Abstract
Most studies agree that males and females respond differently to drugs of abuse. In females, estradiol enhances the behavioral response to cocaine. However, studies on the role of testosterone and the locomotor response to psychostimulants in the male rat are inconclusive. Our study was designed to determine the behavioral effects of testosterone on the development and persistence of cocaine sensitization in male rats. We tested different doses of cocaine (10, 15 and 30mg/kg) to determine which dose induced locomotor sensitization in intact (INT) and gonadectomized (GDX) animals. We also investigated if GDX males with testosterone replacement (GDX-T) showed a similar locomotor response to cocaine as INT males.
Our data showed that gonadectomy enhanced the locomotor response to a single cocaine injection. This effect was not observed in gonadectomized rats that received testosterone replacement. However, GDX rats did not show a progressive increase in their locomotor response to repeated cocaine administration (15 and 30 mg/kg) (sensitization) as did INT and GDX-T animals. It is possible that in GDX males, the initial high locomotor response to cocaine creates a ceiling effect that limits further increase in cocaine-induced hyperactivity. These findings indicate that testosterone not only modulates the behavioral response to a single and to repeated cocaine injections, but is essential for male rats to become sensitized to cocaine.
Keywords: Gonadectomy, Testosterone, Cocaine, Behavioral Sensitization, Locomotor Activity, Castration, Dose Response
1. Introduction
Drug addiction is a mental health problem that affects approximately 9.9 million Americans. The National Survey on Drug Use and Health of 2007 showed that an estimated 2.1 million Americans were cocaine dependent, men having a higher rate of cocaine dependence than women. This difference can be partly attributed, to the greater opportunity that males have to use drugs compared to females [1, 2] (SAMHSA, 2004). When this factor is taken into consideration, approximately an equal amount of men and women that are exposed to drugs of abuse will continue to use them frequently [1, 2] (SAHMSA, 2004).
There is some controversy regarding gender differences and the subjective effects of cocaine. There are studies that find that cocaine induces a greater sense of “well being” [3], and greater craving [4] in women than in men. In contrast, other studies report that men describe a more intense “high” to cocaine than women [5–8] while other studies find no differences between genders [9–12].
This same dichotomy has been observed when analyzing other parameters. For example, several studies report gender differences in cardiovascular effects of cocaine [8, 13] whereas others find no effect [11, 14]. In other areas of study, the differences between sexes are more consistent, such as the increased reactivity of the hypothalamic-pituitary-adrenal axis to cocaine in females [13, 15]. Gender differences have also been reported in the rate of developing dependence once exposed to cocaine [16, 17], as well in the response to treatment [16, 18, 19]. These gender differences appear to be independent of drug pharmacokinetics [9–11].
Part of the above-mentioned differences between the sexes may be attributed to differences in the gonadal hormonal milieu. For example, in women, the subjective effects of cocaine fluctuate with the menstrual cycle. Stimulant and euphoric effects of cocaine are greater during the follicular phase (when the progesterone/estradiol ratio is low), than during the luteal phase (when the progesterone/estradiol ratio is high) [5, 7, 20, 21].
Sex differences in response to cocaine have also been reported in animal subjects. Cocaine induces higher locomotor activity (LMA) in female than in male rodents [22, 23], as well as a more robust sensitization [24]. Females also acquire the criteria of self administration faster and the number of cocaine infusions they take is greater than that of males [25]. Ovariectomy reduces the behavioral response to chronically administered cocaine [25, 26]. Replacement of the gonadal steroid estradiol increases cocaine-induced LMA and sensitization [23, 26–28], as well as conditioned place preference to cocaine [23] attesting to estradiol’s enhancement of cocaine-induced locomotor sensitization. In contrast, treatment of ovariectomized rats with progesterone diminishes acquisition of cocaine self-administration [29].
Fewer studies have investigated the effect of androgens on the behavioral response to psychostimulants in male rats. Several studies find that gonadectomy did not affect cocaine-induced locomotor activity [30–33], stereotypy [34], rotational behavior [26], cocaine self-administration [29, 35] nor amphetamine stimulated locomotor activity [36]. However, others find that gonadectomy increased cocaine-induced hyperactivity [37] as well as amphetamine-induced hyperactivity [38], rearing [39] and stereotypy [40]. Furthermore, males treated with testosterone show a decrease in cocaine-induced [41] and amphetamine-induced [40] hyperactivity.
The effect of testosterone on behavioral sensitization to repeated cocaine administration in male rats has received little attention. Therefore, our study was designed to determine the behavioral effects of testosterone on the development and persistence of cocaine sensitization in male rats. Behavioral sensitization is defined as an increased response over time to repeated intermittent drug injections [42]. It is characterized by neurochemical changes in the reward pathway of the brain, thus making locomotor sensitization an excellent model to study addiction. The dopaminergic projection from the ventral tegmental area (VTA) to corticolimbic structures is an essential component of the neural circuitry that mediates motivation, as well as the hedonic and psychomotor properties of drugs of abuse. Experiments of intracranial self-stimulation highlight the importance of the VTA as a neural substrate of reward [43, 44]. Indeed, transitory increased excitatory input to the VTA is essential for the development of behavioral sensitization [45]. The nucleus accumbens (NAc) also plays a major role in the expression of sensitization as illustrated by experiments where lesioning of the NAc inhibits expression of this behavior (for review see [46]).
In this study we investigated the effect of gonadectomy on behavioral sensitization to cocaine in male rats. We also tested whether testosterone replacement to GDX animals reinstated behaviors to similar levels as those observed in intact rats. To determine if the effect of gonadectomy was dose-dependent, several doses of cocaine (10, 15 and 30mg/kg) were investigated.
2. Materials and Methods
2.1 Behavioral Studies
2.1.1 Animals
Adult male Sprague-Dawley rats (300–325g) were purchased from Taconic Farms (Germantown, NY, USA). Animals were housed in groups of two in a temperature and humidity controlled room, under a 12-h light-dark cycle with lights off at 7 PM. Water and Harlan Tek® rat chow was provided ad libitum. All animal experiments were approved by the Institutional Animal Care and Use Committee (IACUC) of the University of Puerto Rico, Medical Sciences Campus and adhere to USDA, NIH and AAALAC guidelines.
2.1.2 Drugs and Chemicals
The anesthetics used for surgery were ketamine (65mg/kg) and xylazine (7mg/kg) (Sigma-Aldrich, St. Louis, MO, USA). Three doses of cocaine-HCl (Sigma-Aldrich) were used in this study: 10mg/kg, 15mg/kg and 30mg/kg. All drugs were dissolved in 0.9% sterile saline and injected intraperitoneally (i.p.).
2.1.3 Surgical Procedures
Animals were anesthetized with ketamine/xylazine anesthesia. Intact (INT) animals were sham operated and remained gonadally intact, whereas gonadectomized (GDX) animals had their testes removed. For the testosterone replacement study, a 10mm silastic tube implant (inner diameter of 1.47 mm, 1.97 mm outer diameter, Dow Corning®, Midland, MI, USA) was placed subcutaneously in the midscapular region during surgery. Empty implants were used as controls in INT and GDX animals, while gonadectomized animals with testosterone replacement (GDX-T) received implants filled with testosterone propionate (4-Androsten-17β-ol-3-one 17-propionate) (Sigma-Aldrich, St Louis, MO, USA). This method of testosterone replacement has been successful in restoring copulatory behavior in male rats [47, 48]. Animals were allowed one week of recovery from surgery, after which the behavioral testing began.
2.1.4 Locomotor Activity Chamber
Horizontal (HACTV) and stereotyped (STRCNT) activity were measured with an automated animal activity cage system (Versamax™ system) purchased from AccuScan Instruments (Columbus, Ohio, USA). The activity cages are made from clear acrylic (42 cm × 42 cm × 30 cm), with 16 equally spaced (2.5 cm) infrared beams across the length and width of the cage at a height of 2 cm from the cage floor (horizontal beams). An additional set of 16 infrared beams is located at a height of 10 cm from the cage floor (vertical beams). All beams are connected to a Data AnalyserR that sends information to a personal computer that displays beam data through a WindowsR-based program (VersadatR). The Versamax™ system differentiates between stereotyped and horizontal locomotor activity based on repeated interruption of the same beam or sequential breaking of different beams respectively.
2.1.5 Locomotor Sensitization
LMA was measured in a dimly isolated room with constant humidity (70%) and temperature (25°C). To diminish the effects of novelty, animals were habituated to the activity cage for one hour on the day before receiving the first injection (Day 0) and for 30 min on days 1, 5 and 13 prior to cocaine injection. On days 1, 5 and 13 LMA was measured for 30 minutes prior to injection and then for 60 additional minutes after injection. From days 1–5 the animals received daily i.p. injections of 0.9% sterile saline (Nicolas Carrillo, Baxter, Baxter Healthcare Corporation, Deerfield, IL, USA) or one of the doses of cocaine (10mg/kg, 15mg/kg or 30mg/kg). During days 6–12 the animals remained in their home cages undisturbed. On day 13, animals were challenged with an injection of saline or of the same dose of cocaine. Each session of behavioral testing consisted of at least one animal of each group to minimize intergroup variation that may result from differences in time of testing and/or injections. Animals were sacrificed after behavioral testing on the day of the challenge. Trunk blood was collected from each animal, and blood plasma stored at −70°C until the day of the radioimmunoassay (RIA).
2.2 Radioimmunoassays
All use of radioactive materials was approved by the Radiation Safety Committee of the University of Puerto Rico, Medical Sciences Campus. This committee oversees the compliance of NRC regulations for all UPR MSC laboratories.
2.2.1 Total Testosterone
Total plasma testosterone was determined with a RIA kit purchased from MP Biomedical (Costa Mesa, California). On the day of the assay, plasma samples were thawed at room temperature and a 50ul aliquot from each sample was pipetted into polypropylene 12×75mm culture tubes. Each sample had a duplicate. The standard curve was prepared in triplicate with seven different concentrations of testosterone, ranging from 0 to 10ng/ml. The assay was conducted following the instructions of the manufacturer.
2.2.2 Free Testosterone
Free plasma testosterone was determined using a Coat-A-Count Free Testosterone RIA kit from MP Biomedical (Costa Mesa, California). In this assay, the antibody against testosterone is bound to the walls of the test tubes. Samples were thawed at room temperature and a 50 µl aliquot pipetted into the test tubes with antibody. Each sample had a duplicate. The standard curve consisted of seven standards (0.25, 1.0, 2.5, 10, 25, 50 and 100 pg/ml), and was prepared in triplicates.
After completion of each assay radioactivity was counted in a gamma counter (Beckman Gamma 5500B) for one minute. Hormonal values were interpolated from a standard curve prepared in triplicate using standard calibrators and quality control serum. The calculation of the data reduction was performed by linear regression and logit-log representation with the aid of computer software. All samples were assayed in duplicate.
2.3 Statistical Analysis
The statistical analysis used varied according to the groups to be compared. Since the first 30 min after cocaine injection is where changes in LMA are more robust, we used this time frame for statistical analysis and comparisons among groups (INT, GDX, GDX-T), treatment (saline, cocaine) and days (1, 5 and 13).
For our time course data, a repeated measures two-way ANOVA was used to analyze the first 30 minutes of activity after injection with locomotor activity as the dependent variable and time (in 5 minute intervals) and days (Day 1, 5 and 13) as the independent variables. A post-hoc Bonferroni test was conducted to determine the time points that were significantly different. Animals that showed an overall significant increase (p<0.05) in LMA during the first 30 minutes after repeated cocaine administration were considered sensitized.
Data presented in bar graphs, was analyzed using an unpaired t-test or a oneway ANOVA, depending whether the comparison was within the same group or different groups of animals. All data were analyzed using GraphPad Prism version 5.00 for Windows (GraphPad Software, San Diego California USA, www.graphpad.com) computer software. Data are presented as the mean ± standard error of the mean (SEM). A p-value (p<0.05) was considered statistically significant.
3. Results
3.1 Basal locomotor activity
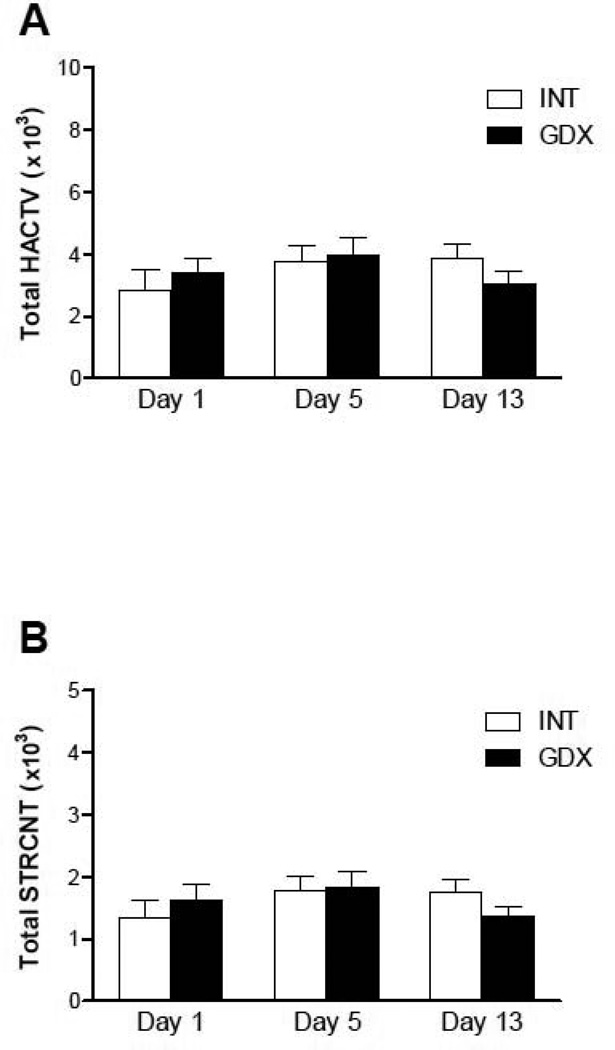
Gonadectomy did not alter basal LMA on any of the days tested as compared to INT animals (Fig. 1). In addition, basal LMA remained similar throughout the testing period (i.e. did not increase or decrease with time) in all groups tested.
Figure 1. Basal locomotor activity of intact and gonadectomized male rats.
Total horizontal (A) and stereotyped (B) activity of intact (INT) (white bars) and gonadectomized (GDX) (black bars) adult male rats is presented as the mean sum ± SEM of the first 30 minutes after saline injection on days 1, 5 and 13; N: INT = 8, GDX = 8. Gonadectomy did not alter basal locomotor activity on any of the days. Repeated saline injections did not alter basal LMA throughout the testing period.
3.2 Locomotor response to cocaine
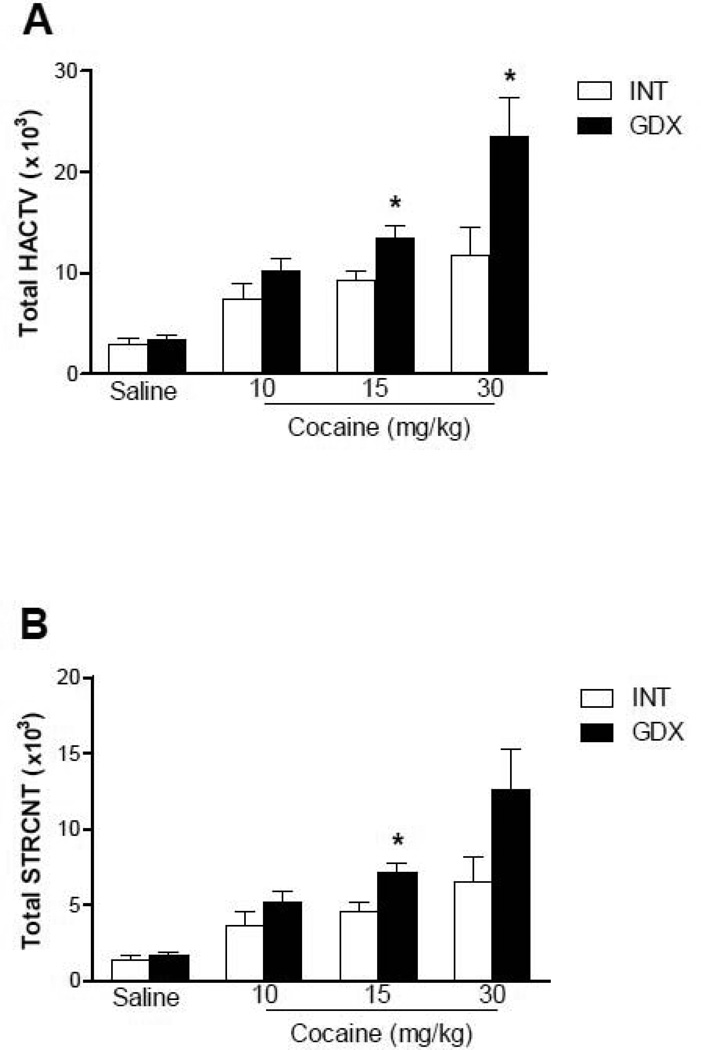
A single cocaine injection significantly increased LMA of all animals, as compared to their saline counterparts. This hyperlocomotion was further enhanced in GDX animals at higher doses of cocaine, such as 15 and 30 mg/kg (Fig. 2, p<0.05). On days 5 and 13 there was no significant difference between INT and GDX animals receiving cocaine (p>0.05).
Figure 2. Locomotor response of intact and gonadectomized male rats to different doses of cocaine.
Total horizontal (A) and stereotyped (B) activity of INT and GDX male rats is presented as the mean sum ± SEM of the first 30 minutes after a single injection of saline (0.9%) or cocaine (10, 15 or 30mg/kg); N: Saline: INT = 8, GDX = 8; 10mg/kg: INT = 11, GDX = 10; 15mg/kg: INT = 30 (STRCNT) 31(HACTV), GDX = 29; 30mg/kg: INT = 9, GDX = 9. Cocaine increased locomotor activity of all animals compared to their saline counterpart. Gonadectomy enhanced the locomotor response to 15 and 30mg/kg of cocaine when compared to intact males. * p<0.05 compared to LMA of INT animals with the same dose of cocaine.
3.3 Behavioral sensitization to cocaine
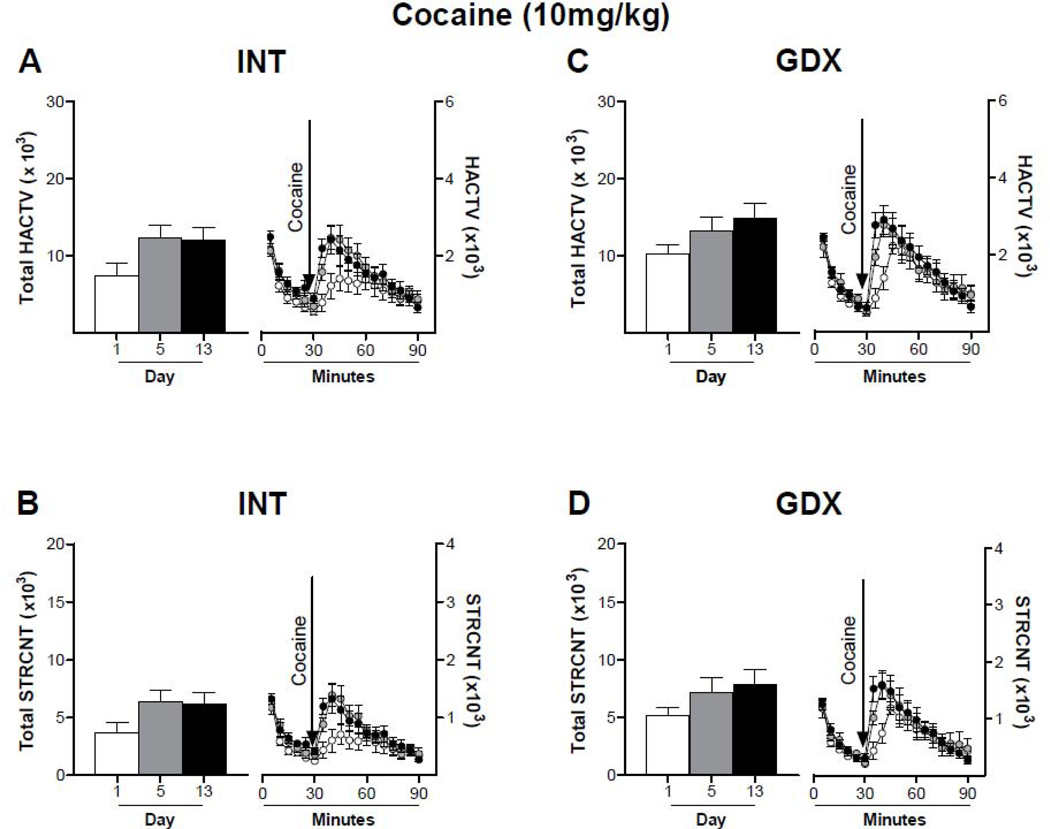
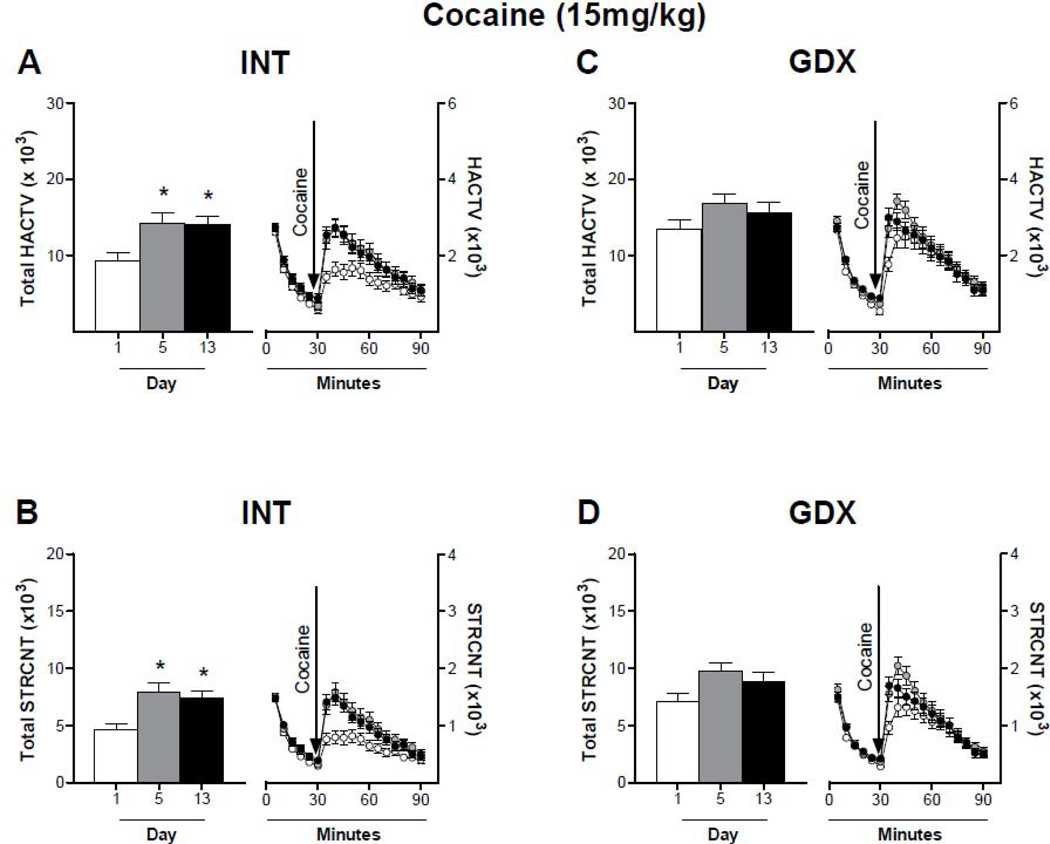
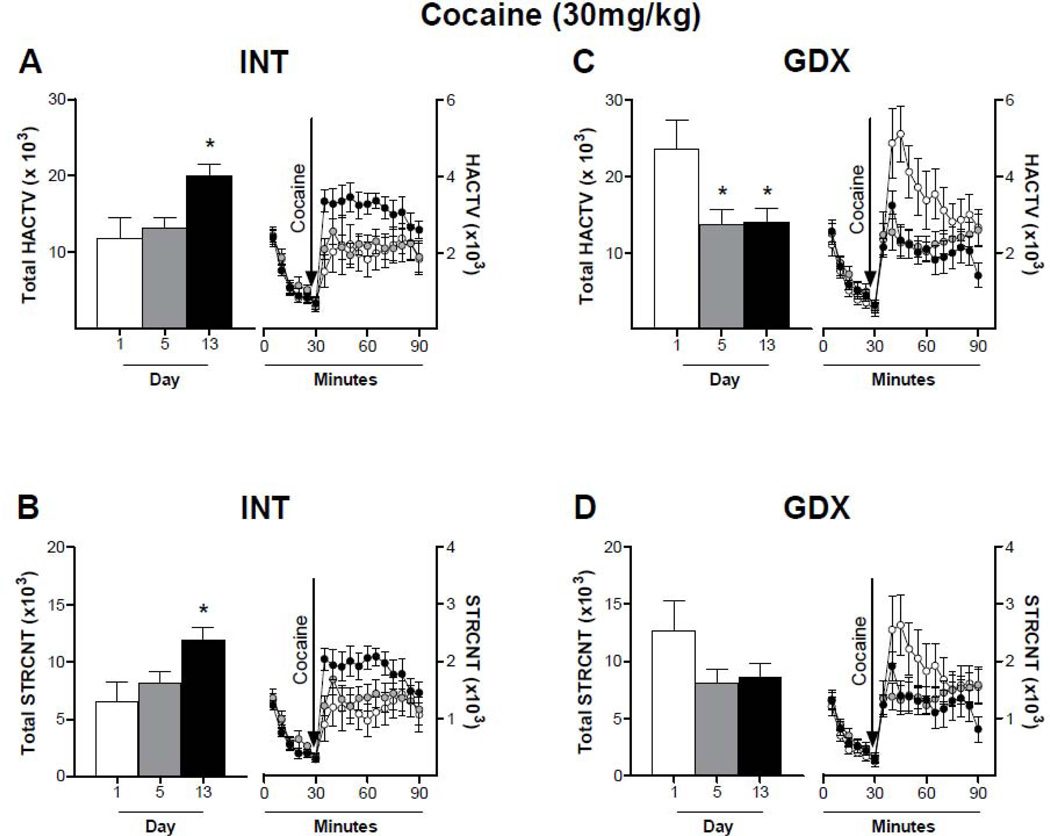
The locomotor response to repeated cocaine was altered differently according to cocaine dose and hormone treatment. When comparing the response to cocaine during the first 30 minutes after cocaine administration, a low dose of cocaine (10mg/kg) did not induce sensitization in INT (Fig. 3A: Two-way ANOVA, p > 0.05, F = 2.747; Bonferroni post test, D1 vs D5: 40min; D1 vs D13: 35–40 min; Fig. 3B: Two-way ANOVA, p > 0.05, F = 2.704; Bonferroni post test, D1 vs D5: 40min; D1 vs D13: 35–40 min) nor GDX animals (Fig. 3C: Two-way ANOVA, p > 0.05, F = 1.978; Bonferroni post test, D1 vs D5: 40min; D1 vs D13: 35–40 min; Fig. 3D, Two-way ANOVA, p > 0.05, F = 1.587; Bonferroni post test, D1 vs D5: 40min; D1 vs D13: 35–40 min), although the first 10 minutes after injection are significantly different. On the other hand, 15mg/kg cocaine induced sensitization in INT male rats (Fig. 4A: Two-way ANOVA, p < 0.05, F = 5.905; Bonferroni post test, D1 vs D5: 35–45min; D1 vs D13: 35–45 min; Fig. 4B: Two-way ANOVA, p < 0.05, F = 6.647; Bonferroni post test, D1 vs D5: 35–45min; D1 vs D13: 35–45 min). This effect was suppressed by gonadectomy (Fig. 4C: Two-way ANOVA, p > 0.05, F = 1.948; Bonferroni post test, D1 vs D5: 35–40min; D1 vs D13: 35 min; Fig. 4D: Two-way ANOVA, p > 0.05, F = 2.901; Bonferroni post test, D1 vs D5: 35–40min; D1 vs D13: 35 min). Repeated administrations of 30mg/kg cocaine had opposite effects on INT and GDX animals (Fig. 5). In INT rats cocaine-induced LMA remained the same throughout days 1–5 and increased after a withdrawal period of 7 days (Fig. 5A: Two-way ANOVA, p < 0.05, F = 4.700; Bonferroni post test, D1 vs D13: 35–60 min; Fig. 5B: Two-way ANOVA, p < 0.05, F = 4.413; Bonferroni post test, D1 vs D13: 35, 60 min). In contrast, cocaine-induced horizontal activity of GDX rats was highest at day 1, and decreased during days 5 and 13 (Fig. 5C: Two-way ANOVA, p < 0.05, F = 4.273; Bonferroni post test, D1 vs D5: 40–45min; D1 vs D13: 45 min; Fig. 5D: Two-way ANOVA, p < 0.05, F = 1.879; Bonferroni post test, D1 vs D5: 45min).
Figure 3. Locomotor response of intact and gonadectomized male rats to repeated cocaine (10mg/kg) administration.
Animals received daily injections of cocaine (10mg/kg) for 5 consecutive days and a challenge injection on day 13. Horizontal (A and C) and stereotyped (B and D) activity was recorded on days 1 (white bar and circle), 5 (grey bar and circle) and 13 (black bar and circle) for 30 min prior to injection (0 – 30 min) and 1 hour after injection (31 – 90 min). Data are presented on the left of each panel as the mean sum ± SEM of the first 30 minutes after cocaine injection and on the right of each panel as mean ± SEM in 5 min intervals; N: INT = 11, GDX = 10. Locomotor activity was not significantly different on days 5 and 13 from day 1, indicating that neither INT nor GDX male rats become sensitized with a 10mg/kg dose of cocaine.
Figure 4. Locomotor response of intact and gonadectomized male rats to repeated cocaine (15mg/kg) administration.
Animals received daily injections of 15mg/kg cocaine for 5 consecutive days and a challenge injection on day 13. Horizontal (A and C) and stereotyped (B and D) activity was recorded on days 1 (white bar and circle), 5 (grey bar and circle) and 13 (black bar and circle) for 30 min prior to injection (0 – 30 min) and 1 hour after injection (31 – 90 min). Data are presented on the left of each panel as the mean sum ± SEM of the first 30 minutes after cocaine injection and on the right of each panel as mean ± SEM in 5 min intervals; N: INT = 30 (STRCNT) – 31 (HACTV), GDX = 29. INT male rats show an increased locomotor response after repeated injections, while GDX animals do not. These results indicate that 15mg/kg of cocaine induces sensitization in INT but not in GDX male rats. * p<0.05 compared to LMA on day 1.
Figure 5. Locomotor response of intact and gonadectomized male rats to repeated cocaine (30mg/kg) administration.
Animals received daily injections of 30mg/kg cocaine for 5 consecutive days and a challenge injection on day 13. Horizontal (A and C) and stereotyped (B and D) activity was recorded on days 1 (white bar and circle), 5 (grey bar and circle) and 13 (black bar and circle) for 30 min prior to injection (0 – 30 min) and 1 hour after injection (31 – 90 min). Data are presented on the left of each panel as the mean sum ± SEM of the first 30 minutes after cocaine injection and on the right of each panel as mean ± SEM in 5 min intervals; N: INT = 9, GDX = 9. INT male rats show an increase locomotor response after a withdrawal period. On the other hand, GDX animals show decreased horizontal activity after repeated injections when compared to day 1. * p<0.05 compared to LMA on day 1.
3.4 Testosterone Replacement
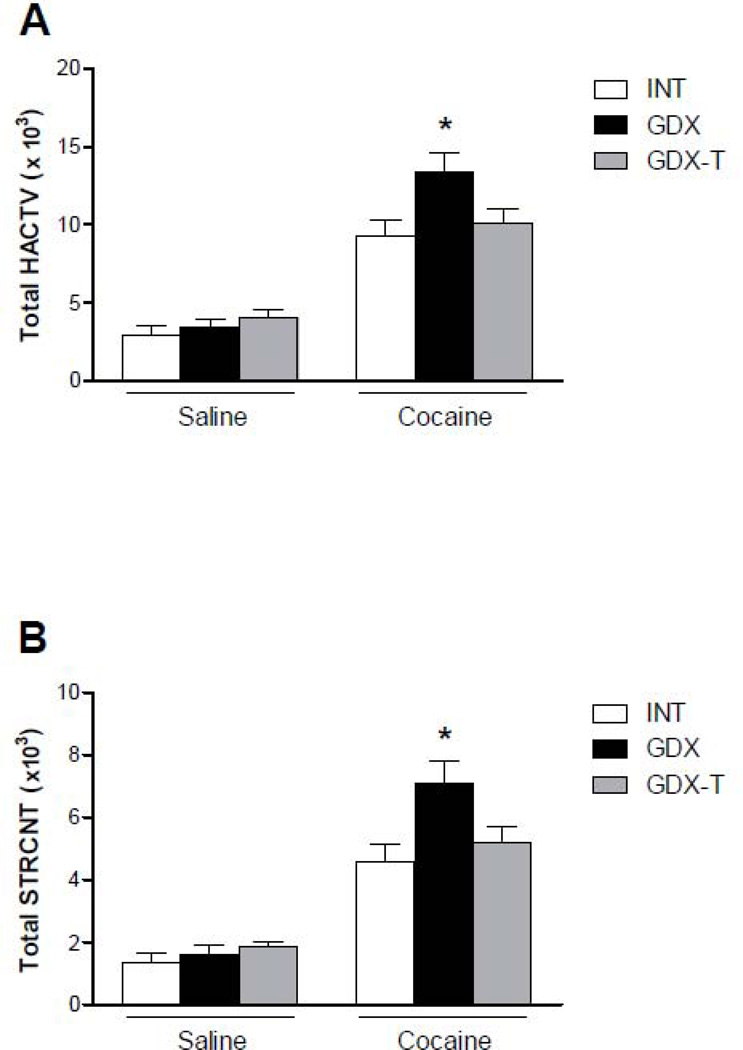
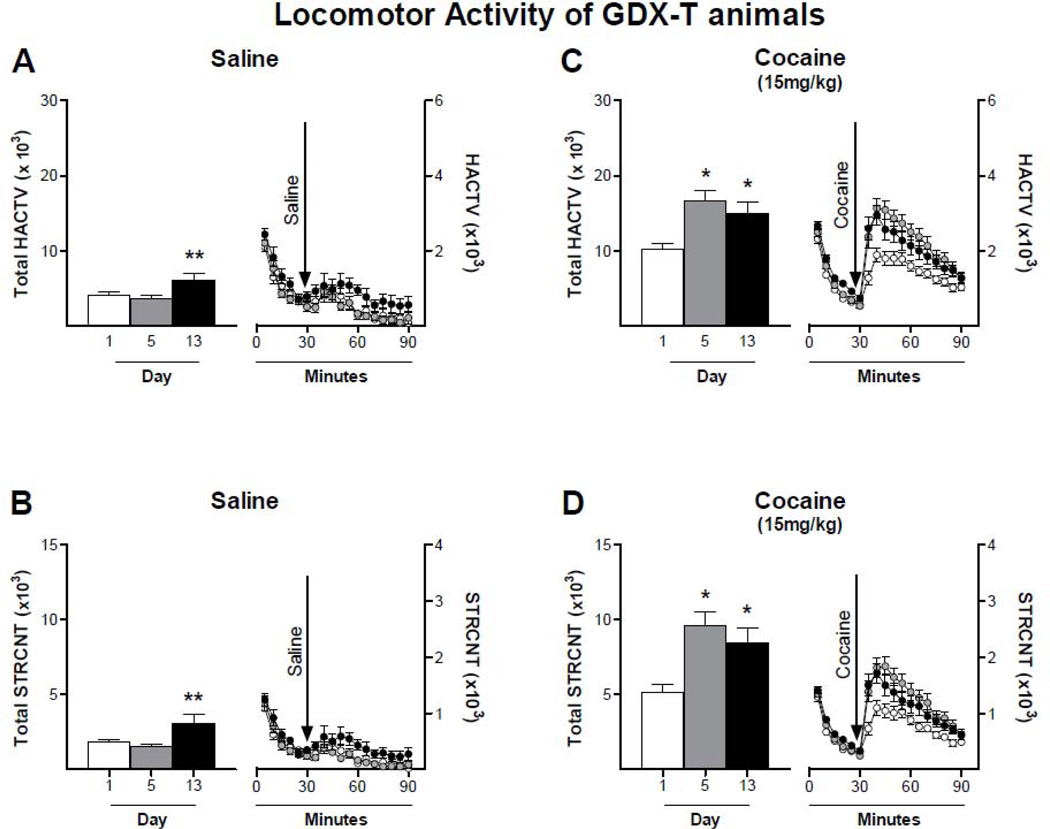
Basal LMA of saline treated rats did not differ among the groups (Fig. 6). These data show that gonadectomy, nor testosterone replacement, altered locomotor activity of adult male rats. A single 15 mg/kg cocaine injection increased LMA of all animals compared to their saline counterparts. As previously illustrated, gonadectomy enhanced the locomotor response to a single cocaine injection; however, this effect was reversed by testosterone administration (Fig. 6) (One way ANOVA, p < 0.05). Similar to what we observed with INT males treated with 15 mg/kg of cocaine, cocaine-induced LMA in GDX-T rats was higher on days 5 and 13 than on day 1, indicative of sensitization ( Fig. 7C: Two-way ANOVA, p < 0.05, F = 7.128; Bonferroni post test, D1 vs D5: 35–60min; D1 vs D13: 35–40 min; Fig. 7D: Two-way ANOVA, p < 0.05, F = 4.562; Bonferroni post test, D1 vs D5: 35–50min; D1 vs D13: 35min). We also observed that basal LMA of GDX-T rats was higher on day 13 than on day 1 and day 5 (Fig. 7A: Two-way ANOVA, p < 0.05, F = 4.052; Bonferroni post test not significant; Fig. 7B: Two-way ANOVA, p < 0.05, F = 4.563; Bonferroni post test not significant). To ascertain whether the increase in cocaine treated animals was due to the increase in basal LMA, we subtracted total basal LMA from total cocaine-induced LMA in GDX-T rats (HACTV: day 1: 6497±883.3; day 5: 12609±1326; day 13: 8930±1540; STRCNT: day 1: 3313±511.3; day 5: 8040±912.6; day 13: 6263±1110). Although the higher basal LMA on day 13 attenuated the increased response to cocaine, there was still a significant increase in stereotyped activity and a trend towards increased horizontal activity (t-test, day 1 vs day 13: HACTV: p=0.1762; STRCNT: p=0.0127).
Figure 6. Locomotor response of intact, gonadectomized and gonadectomized male rats with testosterone replacement to a single saline or cocaine (15mg/kg) injection.
Total horizontal (A) and stereotyped (B) activity of INT (white bars), GDX (black bars) and GDX-T (grey bars) male rats, presented as the mean sum ± SEM of the first 30 minutes after a single injection of saline (0.9%) or cocaine (15mg/kg); N: Saline: INT = 8, GDX = 8, GDX-T = 7; Cocaine: INT = 30 (STRCNT) 31(HACTV), GDX = 29, GDX-T = 28. Testosterone treatment of GDX animals did not alter basal locomotor activity. Cocaine administration increased LMA of all animal groups. Hormone treatment altered the locomotor response to a single cocaine injection. The increased locomotor response of GDX male rats to a single 15mg/kg cocaine injection was attenuated by testosterone replacement.
* p<0.05 compared to LMA of INT animals with the same dose of cocaine.
Figure 7. Basal and cocaine-induced locomotor activity of gonadectomized male rats with testosterone replacement.
Animals received daily injections of saline or 15mg/kg cocaine for 5 consecutive days and a challenge injection on day 13. Horizontal (A and B) and stereotyped (C and D) activity was recorded on days 1 (white bar and circle), 5 (grey bar and circle) and 13 (black bar and circle) for 30 min prior to injection (0 – 30 min) and 1 hour after injection (31 – 90 min). Data are presented on the left of each panel as the mean sum ± SEM of the first 30 minutes after cocaine injection and on the right of each panel as mean ± SEM in 5 min intervals; N : Saline = 7; Cocaine = 28. Basal LMA was increased on day 13 compared to days 1 and 5. GDX-T male rats show an increased locomotor response after repeated cocaine administrations. * p<0.05 compared to LMA on day 1; ** p<0.05 compared to LMA on day 1 and day 5.
3.5 Plasma levels of Testosterone
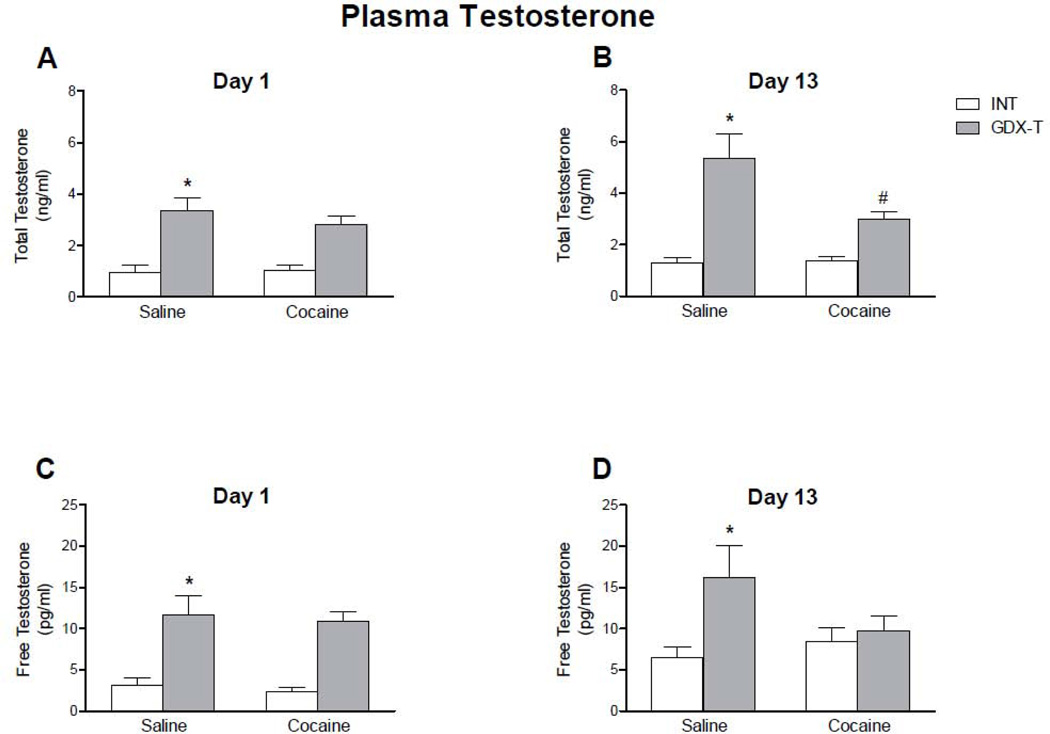
Total and free testosterone levels varied according to hormone treatments. As expected, GDX animals had negligible levels of both total and free testosterone (data not shown). On the other hand, GDX-T rats had approximately twice the amount of free and total testosterone plasma levels than INT males (t-test, p<0.05).
A single cocaine injection (15 mg/kg) had no effect on total or free testosterone plasma levels. However, repeated cocaine injections decreased plasma testosterone of GDX-T compared to saline controls, although only total testosterone reached statistical significance (t-test, Total T: p < 0.01; Free T: p > 0.05).
4. Discussion
Our data shows that in drug naïve rats, gonadectomy enhanced the locomotor response to a cocaine injection. This effect was not observed in gonadectomized rats that received testosterone replacement. In addition, GDX rats did not show a progressive increase in their locomotor response to repeated cocaine administration (15 and 30 mg/kg) (sensitization) as did INT and GDX-T males. These findings show that testosterone modulates the behavioral response to a single and to repeated cocaine injections.
Previous studies in this area report either no effect of testosterone [26, 29–36], or attenuation of the behavioral effects of psychostimulants in drug naïve rats [37–41], similar to what we have found in this study. It could be argued that these discrepancies result from differences strain of rats used [33], route of cocaine administration [30, 31], and/or the behavioral parameter assessed [26], among others. However, we consider that the dose of cocaine used is of the utmost importance. The effect of gonadectomy on cocaine-induced LMA is dose dependent, seen only at the higher cocaine doses and not with 10mg/kg cocaine [33, 37, 49]. Notably, this effect may be specific to the behavioral parameter assessed, given that no difference was reported between INT and GDX male rats when higher doses of cocaine (20mg/kg) were administered and when rotational behavior was ascertained after a 6-hydroxy dopamine lesion in the nigrostriatal pathway [26].
This is the first dose response study of cocaine-induced locomotor sensitization in INT and GDX rats. Several doses of cocaine are effective in inducing behavioral sensitization in gonadally intact males, and the effective dose can vary according to the behavioral parameter measured. Although most studies use 15 mg/kg of cocaine to induce sensitization [27, 33, 50, 51] doses of 5, 10 and 20 mg/kg have also proven to be effective when measuring rotational behavior [26].
In our study, cocaine doses of 15 and 30 mg/kg were effective in inducing sensitization in INT but not in GDX male rats. Curiously, at a dose of 10 mg/kg, cocaine-induced hyperactivity during the first 10 minutes after injection was higher on day 13 than on day 1 in INT and in GDX males. However, total locomotor activity during the first 30 minutes after cocaine injection was not. Interestingly, the dose of 30 mg/kg of cocaine induced sensitization in INT rats only after a 7 day withdrawal period. In contrast, in GDX rats it reduced the locomotor response, a behavior reminiscent of the tolerance observed with continuous infusion of cocaine [52–56]. Sensitization to a particular drug may persist from months to years after discontinuing drug treatment [42, 57]. In fact, cocaine-induced hyperactivity is enhanced in sensitized rats following a drug-free period [58–60].
A vast number of studies show that 5 daily injections of cocaine induced sensitization in gonadally intact male rats [61–64]. The present study found the same results in INT and GDX-T rats, but not in GDX rats. It is possible that GDX male rats require a longer treatment period to develop sensitization or a lower dose.
Previous studies investigating the effects of gonadectomy on cocaine-induced sensitization report that both INT and GDX male rats sensitize to various doses of cocaine [26, 33, 34]. Notably, these studies did not find that gonadectomy enhanced the initial locomotor response to cocaine, which has been shown to predict the subsequent response to cocaine as well as the development of sensitization [65]. For example, an acute cocaine injection (10mg/kg, i.p.) may induce either a high or low locomotor response. High cocaine responders (HCR) do not show an increase in the locomotor response with repeated cocaine administration, while low cocaine responders (LCR) become sensitized to repeated cocaine administration [65]. In our study, GDX male rats exhibited an initial higher response to cocaine than INT rats but they did not become sensitized, thus behaving as HCR animals.
Cocaine is known to alter plasma sex steroid levels. A single injection of cocaine (15 or 40mg/kg) increases testosterone up to 2 hours after injection, followed by a subsequent decline [66, 67] that continues after repeated administration of cocaine [67]. Our results indicate that on day 13, plasma levels of testosterone are lower in GDX-T rats that received cocaine repeatedly than in GDX-T saline treated rats. However, this decrease is not observed in INT animals. These data suggest that cocaine may be altering testosterone clearance by hepatic or renal tissue. The fact that we did not observe changes in INT animals may be attributed to the higher testosterone released by the silastic implants. Another possibility is that INT animals may compensate the increased clearance by increasing testosterone synthesis, returning testosterone levels to normal.
Androgens modulate the mesolimbic dopaminergic system, an essential component of the brain reward circuitry associated with drug reward and sensitization. Release of dopamine (DA) in the NAc is necessary for the drug ‘high’, for the initiation of addiction [68] and is associated with increased LMA [69, 70]. Extracellular DA concentration is regulated by several factors, including reuptake by dopamine transporters (DAT) and decreased DA release by activation of dopamine autoreceptors (D2 receptors). Androgens can alter DA concentrations. Nandrolone decanoate, an anabolic androgenic steroid, has been found to upregulate D2 receptor in the VTA, NAc core and caudate putamen [71] and decrease DAT density in the striatum and caudate putamen [72]. Although there is still controversy, several studies show that repeated cocaine decreases DAT mRNA expression [73–75] and activity [65] in the reward system. Decreased expression/activity of DAT decreases DA clearance leading to an increased locomotor response to repeated administration of cocaine, just like we observed in INT animals.
On the other hand, we report that GDX rats respond more robustly to a single cocaine injection than INT rats, similar to the HCR animals described by Sabeti et al. [70]. In their study, the initial higher response of the HCR animals was correlated with increased DAT inhibition, which leads to increased extracellular DA in the NAc. In addition, HCR animals do not become sensitized to repeated cocaine administrations correlating with no further DAT inhibition, while both an increase in DAT inhibition and sensitization are observed in LCR.
The initial high response to cocaine may be a ceiling effect limiting further increase in locomotor response with repeated cocaine administration. Therefore it is possible that testosterone increases DAT density in the reward pathway, thus increasing DA reuptake which in turn decreases extracellular DA in the NAc resulting in a reduced initial locomotor response to cocaine by both in INT and GDX-T animals. With repeated cocaine administrations, DAT expression/activity would decrease, resulting in sensitization of animals with testosterone. Conversely, gonadectomy can increase extracellular DA response by decreasing DA reuptake due to lower DAT density, resulting in an increased initial response to cocaine. There would be no further change in DAT activity/expression with repeated cocaine administration resulting in no sensitization of male rats lacking testosterone. This of course is speculative and requires further experimentation.
In summary, this study shows that testosterone modulates the locomotor response to cocaine in male rats. Initially, GDX rats respond with higher hyperactivity to cocaine. However, after repeated exposure to cocaine, the response of these animals does not increase further, as if they had reached a plateau. However, INT and GDX-T male rats show a progressive increase in locomotor response, or sensitization, different from the “ceiling effect” observed in GDX rats. Further studies are warranted to elucidate the neural substrates and mechanisms involved in testosterone modulation of cocaine-induced LMA. These data suggest that testosterone is a required substrate that participates in neuroadaptative processes triggered by repeated exposure to cocaine. They also attest to the relevance of evaluating androgen receptors as potential candidates for treatment of addictive disorders.
Figure 8. Plasma levels of total and free testosterone on the first and last day of injection of intact and gonadectomized male rats with testosterone replacement.
Total (A and B) and free (C and D) testosterone levels were measured by radioimmunoassay in INT (white bars) and GDX-T (grey bars) animals. Data are presented as mean ± SEM; N: Total Testosterone: Day 1: Saline: INT = 9, GDX = 8; Cocaine: INT = 8, GDX-T = 9; Day 13: Saline: INT = 22, GDX-T = 21; Cocaine; INT = 32, GDX-T = 33; Free Testosterone: Day 1: Saline: INT = 9, GDX = 8; Cocaine: INT = 8, GDX-T = 9; Day 13: Saline: INT = 10, GDX-T = 9; Cocaine; INT = 10, GDX-T = 10. Values for GDX animals were negligible and not included in the graph. GDX-T animals had higher total and free testosterone levels than intact male rats when treated with saline. A single cocaine injection (Day 1) did not alter total (A) or free (C) testosterone plasma levels when compared to their saline counterpart. Repeated cocaine administration (Day 13; B and D) decreased plasma testosterone of GDX-T compared to their saline counterparts, although only total testosterone reached statistical significance. * p<0.05 compared to INT animals with the same drug treatment; # p < 0.01 compared to the saline counterpart.
Gonadectomy of male rats enhanced cocaine-induced hyperactivity.
Gonadectomized male rats did not sensitize with repeated cocaine administration.
Gonadectomized male rats with testosterone show sensitization to cocaine.
Testosterone is essential for cocaine-induced sensitization in male rats.
Acknowledgements
The authors would like to thank Natasha Lugo-Escobar, Anabel Puig-Ramos, Gladys Santiago, Yvonne Torres-Díaz and Rafael Vázquez for their technical assistance and Nildris Cruz, Natasha Lugo-Escobar, Dr. Joyce M. Vélez and Dr. Carlos A. Jiménez-Rivera for their revision of the manuscript. This work was supported by: NIH grants: RR03051, RR11126 (RCMI); U54NS39405 (SNRP); SO6-GM08224 (MBRS/SCORE); and R25GM061838 (MBRS/RISE).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errorsmaybe discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Van Etten ML, Anthony JC. Comparative epidemiology of initial drug opportunities and transitions to first use: marijuana, cocaine, hallucinogens and heroin. Drug Alcohol Depend. 1999 Apr 1;54(2):117–125. doi: 10.1016/s0376-8716(98)00151-3. [DOI] [PubMed] [Google Scholar]
- 2.Van Etten ML, Neumark YD, Anthony JC. Male-female differences in the earliest stages of drug involvement. 1999 Sep;94(9):1413–1419. doi: 10.1046/j.1360-0443.1999.949141312.x. Addiction. [DOI] [PubMed] [Google Scholar]
- 3.McCance-Katz EF, Hart CL, Boyarsky B, Kosten T, Jatlow P. Gender effects following repeated administration of cocaine and alcohol in humans. Subst Use Misuse. 2005;40(4):511–528. doi: 10.1081/ja-200030693. [DOI] [PubMed] [Google Scholar]
- 4.Robbins SJ, Ehrman RN, Childress AR, O’Brien CP. Comparing levels of cocaine cue reactivity in male and female outpatients. Drug Alcohol Depend. 1999 Feb 1;53(3):223–230. doi: 10.1016/s0376-8716(98)00135-5. [DOI] [PubMed] [Google Scholar]
- 5.Lukas SE, Sholar M, Lundahl LH, Lamas X, Kouri E, Wines JD, et al. Sex differences in plasma cocaine levels and subjective effects after acute cocaine administration in human volunteers. Psychopharmacology (Berl) 1996 Jun;125(4):346–354. doi: 10.1007/BF02246017. [DOI] [PubMed] [Google Scholar]
- 6.Haney M, Foltin RW, Fischman MW. Effects of pergolide on intravenous cocaine self- administration in men and women. Psychopharmacology (Berl) 1998 May;137(1):15–24. doi: 10.1007/s002130050588. [DOI] [PubMed] [Google Scholar]
- 7.Sofuoglu M, Dudish-Poulsen S, Nelson D, Pentel PR, Hatsukami DK. Sex and menstrual cycle differences in the subjective effects from smoked cocaine in humans. Exp Clin Psychopharmacol. 1999 Aug;7(3):274–283. doi: 10.1037//1064-1297.7.3.274. [DOI] [PubMed] [Google Scholar]
- 8.Lynch WJ, Kalayasiri R, Sughondhabirom A, Pittman B, Coric V, Morgan PT, et al. Subjective responses and cardiovascular effects of self-administered cocaine in cocaine-abusing men and women. Addict Biol. 2008 Sep;13:403–410. doi: 10.1111/j.1369-1600.2008.00115.x. (3–4) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mendelson JH, Mello NK, Sholar MB, Siegel AJ, Kaufman MJ, Levin JM, et al. Cocaine pharmacokinetics in men and in women during the follicular and luteal phases of the menstrual cycle. Neuropsychopharmacology. 1999 Aug;21(2):294–303. doi: 10.1016/S0893-133X(99)00020-2. [DOI] [PubMed] [Google Scholar]
- 10.Evans SM, Haney M, Fischman MW, Foltin RW. Limited sex differences in response to “binge” smoked cocaine use in humans. Neuropsychopharmacology. 1999 Sep;21(3):445–454. doi: 10.1016/S0893-133X(98)00120-1. [DOI] [PubMed] [Google Scholar]
- 11.Collins SL, Evans SM, Foltin RW, Haney M. Intranasal cocaine in humans: effects of sex and menstrual cycle. Pharmacol Biochem Behav. 2007 Jan;86(1):117–124. doi: 10.1016/j.pbb.2006.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Waldrop AE, Price KL, Desantis SM, Simpson AN, Back SE, McRae AL, et al. Community-dwelling cocaine-dependent men and women respond differently to social stressors versus cocaine cues. Psychoneuroendocrinology. 2010 Jul;35(6):798–806. doi: 10.1016/j.psyneuen.2009.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fox HC, Garcia M, Jr, Kemp K, Milivojevic V, Kreek MJ, Sinha R. Gender differences in cardiovascular and corticoadrenal response to stress and drug cues in cocaine dependent individuals. Psychopharmacology (Berl) 2006 Apr;185(3):348–357. doi: 10.1007/s00213-005-0303-1. [DOI] [PubMed] [Google Scholar]
- 14.Braun BL, Murray DM, Sidney S. Lifetime cocaine use and cardiovascular characteristics among young adults: the CARDIA study. Am J Public Health. 1997 Apr;87(4):629–634. doi: 10.2105/ajph.87.4.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fox HC, Hong KI, Siedlarz KM, Bergquist K, Anderson G, Kreek MJ, et al. Sex-specific dissociations in autonomic and HPA responses to stress and cues in alcohol-dependent patients with cocaine abuse. Alcohol Alcohol. 2009;44(6):575–585. doi: 10.1093/alcalc/agp060. Nov-Dec. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McCance-Katz EF, Carroll KM, Rounsaville BJ. Gender differences in treatment-seeking cocaine abusers-implications for treatment and prognosis. Am J Addict. 1999;8(4):300–311. doi: 10.1080/105504999305703. Fall. [DOI] [PubMed] [Google Scholar]
- 17.Griffin ML, Weiss RD, Mirin SM, Lange U. A comparison of male and female cocaine abusers. Arch Gen Psychiatry. 1989 Feb;46(2):122–126. doi: 10.1001/archpsyc.1989.01810020024005. [DOI] [PubMed] [Google Scholar]
- 18.Weiss RD, Martinez-Raga J, Griffin ML, Greenfield SF, Hufford C. Gender differences in cocaine dependent patients: a 6 month follow-up study. Drug Alcohol Depend. 1997 Jan 10;44(1):35–40. doi: 10.1016/s0376-8716(96)01319-1. [DOI] [PubMed] [Google Scholar]
- 19.Najavits LM, Lester KM. Gender differences in cocaine dependence. Drug Alcohol Depend. 2008 Sep 1;97:190–194. doi: 10.1016/j.drugalcdep.2008.04.012. (1–2) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Evans SM, Foltin RW. Exogenous progesterone attenuates the subjective effects of smoked cocaine in women, but not in men. Neuropsychopharmacology. 2006 Mar;31(3):659–674. doi: 10.1038/sj.npp.1300887. [DOI] [PubMed] [Google Scholar]
- 21.Evans SM, Haney M, Foltin RW. The effects of smoked cocaine during the follicular and luteal phases of the menstrual cycle in women. Psychopharmacology (Berl) 2002 Feb;159(4):397–406. doi: 10.1007/s00213-001-0944-7. [DOI] [PubMed] [Google Scholar]
- 22.van Haaren F, Meyer ME. Sex differences in locomotor activity after acute and chronic cocaine administration. Pharmacol Biochem Behav. 1991 Aug;39(4):923–927. doi: 10.1016/0091-3057(91)90054-6. [DOI] [PubMed] [Google Scholar]
- 23.Segarra AC, Agosto-Rivera JL, Febo M, Lugo-Escobar N, Menendez-Delmestre R, Puig-Ramos A, et al. Estradiol: a key biological substrate mediating the response to cocaine in female rats. Horm Behav. 2010 Jun;58(1):33–43. doi: 10.1016/j.yhbeh.2009.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cailhol S, Mormede P. Strain and sex differences in the locomotor response and behavioral sensitization to cocaine in hyperactive rats. Brain Res. 1999 Sep 18;842(1):200–205. doi: 10.1016/s0006-8993(99)01742-4. [DOI] [PubMed] [Google Scholar]
- 25.Hu M, Crombag HS, Robinson TE, Becker JB. Biological basis of sex differences in the propensity to self-administer cocaine. Neuropsychopharmacology. 2004 Jan;29(1):81–85. doi: 10.1038/sj.npp.1300301. [DOI] [PubMed] [Google Scholar]
- 26.Hu M, Becker JB. Effects of sex and estrogen on behavioral sensitization to cocaine in rats. J Neurosci. 2003 Jan 15;23(2):693–699. doi: 10.1523/JNEUROSCI.23-02-00693.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Febo M, Gonzalez-Rodriguez LA, Capo-Ramos DE, Gonzalez-Segarra NY, Segarra AC. Estrogen-dependent alterations in D2/D3-induced G protein activation in cocaine-sensitized female rats. J Neurochem. 2003 Jul;86(2):405–412. doi: 10.1046/j.1471-4159.2003.01858.x. [DOI] [PubMed] [Google Scholar]
- 28.Puig-Ramos A, Santiago GS, Segarra AC. U-69593, a kappa opioid receptor agonist, decreases cocaine-induced behavioral sensitization in female rats. Behav Neurosci. 2008 Feb;122(1):151–160. doi: 10.1037/0735-7044.122.1.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jackson LR, Robinson TE, Becker JB. Sex differences and hormonal influences on acquisition of cocaine self-administration in rats. Neuropsychopharmacology. 2006 Jan;31(1):129–138. doi: 10.1038/sj.npp.1300778. [DOI] [PubMed] [Google Scholar]
- 30.Harrod SB, Booze RM, Welch M, Browning CE, Mactutus CF. Acute and repeated intravenous cocaine-induced locomotor activity is altered as a function of sex and gonadectomy. Pharmacol Biochem Behav. 2005 Sep;82(1):170–181. doi: 10.1016/j.pbb.2005.08.005. [DOI] [PubMed] [Google Scholar]
- 31.Harrod SB, Mactutus CF, Browning CE, Welch M, Booze RM. Home cage observations following acute and repeated IV cocaine in intact and gonadectomized rats. Neurotoxicol Teratol. 2005;27(6):891–896. doi: 10.1016/j.ntt.2005.07.004. Nov-Dec. [DOI] [PubMed] [Google Scholar]
- 32.Haney M, Castanon N, Cador M, Le Moal M, Mormede P. Cocaine sensitivity in Roman High and Low Avoidance rats is modulated by sex and gonadal hormone status. Brain Res. 1994 May 9;645:179–185. doi: 10.1016/0006-8993(94)91651-9. (1–2) [DOI] [PubMed] [Google Scholar]
- 33.Chin J, Sternin O, Wu HB, Burrell S, Lu D, Jenab S, et al. Endogenous gonadal hormones modulate behavioral and neurochemical responses to acute and chronic cocaine administration. Brain Res. 2002 Jul 26;945(1):123–130. doi: 10.1016/s0006-8993(02)02807-x. [DOI] [PubMed] [Google Scholar]
- 34.Chen R, Osterhaus G, McKerchar T, Fowler SC. The role of exogenous testosterone in cocaine-induced behavioral sensitization and plasmalemmal or vesicular dopamine uptake in castrated rats. Neurosci Lett. 2003 Nov 20;351(3):161–164. doi: 10.1016/j.neulet.2003.07.018. [DOI] [PubMed] [Google Scholar]
- 35.Caine SB, Bowen CA, Yu G, Zuzga D, Negus SS, Mello NK. Effect of gonadectomy and gonadal hormone replacement on cocaine self-administration in female and male rats. Neuropsychopharmacology. 2004 May 29;(5):929–942. doi: 10.1038/sj.npp.1300387. [DOI] [PubMed] [Google Scholar]
- 36.Menniti FS, Baum MJ. Differential effects of estrogen and androgen on locomotor activity induced in castrated male rats by amphetamine, a novel environment, or apomorphine. Brain Res. 1981 Jul 6;216(1):89–107. doi: 10.1016/0006-8993(81)91280-4. [DOI] [PubMed] [Google Scholar]
- 37.Walker QD, Cabassa J, Kaplan KA, Li ST, Haroon J, Spohr HA, et al. Sex differences in cocaine-stimulated motor behavior: disparate effects of gonadectomy. Neuropsychopharmacology. 2001 Jul;25(1):118–130. doi: 10.1016/S0893-133X(00)00248-7. [DOI] [PubMed] [Google Scholar]
- 38.Forgie ML, Stewart J. Six differences in the locomotor-activating effects of amphetamine: role of circulating testosterone in adulthood. Physiol Behav. 1994 Apr;55(4):639–644. doi: 10.1016/0031-9384(94)90038-8. [DOI] [PubMed] [Google Scholar]
- 39.Dluzen DE, Green MA, Ramirez VD. The effect of hormonal condition on dose- dependent amphetamine-stimulated behaviors in the male rat. Horm Behav. 1986 Mar;20(1):1–6. doi: 10.1016/0018-506x(86)90024-3. [DOI] [PubMed] [Google Scholar]
- 40.Beatty WW, Dodge AM, Traylor KL. Stereotyped behavior elicited by amphetamine in the rat: influences of the testes. Pharmacol Biochem Behav. 1982 Apr;16(4):565–568. doi: 10.1016/0091-3057(82)90416-6. [DOI] [PubMed] [Google Scholar]
- 41.Long SF, Dennis LA, Russell RK, Benson KA, Wilson MC. Testosterone implantation reduces the motor effects of cocaine. Behav Pharmacol. 1994 Feb;5(1):103–106. doi: 10.1097/00008877-199402000-00012. [DOI] [PubMed] [Google Scholar]
- 42.Robinson TE, Berridge KC. Addiction. Annu Rev Psychol. 2003;54:25–53. doi: 10.1146/annurev.psych.54.101601.145237. [DOI] [PubMed] [Google Scholar]
- 43.Fibiger HC, LePiane FG, Jakubovic A, Phillips AG. The role of dopamine in intracranial self-stimulation of the ventral tegmental area. J Neurosci. 1987 Dec;7(12):3888–3896. doi: 10.1523/JNEUROSCI.07-12-03888.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wise RA. Opiate reward: sites and substrates. Neurosci Biobehav Rev. 1989;13:129–133. doi: 10.1016/s0149-7634(89)80021-1. Summer- Fall. (2–3) [DOI] [PubMed] [Google Scholar]
- 45.Wolf ME, Xue CJ. Amphetamine and D1 dopamine receptor agonists produce biphasic effects on glutamate efflux in rat ventral tegmental area: modification by repeated amphetamine administration. J Neurochem. 1998 Jan;70(1):198–209. doi: 10.1046/j.1471-4159.1998.70010198.x. [DOI] [PubMed] [Google Scholar]
- 46.Chen JC, Chen PC, Chiang YC. Molecular mechanisms of psychostimulant addiction. Chang Gung Med J. 2009;32(2):148–154. Mar-Apr. [PubMed] [Google Scholar]
- 47.McGinnis MY, Dreifuss RM. Evidence for a role of testosterone-androgen receptor interactions in mediating masculine sexual behavior in male rats. Endocrinology. 1989 Feb;124(2):618–626. doi: 10.1210/endo-124-2-618. [DOI] [PubMed] [Google Scholar]
- 48.Baum MJ, Melamed E, Globus M. Dissociation of the effects of castration and testosterone replacement on sexual behavior and neural metabolism of dopamine in the male rat. Brain Res Bull y1986. Feb;16(2):145–148. doi: 10.1016/0361-9230(86)90025-0. [DOI] [PubMed] [Google Scholar]
- 49.Martinez-Sanchis S, Aragon CM, Salvador A. Cocaine-induced locomotor activity is enhanced by exogenous testosterone. Physiol Behav. 2002 Aug;76:605–609. doi: 10.1016/s0031-9384(02)00764-3. (4–5) [DOI] [PubMed] [Google Scholar]
- 50.Febo M, Jimenez-Rivera CA, Segarra AC. Estrogen and opioids interact to modulate the locomotor response to cocaine in the female rat. Brain Res. 2002 Jul 5;943(1):151–161. doi: 10.1016/s0006-8993(02)02748-8. [DOI] [PubMed] [Google Scholar]
- 51.Li Y, Acerbo MJ, Robinson TE. The induction of behavioural sensitization is associated with cocaine-induced structural plasticity in the core (but not shell) of the nucleus accumbens. Eur J Neurosci. 2004 Sep;20(6):1647–1654. doi: 10.1111/j.1460-9568.2004.03612.x. [DOI] [PubMed] [Google Scholar]
- 52.Izenwasser S, French D, Carroll FI, Kunko PM. Continuous infusion of selective dopamine uptake inhibitors or cocaine produces time-dependent changes in rat locomotor activity. Behav Brain Res. 1999 Mar;99(2):201–208. doi: 10.1016/s0166-4328(98)00104-1. [DOI] [PubMed] [Google Scholar]
- 53.Inada T, Polk K, Purser C, Hume A, Hoskins B, Ho IK, et al. Behavioral and neurochemical effects of continuous infusion of cocaine in rats. Neuropharmacology. 1992 Jul;31(7):701–708. doi: 10.1016/0028-3908(92)90149-j. [DOI] [PubMed] [Google Scholar]
- 54.Reith ME, Benuck M, Lajtha A. Cocaine disposition in the brain after continuous or intermittent treatment and locomotor stimulation in mice. J Pharmacol Exp Ther. 1987 Oct;243(1):281–287. [PubMed] [Google Scholar]
- 55.Johansson EK, Tucker SM, Ginn HB, Martin BR, Aceto MD. Functional and Pharmacokinet. 1992;17(2):155–162. doi: 10.1007/BF03188784. Apr-Jun. [DOI] [PubMed] [Google Scholar]
- 56.King GR, Joyner C, Lee T, Kuhn C, Ellinwood EH., Jr Intermittent and continuous cocaine administration: residual behavioral states during withdrawal. Pharmacol Biochem Behav. 1992 Sep;43(1):243–248. doi: 10.1016/0091-3057(92)90664-2. [DOI] [PubMed] [Google Scholar]
- 57.Paulson PE, Camp DM, Robinson TE. Time course of transient behavioral depression and persistent behavioral sensitization in relation to regional brain monoamine concentrations during amphetamine withdrawal in rats. Psychopharmacology (Berl) 1991;103(4):480–492. doi: 10.1007/BF02244248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kolta MG, Shreve P, De Souza V, Uretsky NJ. Time course of the development of the enhanced behavioral and biochemical responses to amphetamine after pretreatment with amphetamine. Neuropharmacology. 1985 Sep;24(9):823–829. doi: 10.1016/0028-3908(85)90032-2. [DOI] [PubMed] [Google Scholar]
- 59.Robinson TE, Becker JB. Enduring changes in brain and behavior produced by chronic amphetamine administration: a review and evaluation of animal models of amphetamine psychosis. Brain Res. 1986 Jun;396(2):157–198. doi: 10.1016/s0006-8993(86)80193-7. [DOI] [PubMed] [Google Scholar]
- 60.Kalivas PW, Duffy P. Time course of extracellular dopamine and behavioral sensitization to cocaine. I. Dopamine axon terminals. J Neurosci. 1993 Jan;13(1):266–275. doi: 10.1523/JNEUROSCI.13-01-00266.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Partridge B, Schenk S. Context-independent sensitization to the locomotor-activating effects of cocaine. Pharmacol Biochem Behav. 1999 Aug;63(4):543–548. doi: 10.1016/s0091-3057(99)00013-1. [DOI] [PubMed] [Google Scholar]
- 62.Chefer VI, Czyzyk T, Bolan EA, Moron J, Pintar JE, Shippenberg TS. Endogenous kappa-opioid receptor systems regulate mesoaccumbal dopamine dynamics and vulnerability to cocaine. J Neurosci. 2005 May 18;25(20):5029–5037. doi: 10.1523/JNEUROSCI.0854-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Heidbreder CA, Shippenberg TS. Evidence for an involvement of muscarinic cholinergic systems in the induction but not expression of behavioral sensitization to cocaine. Synapse. 1996 Oct;24(2):182–192. doi: 10.1002/(SICI)1098-2396(199610)24:2<182::AID-SYN10>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 64.Shippenberg TS, Rea W, Slusher BS. Modulation of behavioral sensitization to cocaine by NAALADase inhibition. Synapse. 2000 Nov;38(2):161–166. doi: 10.1002/1098-2396(200011)38:2<161::AID-SYN7>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 65.Sabeti J, Gerhardt GA, Zahniser NR. Individual differences in cocaine-induced locomotor sensitization in low and high cocaine locomotor-responding rats are associated with differential inhibition of dopamine clearance in nucleus accumbens. J Pharmacol Exp Ther. 2003 Apr;305(1):180–190. doi: 10.1124/jpet.102.047258. [DOI] [PubMed] [Google Scholar]
- 66.Gordon LA, Mostofsky DI, Gordon GG. Changes in testosterone levels in the rat following intraperitoneal cocaine HCl. Int J Neurosci. 1980;11(2):139–141. doi: 10.3109/00207458009150338. [DOI] [PubMed] [Google Scholar]
- 67.Berul CI, Harclerode JE. Effects of cocaine hydrochloride on the male reproductive system. Life Sci. 1989;45(1):91–95. doi: 10.1016/0024-3205(89)90440-2. [DOI] [PubMed] [Google Scholar]
- 68.Kalivas PW, Volkow ND. The neural basis of addiction: a pathology of motivation and choice. Am J Psychiatry. 2005 Aug;162(8):1403–1413. doi: 10.1176/appi.ajp.162.8.1403. [DOI] [PubMed] [Google Scholar]
- 69.Chefer VI, Zakharova I, Shippenberg TS. Enhanced responsiveness to novelty and cocaine is associated with decreased basal dopamine uptake and release in the nucleus accumbens: quantitative microdialysis in rats under transient conditions. J Neurosci. 2003 Apr 1;23(7):3076–3084. doi: 10.1523/JNEUROSCI.23-07-03076.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sabeti J, Gerhardt GA, Zahniser NR. Acute cocaine differentially alters accumbens and striatal dopamine clearance in low and high cocaine locomotor responders: behavioral and electrochemical recordings in freely moving rats. J Pharmacol Exp Ther. 2002 Sep;302(3):1201–1211. doi: 10.1124/jpet.102.035816. [DOI] [PubMed] [Google Scholar]
- 71.Kindlundh AM, Lindblom J, Bergstrom L, Wikberg JE, Nyberg F. The anabolic- androgenic steroid nandrolone decanoate affects the density of dopamine receptors in the male rat brain. Eur J Neurosci. 2001 Jan;13(2):291–296. doi: 10.1046/j.0953-816x.2000.01402.x. [DOI] [PubMed] [Google Scholar]
- 72.Kindlundh AM, Rahman S, Lindblom J, Nyberg F. Increased dopamine transporter density in the male rat brain following chronic nandrolone decanoate administration. Neurosci Lett. 2004 Feb 12;356(2):131–134. doi: 10.1016/j.neulet.2003.11.040. [DOI] [PubMed] [Google Scholar]
- 73.Belin D, Deroche-Gamonet V, Jaber M. Cocaine-induced sensitization is associated with altered dynamics of transcriptional responses of the dopamine transporter, tyrosine hydroxylase, and dopamine D2 receptors in C57Bl/6J mice. Psychopharmacology (Berl) 2007 Sep;193(4):567–578. doi: 10.1007/s00213-007-0790-3. [DOI] [PubMed] [Google Scholar]
- 74.Burchett SA, Bannon MJ. Serotonin, dopamine and norepinephrine transporter mRNAs: heterogeneity of distribution and response to ‘binge’ cocaine administration. Brain Res Mol Brain Res. 1997 Oct 3;49:95–102. doi: 10.1016/s0169-328x(97)00131-9. (1–2) [DOI] [PubMed] [Google Scholar]
- 75.Letchworth SR, Daunais JB, Hedgecock AA, Porrino LJ. Effects of chronic cocaine administration on dopamine transporter mRNA and protein in the rat. Brain Res. 1997 Mar 7;750:214–222. doi: 10.1016/s0006-8993(96)01384-4. (1–2) [DOI] [PubMed] [Google Scholar]