highly variable between individuals, the prognoses for SAPHO syndrome and CRMO is relatively good, and disabling complications are rare 48, 53. Peripheral arthritis may lead to erosive joint disease in adults. Adults may also suffer from bony deformities and limb length discrepancies. CRMO is generally considered to be a self-limiting condition with healing of sclerotic bone lesions and normalization of bone within 5 years of disease remission58.
The pathogenesis of SAPHO is poorly understood and, as such, a variety of therapies have been employed with variable benefit. Nonsteroidal anti-inflammatory agents and intra-articular corticosteroids have been useful in the management of joint inflammation37, 48. Bisphosphonates may achieve pain relief and sustained remission of bony disease, presumably due to inhibition of bone resorption and anti-inflammatory properties, however responses are variable 59–63. Systemic corticosteroids in combination with disease modifying anti-rheumatic drugs (DMARDs) such as methotrexate and azathioprine have been used with some benefit for the management of bony and cutaneous disease48, 56. Anti-TNFα agents including etanercept, adalimumab and infliximab have shown promise in the management of bone disease in SAPHO syndrome, with generally rapid improvement in bone pain as early as after the first treatment64–67. TNFα blockade has also been beneficial for the management of pustular dermatoses associated with SAPHO syndrome. Notably, however, the development of SAPHO syndrome was reported in a patient with inflammatory bowel disease treated with infliximab68. The paradoxical induction of skin diseases treated with anti-TNFα agents is a well-known phenomenon69. In 6 of 7 patients with SAPHO, improvement in cutaneous and bony manifestations have been reported with the IL-1 antagonist anakinra, suggesting a potential targeted therapy for this syndrome70, 71.
PUSTULAR PSORIASIS AND CLINICAL VARIANTS
Overview
Genetic advances in monogenic pustular diseases such as DIRA and DITRA have implicated IL-1 family proteins in the development of pustular disease, and have led to the successful use of targeted IL-1 blocking agents for these conditions. These insights may provide clues to the pathogenesis of other pustular dermatoses which are thought to have a polygenic etiology, such as pustular psoriasis and its variants, and to the potential application of similar targeted therapy for these recalcitrant skin conditions.
Pustular psoriasis is characterized by non-infective macroscopic pustules. In 85% of patients, typical plaque psoriasis precedes the appearance of generalized pustular psoriasis (GPP)72. Twenty-five percent of children with pustular psoriasis have a family history of psoriasis, suggesting a strong genetic component. In adults, pustular psoriasis is much less common than plaque-type psoriasis, with an estimated prevalence of 0.1% in the general population and a female predominance (F:M 3:2)73, 74. In children, however, a male predominance is seen (M:F 3:2). Male children have an earlier age of disease onset (3.5 months of age), as compared with females (8 years of age)75. Although pustular psoriasis has long been thought to be a variant of psoriasis, pustular psoriasis and its variants have distinct HLA susceptibility loci that suggest that these two categories of disease may be genetically distinct76, 77. Whereas skin manifestations of psoriasis vulgaris are strongly associated with HLA-Cw6, as well as HLA-DRB1*0701/2, HLA-B13, HLA-B17 and HLA-B3778, these alleles are not associated with GPP, acrodermatitis continua of Hallopeau (ACH) or palmoplantar pustulosis (PPP)77, 79. Peripheral psoriatic arthritis alone has been associated with HLA-B27, however this allele is most strongly associated with radiographic sacroileitis80. HLA-B27 is also associated with GPP and ACH in the setting of axial arthritis77, 81, as well as PPP in the context of SAPHO syndrome54, 82 and reactive arthritis, which is characterized by uveitis, urethritis and spondyloarthritis83.
In 1968, Baker and Ryan described two distinct clinical presentations of pustular psoriasis73, 84. The first group was characterized by onset of plaque psoriasis before age 40. Individuals developed pustular psoriasis following exposure to a precipitating factor (List 1), and lesions tended to resolve with a return to more classic plaque-type psoriasis following treatment. In contrast, the second group was characterized by disease onset later in life, with a peak incidence between ages 41 and 60 years73. Initial psoriatic lesions in this group tended to occur in atypical distributions such as the flexural areas, palmoplantar surfaces and/or the fingertips. These lesions were followed shortly thereafter by pustular lesions that progressed rapidly, often with accompanying systemic manifestations including fever and leukocytosis. Of note, a significant subset of this group presented with pustular psoriasis without antecedent plaque disease. External precipitating factors were less common in this group, and individuals typically had significant morbidity and mortality attributable to both the aggressiveness of the disease and advanced age of some patients. Baker and Ryan also noted that localized and generalized forms of pustular psoriasis could overlap with one another over time73.
List 1. Precipitants of pustular psoriasis.
| Infection (most notably Streptococcal infection, vaccines*) |
Drugs
|
| Pregnancy |
| Menstruation |
| Stress |
| Surgery |
| Alcohol |
| Sunlight, sunburn |
| Exertion |
| Seasonal variation |
| Birch pollen* |
| Milk |
| Pork |
| * denotes precipitant associated with juvenile GPP |
Pustular psoriasis can also be divided into two phenotypic categories, localized and generalized disease. Other more specific classification systems have been proposed, however one system has not been uniformly utilized73, 84, 85.
Generalized pustular psoriasis
Generalized pustular psoriasis (GPP) consists of von Zumbusch type generalized pustular psoriasis and annular pustular psoriasis. In pregnant women, von Zumbusch type GPP is termed impetigo herpetiformis. Subcorneal pustular dermatosis (SCPD) is a controversial variant of pustular psoriasis characterized by superficial pustules forming annular and gyrate patterns.
von Zumbusch-type generalized pustular psoriasis
In 1910, Leo Ritter von Zumbusch described a fulminant variant of GPP characterized by sudden-onset, inflamed plaques studded with sterile pustules86. Clinical diagnostic criteria for GPP have been proposed87. (Table 3)
Table 3.
Diagnostic criteria for generalized pustular psoriasis87
| All 5 criteria must be met for diagnosis. |
|---|
|
The first manifestations of von Zumbusch type GPP are typically fever and leukocytosis for 1–2 days, followed by skin edema and erythema88. Pustules appear shortly thereafter within erythematous plaques in crops which coalesce into ‘lakes of pus.’ Generalized erythroderma may occur. Skin pain, burning, diaphoresis and pruritus are common symptoms. Systemic findings, including malaise, fatigue, anorexia and arthritis parallel skin disease in adults. Arthritis develops in approximately one-third of patients, often involving the distal interphalangeal joints but may also involve the sacroiliac and other joints73. Joint disease is uncommon in children with pustular psoriasis89, 90. Nail findings typical of psoriasis, including hypertrophy, thickening, subungual pustulation, nail pitting and onycholysis, are commonly seen in von Zumbusch type GPP 11, 89–91. Although cutaneous infections are uncommon, pulmonary infections, pyogenic abcesses and cystitis may occur88.
Oral findings in von Zumbusch type GPP include migratory arcuate and circinate plaques on the dorsal tongue consistent with geographic tongue. Similar findings may occur on the buccal mucosa92, 93. Some speculate that these oral manifestations may represent a forme fruste of pustular psoriasis92, while others believe oral mucosal manifestations are a harbinger of GPP91, 94. The most commonly reported ocular manifestation is purulent sterile conjunctivitis11, 12, 95, 96, however iridocyclitis12, corneal ulceration12 and corneal exfoliation97 may develop.
Leukocytosis is the most common laboratory abnormality in von Zumbusch type GPP. Decreased serum calcium concentration is a result of hypoalbuminemia73, 98. Ionized calcium is typically normal and patients are therefore not symptomatic. Elevated alkaline phosphatase, transaminases and bilirubin may also be seen73, 95. Absolute hypovolemia secondary to fluid losses may lead to pre-renal azotemia91, 98. Gram positive septicemia and herpes simplex virus conjunctivitis have been reported in juvenile cases75. Mortality in patients with von Zumbusch type GPP is most commonly due to cardiac failure, sepsis or acute respiratory distress syndrome84,99–102.
The course of von Zumbusch type GPP is episodic, and symptoms may recur several times a year or only upon exposure to precipitants. Cutaneous and systemic symptoms may spontaneously remit or may require prolonged therapy to induce remission. Despite the significant morbidity associated with acute episodes of von Zumbusch type GPP in children, the prognosis of affected patients is generally quite good. Conservative topical management with corticosteroids and topical calcipotriol, or narrowband UVB in combination with topical therapy, is the preferred first-line therapies for management of von Zumbusch type GPP in children, and resolution with supportive conservative topical management can usually be achieved in this patient population103. Second-line agents include cyclosporine, methotrexate and systemic retinoids104. Methotrexate 0.2mg/kg/week has been shown to be effective in the management of von Zumbusch type GPP in children105–107. Etanercept has also been used safely for von Zumbusch type GPP in children, and may be considered for recalcitrant disease108.
In adults, systemic retinoids are considered by some to be first-line therapy for von Zumbusch type GPP87, 109–111. Isotretinoin may be preferred to acitretin in women with von Zumbusch type GPP of child-bearing age given its short half-life, however it may be less efficacious than acitretin112. Response is typically seen within several days to weeks. Methotrexate, cyclosporine and infliximab are alternative options113, 114. Given the rapid response following cyclosporine and infliximab treatment, many clinicians now consider these first-line agents for the management of von Zumbusch type GPP. Care should be exercised with methotrexate use in the elderly and individuals with hepatic disease, and methotrexate and cyclosporine must be used with caution in patients with renal dysfunction. Psoralen plus ultraviolet A (PUVA) has also been combined with acitretin for management of von Zumbusch type GPP110.
Although systemic corticosteroid taper may induce a flare of pustular disease, judicious use of corticosteroids in the setting of disease flare may be helpful for acute management. Corticosteroids are best used in combination with steroid-sparing agents such as biologics or DMARDs and require slow tapering87. Adalimumab, etanercept and alefacept have been used with some success in the management of GPP, but are considered second line agents for von Zumbusch type GPP115, 116. The IL-1 antagonist anakinra and IL-12/23 antagonist ustekinumab have been used in isolated cases, but have not been systematically studied in this population117, 118.
Impetigo herpetiformis
Impetigo herpetiformis refers to the development of von Zumbusch type GPP during pregnancy. A personal or family history of psoriasis may be present in these patients91, 119, 120. Disease onset is typically in the third trimester, although it may occur earlier in pregnancy, and both cutaneous and systemic symptoms resolve gradually following delivery or termination of pregnancy15, 91, 120–122. Recurrence may occur with subsequent pregnancy, ovulation or oral contraceptive use, suggesting a role for progesterone in disease precipitation 15, 91, 120, 121, 123, 124. In addition, von Zumbusch type GPP has been reported in the setting of progesterone challenge95 .
Like von Zumbusch type GPP, cutaneous and systemic findings of impetigo herpetiformis are abrupt in onset. Unlike von Zumbusch type GPP, however, early involvement of the flexural surfaces and healing with hyperpigmentation is common in impetigo herpetiformis91, 120, 122, 125. Erosive and circinate plaques involving the tongue, buccal mucosa and esophagus may occur. Subungual pustules may result in onycholysis126. Symptomatic hypocalcemia is common and necessitates close monitoring of mother and fetus. Fetal mortality rates as high as 89% have been reported15. Placental insufficiency is the most frequently cited cause for fetal mortality15, 122.
First-line therapy for impetigo herpetiformis includes topical calcipotriol, topical and oral corticosteroids, and cyclosporine. Cyclosporine is FDA pregnancy category C, however, it has been utilized in the setting of acute disease flares with significant systemic manifestations111, 127, 128. Narrowband UVB in combination with topical steroids and cyclosporine has also been used during pregnancy129. Biologics including etanercept, adalimumab and infliximab are FDA pregnancy category B, and data for their use in this clinical scenario is limited. Infliximab has been used successfully for impetigo herpetiformis in the post-partum period130. Topical and systemic retinoids and methotrexate are FDA pregnancy category X and thus contraindicated in pregnancy. Patients treated in the postpartum period with these agents should be advised not to breastfeed while receiving therapy131–134. PUVA has been used with success in one case but is not advisable given fetal exposure to psoralens135.
Annular pustular psoriasis
Annular pustular psoriasis (APP), also known as erythema circine recidivants, is a rare variant of GPP with a recurrent course but good overall prognosis136. Although APP does not have a predilection for childhood, it is the most frequent presentation of pustular psoriasis in childhood, with mean age of onset of 11 years in children136, 137. Adult-onset APP typically presents during the sixth or seventh decades of life and appears to have a greater predilection for females than males73, 93.
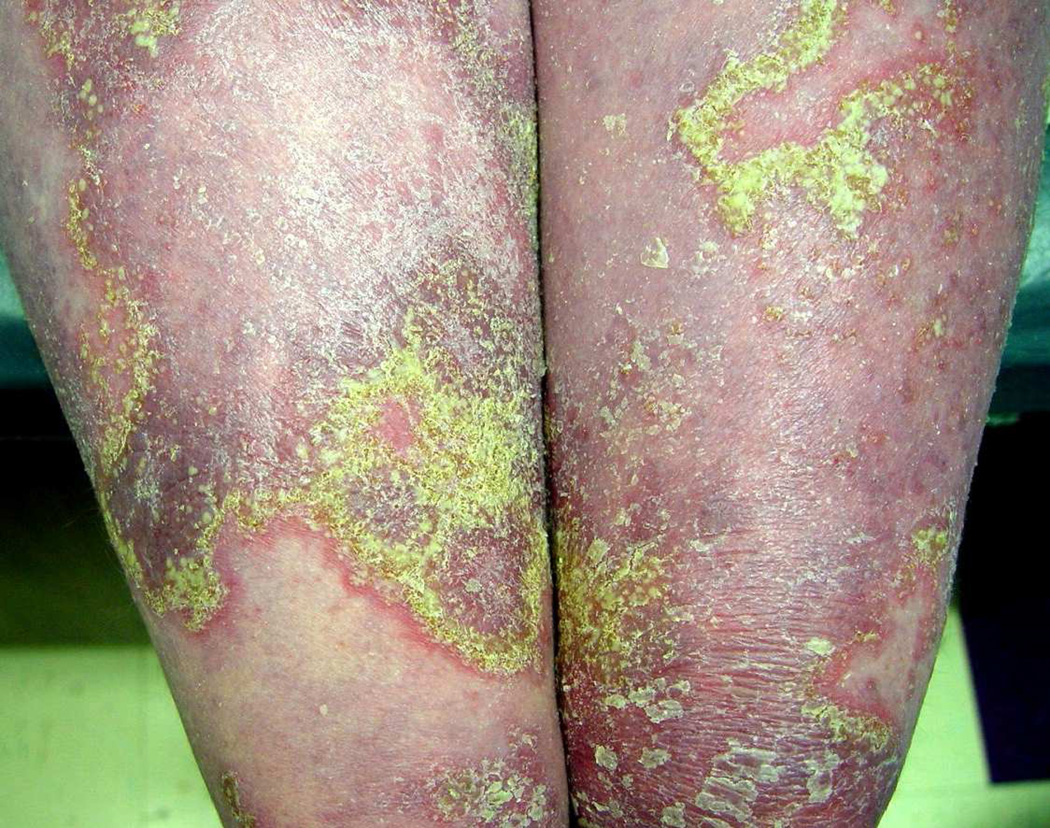
APP onset is not typically preceded or followed by plaque psoriasis. Unlike von Zumbusch type GPP and impetigo herpetiformis, APP has a less severe course characterized by diffuse, slowly migrating, annular and gyrate erythematous plaques with sterile pustules along the advancing edges involving the trunk, neck and extremities73, 138. (Figure 4) Polyarthritis can develop. Pruritus may be present, however other systemic symptoms and laboratory abnormalities, including leukocytosis, hypocalcemia and elevated acute phase reactants are typically absent in both children and adults84. Recurrent courses may occur over decades, but episodes are typically much less severe than seen in von Zumbusch type GPP. Of note, features of von Zumbusch type GPP and APP may overlap within the same patient.
Figure 4. Annular pustular psoriasis.
Gyrate plaques of annular pustular psoriasis notable for central sparing and erythematous border studded with pustules and yellow crusting. Photo courtesy of Jeffrey Callen.
Given the subacute nature of APP, some patients with mild disease respond well to topical corticosteroids and warm water compresses, while others may require systemic therapy. Acitretin139, dapsone138, 140, 141, and methotrexate have been successful in patients requiring systemic therapy. Low-dose methotrexate has been used with success in the pediatric setting105. Success with systemic retinoids has also been reported, but must be used with care in the pediatric population142, 143.
Subcorneal pustular dermatosis
Subcorneal pustular dermatosis (SCPD), or Sneddon-Wilkinson disease, was first described by Sneddon and Wilkinson in 1956144. Since SCPD was first described, controversy has ensued regarding its classification145–148. Some experts contend that SCPD is not a distinct entity but rather part of the spectrum of psoriasis and pustular psoriasis, resembling the annular pattern of pustular psoriasis145. In a review of 103 patients, Baker and Ryan reported 10 patients with annular pustular psoriasis, some of whom exhibited subcorneal pustules resembling SCPD73. One longitudinal study reported that 10 of 23 patients with SCPD progressed to clinical and histopathologic evidence of pustular psoriasis or plaque psoriasis 3–40 years after initial diagnosis of SCPD149. Four of these 10 patients had a family history of psoriasis or pustular psoriasis. These data suggest that SPCD and pustular psoriasis may be closely related conditions.
This rare disorder is more common in females than males (F:M 4:1). Onset typically occurs between the 5th and 7th decades of life, although cases in children as young as 3 months of age have been reported150–153. SCPD is clinically characterized by abrupt onset of superficial flaccid pustules distributed in the flexural surfaces including the axillae, groin and inframammary folds, which progress over 24 to 48 hours to form annular and gyrate patterns in a generalized distribution. Pustules are typically seen in the setting of normal-appearing skin, but may be found in the setting of erythematous skin. The disease course is chronic, characterized by exacerbations and remissions sometimes for many years. (Figure 5) Acral skin, face, nails and mucosal surfaces are rarely involved. Associated symptoms include irritation and skin pain, but patients rarely complain of pruritus144, 154. Lesions heal with hyperpigmentation without scarring. SCPD has been associated with pyoderma gangrenosum155, 156, inflammatory bowel disease148, 149, 157 and IgA monoclonal gammopathy, including IgA myeloma 155, 158, 159,. Infectious precipitants including urinary tract infections and upper respiratory infections have also been described160. Systemic manifestations including fever, malaise, fatigue and arthralgias are rare in SCPD149. In the first reported cases in children, fever and leukocytosis were noted, but subsequent case reports have indicated that there is no difference in clinicopathologic features and prognosis of SPCD between adults and children152, 153.
Figure 5. Subcorneal pustular dermatosis.
Superficial pustules and scale collarettes in annular and gyrate patterns overlying a relatively non-inflammatory background.
Histopathologically, SCPD is characterized by accumulation of subcorneal neutrophils atop fairly normal appearing epidermis in which spongiosis and acantholysis are absent144. In contrast to pustular psoriasis, neutrophils may migrate through the epidermis, but do not form spongiform pustules. Spongiosis, microabscesses, acanthosis and regular elongation of rete ridges are not observed. SCPD can be differentiated from pemphigus and benign familial pemphigus by the lack of acantholysis, although acantholysis can be seen in older lesions161. Bacteria and fungal elements are absent. Direct and indirect immunofluorescence studies are negative. Flexural distribution, subcorneal (rather than subepidermal) pustules and lack of pruritus distinguish SPCD from dermatitis herpetiformis.
Cases clinically resembling SCPD but showing IgA deposition in the skin and circulating IgA antibodies have been reported and termed IgA pemphigus, intraepidermal IgA pustulosis, intraepidermal neutrophilic IgA dermatosis and intercellular IgA dermatosis. Controversy exists over whether these cases represent a subgroup of SCPD or are a distinct entity162. Clinically, IgA pemphigus frequently involves the scalp and face which may aid in distinguishing this condition from SCPD. Further confusing matters, some reports have noted that IgA deposits may not be seen by immunofluorescence early in disease onset, and multiple skin biopsies may be required to make a diagnosis of IgA pemphigus163. Serum and urine protein electrophoresis are prudent components of the evaluation of SCPD patients.
Most but not all patients with SCPD respond to sulfones, presumably due to their ability to inhibit neutrophil chemotaxis. Dapsone should be used with caution and close monitoring in pediatric patients given hematologic adverse effects. In addition to dapsone, other anti-neutrophilic drugs including colchicine, sulfapyridine and sulfamethoxypyrazine have also been employed with variable success149, 152, 164. Topical steroids have also been used successfully as monotherapy or in combination with dapsone165. Variable results have been reported with oral retinoids166, 167. Broad and narrow band UVB, PUVA and re-PUVA have been reported to be successful in the management of SCPD, although PUVA was found ineffective in one case124, 159, 168–170. Recalcitrant disease has been managed successfully with infliximab171, 172.
Localized pustular psoriasis
Localized pustular psoriasis can be quite debilitating because of the sites affected, but unlike GPP, it is not commonly associated with systemic symptoms. This group of diseases includes acrodermatitis continua of Hallopeau, palmoplantar pustulosis and palmoplantar pustular psoriasis.
Acrodermatitis continua of Hallopeau
In 1890, Francois Henri Hallopeau described a painful acral pustular condition characterized by sterile pustules involving the distal fingers and, less often, the toes173. This rare and often disabling condition, termed acrodermatitis continua of Hallopeau (ACH) or dermatitis repens, is most common in middle-aged women174. Disease onset often occurs after localized trauma or infection involving a single digit174. Pustules develop with hyperemia involving the distal aspect of one or two digits, and progress proximally. Pustulation may involve the nail bed and nail matrix, leading to onychodystrophy, destruction of the nail plate and anonychia. Inflammatory paronychia is common. ACH may progress to involve the hands, forearms and feet. Skin atrophy, dermal sclerosis, osteolysis of the distal phalanges and arthropathy of the interphalangeal joints are seen in prolonged, refractory or untreated cases175. ACH has a chronic relapsing course with intermittent episodes of acute pustulation that is frequently refractory to therapy. Spontaneous remission is uncommon. In rare instances, syndactyly and involvement of the nasal tip may occur176. In the setting of long-standing disease, most often in the elderly, subsequent episodes of generalized pustular eruption have been reported84, 177.
ACH is often refractory to therapy. Attempts to treat with numerous different topical, systemic and biologic agents have met with mixed results. (List 2)
List 2. Potential therapies for acrodermatitis continua of Hallopeau.
| Systemic antimicrobials176, 177 |
| Tetracyclines |
| Azithromycin |
| Dapsone |
| Topical medications176, 177, 209 |
| Topical steroids |
| Calcipotriol |
| Tazarotene |
| Betamethasone |
| Topical 5-fluorouracil |
| Topical tacrolimus |
| Phototherapy176, 209–211 |
| Narrow band UVB |
| Psoralen ultraviolet A |
| Grenz rays |
| Immunomodulators 176, 209, 211 |
| Prednisone |
| Acitretin |
| Hydroxycarbamide |
| Colchicine |
| Methotrexate |
| Cyclosporine |
| Biologic agents |
| Adalimumab- ^6 cases211–215 |
| Infliximab- 2 cases211, 216, 217 |
| Etanercept- #5 cases211, 218–222 |
| Ustekinumab-1 case 211 |
| Anakinra-1 case 211 |
| ^ Some patients treated in combination with topical steroids and cyclosporine |
| # Some patients treated in combination with topical steroids or acitretin |
Palmoplantar pustulosis and palmoplantar pustular psoriasis
Palmoplantar pustulosis (PPP) is a painful, debilitating inflammatory skin condition characterized by crops of sterile pustules localized to the palms and soles. Disease onset tends to occur between ages 30 and 50, with a female predilection (F:M 3:2). A higher prevalence of smoking is noted in patients with PPP than in patients with other dermatoses, and a higher prevalence of PPP is noted in smokers as compared with nonsmokers178–181. Other precipitating factors include trauma, stress, warm weather and upper respiratory infection182.
The majority of cases of PPP are chronic, however acute cases of PPP following febrile illness with sudden onset (24–48 hours) and rapid resolution (2–3 weeks) have been reported and seem to portend a favorable prognosis183. Whereas acute PPP may last several days to weeks, chronic PPP may last several decades, with periods of resolution lasting less than 1 year in the majority of patients182.
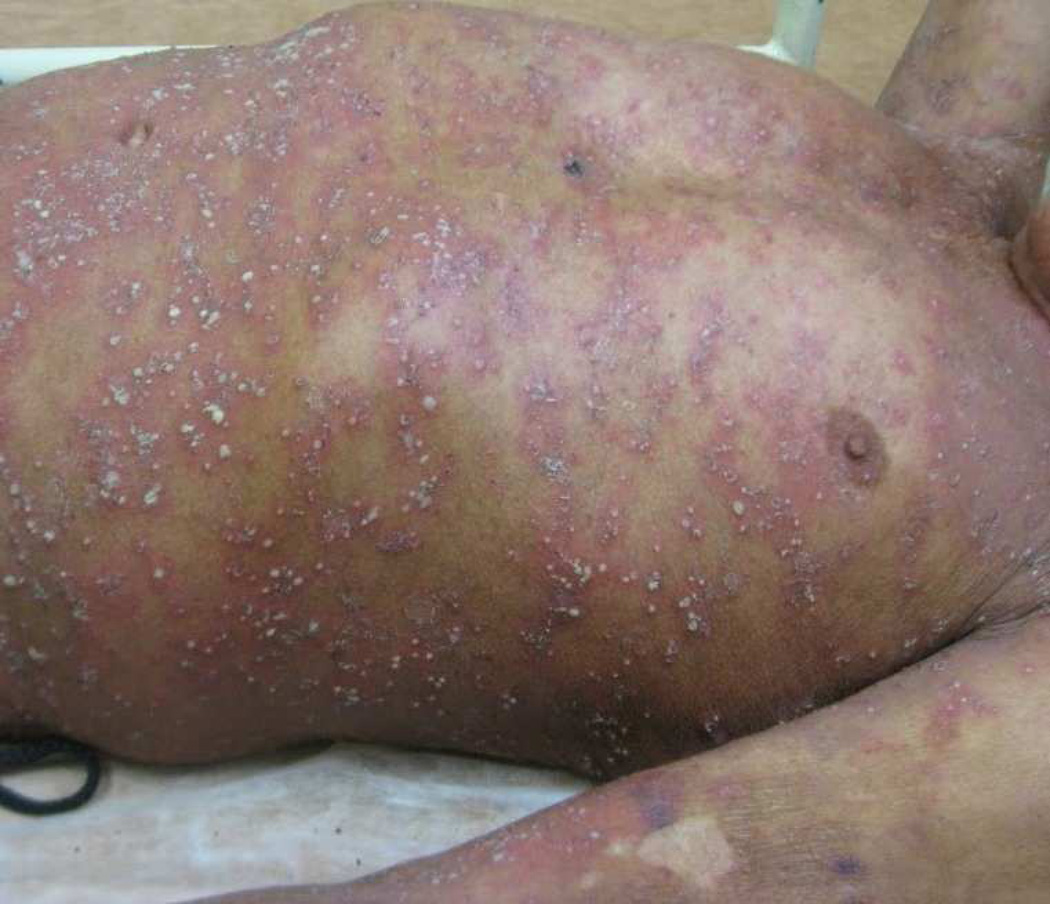
PPP is characterized by pustules in a background of normal-appearing or inflamed skin on the palmar or plantar surfaces182. (Figure 6) PPP can be disabling secondary to painful fissures, pruritus and burning sensation of the skin. Nail dystrophy from subungual pustulation can progress to nail destruction, and onycholysis may occur in 1/3 of patients184, 185. Both arthritis and arthralgias have also been reported in the setting of PPP186, 187. Aseptic osteitis of the sternoclavicular joint has been reported in association with PPP in the context of SAPHO syndrome37. An increased prevalence of antithyroid antibodies and thyroid disease are noted in patients with PPP188,189.
Figure 6. Palmoplantar pustular psoriasis.
Pustules, scaling and painful erosions with overlying hemorrhagic crust on the palmar surfaces. Photo courtesy of Kristina Callis-Duffin.
Approximately 10–20% of PPP patients have plaque psoriasis on other parts of the body, a distinction termed palmoplantar pustular psoriasis. The dorsal surfaces of the hands and feet may also be involved in these cases. The relationship between PPP and palmoplantar pustular psoriasis is controversial—whereas some clinicians speculate that both conditions lie on a spectrum of pustular psoriasis, others assert they are distinct entities. Similarities in histopathology, neutrophil dysfunction and chemokines profiles have been reported in PPP and psoriasis, suggesting a common pathogenic mechanism190, however, as mentioned earlier, genetic predisposing factors differ between PPP and psoriasis191.
Disease clearance can be achieved with medium-potency topical corticosteroids under hydrocolloid dressing occlusion, however recurrence is common upon cessation of therapy192. UVA can induce clearance in approximately 40% of people with PPP193. Acitretin has been shown to improve disease in about two-thirds of people with PPP, but may require continued treatment after achieving remission to avoid recurrent symptoms194, 195. Low-dose cyclosporine (1–2.5mg/kg/day) led to moderate objective improvement in about two-thirds of PPP patients within one month196, 197. Tetracycline antibiotics also produced objective improvement in about one-half of patients with PPP, however, complete clearance is rarely seen 198, 199. There are no randomized controlled trials supporting the use of methotrexate, although one uncontrolled prospective study showed benefit in one-third of treated patients, who primarily represented individuals with evidence of psoriasis at other sites 200.
Studies of biologic agents in PPP have resulted in conflicting data. A placebo-controlled trial of etanercept in 15 patients did not demonstrate a statistical benefit201. Adalimumab was associated with improvement in cutaneous and articular disease in a single patient202. Sequential therapy with adalimumab and infliximab was effective in the management of refractory PPP in 1 case203. Caution must be exercised with the administration of TNF inhibitors as paradoxical induction of psoriasis, pustulosis and PPP have been reported with these agents204, 205. More recently, ustekinumab resulted in disease clearance in 6 of 9 PPP patients111.
Acropustulosis of infancy
Acropustulosis of infancy (AI) is one of the most common forms of pustular psoriasis presenting in childhood75, 136, 206. This condition predominates in male children of African descent, however may occur in both sexes and in all races207, 208. One series reported acropustulosis in 4.7% of juvenile psoriasis patients, with approximately two-thirds of cases occurring in children less than 5 years of age206.
AI is characterized by intermittent crops of intensely pruritic vesiculopustules occurring on the acral surfaces. Vesiculopustules do not coalesce. Disease onset typically occurs prior to 10 months of age, and lesions tend to persist for about two years, resolving by age 3 207, 208. Although AI will spontaneously remit, potent topical steroids are useful for disease management. Pustular lesions show a striking response to sulfones, particularly dapsone,208 however the risks of methemoglobinemia and other hematologic adverse events may outweigh its benefit for a self-limited condition.
Histopathology of pustular psoriasis and variants
Neutrophils are the predominant feature upon histopathologic examination of pustular psoriasis and its variants in both children and adults. The epidermis is notable for variable hyperplasia, absent granular layer, parakeratosis, suprapapillary thinning, intracorneal aggregates of neutrophils (Munro microabscesses) and epidermal spongiosis with neutrophils (spongiform pustules of Kogoj). Prominent and dilated vessels are noted in the superficial dermis, with sparse mononuclear cell infiltrate and scattered neutrophils in the dermis161. Special stains for bacteria or fungal elements are negative. In annular pustular psoriasis, subcorneal pustules may be observed136. In SCPD, subcorneal neutrophils accumulate atop fairly normal appearing epidermis in which spongiosis, spongiform pustules, microabscesses, acanthosis and acantholysis are absent144. In palmoplantar pustulosis, eosinophils and mast cells may be seen surrounding pustules in the upper dermis, and the normal spiral columnar architecture of eccrine ducts is absent 182. In acropustulosis of infancy, both neutrophils and eosinophils may be seen within intraepidermal vesicles both on histopathology and smear208.
Differential diagnosis of pustular psoriasis and variants
The differential diagnosis for generalized pustular psoriasis includes acute generalized exanthematous pustulosis (AGEP), subcorneal pustular dermatosis (SCPD), reactive arthritis and cutaneous infections including impetigo, folliculitis, miliary tuberculosis and generalized candidiasis. In addition, tinea corporis and gyrate erythemas should be considered in the differential diagnosis of annular pustular psoriasis. In children, childhood bullous dermatosis, miliaria pustulosa, staphylococcal scalded skin syndrome and generalized seborrheic dermatitis should also be considered. IgA pemphigus, pemphigus foliaceus and dermatitis herpetiformis should be considered on the differential for SCPD. (Table 4)
Table 4.
Differential diagnosis of pustular psoriasis and its variants
| Condition | Differential Diagnosis |
|---|---|
| von Zumbusch type generalized pustular psoriasis |
Acute generalized exanthematous pustulosis |
| Subcorneal pustular dermatosis | |
| Diffuse impetigo | |
| Impetigo herpetiformis | Folliculitis |
| Miliary tuberculosis | |
| Tinea corporis | |
| Cutaneous candidiasis | |
| Subcorneal pustular dermatosis | |
| Generalized seborrheic dermatitis | |
| Reactive arthritis | |
| Childhood bullous dermatosis* | |
| Miliaria pustulosa* | |
| Staphylococcal scalded skin syndrome* | |
| Annular pustular psoriasis | Erythema annular centrifugum |
| Erythema gyratum repens | |
| Tinea corporis | |
| Granuloma annulare | |
| Urticaria | |
| Erythema multiforme | |
| Erythema chronicum migrans, | |
| Subcutaneous lupus erythematosus | |
| Annular erythema of infancy* | |
| Subcorneal pustular dermatosis | IgA pemphigus |
| Annular pustular psoriasis | |
| Dermatitis herpetiformis | |
| Tinea corporis | |
| Acrodermatitis of Hallopeau | Herpetic whitlow |
| Tinea manuum or pedis | |
| Dishidrotic eczema | |
| Bacterial or fungal paronychia | |
| Secondarily-infected malignancy | |
| Secondarily-infected contact dermatitis | |
| Palmoplantar pustulosis | Tinea mannum/pedis/unguium |
| Palmoplantar pustular psoriasis | Dishidrotic eczema |
| Contact dermatitis | |
| Bacterial or fungal infection | |
consider especially in children
The differential diagnosis of acral pustulosis includes cutaneous fungal infection, bacterid eruption and dishidrotic eczema. If pustulosis is limited to the digits, herpetic whitlow, secondarily-infected malignancy and chronic bacterial, fungal or viral paronychia should also be considered. In children, acral pustular eruptions should prompt skin examination for scabies.
CONCLUSION
Pustular psoriasis and its variants share a number of overlapping cutaneous, systemic and osteo-articular features. The classification of these diseases will continue to evolve over the coming years as underlying biologic pathways are elucidated, allowing differentiation based on shared mechanisms of disease rather than clinical similarities alone. Recent genetic insights into several rare monogenic forms of pustular disease have already revealed new autoinflammatory pathways and highlight the potential for new targeted interventions for these challenging conditions.
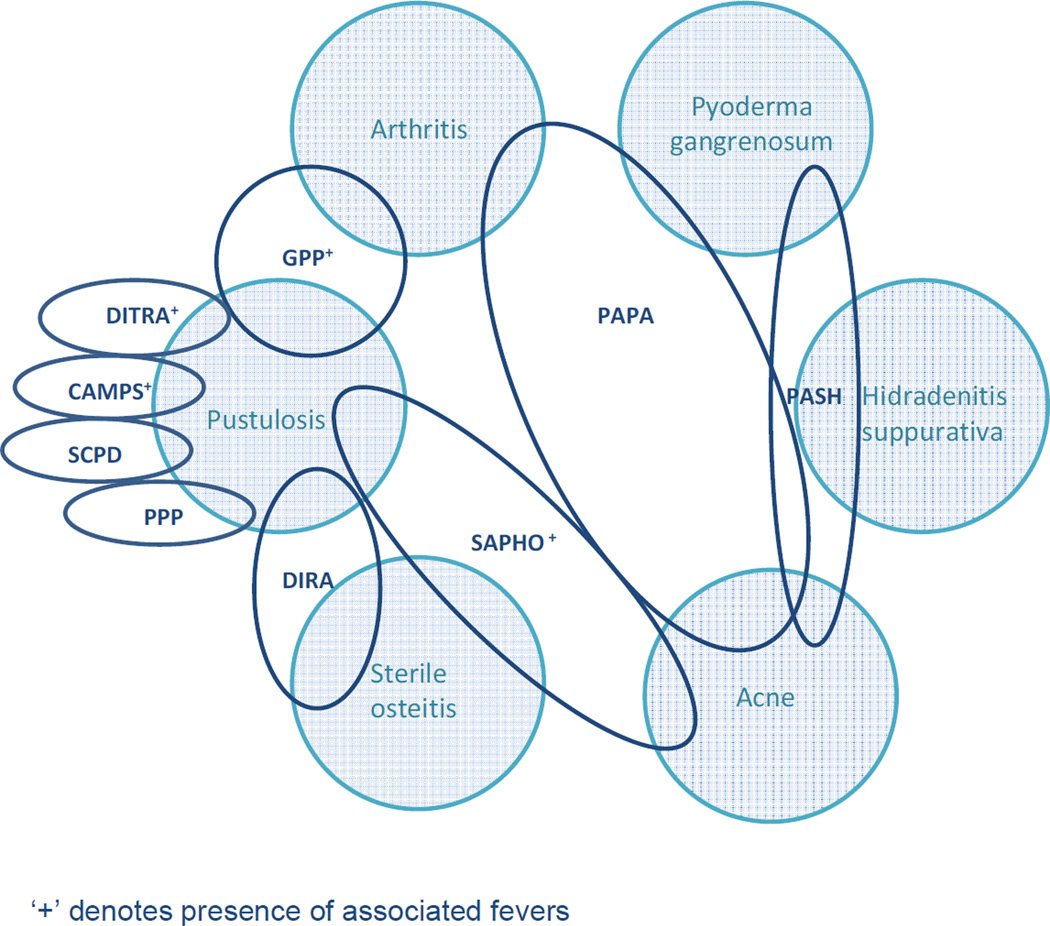
Figure 1. Overlapping clinical features of pustular dermatoses.
CARD 14-mediated pustular psoriasis (CAMPS); deficiency of IL-1 receptor antagonist (DIRA); deficiency of the IL-36 receptor antagonist (DITRA); generalized pustular psoriasis (GPP); pyogenic arthritis, pyoderma gangrenosum, and acne (PAPA); pyoderma, acne and suppurative hidradenitis (PASH); palmoplantar pustulosis (PPP); synovitis, acne, pustulosis, hyperostosis and osteitis (SAPHO); subcorneal pustular dermatosis (SCPD).
KEY POINTS.
Deficiency of the IL-1 receptor antagonist (DIRA) is an autosomal recessive autoinflammatory disease characterized by perinatal onset pustular dermatosis resembling pustular psoriasis, multifocal aseptic osteomyelitis and periostitis. It can be effectively treated with IL-1 receptor antagonists.
Pyogenic arthritis, pyoderma gangrenosum and acne comprise PAPA syndrome, an autosomal dominant autoinflammatory syndrome caused by mutations in the PSTPIP1 gene.
Synovitis, acne, pustulosis, hyperostosis and osteitis comprise the autoinflammatory syndrome known as SAPHO. Chronic recurrent multifocal osteomyelitis (CRMO) is likely a subtype of SAPHO that predominantly affects children.
Pustular psoriasis constitutes a spectrum of inflammatory pustular dermatoses ranging from localized acrodermatitis continua of Hallopeau and palmoplantar pustulosis to generalized disorders including von Zumbusch pustular psoriasis and impetigo herpetiformis.
The clinical similarities between defined autoinflammatory diseases with neutrophilic pustules and pustular psoriasis provides potential new mechanisms of treatment with biologic agents targeting autoinflammatory pathways.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
DISCLOSURES
Dr. Cowen: None
Dr. Naik: None
REFERENCES
- 1.Aksentijevich I, Masters SL, Ferguson PJ, et al. An autoinflammatory disease with deficiency of the interleukin-1-receptor antagonist. N Engl J Med. 2009 Jun 4;360(23):2426–2437. doi: 10.1056/NEJMoa0807865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jesus AA, Osman M, Silva CA, et al. A novel mutation of IL1RN in the deficiency of interleukin-1 receptor antagonist syndrome: description of two unrelated cases from Brazil. Arthritis Rheum. 2011 Dec;63(12):4007–4017. doi: 10.1002/art.30588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Levenson D. New inherited immune disorder revealed. American journal of medical genetics. Part A. 2009;149(9) doi: 10.1002/ajmg.a.33102. fm v. [DOI] [PubMed] [Google Scholar]
- 4.Minkis K, Aksentijevich I, Goldbach-Mansky R, et al. Interleukin 1 Receptor Antagonist Deficiency Presenting as Infantile Pustulosis Mimicking Infantile Pustular Psoriasis. Arch Dermatol. 2012 Mar 19; doi: 10.1001/archdermatol.2011.3208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Reddy S, Jia S, Geoffrey R, et al. An autoinflammatory disease due to homozygous deletion of the IL1RN locus. N Engl J Med. 2009 Jun 4;360(23):2438–2444. doi: 10.1056/NEJMoa0809568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stenerson M, Dufendach K, Aksentijevich I, Brady J, Austin J, Reed AM. The first reported case of compound heterozygous IL1RN mutations causing deficiency of the interleukin-1 receptor antagonist. Arthritis & Rheumatism. 2011;63(12):4018–4022. doi: 10.1002/art.30565. [DOI] [PubMed] [Google Scholar]
- 7.Schnellbacher C, Ciocca G, Menendez R, et al. Deficiency of Interleukin-1 Receptor Antagonist Responsive to Anakinra. Pediatr Dermatol. 2012 Apr 4; doi: 10.1111/j.1525-1470.2012.01725.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hubler WR., Jr Lingual lesions of generalized pustular psoriasis Report of five cases and a review of the literature. J Am Acad Dermatol. 1984 Dec;11(6):1069–1076. doi: 10.1016/s0190-9622(84)70261-1. [DOI] [PubMed] [Google Scholar]
- 9.Jordan CT, Cao L, Roberson ED, et al. Rare and Common Variants in CARD14, Encoding an Epidermal Regulator of NF-kappaB, in Psoriasis. Am J Hum Genet. 2012 May 4;90(5):796–808. doi: 10.1016/j.ajhg.2012.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jordan CT, Cao L, Roberson ED, et al. PSORS2 Is Due to Mutations in CARD14. Am J Hum Genet. 2012 May 4;90(5):784–795. doi: 10.1016/j.ajhg.2012.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Landry M, Muller SA. Generalized pustular psoriasis Observations on the course of the disease in a familial occurrence. Arch Dermatol. 1972 May;105(5):711–716. doi: 10.1001/archderm.105.5.711. [DOI] [PubMed] [Google Scholar]
- 12.Lindgren S, Groth O. Generalized pustular psoriasis A report on thirteen patients. Acta Derm Venereol. 1976;56(2):139–147. [PubMed] [Google Scholar]
- 13.Marrakchi S, Guigue P, Renshaw BR, et al. Interleukin-36-receptor antagonist deficiency and generalized pustular psoriasis. N Engl J Med. 2011 Aug 18;365(7):620–628. doi: 10.1056/NEJMoa1013068. [DOI] [PubMed] [Google Scholar]
- 14.Onoufriadis A, Simpson MA, Pink AE, et al. Mutations in IL36RN/IL1F5 are associated with the severe episodic inflammatory skin disease known as generalized pustular psoriasis. Am J Hum Genet. 2011 Sep 9;89(3):432–437. doi: 10.1016/j.ajhg.2011.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Oumeish OY, Farraj SE, Bataineh AS. Some aspects of impetigo herpetiformis. Arch Dermatol. 1982 Feb;118(2):103–105. [PubMed] [Google Scholar]
- 16.Sugiura K, Takeichi T, Kono M, et al. A novel IL36RN/IL1F5 homozygous nonsense mutation, p. Arg10X, in a Japanese patient with adult-onset generalized pustular psoriasis. British Journal of dermatology. 2012 doi: 10.1111/j.1365-2133.2012.10953.x. [DOI] [PubMed] [Google Scholar]
- 17.Fuchs-Telem D, Sarig O, van Steensel MA, et al. Familial pityriasis rubra pilaris is caused by mutations in CARD14. Am J Hum Genet. 2012 Jul 13;91(1):163–170. doi: 10.1016/j.ajhg.2012.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Habal NC, Chen Y, Jordan C, et al. Pathogenesis Study of Infantile-Onset, Severe Pustular Psoriasis Reveals a De Novo Mutation in CARD14 Causing Psoriasis Which Responds Clinically to IL-12/23 Blocking Treatment with Ustekinumab. Arthritis and Rheumatism. 2011:63. [Google Scholar]
- 19.Lindor NM, Arsenault TM, Solomon H, Seidman CE, McEvoy MT. A new autosomal dominant disorder of pyogenic sterile arthritis, pyoderma gangrenosum, and acne: PAPA syndrome. Mayo Clin Proc. 1997 Jul;72(7):611–615. doi: 10.1016/S0025-6196(11)63565-9. [DOI] [PubMed] [Google Scholar]
- 20.Jacobs JC, Goetzl EJ. "Streaking leukocyte factor," arthritis, and pyoderma gangrenosum. Pediatrics. 1975 Oct;56(4):570–578. [PubMed] [Google Scholar]
- 21.Waite AL, Schaner P, Richards N, et al. Pyrin Modulates the Intracellular Distribution of PSTPIP1. PLoS One. 2009;4(7):e6147. doi: 10.1371/journal.pone.0006147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Demidowich AP, Freeman AF, Kuhns DB, et al. Brief report: genotype, phenotype, and clinical course in five patients with PAPA syndrome (pyogenic sterile arthritis, pyoderma gangrenosum, and acne) Rheum Arthritis. 2012 Jun;64(6):2022–2027. doi: 10.1002/art.34332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shoham NG, Centola M, Mansfield E, et al. Pyrin binds the PSTPIP1/CD2BP1 protein, defining familial Mediterranean fever and PAPA syndrome as disorders in the same pathway. Proc Natl Acad Sci U S A. 2003 Nov 11;100(23):13501–13506. doi: 10.1073/pnas.2135380100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yu JW, Fernandes-Alnemri T, Datta P, et al. Pyrin activates the ASC pyroptosome in response to engagement by autoinflammatory PSTPIP1 mutants. Mol Cell. 2007 Oct 26;28(2):214–227. doi: 10.1016/j.molcel.2007.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schellevis MA, Stoffels M, Hoppenreijs EP, Bodar E, Simon A, van der Meer JW. Variable expression and treatment of PAPA syndrome. Ann Rheum Dis. 2011 Jun;70(6):1168–1170. doi: 10.1136/ard.2009.126185. [DOI] [PubMed] [Google Scholar]
- 26.Brenner M, Ruzicka T, Plewig G, Thomas P, Herzer P. Targeted treatment of pyoderma gangrenosum in PAPA (pyogenic arthritis, pyoderma gangrenosum and acne) syndrome with the recombinant human interleukin-1 receptor antagonist anakinra. Br J Dermatol. 2009 Nov;161(5):1199–1201. doi: 10.1111/j.1365-2133.2009.09404.x. [DOI] [PubMed] [Google Scholar]
- 27.Cortis E, De Benedetti F, Insalaco A, et al. Abnormal production of tumor necrosis factor (TNF)--alpha and clinical efficacy of the TNF inhibitor etanercept in a patient with PAPA syndrome [corrected] The Journal of pediatrics. 2004;145(6):851. doi: 10.1016/j.jpeds.2004.08.001. [DOI] [PubMed] [Google Scholar]
- 28.Dierselhuis MP, Frenkel J, Wulffraat NM, Boelens JJ. Anakinra for flares of pyogenic arthritis in PAPA syndrome. Rheumatology (Oxford) 2005 Mar;44(3):406–408. doi: 10.1093/rheumatology/keh479. [DOI] [PubMed] [Google Scholar]
- 29.Hong JB, Su YN, Chiu HC. Pyogenic arthritis, pyoderma gangrenosum, and acne syndrome (PAPA syndrome): report of a sporadic case without an identifiable mutation in the CD2BP1 gene. Journal of the American Academy of Dermatology. 2009;61(3):533. doi: 10.1016/j.jaad.2008.11.017. [DOI] [PubMed] [Google Scholar]
- 30.Tallon B, Corkill M. Peculiarities of PAPA syndrome. Rheumatology. 2006;45(9):1140–1143. doi: 10.1093/rheumatology/kei178. [DOI] [PubMed] [Google Scholar]
- 31.Wise CA, Gillum JD, Seidman CE, et al. Mutations in CD2BP1 disrupt binding to PTP PEST and are responsible for PAPA syndrome, an autoinflammatory disorder. Hum Mol Genet. 2002 Apr 15;11(8):961–969. doi: 10.1093/hmg/11.8.961. [DOI] [PubMed] [Google Scholar]
- 32.Yeon HB, Lindor NM, Seidman J, Seidman CE. Pyogenic arthritis, pyoderma gangrenosum, and acne syndrome maps to chromosome 15q. American journal of human genetics. 2000;66(4):1443. doi: 10.1086/302866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Braun-Falco M, Kovnerystyy O, Lohse P, Ruzicka T. Pyoderma gangrenosum, acne, and suppurative hidradenitis (PASH)-a new autoinflammatory syndrome distinct from PAPA syndrome. Journal of the American Academy of Dermatology. 2012;66(3):409–415. doi: 10.1016/j.jaad.2010.12.025. [DOI] [PubMed] [Google Scholar]
- 34.Smith EJ, Allantaz F, Bennett L, et al. Clinical, molecular, and genetic characteristics of PAPA syndrome: A review. Current Genomics. 2010;11(7):519. doi: 10.2174/138920210793175921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stichweh DS, Punaro M, Pascual V. Dramatic improvement of pyoderma gangrenosum with infliximab in a patient with PAPA syndrome. Pediatr Dermatol. 2005 May-Jun;22(3):262–265. doi: 10.1111/j.1525-1470.2005.22320.x. [DOI] [PubMed] [Google Scholar]
- 36.Tofteland ND, Shaver TS. Clinical efficacy of etanercept for treatment of PAPA syndrome. J Clin Rheumatol. 2010 Aug;16(5):244–245. doi: 10.1097/RHU.0b013e3181e969b9. [DOI] [PubMed] [Google Scholar]
- 37.Chamot AM, Benhamou CL, Kahn MF, Beraneck L, Kaplan G, Prost A. [Acne-pustulosis-hyperostosisosteitis syndrome. Results of a national survey. 85 cases] Rev Rhum Mal Osteoartic. 1987 Mar;54(3):187–196. [PubMed] [Google Scholar]
- 38.Giedion A, Holthusen W, Masel LF, Vischer D. [Subacute and chronic "symmetrical" osteomyelitis] Ann Radiol (Paris) 1972 Mar-Apr;15(3):329–342. [PubMed] [Google Scholar]
- 39.Majeed HA, Al-Tarawna M, El-Shanti H, Kamel B, Al-Khalaileh F. The syndrome of chronic recurrent multifocal osteomyelitis and congenital dyserythropoietic anaemia Report of a new family and a review. Eur J Pediatr. 2001 Dec;160(12):705–710. doi: 10.1007/s004310100799. [DOI] [PubMed] [Google Scholar]
- 40.Majeed HA, El-Shanti H, Al-Rimawi H, Al-Masri N. On mice and men: An autosomal recessive syndrome of chronic recurrent multifocal osteomyelitis and congenital dyserythropoietic anemia. J Pediatr. 2000 Sep;137(3):441–442. doi: 10.1067/mpd.2000.107613. [DOI] [PubMed] [Google Scholar]
- 41.Majeed HA, Kalaawi M, Mohanty D, et al. Congenital dyserythropoietic anemia and chronic recurrent multifocal osteomyelitis in three related children and the association with Sweet syndrome in two siblings. J Pediatr. 1989 Nov;115((5 Pt 1)):730–734. doi: 10.1016/s0022-3476(89)80650-x. [DOI] [PubMed] [Google Scholar]
- 42.Ferguson PJ, Chen S, Tayeh MK, et al. Homozygous mutations in LPIN2 are responsible for the syndrome of chronic recurrent multifocal osteomyelitis and congenital dyserythropoietic anaemia (Majeed syndrome) J Med Genet. 2005 Jul;42(7):551–557. doi: 10.1136/jmg.2005.030759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Depasquale R, Kumar N, Lalam RK, et al. SAPHO: What radiologists should know. Clin Radiol. 2012 Mar;67(3):195–206. doi: 10.1016/j.crad.2011.08.014. [DOI] [PubMed] [Google Scholar]
- 44.Earwaker JW, Cotten A. SAPHO: syndrome or concept? Imaging findings. Skeletal Radiol. 2003 Jun;32(6):311–327. doi: 10.1007/s00256-003-0629-x. [DOI] [PubMed] [Google Scholar]
- 45.Beretta-Piccoli BC, Sauvain MJ, Gal I, et al. Synovitis, acne, pustulosis, hyperostosis, osteitis (SAPHO) syndrome in childhood: a report of ten cases and review of the literature. European journal of pediatrics. 2000;159(8):594–601. doi: 10.1007/s004310000500. [DOI] [PubMed] [Google Scholar]
- 46.Huber AM, Lam PY, Duffy CM, et al. Chronic recurrent multifocal osteomyelitis: clinical outcomes after more than five years of follow-up. J Pediatr. 2002 Aug;141(2):198–203. doi: 10.1067/mpd.2002.126457. [DOI] [PubMed] [Google Scholar]
- 47.Khanna G, Sato TS, Ferguson P. Imaging of chronic recurrent multifocal osteomyelitis. Radiographics. 2009 Jul-Aug;29(4):1159–1177. doi: 10.1148/rg.294085244. [DOI] [PubMed] [Google Scholar]
- 48.Hayem G, Bouchaud-Chabot A, Benali K, et al. SAPHO syndrome: a long-term follow-up study of 120 cases; Paper presented at: Seminars in arthritis and rheumatism; 1999. [DOI] [PubMed] [Google Scholar]
- 49.Kahn MF, Bouvier M, Palazzo E, Tebib JG, Colson F. Sternoclavicular pustulotic osteitis (SAPHO) 20-year interval between skin and bone lesions. J Rheumatol. 1991 Jul;18(7):1104–1108. [PubMed] [Google Scholar]
- 50.Sonozaki H, Mitsui H, Miyanaga Y, et al. Clinical features of 53 cases with pustulotic arthro-osteitis. Ann Rheum Dis. 1981 Dec;40(6):547–553. doi: 10.1136/ard.40.6.547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bhalla R, Sequeira W. Arthritis associated with hidradenitis suppurativa. Ann Rheum Dis. 1994 Jan;53(1):64–66. doi: 10.1136/ard.53.1.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rosner IA, Burg CG, Wisnieski JJ, Schacter BZ, Richter DE. The clinical spectrum of the arthropathy associated with hidradenitis suppurativa and acne conglobata. J Rheumatol. 1993 Apr;20(4):684–687. [PubMed] [Google Scholar]
- 53.Colina M, Govoni M, Orzincolo C, Trotta F. Clinical and radiologic evolution of synovitis, acne, pustulosis, hyperostosis, and osteitis syndrome: a single center study of a cohort of 71 subjects. Arthritis Rheum. 2009 Jun 15;61(6):813–821. doi: 10.1002/art.24540. [DOI] [PubMed] [Google Scholar]
- 54.Kahn MF, Bouchon JP, Chamot AM, Palazzo E. [Chronic enterocolopathies and SAPHO syndrome 8 cases] Rev Rhum Mal Osteoartic. 1992 Feb;59(2):91–94. [PubMed] [Google Scholar]
- 55.Yamasaki O, Iwatsuki K, Kaneko F. A case of SAPHO syndrome with pyoderma gangrenosum and inflammatory bowel disease masquerading as Behcet's disease. Adv Exp Med Biol. 2003;528:339–341. doi: 10.1007/0-306-48382-3_69. [DOI] [PubMed] [Google Scholar]
- 56.Jansson A, Renner ED, Ramser J, et al. Classification of non-bacterial osteitis: retrospective study of clinical, immunological and genetic aspects in 89 patients. Rheumatology (Oxford) 2007 Jan;46(1):154–160. doi: 10.1093/rheumatology/kel190. [DOI] [PubMed] [Google Scholar]
- 57.Benhamou CL, Chamot AM, Kahn MF. Synovitis-acne-pustulosis hyperostosisosteomyelitis syndrome (SAPHO). A new syndrome among the spondyloarthropathies? Clin Exp Rheumatol. 1988 Apr-Jun;6(2):109–112. [PubMed] [Google Scholar]
- 58.Jurik AG, Helmig O, Ternowitz T, Moller BN. Chronic recurrent multifocal osteomyelitis: a follow-up study. J Pediatr Orthop. 1988 Jan-Feb;8(1):49–58. doi: 10.1097/01241398-198801000-00012. [DOI] [PubMed] [Google Scholar]
- 59.Amital H, Applbaum Y, Aamar S, Daniel N, Rubinow A. SAPHO syndrome treated with pamidronate: an open-label study of 10 patients. Rheumatology. 2004;43(5):658–661. doi: 10.1093/rheumatology/keh149. [DOI] [PubMed] [Google Scholar]
- 60.Guignard S, Job-Deslandre C, Sayag-Boukris V, Kahan A. Pamidronate treatment in SAPHO syndrome. Joint Bone Spine. 2002 Jun;69(4):392–396. doi: 10.1016/s1297-319x(02)00419-0. [DOI] [PubMed] [Google Scholar]
- 61.Solau-Gervais E, Soubrier M, Gerot I, et al. The usefulness of bone remodelling markers in predicting the efficacy of pamidronate treatment in SAPHO syndrome. Rheumatology (Oxford) 2006 Mar;45(3):339–342. doi: 10.1093/rheumatology/kei160. [DOI] [PubMed] [Google Scholar]
- 62.Colina M, La Corte R, Trotta F. Sustained remission of SAPHO syndrome with pamidronate: a follow-up of fourteen cases and a review of the literature. Clinical & Experimental Rheumatology. 2009;27(1):112. [PubMed] [Google Scholar]
- 63.Kopterides P, Pikazis D, Koufos C. Successful treatment of SAPHO syndrome with zoledronic acid. Arthritis & Rheumatism. 2004;50(9):2970–2973. doi: 10.1002/art.20464. [DOI] [PubMed] [Google Scholar]
- 64.Abdelghani KB, Dran DG, Gottenberg JE, Morel J, Sibilia J, Combe B. Tumor Necrosis Factor-α Blockers in SAPHO Syndrome. The Journal of rheumatology. 2010;37(8):1699–1704. doi: 10.3899/jrheum.091086. [DOI] [PubMed] [Google Scholar]
- 65.Aieska De Souza M, Solomon GE, Strober BE. SAPHO Syndrome Associated With Hidradenitis Suppurativa Successfully Treated with Infliximab and Methotrexate. Bulletin of the NYU Hospital for Joint Diseases. 2011;69(2):185–187. [PubMed] [Google Scholar]
- 66.Castellví I, Bonet M, Narváez JA, Molina-Hinojosa JC. Successful treatment of SAPHO syndrome with adalimumab: a case report. Clinical rheumatology. 2010;29(10):1205–1207. doi: 10.1007/s10067-010-1476-5. [DOI] [PubMed] [Google Scholar]
- 67.Vilar-Alejo J, Dehesa L, de la Rosa-del Rey P, Novoa-Medina J, Almazán PV, Medina NS. SAPHO syndrome with unusual cutaneous manifestations treated successfully with etanercept. Acta dermato-venereologica. 2010;90(5):531. doi: 10.2340/00015555-0895. [DOI] [PubMed] [Google Scholar]
- 68.Van Den Eynde M, Lecluyse K, Chioccioli C, Brouckaert M, Caussin E, Lammens P. [Crohn's disease and the SAPHO syndrome during treatment with infliximab: a case report and review of literature] Gastroenterol Clin Biol. 2007 Jun-Jul;31(6–7):607–610. doi: 10.1016/s0399-8320(07)89438-3. [DOI] [PubMed] [Google Scholar]
- 69.Sfikakis P, Iliopoulos A, Elezoglou A, Kittas C, Stratigos A. Psoriasis induced by anti-tumor necrosis factor therapy: a paradoxical adverse reaction. Arthritis & Rheumatism. 2005;52(8):2513–2518. doi: 10.1002/art.21233. [DOI] [PubMed] [Google Scholar]
- 70.Colina M, Pizzirani C, Khodeir M, et al. Dysregulation of P2X7 receptor-inflammasome axis in SAPHO syndrome: successful treatment with anakinra. Rheumatology. 2010;49(7):1416–1418. doi: 10.1093/rheumatology/keq074. [DOI] [PubMed] [Google Scholar]
- 71.Wendling D, Prati C, Aubin F. Anakinra treatment of SAPHO syndrome: short-term results of an open study. Ann Rheum Dis. 2012 Jun;71(6):1098–1100. doi: 10.1136/annrheumdis-2011-200743. [DOI] [PubMed] [Google Scholar]
- 72.Koch F. Zur Frage der Identität von Impetigo herpetiformis, Psoriasis pustulosa und Psoriasis vulgaris. Hautarzt. 1952;3:165–168. [Google Scholar]
- 73.Baker H, Ryan TJ. Generalized pustular psoriasis. A clinical and epidemiological study of 104 cases. Br J Dermatol. 1968 Dec;80(12):771–793. doi: 10.1111/j.1365-2133.1968.tb11947.x. [DOI] [PubMed] [Google Scholar]
- 74.De Oliveira ST, Maragno L, Arnone M, Fonseca Takahashi MD, Romiti R. Generalized pustular psoriasis in childhood. Pediatric dermatology. 2010;27(4):349–354. doi: 10.1111/j.1525-1470.2010.01084.x. [DOI] [PubMed] [Google Scholar]
- 75.Zelickson BD, Muller SA. Generalized pustular psoriasis in childhood. Report of thirteen cases. J Am Acad Dermatol. 1991 Feb;24((2 Pt 1)):186–194. doi: 10.1016/0190-9622(91)70025-w. [DOI] [PubMed] [Google Scholar]
- 76.Ward JM, Barnes RMR. HLA antigens in persistent palmoplantar pustulosis and its relationship to psoriasis. British Journal of dermatology. 2006;99(5):477–483. doi: 10.1111/j.1365-2133.1978.tb02013.x. [DOI] [PubMed] [Google Scholar]
- 77.Zachariae H, Overgaard Petersen H, Kissmeyer Nielsen F, Lamm L. HL-A antigens in pustular psoriasis. Dermatologica. 1977;154(2):73–77. doi: 10.1159/000251035. [DOI] [PubMed] [Google Scholar]
- 78.Brenner W, Gschnait F, Mayr WR. HLA B13, B17, B37 and Cw6 in psoriasis vulgaris: association with the age of onset. Arch Dermatol Res. 1978 Aug 28;262(3):337–339. doi: 10.1007/BF00447371. [DOI] [PubMed] [Google Scholar]
- 79.Karvonen J, Tiilikainen A, Lassus A. HL-A antigens in patients with persistent palmoplantar pustulosis and pustular psoriasis. Ann Clin Res. 1975 Apr;7(2):112–115. [PubMed] [Google Scholar]
- 80.Woodrow JC, Ilchysyn A. HLA antigens in psoriasis and psoriatic arthritis. J Med Genet. 1985 Dec;22(6):492–495. doi: 10.1136/jmg.22.6.492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Svejgaard A, Svejgaard E, Nielsen LS, Jacobsen B. Some speculations on the associations between HL-A and disease based on studies of psoriasis patients and their families. Transplant Proc. 1973 Dec;5(4):1797–1798. [PubMed] [Google Scholar]
- 82.Szanto E, Linse U. Arthropathy associated with palmoplantar pustulosis. Clin Rheumatol. 1991 Jun;10(2):130–135. doi: 10.1007/BF02207650. [DOI] [PubMed] [Google Scholar]
- 83.Keat A, Maini R, Nkwazi G, Pegrum G, Ridgway G, Scott J. Role of Chlamydia trachomatis and HLA-B27 in sexually acquired reactive arthritis. British medical journal. 1978;1(6113):605–607. doi: 10.1136/bmj.1.6113.605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ryan TJ, Baker H. The prognosis of generalized pustular psoriasis. Br J Dermatol. 1971 Nov;85(5):407–411. doi: 10.1111/j.1365-2133.1971.tb14044.x. [DOI] [PubMed] [Google Scholar]
- 85.Tolman MM, Moschella SL. Pustular psoriasis (Zumbusch) Arch Dermatol. 1960 Mar;81:400–404. doi: 10.1001/archderm.1960.03730030058009. [DOI] [PubMed] [Google Scholar]
- 86.von Zumbusch LR. Psoriasis und pustulöses Exanthem. Archives of Dermatological Research. 1909;99(1):335–346. [Google Scholar]
- 87.Umezawa Y, Ozawa A, Kawasima T, et al. Therapeutic guidelines for the treatment of generalized pustular psoriasis (GPP) based on a proposed classification of disease severity. Arch Dermatol Res. 2003 Apr;295(Suppl 1):S43–S54. doi: 10.1007/s00403-002-0371-6. [DOI] [PubMed] [Google Scholar]
- 88.Kingery FA, Chinn HD, Saunders TS. Generalized pustular psoriasis. Arch Dermatol. 1961 Dec;84:912–919. doi: 10.1001/archderm.1961.01580180028003. [DOI] [PubMed] [Google Scholar]
- 89.al-Fouzan AS, Nanda A. A survey of childhood psoriasis in Kuwait. Pediatr Dermatol. 1994 Jun;11(2):116–119. doi: 10.1111/j.1525-1470.1994.tb00563.x. [DOI] [PubMed] [Google Scholar]
- 90.Beylot C, Bioulac P, Grupper C. Generalized pustular psoriasis in infants and children: report of 27cases. In: Farber AC EM, Jacobs PH, editors. Psoriasis. New York: Yorke Medical books; 1977. pp. 171–179. [Google Scholar]
- 91.Braverman IM, Cohen I, O'Keefe E. Metabolic and ultrastructural studies in a patient with pustular psoriasis (von Zumbusch) Arch Dermatol. 1972 Feb;105(2):189–196. [PubMed] [Google Scholar]
- 92.Dawson TA. Tongue lesions in generalized pustular psoriasis. Br J Dermatol. 1974 Oct;91(4):419–424. doi: 10.1111/j.1365-2133.1974.tb13080.x. [DOI] [PubMed] [Google Scholar]
- 93.Zelickson BD, Muller SA. Generalized pustular psoriasis. A review of 63 cases. Arch Dermatol. 1991 Sep;127(9):1339–1345. [PubMed] [Google Scholar]
- 94.O'Keefe E, Braverman IM, Cohen I. Annulus migrans Identical lesions in pustular psoriasis, Reiter's syndrome, and geographic tongue. Arch Dermatol. 1973 Feb;107(2):240–244. doi: 10.1001/archderm.107.2.240. [DOI] [PubMed] [Google Scholar]
- 95.Shelley WB. Generalized pustular psoriasis induced by potassium iodide. A postulated role for dihydrofolic reductase. JAMA. 1967 Sep 25;201(13):1009–1014. [PubMed] [Google Scholar]
- 96.Wagner G, Luckasen JR, Goltz RW. Mucous membrane involvement in generalized pustular psoriasis: Report of three cases and review of the literature. Arch Dermatol. 1976 Jul;112(7):1010–1014. [PubMed] [Google Scholar]
- 97.Gordon M, Pearlstein HH, Burgoon CF., Jr Pustular psoriasis (zumbusch) Dermatologica. 1969;138(2):65–74. doi: 10.1159/000253967. [DOI] [PubMed] [Google Scholar]
- 98.Warren DJ, Winney RJ, Beveridge GW. Oligaemia, renal failure, and jaundice associated with acute pustular psoriasis. Br Med J. 1974 May 25;2(5916):406–408. doi: 10.1136/bmj.2.5916.406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Abou-Samra T, Constantin JM, Amarger S, et al. Generalized pustular psoriasis complicated by acute respiratory distress syndrome. Br J Dermatol. 2004 Feb;150(2):353–356. doi: 10.1111/j.1365-2133.2004.05777.x. [DOI] [PubMed] [Google Scholar]
- 100.Doval IG, Peteiro C, Toribio J. Acute respiratory distress syndrome and generalized pustular psoriasis: another case report. Archives of dermatology. 1998;134(1):103. doi: 10.1001/archderm.134.1.103. [DOI] [PubMed] [Google Scholar]
- 101.Griffiths M, Porter W, Fergusson-Wood L, Adriaans B. Generalized pustular psoriasis complicated by acute respiratory distress syndrome. British Journal of dermatology. 2006;155(2):496–497. doi: 10.1111/j.1365-2133.2006.07364.x. [DOI] [PubMed] [Google Scholar]
- 102.Sadeh JS, Rudikoff D, Gordon ML, Bowden J, Goldman BD, Lebwohl M. Pustular and erythrodermic psoriasis complicated by acute respiratory distress syndrome. Archives of dermatology. 1997;133(6):747. [PubMed] [Google Scholar]
- 103.Khan SA, Peterkin GA, Mitchell PC. Juvenile generalized pustular psoriasis A report of five cases and a review of the literature. Arch Dermatol. 1972 Jan;105(1):67–72. doi: 10.1001/archderm.105.1.67. [DOI] [PubMed] [Google Scholar]
- 104.Mahe E, Bodemer C, Pruszkowski A, Teillac-Hamel D, de Prost Y. Cyclosporine in childhood psoriasis. Archives of dermatology. 2001;137(11):1532. [PubMed] [Google Scholar]
- 105.Kalla G, Goyal AM. Juvenile generalized pustular psoriasis. Pediatr Dermatol. 1996 Jan-Feb;13(1):45–46. doi: 10.1111/j.1525-1470.1996.tb01187.x. [DOI] [PubMed] [Google Scholar]
- 106.Kumar B, Dhar S, Handa S, Kaur I. Methotrexate in childhood psoriasis. Pediatr Dermatol. 1994 Sep;11(3):271–273. doi: 10.1111/j.1525-1470.1994.tb00602.x. [DOI] [PubMed] [Google Scholar]
- 107.Ryan TJ, Baker H. Systemic corticosteroids and folic acid antagonists in the treatment of generalized pustular psoriasis. Evaluation and prognosis based on the study of 104 cases. Br J Dermatol. 1969 Feb;81(2):134–145. doi: 10.1111/j.1365-2133.1969.tb15995.x. [DOI] [PubMed] [Google Scholar]
- 108.Pereira T, Vieira A, Fernandes J, Antunes H, Basto A. Anti-TNF-a therapy in childhood pustular psoriasis. Dermatology. 2006;213(4):350–352. doi: 10.1159/000096202. [DOI] [PubMed] [Google Scholar]
- 109.Mengesha YM, Bennett ML. Pustular skin disorders: diagnosis and treatment. Am J Clin Dermatol. 2002;3(6):389–400. doi: 10.2165/00128071-200203060-00003. [DOI] [PubMed] [Google Scholar]
- 110.Ozawa A, Ohkido M, Haruki Y, et al. Treatments of generalized pustular psoriasis: a multicenter study in Japan. J Dermatol. 1999 Mar;26(3):141–149. doi: 10.1111/j.1346-8138.1999.tb03444.x. [DOI] [PubMed] [Google Scholar]
- 111.Robinson A, Van Voorhees AS, Hsu S, et al. Treatment of pustular psoriasis: from the Medical Board of the National Psoriasis Foundation. J Am Acad Dermatol. 2012 Aug;67(2):279–288. doi: 10.1016/j.jaad.2011.01.032. [DOI] [PubMed] [Google Scholar]
- 112.Moy RL, Kingston TP, Lowe NJ. Isotretinoin vs etretinate therapy in generalized pustular and chronic psoriasis. Arch Dermatol. 1985 Oct;121(10):1297–1301. [PubMed] [Google Scholar]
- 113.Collins P, Rogers S. The efficacy of methotrexate in psoriasis--a review of 40 cases. Clin Exp Dermatol. 1992 Jul;17(4):257–260. doi: 10.1111/j.1365-2230.1992.tb02161.x. [DOI] [PubMed] [Google Scholar]
- 114.Poulalhon N, Begon E, Lebbe C, et al. A follow-up study in 28 patients treated with infliximab for severe recalcitrant psoriasis: evidence for efficacy and high incidence of biological autoimmunity. Br J Dermatol. 2007 Feb;156(2):329–336. doi: 10.1111/j.1365-2133.2006.07639.x. [DOI] [PubMed] [Google Scholar]
- 115.Callen JP, Jackson JH. Adalimumab effectively controlled recalcitrant generalized pustular psoriasis in an adolescent. J Dermatolog Treat. 2005;16(5–6):350–352. doi: 10.1080/09546630500430604. [DOI] [PubMed] [Google Scholar]
- 116.Kamarashev J, Lor P, Forster A, Heinzerling L, Burg G, Nestle F. Generalised pustular psoriasis induced by cyclosporin a withdrawal responding to the tumour necrosis factor alpha inhibitor etanercept . Djermatology. 2002;205(2):213–216. doi: 10.1159/000063919. [DOI] [PubMed] [Google Scholar]
- 117.Dauden E, Santiago-et-Sanchez-Mateos D, Sotomayor-Lopez E, Garcia-Diez A. Ustekinumab: effective in a patient with severe recalcitrant generalized pustular psoriasis. Br J Dermatol. 2010 Dec;163(6):1346–1347. doi: 10.1111/j.1365-2133.2010.09995.x. [DOI] [PubMed] [Google Scholar]
- 118.Viguier M, Guigue P, Pages C, Smahi A, Bachelez H. Successful treatment of generalized pustular psoriasis with the interleukin-1-receptor antagonist Anakinra: lack of correlation with IL1RN mutations. Ann Intern Med. 2010 Jul 6;153(1):66–67. doi: 10.7326/0003-4819-153-1-201007060-00030. [DOI] [PubMed] [Google Scholar]
- 119.Hubler WR., Jr Familial juvenile generalized pustular psoriasis. Arch Dermatol. 1984 Sep;120(9):1174–1178. [PubMed] [Google Scholar]
- 120.Oosterling RJ, Nobrega RE, Du Boeuff JA, Van Der Meer JB. Impetigo herpetiformis or generalized pustular psoriasis? Arch Dermatol. 1978 Oct;114(10):1527–1529. [PubMed] [Google Scholar]
- 121.Bajaj AK, Swarup V, Gupta OP, Gupta SC. Impetigo herpetiformis. Dermatologica. 1977;155(5):292–295. doi: 10.1159/000250980. [DOI] [PubMed] [Google Scholar]
- 122.Beveridge GW, Harkness RA, Livingstone JR. Impetigo herpetiformis in two successive pregnancies. Br J Dermatol. 1966 Feb;78(2):106–112. doi: 10.1111/j.1365-2133.1966.tb12183.x. [DOI] [PubMed] [Google Scholar]
- 123.Murphy FR, Stolman LP. Generalized pustular psoriasis. Arch Dermatol. 1979 Oct;115(10):1215–1216. [PubMed] [Google Scholar]
- 124.Orton DI, George SA. Subcorneal pustular dermatosis responsive to narrowband (TL-01) UVB phototherapy. Br J Dermatol. 1997 Jul;137(1):149–150. doi: 10.1111/j.1365-2133.1997.tb03720.x. [DOI] [PubMed] [Google Scholar]
- 125.Pierard GE, Pierard-Franchimont C, de la Brassinne M. Impetigo herpetiformis and pustular psoriasis during pregnancy. Am J Dermatopathol. 1983 Jun;5(3):215–220. doi: 10.1097/00000372-198306000-00004. [DOI] [PubMed] [Google Scholar]
- 126.Sauer GC, Geha BJ. Impetigo herpetiformis. Report of a case treated with corticosteroid-review of the literature. Arch Dermatol. 1961 Jan;83:119–126. doi: 10.1001/archderm.1961.01580070125014. [DOI] [PubMed] [Google Scholar]
- 127.Kapoor R, Kapoor JR. Cyclosporine resolves generalized pustular psoriasis of pregnancy. Arch Dermatol. 2006 Oct;142(10):1373–1375. doi: 10.1001/archderm.142.10.1373. [DOI] [PubMed] [Google Scholar]
- 128.Shaw CJ, Wu P, Sriemevan A. First trimester impetigo herpetiformis in multiparous female successfully treated with oral cyclosporine. BMJ Case Reports. 2011:2011. doi: 10.1136/bcr.02.2011.3915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Vun YY, Jones B, Al-Mudhaffer M, Egan C. Generalized pustular psoriasis of pregnancy treated with narrowband UVB and topical steroids. Journal of the American Academy of Dermatology. 2006;54(2):S28–S30. doi: 10.1016/j.jaad.2005.04.041. [DOI] [PubMed] [Google Scholar]
- 130.Sheth N, Greenblatt DT, Acland K, Barker J, Teixeira F. Generalized pustular psoriasis of pregnancy treated with infliximab. Clin Exp Dermatol. 2009 Jun;34(4):521–522. doi: 10.1111/j.1365-2230.2008.02963.x. [DOI] [PubMed] [Google Scholar]
- 131.Breier-Maly J, Ortel B, Breier F, Schmidt JB, Honigsmann H. Generalized pustular psoriasis of pregnancy (impetigo herpetiformis) Dermatology. 1999;198(1):61–64. doi: 10.1159/000018066. [DOI] [PubMed] [Google Scholar]
- 132.Bukhari IA. Impetigo herpetiformis in a primigravida: successful treatment with etretinate. J Drugs Dermatol. 2004 Jul-Aug;3(4):449–451. [PubMed] [Google Scholar]
- 133.Gimenez Garcia R, Gimenez Garcia MC, Llorente de la Fuente A. Impetigo herpetiformis: response to steroids and etretinate. Int J Dermatol. 1989 Oct;28(8):551–552. doi: 10.1111/j.1365-4362.1989.tb04616.x. [DOI] [PubMed] [Google Scholar]
- 134.Winzer M, Wolff HH. [Impetigo herpetiformis] Hautarzt. 1988 Feb;39(2):110–113. [PubMed] [Google Scholar]
- 135.Lohrisch I, Heilmann S, Haustein UF. [Impetigo herpetiformis and PUVA-treatment (author's transl)] Dermatol Monatsschr. 1979 Sep;165(9):648–652. [PubMed] [Google Scholar]
- 136.Liao PB, Rubinson R, Howard R, Sanchez G, Frieden IJ. Annular pustular psoriasis--most common form of pustular psoriasis in children: report of three cases and review of the literature. Pediatr Dermatol. 2002 Jan-Feb;19(1):19–25. doi: 10.1046/j.1525-1470.2002.00026.x. [DOI] [PubMed] [Google Scholar]
- 137.Staughton R. Infantile generalized pustular psoriasis responding to dapsone. Proc R Soc Med. 1977 Apr;70(4):286–287. [PMC free article] [PubMed] [Google Scholar]
- 138.Adler DJ, Rower JM, Hashimoto K. Annular pustular psoriasis. Arch Dermatol. 1981 May;117(5):313–314. [PubMed] [Google Scholar]
- 139.Vocks E, Worret WI, Ring J. Erythema annulare centrifugum-type psoriasis: a particular variant of acute-eruptive psoriasis. J Eur Acad Dermatol Venereol. 2003 Jul;17(4):446–448. doi: 10.1046/j.1468-3083.2003.00715.x. [DOI] [PubMed] [Google Scholar]
- 140.Singh N, Thappa DM. Circinate pustular psoriasis localized to glans penis mimicking'circinate balanitis' and responsive to dapsone. Indian Journal of Dermatology, Venereology, and Leprology. 2008;74(4):388. [PubMed] [Google Scholar]
- 141.Park YM, Kang H, Cho BK. Annular pustular psoriasis localized to the dorsa of the feet. Acta Derm Venereol. 1999 Mar;79(2):161–162. doi: 10.1080/000155599750011444. [DOI] [PubMed] [Google Scholar]
- 142.Rosinska D, Wolska H, Jablonska S, Konca I. Etretinate in severe psoriasis of children. Pediatr Dermatol. 1988 Nov;5(4):266–272. doi: 10.1111/j.1525-1470.1988.tb00902.x. [DOI] [PubMed] [Google Scholar]
- 143.Shelnitz LS, Esterly NB, Honig PJ. Etretinate therapy for generalized pustular psoriasis in children. Archives of dermatology. 1987;123(2):230. [PubMed] [Google Scholar]
- 144.Sneddon IB, Wilkinson DS. Subcorneal pustular dermatosis. Br J Dermatol. 1956 Dec;68(12):385–394. doi: 10.1111/j.1365-2133.1956.tb12774.x. [DOI] [PubMed] [Google Scholar]
- 145.Chimenti S, Ackerman AB. Is subcorneal pustular dermatosis of Sneddon and Wilkinson an entity sui generis? Am J Dermatopathol. 1981 Winter;3(4):363–376. doi: 10.1097/00000372-198100340-00007. [DOI] [PubMed] [Google Scholar]
- 146.Pinkus H. Is subcorneal pustular dermatosis of Sneddon and Wilkinson and entity sui generis? Am J Dermatopathol. 1981 Winter;3(4):379–80. doi: 10.1097/00000372-198100340-00009. [DOI] [PubMed] [Google Scholar]
- 147.Sneddon I, Wilkinson DS. Subcorneal pustular dermatosis differs from subcorneal pustulosis. Am J Dermatopathol. 1981 Winter;3(4):377. doi: 10.1097/00000372-198100340-00008. [DOI] [PubMed] [Google Scholar]
- 148.Wolff K. Subcorneal pustular dermatosis is not pustular psoriasis. Am J Dermatopathol. 1981 Winter;3(4):381–382. doi: 10.1097/00000372-198100340-00010. [DOI] [PubMed] [Google Scholar]
- 149.Sanchez NP, Perry HO, Muller SA, Winkelmann RK. Subcorneal pustular dermatosis and pustular psoriasis. A clinicopathologic correlation. Arch Dermatol. 1983 Sep;119(9):715–721. [PubMed] [Google Scholar]
- 150.Apted J. Subcorneal pustular dermatosis is seen in early infancy. Pediatr Dermatol. 1976;1:1–3. [Google Scholar]
- 151.Beck AL, Jr, Kipping HL, Crissey JT. Subcorneal pustular dermatosis: Report of a case. Archives of dermatology. 1961;83(4):627. doi: 10.1001/archderm.1961.01580100091011. [DOI] [PubMed] [Google Scholar]
- 152.Johnson SAM, Cripps DJ. Subcorneal pustular dermatosis in children. Archives of dermatology. 1974;109(1):73. [PubMed] [Google Scholar]
- 153.Kocak M, Birol A, Erkek E, Bozdoğan Ö, Atasoy P. Juvenile subcorneal pustular dermatosis: a case report. Pediatric dermatology. 2003;20(1):57–59. doi: 10.1046/j.1525-1470.2003.03013.x. [DOI] [PubMed] [Google Scholar]
- 154.Sneddon IB, Wilkinson DS. Subcorneal pustular dermatosis. Br J Dermatol. 1979 Jan;100(1):61–68. doi: 10.1111/j.1365-2133.1979.tb03570.x. [DOI] [PubMed] [Google Scholar]
- 155.Marsden JR, Millard LG. Pyoderma gangrenosum, subcorneal pustular dermatosis and IgA paraproteinaemia. Br J Dermatol. 1986 Jan;114(1):125–129. doi: 10.1111/j.1365-2133.1986.tb02787.x. [DOI] [PubMed] [Google Scholar]
- 156.Scerri L, Zaki I, Allen BR. Pyoderma gangrenosum and subcorneal pustular dermatosis, without monoclonal gammopathy. Br J Dermatol. 1994 Mar;130(3):398–399. doi: 10.1111/j.1365-2133.1994.tb02941.x. [DOI] [PubMed] [Google Scholar]
- 157.Delaporte E, Colombel JF, Nguyen-Mailfer C, Piette F, Cortot A, Bergoend H. Subcorneal pustular dermatosis in a patient with Crohn's disease. Acta Derm Venereol. 1992 Aug;72(4):301–302. [PubMed] [Google Scholar]
- 158.Cream JJ, Grimes SM, Roberts PD. Subcorneal pustulosis and IgA myelomatosis. Br Med J. 1977 Feb 26;1(6060):550. doi: 10.1136/bmj.1.6060.550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Todd DJ, Bingham EA, Walsh M, Burrows D. Subcorneal pustular dermatosis and IgA paraproteinaemia: response to both etretinate and PUVA. Br J Dermatol. 1991 Oct;125(4):387–389. doi: 10.1111/j.1365-2133.1991.tb14179.x. [DOI] [PubMed] [Google Scholar]
- 160.Ellis FA. Subcorneal pustular dermatosis. AMA Arch Derm. 1958 Nov;78(5):580–588. doi: 10.1001/archderm.1958.01560110028003. [DOI] [PubMed] [Google Scholar]
- 161.Weedon D. Weedon's Skin Pathology. 3 ed. Vol 1. Churchill Livingston; 2009. [Google Scholar]
- 162.Reed J, Wilkinson J. Subcorneal pustular dermatosis. Clin Dermatol. 2000 May-Jun;18(3):301–313. doi: 10.1016/s0738-081x(99)00121-2. [DOI] [PubMed] [Google Scholar]
- 163.Hashimoto T, Yasumoto S, Nagata Y, Okamoto T, Fujita S. Clinical, histopathological and immunological distinction in two cases of IgA pemphigus. Clin Exp Dermatol. 2002 Nov;27(8):636–640. doi: 10.1046/j.1365-2230.2002.01061.x. [DOI] [PubMed] [Google Scholar]
- 164.Harvath L, Yancey KB, Katz SI. Selective inhibition of human neutrophil chemotaxis to N-formyl-methionylleucyl-phenylalanine by sulfones. J Immunol. 1986 Aug 15;137(4):1305–1311. [PubMed] [Google Scholar]
- 165.Walkden V, Roberts A, Wilkinson J. Two cases of subcorneal pustular dermatosis Response to use of intermittent clobetasol propionate cream. EJD. European journal of dermatology. 1994;4(1):44–46. [Google Scholar]
- 166.Folkers E, Tafelkruyer J. Subcorneal pustular dermatosis (Sneddon-Wilkinson disease)--therapeutic problems. Br J Dermatol. 1978 Jun;98(6):681–684. doi: 10.1111/j.1365-2133.1978.tb03588.x. [DOI] [PubMed] [Google Scholar]
- 167.Iandoli R, Monfrecola G. Treatment of subcorneal pustulosis by etretinate. Dermatologica. 1987;175(5):235–238. doi: 10.1159/000248910. [DOI] [PubMed] [Google Scholar]
- 168.Cameron H, Dawe RS. Subcorneal pustular dermatosis (Sneddon-Wilkinson disease) treated with narrowband (TL-01) UVB phototherapy. Br J Dermatol. 1997 Jul;137(1):150–151. doi: 10.1111/j.1365-2133.1997.tb03721.x. [DOI] [PubMed] [Google Scholar]
- 169.Park YK, Park HY, Bang DS, Cho CK. Subcorneal pustular dermatosis treated with phototherapy. Int J Dermatol. 1986 Mar;25(2):124–126. doi: 10.1111/j.1365-4362.1986.tb04556.x. [DOI] [PubMed] [Google Scholar]
- 170.Bauwens M, De Coninck A, Roseeuw D. Subcorneal pustular dermatosis treated with PUVA therapy. A case report and review of the literature. Dermatology. 1999;198(2):203–205. doi: 10.1159/000018113. [DOI] [PubMed] [Google Scholar]
- 171.Bonifati C, Trento E, Cordiali Fei P, Muscardin L, Amantea A, Carducci M. Early but not lasting improvement of recalcitrant subcorneal pustular dermatosis (Sneddon-Wilkinson disease) after infliximab therapy: relationships with variations in cytokine levels in suction blister fluids. Clin Exp Dermatol. 2005 Nov;30(6):662–665. doi: 10.1111/j.1365-2230.2005.01902.x. [DOI] [PubMed] [Google Scholar]
- 172.Voigtlander C, Luftl M, Schuler G, Hertl M. Infliximab (anti-tumor necrosis factor alpha antibody): a novel, highly effective treatment of recalcitrant subcorneal pustular dermatosis (Sneddon-Wilkinson disease) Arch Dermatol. 2001 Dec;137(12):1571–1574. doi: 10.1001/archderm.137.12.1571. [DOI] [PubMed] [Google Scholar]
- 173.Hallopeau FH. Sur une asphyxie locale des extrémités avec polydactylie suppurative chronique et poussées éphémères de dermatite pustuleuse disséminée et symétrique. Bulletin de la Société française de dermatologie et de syphiligraphie. 1:39–45. [Google Scholar]
- 174.Yerushalmi J, Grunwald MH, Hallel-Halevy D, Avinoach I, Halevy S. Chronic pustular eruption of the thumbs Diagnosis: acrodermatitis continua of Hallopeau (ACH)Arch. Dermatol. 2000 Jul;136(7):925–930. doi: 10.1001/archderm.136.7.925-c. [DOI] [PubMed] [Google Scholar]
- 175.Moschella SL. Review of so-called aseptic neutrophilic dermatoses. Australas J Dermatol. 1983 Aug;24(2):55–62. doi: 10.1111/j.1440-0960.1983.tb00252.x. [DOI] [PubMed] [Google Scholar]
- 176.Kirkup ME, Lovell CR. Acquired syndactyly secondary to acrodermatitis continua of Hallopeau. Br J Dermatol. 2005 May;152(5):1083–1084. doi: 10.1111/j.1365-2133.2005.06580.x. [DOI] [PubMed] [Google Scholar]
- 177.Waller JM, Wu JJ, Murase JE, Dyson SW, Kelly KM. Chronically painful right thumb with pustules and onycholysis Diagnosis: acrodermatitis continua of Hallopeau. Clin Exp Dermatol. 2007 Sep;32(5):619–620. doi: 10.1111/j.1365-2230.2007.02440.x. [DOI] [PubMed] [Google Scholar]
- 178.Akiyama T, Seishima M, Watanabe H, Nakatani A, Mori S, Kitajima Y. The relationships of onset and exacerbation of pustulosis palmaris et plantaris to smoking and focal infections. J Dermatol. 1995 Dec;22(12):930–934. doi: 10.1111/j.1346-8138.1995.tb03948.x. [DOI] [PubMed] [Google Scholar]
- 179.Kubeyinje EP, Belagavi CS. Risk factors for palmo-plantar pustulosis in a developing country. East Afr Med J. 1997 Jan;74(1):54–55. [PubMed] [Google Scholar]
- 180.Michaelsson G, Gustafsson K, Hagforsen E. The psoriasis variant palmoplantar pustulosis can be improved after cessation of smoking. J Am Acad Dermatol. 2006 Apr;54(4):737–738. doi: 10.1016/j.jaad.2005.07.024. [DOI] [PubMed] [Google Scholar]
- 181.Rosen K, Mobacken H, Swanbeck G. PUVA, etretinate, and PUVA-etretinate therapy for pustulosis palmoplantaris A placebo-controlled comparative trial. Arch Dermatol. 1987 Jul;123(7):885–889. doi: 10.1001/archderm.1987.01660310053013. [DOI] [PubMed] [Google Scholar]
- 182.Eriksson MO, Hagforsen E, Lundin IP, Michaelsson G. Palmoplantar pustulosis: a clinical and immunohistological study. Br J Dermatol. 1998 Mar;138(3):390–398. doi: 10.1046/j.1365-2133.1998.02113.x. [DOI] [PubMed] [Google Scholar]
- 183.Burge S, Ryan T. Acute palmoplantar pustulosis. British Journal of dermatology. 1985;113(1):77–83. doi: 10.1111/j.1365-2133.1985.tb02046.x. [DOI] [PubMed] [Google Scholar]
- 184.Burden AD, Kemmett D. The spectrum of nail involvement in palmoplantar pustulosis. Br J Dermatol. 1996 Jun;134(6):1079–1082. [PubMed] [Google Scholar]
- 185.Piraccini B, Tosti A, Iorizzo M, Misciali C. Pustular psoriasis of the nails: treatment and long-term follow-up of 46 patients. British Journal of dermatology. 2002;144(5):1000–1005. doi: 10.1046/j.1365-2133.2001.04189.x. [DOI] [PubMed] [Google Scholar]
- 186.Chamot A, Vion B, Gerster J. Acute pseudoseptic arthritis and palmoplantar pustulosis. Clinical rheumatology. 1986;5(1):118–123. doi: 10.1007/BF02030980. [DOI] [PubMed] [Google Scholar]
- 187.Yamamoto T, Kimura K, Katayama I, Nishioka K. Successful treatment of severe arthralgia associated with palmoplantar pustulosis with low-dose oral cyclosporine A. The Journal of dermatology. 1995;22(7):512. doi: 10.1111/j.1346-8138.1995.tb03435.x. [DOI] [PubMed] [Google Scholar]
- 188.Rosen K, Lindstedt G, Mobacken H, Nystrom E. Thyroid function in patients with pustulosis palmoplantaris. J Am Acad Dermatol. 1988 Dec;19(6):1009–1016. doi: 10.1016/s0190-9622(88)70265-0. [DOI] [PubMed] [Google Scholar]
- 189.Rosen K, Mobacken H, Nilsson LA. Increased prevalence of antithyroid antibodies and thyroid diseases in pustulosis palmoplantaris. Acta Derm Venereol. 1981;61(3):237–240. [PubMed] [Google Scholar]
- 190.Lundin A, Hakansson L, Michaelsson G, Venge P. Neutrophil locomotion and serum chemotactic and chemokinetic activities in pustulosis palmoplantaris compared with psoriasis. Arch Dermatol Res. 1987;279(6):385–391. doi: 10.1007/BF00412624. [DOI] [PubMed] [Google Scholar]
- 191.Asumalahti K, Ameen M, Suomela S, et al. Genetic analysis of PSORS1 distinguishes guttate psoriasis and palmoplantar pustulosis. Journal of investigative dermatology. 2003;120(4):627–632. doi: 10.1046/j.1523-1747.2003.12094.x. [DOI] [PubMed] [Google Scholar]
- 192.Kragballe K, Larsen FG. A hydrocolloid occlusive dressing plus triamcinolone acetonide cream is superior to clobetasol cream in palmo-plantar pustulosis. Acta Derm Venereol. 1991;71(6):540–542. [PubMed] [Google Scholar]
- 193.Murray D, Corbett MF, Warin AP. A controlled trial of photochemotherapy for persistent palmoplantar pustulosis. Br J Dermatol. 1980 Jun;102(6):659–663. doi: 10.1111/j.1365-2133.1980.tb06565.x. [DOI] [PubMed] [Google Scholar]
- 194.Ettler K, Richards B. Acitretin therapy for palmoplantar pustulosis combined with UVA and topical 8-MOP. International Journal of Dermatology. 2001;40(8):541–542. doi: 10.1046/j.1365-4362.2001.01094-3.x. [DOI] [PubMed] [Google Scholar]
- 195.Lassus A, GEIGER JM. Acitretin and etretinate in the treatment of palmoplantar pustulosis: a double-blind comparative trial. British Journal of dermatology. 1988;119(6):755–759. doi: 10.1111/j.1365-2133.1988.tb03499.x. [DOI] [PubMed] [Google Scholar]
- 196.Erkko P, Granlund H, Remitz A, et al. Double-blind placebo-controlled study of long-term low-dose cyclosporin in the treatment of palmoplantar pustulosis. Br J Dermatol. 1998 Dec;139(6):997–1004. doi: 10.1046/j.1365-2133.1998.02555.x. [DOI] [PubMed] [Google Scholar]
- 197.Reitamo S, Erkko P, Remitz A, Lauerma AI, Montonen O, Harjula K. Cyclosporine in the treatment of palmoplantar pustulosis A randomized, double-blind, placebo-controlled study. Arc Dermatol. 1993 Octh;129(10):1273–1279. [PubMed] [Google Scholar]
- 198.Thomsen K, Osterbye P. Pustulosis palmaris et plantaris. Br J Dermatol. 1973 Sep;89(3):293–296. doi: 10.1111/j.1365-2133.1973.tb02977.x. [DOI] [PubMed] [Google Scholar]
- 199.Ward JM, Corbett MF, Hanna MJ. A double-blind trial of clomocycline in the treatment of persistent palmoplantar pustulosis. Br J Dermatol. 1976 Sep;95(3):317–322. doi: 10.1111/j.1365-2133.1976.tb07020.x. [DOI] [PubMed] [Google Scholar]
- 200.Thomsen K. Pustulosis palmaris et plantaris treated with methotrexate. Acta Derm Venereol. 1971;51(5):397–400. [PubMed] [Google Scholar]
- 201.Bissonnette R, Poulin Y, Bolduc C, et al. Etanercept in the treatment of palmoplantar pustulosis. Journal of drugs in dermatology: JDD. 2008;7(10):940. [PubMed] [Google Scholar]
- 202.Ghate JV, Alspaugh CD. Adalimumab in the management of palmoplantar psoriasis. J Drugs Dermatol. 2009 Dec;8(12):1136–1139. [PubMed] [Google Scholar]
- 203.Yawalkar N, Hunger RE. Successful treatment of recalcitrant palmoplantar pustular psoriasis with sequential use of infliximab and adalimumab. Dermatology. 2009;218(1):79–83. doi: 10.1159/000167802. [DOI] [PubMed] [Google Scholar]
- 204.Mössner R, Thaci D, Mohr J, et al. Manifestation of palmoplantar pustulosis during or after infliximab therapy for plaque-type psoriasis: report on five cases. Archives of Dermatological Research. 2008;300(3):101–105. doi: 10.1007/s00403-008-0831-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Shmidt E, Wetter DA, Ferguson SB, Pittelkow MR. Psoriasis and palmoplantar pustulosis associated with tumor necrosis factor-alpha inhibitors: The Mayo Clinic experience, 1998 to 2010. J Am Acad Dermatol. 2011 Jul 11; doi: 10.1016/j.jaad.2011.05.038. [DOI] [PubMed] [Google Scholar]
- 206.Morris A, Rogers M, Fischer G, Williams K. Childhood psoriasis: a clinical review of 1262 cases. Pediatr Dermatol. 2001 May-Jun;18(3):188–198. doi: 10.1046/j.1525-1470.2001.018003188.x. [DOI] [PubMed] [Google Scholar]
- 207.Jarratt M, Ramsdell W. Infantile acropustulosis. Arch Dermatol. 1979 Jul;115(7):834–836. [PubMed] [Google Scholar]
- 208.Kahn G, Rywlin AM. Acropustulosis of infancy. Archives of dermatology. 1979;115(7):831. [PubMed] [Google Scholar]
- 209.Wilsmann-Theis D, Hagemann T, Dederer H, Wenzel J, Bieber T, Novak N. Successful treatment of acrodermatitis continua suppurativa with topical tacrolimus 0.1% ointment. Br J Dermatol. 2004 Jun;150(6):1194–1197. doi: 10.1111/j.1365-2133.2004.05992.x. [DOI] [PubMed] [Google Scholar]
- 210.Bordignon M, Zattra E, Albertin C, Belloni-Fortina A. Successful treatment of a 9-year-old boy affected by acrodermatitis continua of Hallopeau with targeted ultraviolet B narrow-band phototherapy. Photodermatol Photoimmunol Photomed. 2010 Feb;26(1):41–43. doi: 10.1111/j.1600-0781.2009.00468.x. [DOI] [PubMed] [Google Scholar]
- 211.Lipsker D, Perrigouard C, Foubert A, Cribier B. Anakinra for difficult-to-treat neutrophilic panniculitis: IL-1 blockade as a promising treatment option for neutrophil-mediated inflammatory skin disease. Dermatology. 2010;220(3):264–267. doi: 10.1159/000280436. [DOI] [PubMed] [Google Scholar]
- 212.Mueller RB, Ogilvie A, Schwarz S, Kern P, Schett G, Sticherling M. Adalimumab treatment of a patient with psoriasis suppurativa Hallopeau associated osteoarthropathy. Clin Exp Rheumatol. 2009 Sep-Oct;27(5):887. [PubMed] [Google Scholar]
- 213.Puig L, Barco D, Vilarrasa E, Alomar A. Treatment of acrodermatitis continua of Hallopeau with TNF-blocking agents: case report and review. Dermatology. 2010;220(2):154–158. doi: 10.1159/000277415. [DOI] [PubMed] [Google Scholar]
- 214.Ryan C, Collins P, Kirby B, Rogers S. Treatment of acrodermatitis continua of Hallopeau with adalimumab. Br J Dermatol. 2009 Jan;160(1):203–205. doi: 10.1111/j.1365-2133.2008.08893.x. [DOI] [PubMed] [Google Scholar]
- 215.Sopkovich JA, Anetakis Poulos G, Wong HK. Acrodermatitis continua of hallopeau successfully treated with adalimumab. J Clin Aesthet Dermatol. 2012 Feb;5(2):60–62. [PMC free article] [PubMed] [Google Scholar]
- 216.Mang R, Ruzicka T, Stege H. Successful treatment of acrodermatitis continua of Hallopeau by the tumour necrosis factor-alpha inhibitor infliximab (Remicade) Br J Dermatol. 2004 Feb;150(2):379–380. doi: 10.1111/j.1365-2133.2003.05761.x. [DOI] [PubMed] [Google Scholar]
- 217.Rubio C, Martin MA, Arranz Sanchez DM, Vidaurrazaga C, Casado M. Excellent and prolonged response to infliximab in a case of recalcitrant acrodermatitis continua of Hallopeau. J Eur Acad Dermatol Venereol. 2009 Jun;23(6):707–708. doi: 10.1111/j.1468-3083.2008.02998.x. [DOI] [PubMed] [Google Scholar]
- 218.Bonish B, Rashid RM, Swan J. Etanercept responsive acrodermatitis continua of Hallopeau: is a pattern developing? J Drugs Dermatol. 2006 Oct;5(9):903–904. [PubMed] [Google Scholar]
- 219.Kazinski K, Joyce KM, Hodson D. The successful use of etanercept in combination therapy for treatment of acrodermatitis continua of hallopeau. J Drugs Dermatol. 2005 May-Jun;4(3):360–364. [PubMed] [Google Scholar]
- 220.Nikkels AF, Pierard GE. Etanercept and recalcitrant acrodermatitis continua of Hallopeau. J Drugs Dermatol. 2006 Sep;5(8):705–706. [PubMed] [Google Scholar]
- 221.Thielen AM, Barde C, Marazza G, Saurat JH. Long-term control with etanercept (Enbrel) of a severe acrodermatitis continua of Hallopeau refractory to infliximab (Remicade) Dermatology. 2008;217(2):137–139. doi: 10.1159/000134613. [DOI] [PubMed] [Google Scholar]
- 222.Weisshaar E, Diepgen TL. Successful etanercept therapy in therapy refractory acrodermatitis continua suppurativa Hallopeau. J Dtsch Dermatol Ges. 2007 Jun;5(6):489–492. doi: 10.1111/j.1610-0387.2007.06332.x. [DOI] [PubMed] [Google Scholar]