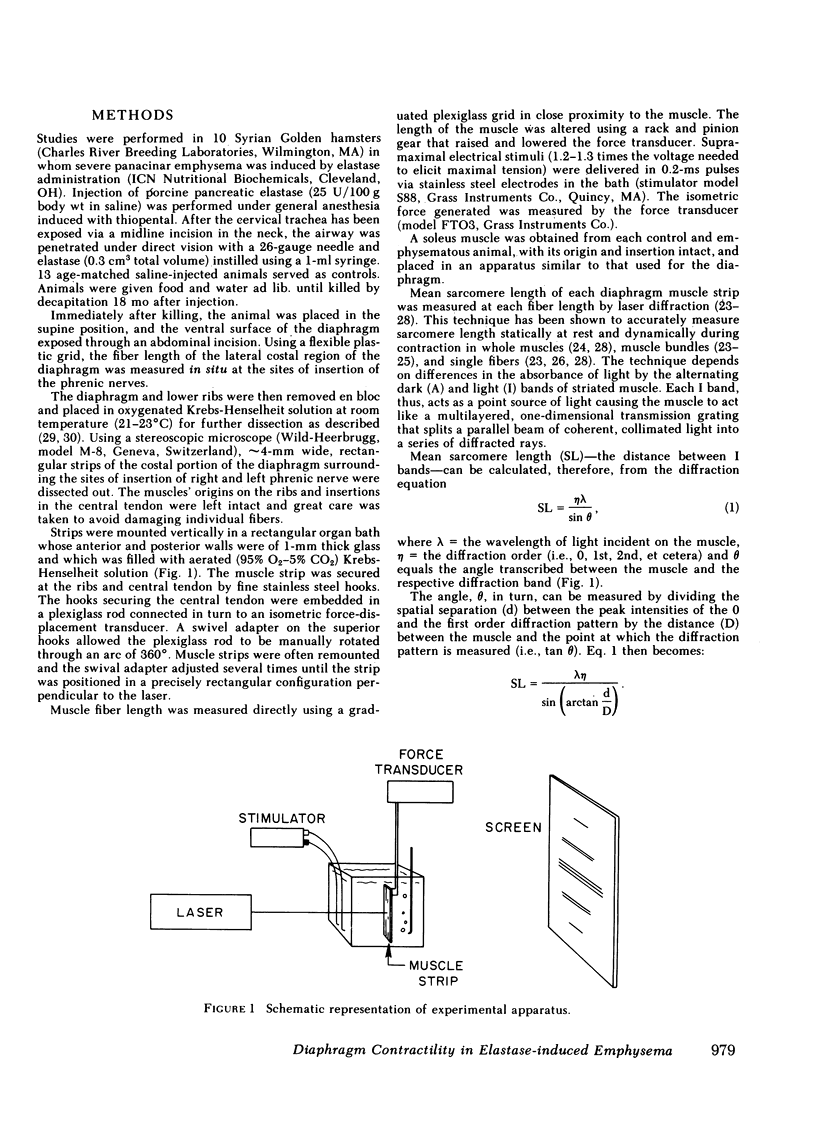
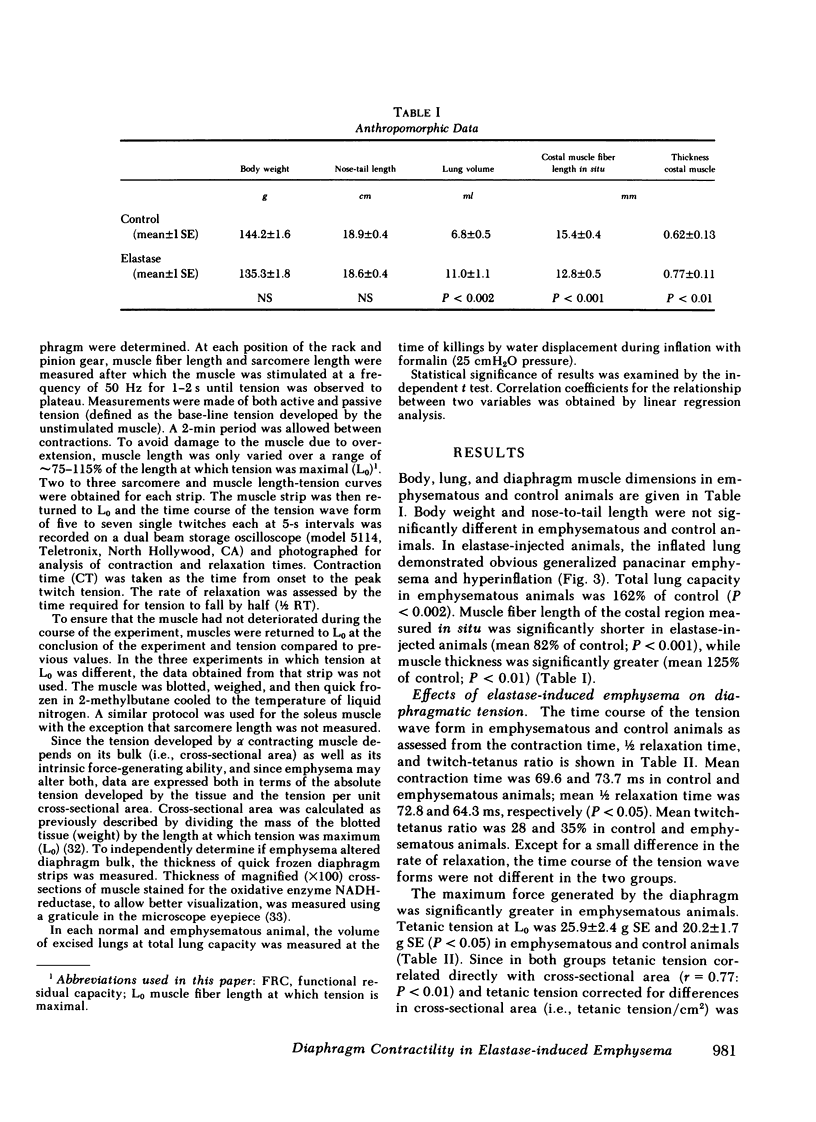
Abstract
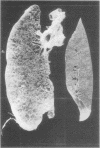
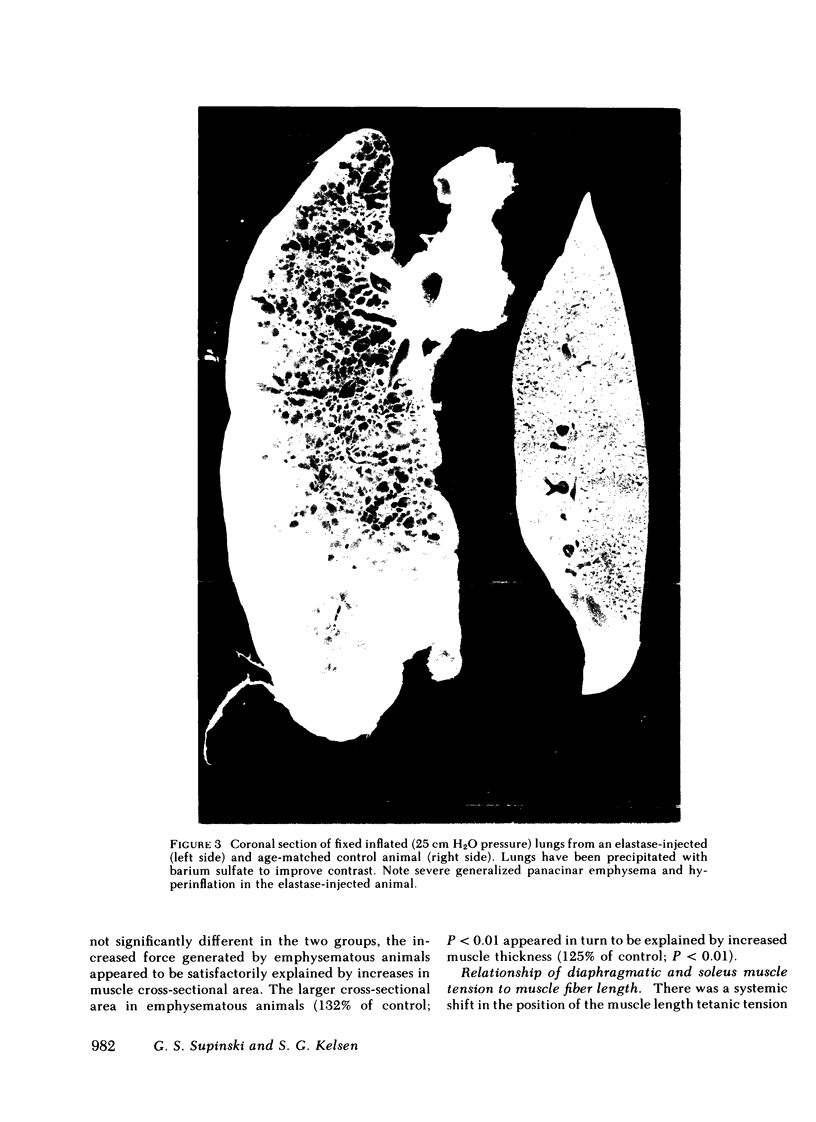
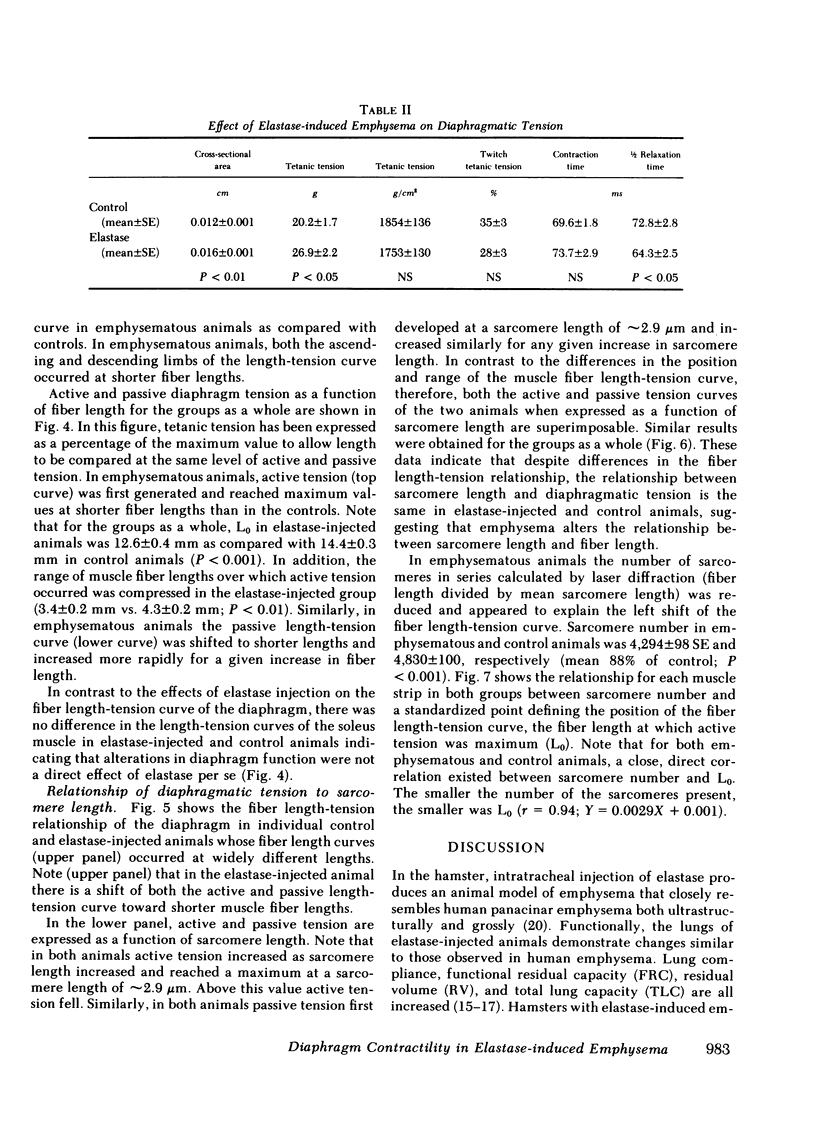
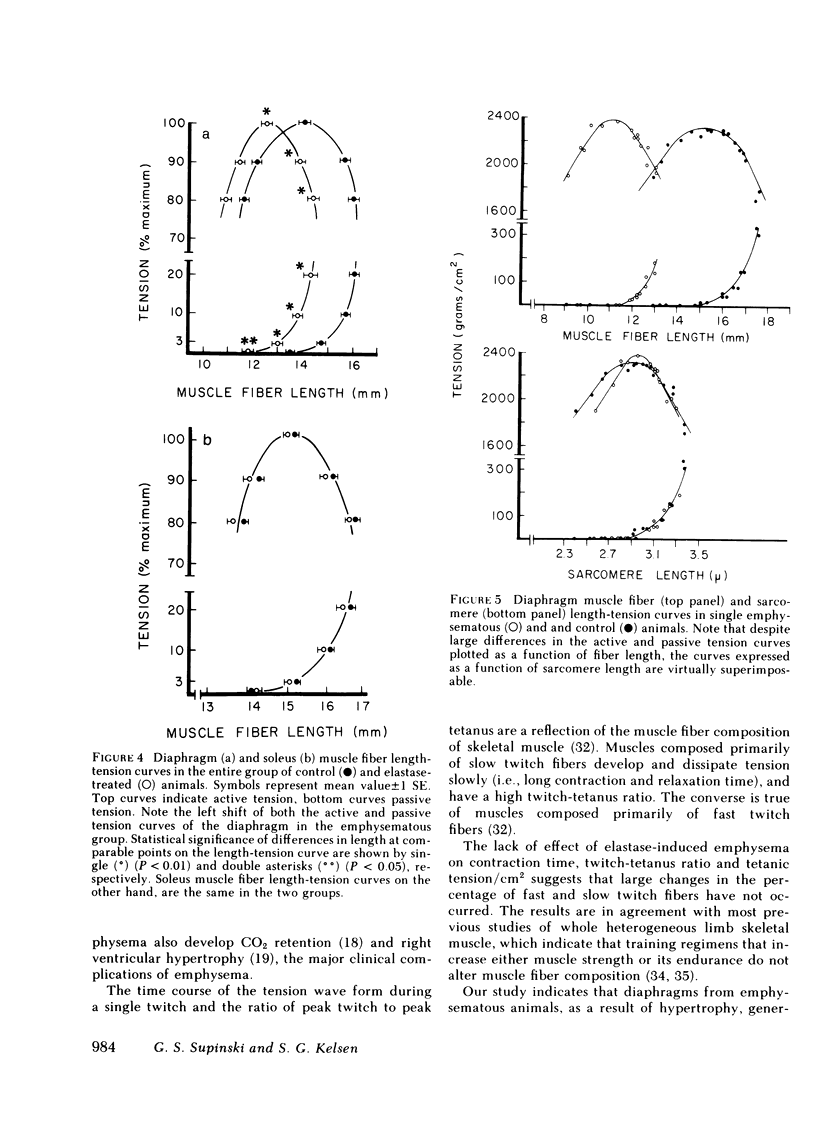
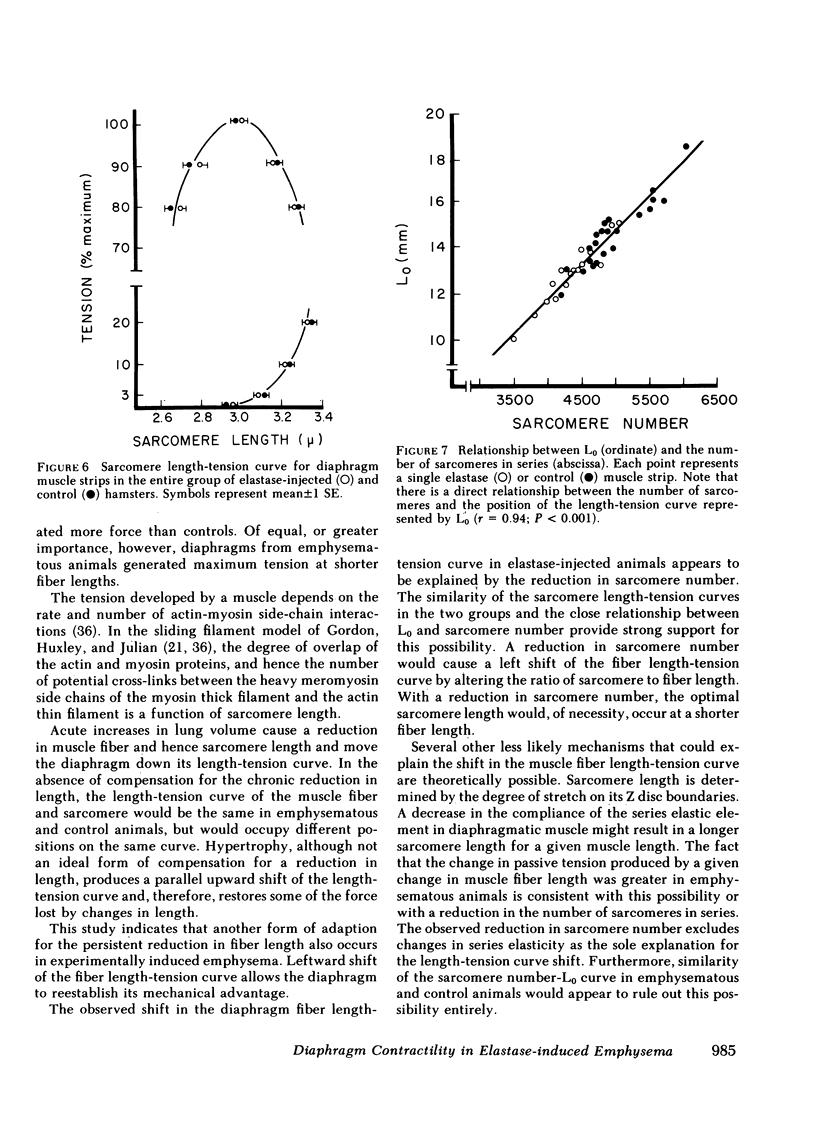
The effect of emphysema on the ability of the diaphragm to generate force was examined in costal diaphragm muscle strips from 10 Golden hamsters killed 18 mo after intratracheal injection of pancreatic elastase in a dose producing hyperinflation (mean total lung capacity [TLC] = 163% of control) and generalized panacinar emphysema. 13 saline-injected normal animals served as controls. The time course of isometric tension and the effect of alterations in muscle fiber and sarcomere length on the isometric tension (T) generated in response to tetanizing electrical stimuli (length-tension [L-T] relationship) were examined. Elastase administration caused an increase in diaphragm muscle thickness and reduction in the length of costal diaphragm muscle fibers measured in situ. Emphysema significantly increased the maximum tetanic tension as a result of hypertrophy. Maximal tension corrected for increases in muscle cross-sectional area (T/cm2), however, was the same in emphysematous (E) and control (C) animals. Emphysema also shifted the muscle fiber L-T curve of the diaphragm but not of a control muscle, the soleus, toward shorter lengths. In contrast to the effects of E on the diaphragm muscle fiber L-T curve, the sarcomere L-T curve was the same in E and C. Since the length at which tension was maximal correlated closely with sarcomere number (r = 0.94; P < 0.001) reduction in the number of sarcomeres in series in muscles from emphysematous animals appeared to explain the shift in the muscle fiber L-T curve. We conclude that in elastase-induced emphysema adaptive changes both in diaphragm cross-sectional area and sarcomere number augment the force-generating ability of the diaphragm. We speculate that changes in sarcomere number compensate for alterations in muscle fiber length resulting from chronic hyperinflation of the thorax, while diaphragmatic muscle hypertrophy represents a response to changes in respiratory load and/or diaphragm configuration (LaPlace relationship).
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arora N. S., Rochester D. F. Effect of body weight and muscularity on human diaphragm muscle mass, thickness, and area. J Appl Physiol Respir Environ Exerc Physiol. 1982 Jan;52(1):64–70. doi: 10.1152/jappl.1982.52.1.64. [DOI] [PubMed] [Google Scholar]
- Bartels E. M., Skydsgaard J. M., Sten-Knudsen O. The time course of the latency relaxation as a function of the sarcomere length in frog and mammalian muscle. Acta Physiol Scand. 1979 Jun;106(2):129–137. doi: 10.1111/j.1748-1716.1979.tb06381.x. [DOI] [PubMed] [Google Scholar]
- Butler C. Diaphragmatic changes in emphysema. Am Rev Respir Dis. 1976 Jul;114(1):155–159. doi: 10.1164/arrd.1976.114.1.155. [DOI] [PubMed] [Google Scholar]
- Byrd R. B., Hyatt R. E. Maximal respiratory pressures in chronic obstructive lung disease. Am Rev Respir Dis. 1968 Nov;98(5):848–856. doi: 10.1164/arrd.1968.98.5.848. [DOI] [PubMed] [Google Scholar]
- CHERNIACK R. M. The oxygen consumption and efficiency of the respiratory muscles in health and emphysema. J Clin Invest. 1959 Mar;38(3):494–499. doi: 10.1172/JCI103826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleworth D. R., Edman K. A. Changes in sarcomere length during isometric tension development in frog skeletal muscle. J Physiol. 1972 Dec;227(1):1–17. doi: 10.1113/jphysiol.1972.sp010016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Close R. I. Dynamic properties of mammalian skeletal muscles. Physiol Rev. 1972 Jan;52(1):129–197. doi: 10.1152/physrev.1972.52.1.129. [DOI] [PubMed] [Google Scholar]
- Delay M. J., Ishide N., Jacobson R. C., Pollack G. H., Tirosh R. Stepwise sarcomere shortening: analysis by high-speed cinemicrography. Science. 1981 Sep 25;213(4515):1523–1525. doi: 10.1126/science.7280674. [DOI] [PubMed] [Google Scholar]
- Derenne J. P., Macklem P. T., Roussos C. The respiratory muscles: Mechanics, control, and pathophysiology. Part 2. Am Rev Respir Dis. 1978 Aug;118(2):373–390. doi: 10.1164/arrd.1978.118.2.373. [DOI] [PubMed] [Google Scholar]
- Evanich M. J., Franco M. J., Lourenço R. V. Force output of the diaphragm as a function of phrenic nerve firing rate and lung volume. J Appl Physiol. 1973 Aug;35(2):208–212. doi: 10.1152/jappl.1973.35.2.208. [DOI] [PubMed] [Google Scholar]
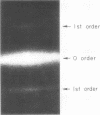
- Fujime S. Optical diffraction study of muscle fibers. Biochim Biophys Acta. 1975 Jan 30;379(1):227–238. doi: 10.1016/0005-2795(75)90026-4. [DOI] [PubMed] [Google Scholar]
- Goldspink G., Tabary C., Tabary J. C., Tardieu C., Tardieu G. Effect of denervation on the adaptation of sarcomere number and muscle extensibility to the functional length of the muscle. J Physiol. 1974 Feb;236(3):733–742. doi: 10.1113/jphysiol.1974.sp010463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon A. M., Huxley A. F., Julian F. J. Tension development in highly stretched vertebrate muscle fibres. J Physiol. 1966 May;184(1):143–169. doi: 10.1113/jphysiol.1966.sp007908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grassino A., Goldman M. D., Mead J., Sears T. A. Mechanics of the human diaphragm during voluntary contraction: statics. J Appl Physiol Respir Environ Exerc Physiol. 1978 Jun;44(6):829–839. doi: 10.1152/jappl.1978.44.6.829. [DOI] [PubMed] [Google Scholar]
- Holloszy J. O., Booth F. W. Biochemical adaptations to endurance exercise in muscle. Annu Rev Physiol. 1976;38:273–291. doi: 10.1146/annurev.ph.38.030176.001421. [DOI] [PubMed] [Google Scholar]
- Huxley A. F. Muscular contraction. J Physiol. 1974 Nov;243(1):1–43. [PMC free article] [PubMed] [Google Scholar]
- Ishikawa S., Hayes J. A. Functional morphotometry of the diaphragm in patients with chronic obstructive lung disease. Am Rev Respir Dis. 1973 Jul;108(1):135–138. doi: 10.1164/arrd.1973.108.1.135. [DOI] [PubMed] [Google Scholar]
- Jolesz F., Sreter F. A. Development, innervation, and activity-pattern induced changes in skeletal muscle. Annu Rev Physiol. 1981;43:531–552. doi: 10.1146/annurev.ph.43.030181.002531. [DOI] [PubMed] [Google Scholar]
- Karlinsky J. B., Snider G. L. Animal models of emphysema. Am Rev Respir Dis. 1978 Jun;117(6):1109–1133. doi: 10.1164/arrd.1978.117.6.1109. [DOI] [PubMed] [Google Scholar]
- Kelsen S. G., Nochomovitz M. L. Fatigue of the mammalian diaphragm in vitro. J Appl Physiol Respir Environ Exerc Physiol. 1982 Aug;53(2):440–447. doi: 10.1152/jappl.1982.53.2.440. [DOI] [PubMed] [Google Scholar]
- Kim M. J., Druz W. S., Danon J., Machnach W., Sharp J. T. Mechanics of the canine diaphragm. J Appl Physiol. 1976 Sep;41(3):369–382. doi: 10.1152/jappl.1976.41.3.369. [DOI] [PubMed] [Google Scholar]
- Kuhn C., 3rd, Tavassoli F. The scanning electron microscopy of elastase-induced emphysema. A comparison with emphysema in man. Lab Invest. 1976 Jan;34(1):2–9. [PubMed] [Google Scholar]
- Lucey E. C., O'Brien J. J., Jr, Pereira W., Jr, Snider G. L. Arterial blood gas values in emphysematous hamsters. Am Rev Respir Dis. 1980 Jan;121(1):83–89. doi: 10.1164/arrd.1980.121.1.83. [DOI] [PubMed] [Google Scholar]
- Mead J. Functional significance of the area of apposition of diaphragm to rib cage [proceedings]. Am Rev Respir Dis. 1979 Feb;119(2 Pt 2):31–32. doi: 10.1164/arrd.1979.119.2P2.31. [DOI] [PubMed] [Google Scholar]
- Muller N., Volgyesi G., Becker L., Bryan M. H., Bryan A. C. Diaphragmatic muscle tone. J Appl Physiol Respir Environ Exerc Physiol. 1979 Aug;47(2):279–284. doi: 10.1152/jappl.1979.47.2.279. [DOI] [PubMed] [Google Scholar]
- Pengelly L. D., Alderson A. M., Milic-Emili J. Mechanics of the diaphragm. J Appl Physiol. 1971 Jun;30(6):797–805. doi: 10.1152/jappl.1971.30.6.797. [DOI] [PubMed] [Google Scholar]
- Robbins N., Olek A., Kelly S. S., Takach P., Christopher M. Quantitative study of motor endplates in muscle fibres dissociated by a simple procedure. Proc R Soc Lond B Biol Sci. 1980 Oct 15;209(1177):555–562. doi: 10.1098/rspb.1980.0112. [DOI] [PubMed] [Google Scholar]
- Rochester D. F., Arora N. S., Braun N. M., Goldberg S. K. The respiratory muscles in chronic obstructive pulmonary disease (COPD). Bull Eur Physiopathol Respir. 1979 Sep-Oct;15(5):951–975. [PubMed] [Google Scholar]
- Scott K. W., Hoy J. The cross sectional area of diaphragmatic muscle fibres in emphysema, measured by an automated image analysis system. J Pathol. 1976 Oct;120(2):121–128. doi: 10.1002/path.1711200208. [DOI] [PubMed] [Google Scholar]
- Sharp J. T., Danon J., Druz W. S., Goldberg N. B., Fishman H., Machnach W. Respiratory muscle function in patients with chronic obstructive pulmonary disease: its relationship to disability and to respiratory therapy. Am Rev Respir Dis. 1974 Dec;110(6 Pt 2):154–168. doi: 10.1164/arrd.1974.110.6P2.154. [DOI] [PubMed] [Google Scholar]
- Sharp J. T., Goldberg N. B., Druz W. S., Fishman H. C., Danon J. Thoracoabdominal motion in chronic obstructive pulmonary disease. Am Rev Respir Dis. 1977 Jan;115(1):47–56. doi: 10.1164/arrd.1977.115.1.47. [DOI] [PubMed] [Google Scholar]
- Snider G. L., Sherter C. B. A one-year study of the evolution of elastase-induced emphysema in hamsters. J Appl Physiol Respir Environ Exerc Physiol. 1977 Oct;43(4):721–729. doi: 10.1152/jappl.1977.43.4.721. [DOI] [PubMed] [Google Scholar]
- Snider G. L., Sherter C. B., Koo K. W., Karlinsky J. B., Hayes J. A., Franzblau C. Respiratory mechanics in hamsters following treatment with endotracrael elastase or collagenase. J Appl Physiol Respir Environ Exerc Physiol. 1977 Feb;42(2):206–215. doi: 10.1152/jappl.1977.42.2.206. [DOI] [PubMed] [Google Scholar]
- Steele R. H., Heard B. E. Size of the diaphragm in chronic bronchitis. Thorax. 1973 Jan;28(1):55–60. doi: 10.1136/thx.28.1.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thurlbeck W. M. Diaphragm and body weight in emphysema. Thorax. 1978 Aug;33(4):483–487. doi: 10.1136/thx.33.4.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittmann T. A., Kelsen S. G. The effect of caffeine on diaphragmatic muscle force in normal hamsters. Am Rev Respir Dis. 1982 Sep;126(3):499–504. doi: 10.1164/arrd.1982.126.3.499. [DOI] [PubMed] [Google Scholar]
- Zite-Ferenczy F., Rüdel R. A diffractometer using a lateral effect photodiode for the rapid determination of sarcomere length changes in cross-striated muscle. Pflugers Arch. 1978 Apr 25;374(1):97–100. doi: 10.1007/BF00585702. [DOI] [PubMed] [Google Scholar]
- ter Keurs H. E., Iwazumi T., Pollack G. H. The sarcomere length-tension relation in skeletal muscle. J Gen Physiol. 1978 Oct;72(4):565–592. doi: 10.1085/jgp.72.4.565. [DOI] [PMC free article] [PubMed] [Google Scholar]